Abstract
BACKGROUND:
Inconsistent associations between modifiable risk factors and chronic back pain (CBP) may be due to the inability of traditional epidemiologic study designs to properly account for an array of potential genetic and environmental confounding factors. The co-twin control research design, comparing modifiable risk factors in twins discordant for CBP, offers a unique way to remove numerous confounding factors.
PURPOSE:
To examine the association of modifiable lifestyle and psychological factors with lifetime CBP.
STUDY DESIGN/SETTING:
Cross-sectional co-twin control study in a nationwide sample of male twin members of the Vietnam Era Twin Registry.
PATIENT SAMPLE:
7,108 participants including 1,308 monozygotic (MZ) pairs and 793 dizygotic pairs.
OUTCOME MEASURE:
Self-reported lifetime history of CBP.
METHODS:
Lifestyle factors included body mass index (BMI), smoking history, alcohol consumption, habitual physical activity, and typical sleep duration. Psychological factors included depression (Patient Health Questionnaire-9 [PHQ9]) and post-traumatic stress disorder (PTSD) symptoms (PTSD Checklist [PCL]). Covariates included age, race, education, and income. Odds ratios (ORs) and 95% confidence intervals (CI) were estimated for the association of risk factors with lifetime CBP when considering twins as individuals, and a within-pair co-twin control analysis that accounted for familial and genetic factors. Funding was through VA grant 5IK2RX001515; there were no study-specific conflicts of interest.
RESULTS:
The mean age of respondents was 62 years and the prevalence of lifetime CBP was 28%. All lifestyle factors were associated with CBP in the individual level analysis. However none of these persisted in the within-pair analyses, except for severe obesity (BMI≥35.0) which was associated with lifetime CBP in both individual-level (OR = 1.6, 95% CI 1.3–1.9) and within-pair analyses (MZ analysis: OR = 3.7, 95% CI 1.2–11.4). PTSD and depression symptoms were strongly associated with lifetime CBP in both the individual-level (moderate/severe depression: OR = 4.2, 95% CI 3.6–4.9 and severe PTSD: OR = 4.8, 95% CI 4.0–5.7) and within-pair (MZ) analyses (moderate/severe depression: OR = 4.6, 95% CI 2.4–8.7 and severe PTSD: OR = 3.2, 95% CI 1.6–6.5).
CONCLUSIONS:
Many associations between modifiable lifestyle risk factors and chronic back pain are due to confounding by familial and genetic factors. Severe obesity, depression, and PTSD should be considered in the development of intervention strategies to reduce the prevalence of chronic back pain.
Keywords: twins, obesity, activity, sleep, smoking, alcohol, musculoskeletal diseases, low back pain, depression, stress disorders
INTRODUCTION
Back pain causes more years lived with disability than any other health condition worldwide.1 Although most adults experience a new (‘acute’) episode of back pain at some point in their lives, the societal burden of back pain is driven by the minority of individuals who do not recover, and go on to develop ‘chronic’ back pain.2 Chronic back pain is most commonly defined as back pain of duration ≥3 months, or ≥6 months.3
Meta-analysis of 11 back pain twin studies using the MaTCH (Meta-analysis of Twin Correlations and Heritability) web application indicate a heritability of 40%, with a pattern of monozygotic (rMZ = 0.56) and dizygotic (rDZ = 0.28) twin correlations indicating an additive genetic model (2rDZ = rMZ).4,5 Back pain heritability appears to be greatest for phenotypes reflecting greater severity as defined by frequency, duration, or functional impact.6 Nevertheless, genetic studies to date attempting to identify specific genetic markers for back pain have been limited to selected candidate genes, 7,8 and no large-scale genome-wide association studies (GWAS) of back pain have been conducted. GWAS may provide insights into the underlying biology of chronic back pain through mechanisms such as intervertebral disc degeneration, pain neurotransmission involving the central and peripheral nervous system, or other processes which have been conceptually linked to back pain without an understanding of the specific mechanisms involved (e.g inflammation,9 endocrine factors,10 vascular disease11,12).
We conducted a meta-analysis of GWAS of chronic back pain in adults of European ancestry from 11 population-based cohort studies from Europe and the United States (US). The aim of the current study was to examine associations between specific genetic markers and chronic back pain.
MATERIAL AND METHODS
Study Populations
The study sample includes members of the Vietnam Era Twin (VET) Registry. The VET Registry was initially constructed in the 1980s from computerized military discharge records. It is a national sample of more than 7,000 male twin pairs from all military branches who served on active duty during the Vietnam era (1965–1975) and were born between 1939 and 1957.[12] The VET Registry was not compiled based on medical or psychiatric diagnoses, health-seeking behaviors, or military service characteristics aside from military discharge. Further details of the VET Registry construction and the zygosity ascertainment process are described elsewhere.[13–15]
Between 2010 and 2012, all living VET Registry members were invited to participate in an observational study of PTSD among Veterans (Cooperative Studies Program #569: The Course and Consequences of PTSD in Vietnam Era Twins, or ‘CSP #569’). This included a mailed questionnaire obtaining information regarding mental and physical health conditions, including back pain. Informed consent was obtained from all participating VET Registry members as part of the parent study CSP #569, and this ancillary study was approved by the Veterans’ Affairs (VA) Puget Sound Institutional Review Board.
Assessment of Potentially Modifiable Lifestyle Factors
We identified potentially modifiable lifestyle factors as risk factors for chronic back pain based on prior literature.[16, 17] Wherever possible, we categorized each lifestyle-related factor using cut points from clinical or public health guidelines. Respondents reported their current height in inches and weight in pounds. BMI was calculated as weight (converted into kilograms) divided by the square of height (converted into meters), and categorized based on the Classification of Overweight and Obesity by the National Heart Lung and Blood institute: Underweight/Normal (BMI <25.0 kg/m2), Overweight (BMI 25.0–29.9 kg/m2), Obesity I (BMI 30.0–34.9 kg/m2), Obesity II (BMI 35.0–39.9 kg/m2), and Obesity III (BMI 40.0+)[18]. For analytic purposes, the categories of Obesity II and Obesity III were combined due to the low number of participants in the Obesity III category. Respondents reported whether they had smoked at least 100 cigarettes during their lifetime, and if so, whether they smoked cigarettes currently. We classified smoking into three mutually exclusive categories: current cigarette smoking, past cigarette smoking (without current or recent use), and never having smoked cigarettes[19]. Physical activity (PA) was assessed using validated survey items from the Behavioral Risk Factor Surveillance Study (BRFSS), which inquire about typical weekly time spent on moderate activities (such as brisk walking, bicycling, vacuuming, gardening, or anything else that causes some increase in breathing or heart rate) and vigorous activities (running, aerobics, heavy yard work, or anything else that causes large increases in breathing or heart rate). These items are reliable and have shown concurrent validity when compared to combined heart rate monitoring with accelerometry[20] and activity logs[21]. We derived ‘moderate-intensity equivalent minutes’ per week using a method applied in recent studies, [22–24] which calculates moderate-intensity equivalent minutes per week (min/wk) by multiplying the minutes of vigorous PA min/wk by 2, and then adds that quantity to moderate PA min/wk to create a composite measure[24]. We then classified moderate-intensity equivalent min/wk into categories based on the 150 min/wk and 300 min/wk recommendations from the 2008 Physical Activity Guidelines for Americans [25]. Due to the large proportion of participants exceeding the minimum recommendations for weekly PA according to the 2008 Guidelines, and in order to have a sufficient number of PA categories to detect possible U-shaped relationships between PA and back pain[26],we defined two separate categories for higher levels of PA activity beyond 300 moderate-intensity equivalent min/wk, based on the distribution of the data. The categories used in the analysis included inactivity (<150 min/wk), low activity (150–299 min/wk), moderate activity (300–899 min/wk), and high activity (≥900 min/wk). Respondents reported on current alcohol consumption as the average number of drinks of an alcoholic beverage consumed in a typical day. We categorized alcohol consumption as no drinks daily, ≤2 drinks daily, or ≥3 drinks daily, based on the definition of moderate alcohol consumption by the National Institute on Alcohol Abuse and Alcoholism[27]. Respondents reported the average number of hours they sleep in a typical night. We categorized sleep duration using common cut points from the literature, including <6 hours/night, 6 to <7 hours/night, 7 to <9 hours/night, and 9+ hours/night. We chose 7–9 hours of sleep per night as the reference category for sleep duration based on the 2015 recommendations of the American Academy of Sleep Medicine and the Sleep Research Society[28].
Assessment of potentially modifiable psychological factors
We assessed current depression symptoms using the Patient Health Questionnaire Depression subscale (PHQ-9), a 9-item measure of depression that grades the frequency of 9 depression symptoms over the past two weeks on a Likert scale.[29] PHQ-9 scores are summed to produce a continuous measure, with scores ranging from 0 (no depression symptoms) to 27 (severe depression symptoms). The PHQ-9 has high test-retest reliability (r=0.84) and internal consistency (α=0.86–89)[29]. Cut points of 5, 10, 15, and 20 reflecting mild, moderate, moderately severe, and severe depressive symptoms are commonly used for research purposes, and a PHQ-9 cut point of 10 has high sensitivity (88%) and specificity (88%) for a diagnosis of major depression[29]. For this analysis, we classified depression symptoms as ‘no depression’ (PHQ-9 continuous score <5), ‘mild depression’ (PHQ-9 continuous score ≥5 and <10) and ‘moderate/severe depression’ (PHQ-9 ≥ 10).
We assessed current PTSD symptoms using the PTSD Checklist Civilian version (PCL).[30] The PCL consists of 17 Likert items that correspond to PTSD symptoms from the Diagnostic and Statistical Manual of Mental Disorders. For each item, respondents report the degree to which they were bothered by symptoms in the past month, ranging from 1 (not at all) to 5 (extremely). Individual PCL items are summed to produce a continuous summary severity score. The PCL has high test-retest reliability (r=0.96 [31]), internal consistency (α=0.96 [32]), and concurrent validity in Veterans when compared to a formal diagnosis of PTSD.[33–35] We used two different PCL cut points at 30 and 50 to define categories of no PTSD symptoms, low-to-moderate PTSD symptoms, and high PTSD symptoms. These values reflect the lowest and highest extremes among commonly used PCL cut points to diagnose PTSD according to the U.S. National Center for PTSD [36], and have been previously validated within the CSP #569 sample as compared to a current PTSD diagnosis[35].
Assessment of Lifetime Chronic Back Pain
Participants reported on whether they had ever experienced chronic back pain, without specification as to the location of pain in the back (lumbar vs. thoracic), pain duration, or pain frequency. The study outcome was participant report of having ever had chronic back pain (‘lifetime chronic back pain’).
Assessment of Potential Confounders
For the primary analysis, we considered as potential confounders selected variables with a conceptual rationale for being associated both with the modifiable risk factors of interest and chronic back pain, but which were unlikely to be on the theoretical causal pathway between the risk factors and chronic back pain (allowing for possible bidirectional effects), or to be a consequence of the risk factors or chronic back pain. These potential confounders included age, race, educational attainment, and household income. Race data obtained from military records were classified as white vs. non-white. Participants reported on educational attainment as the highest grade or year of school completed, and whether degrees were obtained at various educational levels. Educational attainment was categorized as ‘did not complete high school’, ‘high school graduate’, ‘other studies or training beyond college’, and ‘completed 4-year college degree or attended graduate studies’. Participants reported their estimated total combined family income in the prior 12 months before taxes and deductions, choosing from 7 categories ranging from <$15,000 to ≥$150,000. For analytic purposes, income was dichotomized at a cut point of $50,000, corresponding to the median household income in the United States in 2011[37].
Statistical Analysis and Interpretation
We characterized the sample descriptively according to lifetime chronic back pain. Test statistics for the descriptive analysis accounted for clustering due to twinship. We then applied statistical methods for co-twin control studies to examine relationships between potentially modifiable risk factors and lifetime chronic back pain. [10] This modeling approach first treats the twins as individuals, while accounting for the clustered data structure of twin pairs using generalized estimating equations (GEE). This ‘individual-level’ analysis produces measures of association equivalent to that seen in any conventional analysis of unrelated singletons. Next, we perform within-pair analyses of the modifiable risk factors and chronic back pain using conditional logistic regression, restricted to twin pairs discordant for lifetime chronic back pain. This analysis is stratified by twin zygosity to obtain separate estimates of odds ratios for each association in MZ and dizygotic (DZ) twin pairs. The ‘within-MZ-pair’ analysis adjusts completely for genetic and early ‘shared’ family environment common to both twins. The ‘within-DZ-pair analysis’ also adjusts for shared early family environment, but in contrast to the within-MZ-pair analysis, adjusts only for 50% of genetic factors on average. In the individual-level analysis we calculated odds ratios (ORs) and 95% confidence intervals from GEE models for the association of modifiable risk factors with lifetime chronic back pain, after adjusting for the potential confounding influence of age, race, education, and income. In the within-pair analyses we used conditional logistic regression models for matched data; in these models we only adjusted for the confounding factors education and income since age and race were intrinsically adjusted by the co-twin control design.
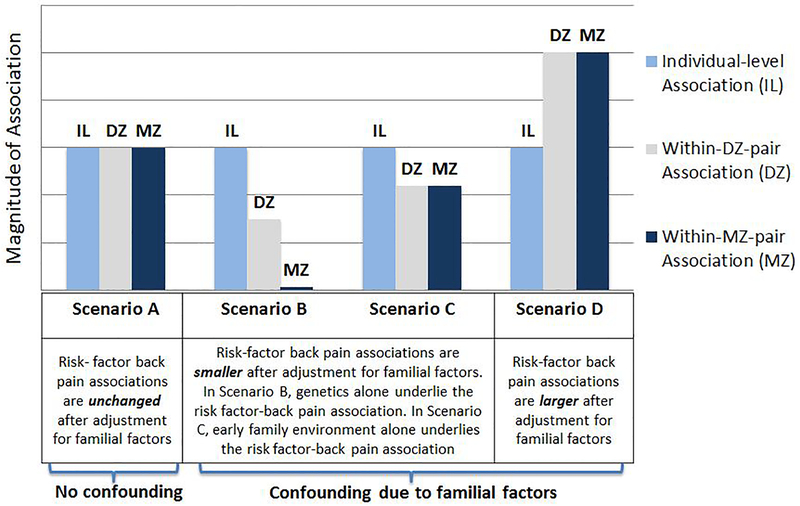
The degree to which within-pair-MZ and within-pair--DZ estimates differ from the individual-level estimates of association can be used to infer the source of confounding as either due to shared genetic factors, early environmental factors, or both[10]. If individual-level, within-DZ-pair, and within-MZ-pair associations are all of similar magnitude, this implies no or minimal confounding due to family factors (Figure 1, Scenario A). If shared genetic factors alone play a major role in the risk factor-back pain association, associations in both the within-MZ-pair and within-DZ-pair analyses will be smaller in magnitude compared with the individual-level analyses, but these changes would be more pronounced in the within-MZ-pair analysis due to the MZ twins’ matched genetics[38] (Figure 1, Scenario B). If early family environment alone plays a major role in the risk factor-back pain association, associations in both the within-MZ-pair and within-DZ-pair analyses will be smaller in magnitude compared with the individual-level analyses (Figure 1, Scenario C), and these differences will be of a generally similar degree since shared early family environmental characteristics are considered to be comparable for DZ twins raised together and MZ twins raised together[38]. In some situations, confounding due to shared genetics and early environmental factors may both be present, and to varying degrees, leading to patterns of risk factor-back associations which combine aspects of both Scenarios B and C. In some instances, adjustment for confounding due to familial factors can even result in larger magnitude associations in within-pair analyses as compared to individual-level analyses[39, 40] (Figure 1, Scenario D).
Figure 1.
Examples of Possible Scenarios of Confounding of Risk Factor-Back Pain Associations due to Familial Factors*. *The term ‘familial factors’ includes both genetic factors and early family environmental factors.
RESULTS
Characteristics of the study sample
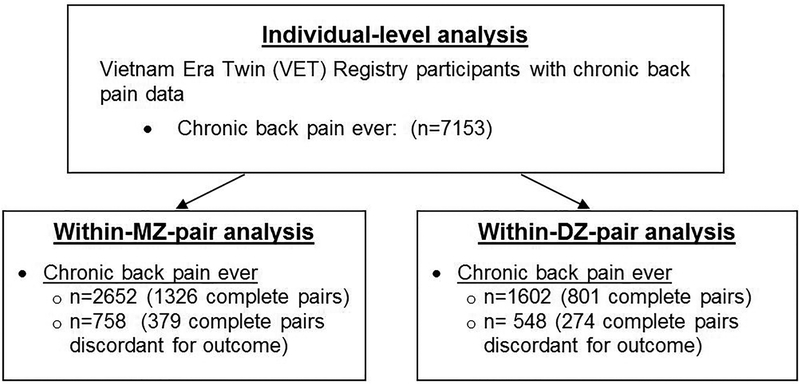
Figure 2 depicts a flowchart of participation in the study. The mean age of study participants was 61.5 years and the lifetime prevalence of chronic back pain was 27.8% (95% CI 26.8–28.9). 25% (95% CI 24.0–26.1) of participants were users of VA health care. As compared to participants without back pain, those reporting lifetime chronic back pain had lower levels of income and education, and were more likely to have ever used VA health care or report ever having applied for VA disability compensation (Table 1). There were significant differences between participants with and without lifetime chronic back pain with respect to all the modifiable risk factors examined, including those with back pain being generally more likely to have severe obesity (BMI ≥35), be a current or past smoker, not drink alcohol, be physically inactive, sleep less than 6 hours a night, and have symptoms of depression or PTSD (Table 1).
Figure 2.
Co-twin control analysis of potentially modifiable risk factors and lifetime chronic back pain
Table 1.
Distribution of sociodemographics and modifiable risk factors according to lifetime chronic back pain*
| Characteristics | Lifetime Chronic Back Pain | ||
|---|---|---|---|
| No (n=5163) |
Yes (n=1990) |
p value* | |
| Sociodemographics | |||
| Age (mean) | 61.7 | 61.0 | <0.001 |
| Race (%) | |||
| White | 93.1% | 92.6% | 0.50 |
| Non-white | 6.9% | 7.4% | |
| Educational attainment | |||
| Did not graduate high school | 4.5% | 8.9% | <0.001 |
| High school graduate | 24.5% | 25.7% | |
| Some college/vocational schoola | 44.2% | 45.0% | |
| Completed college or further studyb | 26.7% | 20.4% | |
| Income (annual family income) | |||
| $50,000 or more | 55.7% | 43.7% | <0.001 |
| VA health care user ever | 23.5% | 35.2% | <0.001 |
| Ever applied for VA disability compensation, for any health condition | 19.8% | 36.8% | <0.001 |
| Modifiable Lifestyle-Related and Psychological Risk Factors | |||
| Body Mass Index | |||
| Normal | 22.1% | 20.3% | <0.001 |
| Overweight | 46.7% | 44.5% | |
| Obesity I | 22.4% | 23.1% | |
| Obesity II/III | 8.8% | 12.2% | |
| Smoking | |||
| Never smoked | 35.4% | 28.0% | |
| Former smoker | 44.7% | 45.3% | <0.001 |
| Current smoker | 20.0% | 26.8% | |
| Alcohol (typical # of drinks per day) | |||
| Never or no current alcohol | 36.2% | 43.6% | <0.001 |
| <2 drinks in typical day | 43.3% | 36.4% | |
| 3 or more drinks | 20.5% | 19.9% | |
| Physical activity [PA] (by moderate-intensity equivalent min/wk) | |||
| Inactive (<150 min/wk) | 32.1% | 39.9% | <0.001 |
| Low PA (150–299 min/wk) | 14.8% | 12.6% | |
| Moderate PA (300–899 min/wk) | 23.2% | 20.4% | |
| High PA >900 min/wk) | 29.9% | 27.1% | |
| Sleep | |||
| <6 hours/night | 25.0% | 40.7% | <0.001 |
| 6 to <7 hours/night | 25.7% | 22.5% | |
| 7 to <9 hours/night [ref] | 45.8% | 32.9% | |
| 9+ hours/night | 3.5% | 3.8% | |
| Depression | |||
| No depression (PHQ<5.0) | 77.6% | 49.0% | |
| Mild Depression (PHQ ≥5.0 and <10.0) | 13.1% | 23.5% | <0.001 |
| Moderate/Severe Depression (PHQ≥10.0) | 9.3% | 27.5% | |
| PTSD | |||
| No PTSD (PCL<30) | 80.1% | 53.6% | <0.001 |
| Low PTSD symptoms (PCL ≥30 and <50) | 14.3% | 26.3% | |
| Severe PTSD symptoms(PCL ≥50) | 5.7% | 20.1% | |
VA= Veterans Affairs, min=minutes, wk=week
p-values adjusted to account for clustering by twinship
Including some college without completion, or having completed vocational school, technical school, or a 2-year college degree
attended and/or completed graduate school\
Co-twin control analysis of potentially modifiable lifestyle factors and lifetime chronic back pain
BMI was significantly associated with chronic back pain (p <0.001, individual-level) and this association persisted in the within-pair-DZ (p=0.02) and MZ (p= 0.02) analysis. Individuals with severe obesity (BMI ≥35) were 1.6 times more likely to report lifetime chronic back pain (95% CI 1.3–1.9) compared to individuals with normal BMI (Table 2). The magnitude of the association between severe obesity and lifetime chronic back pain was substantially larger in the within-DZ-pair (OR 3.7, 95% CI 1.6–8.6) and within-MZ-pair (OR 3.7, 95% CI 1.2–11.4) analyses than seen in the individual-level analysis.
Table 2.
Multivariate-adjusted co-twin control analyses of associations between modifiable risk factors and lifetime chronic back pain*
| Risk Factor | Individual-level analysis | Within-DZ-pair analysis | Within-MZ-pair analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Lifestyle-Related Factors | ||||||
| Body Mass Index | n=6340 | n=436 (218 pairs) | n=616 (308 pairs) | |||
| Normal [ref.] (BMI 18.5–24.9) | 1.0 | 1.0 | 1.0 | |||
| Overweight (BMI 25.0–29.9) | 1.1 (1.0–1.3) | 2.0 (1.1–3.6) | 0.8 (0.5–1.3) | |||
| Obesity I (BMI 30.0– 34.9) | 1.2 (1.0–1.4) | 1.9 (0.9–3.9) | 1.0 (0.5–2.1) | |||
| Obesity II/III (BMI ≥ 35.0) | 1.6 (1.3–1.9) | <0.001 | 3.7 (1.6–8.6) | 0.02 | 3.7 (1.2–11.4) | 0.02 |
| Smoking | n=6343 | n=446 (223 pairs) | n=618 (309 pairs) | |||
| Never smoked [ref.] | 1.0 | 1.0 | 1.0 | |||
| Former smoker | 1.2 (1.1–1.4) | 1.1 (0.7–2.0) | 0.9 (0.5–1.5) | |||
| Current Smoker | 1.4 (1.2–1.6) | 0.004 | 1.1 (0.6–2.1) | 0.91 | 1.1 (0.6–2.0) | 0.80 |
| Alcohol consumption | n=6288 | n=442 (221 pairs) | n=606 (303 pairs) | |||
| None [ref.] | 1.0 | 1.0 | 1.0 | |||
| ≤2 drinks/day | 0.8(0.7–0.9) | 0.8 (0.5–1.3) | 0.7 (0.5–1.1) | |||
| ≥3 drinks/day | 0.8 (0.7–0.9) | 0.003 | 0.8 (0.4–1.4) | 0.57 | 0.7 (0.4–1.2) | 0.27 |
| Physical Activity (PA) | n=6181 | n=402 (201 pairs) | n=606 (303 pairs) | |||
| Inactive [ref.] (<150 min/wk) | 1.0 | 1.0 | 1.0 | |||
| Low PA (150–299 min/wk) | 0.8 (0.7–0.9) | 0.5 (0.2–1.0) | 0.8 (0.5–1.4) | |||
| Moderate PA (300–899 min/wk) | 0.8 (0.7–0.9) | 1.0 (0.6–1.8) | 0.7 (0.4–1.1) | |||
| High PA (>900 min/wk) | 0.8 (0.7–0.9) | 0.002 | 0.8 (0.5–1.4) | 0.15 | 1.0 (0.7–1.6) | 0.38 |
| Sleep | n=6357 | n=446 (223 pairs) | n=616 (308 pairs) | |||
| <6 hours/night | 1.9 (1.7–2.2) | 1.5 (0.9–2.5) | 1.5 (0.9–2.4) | |||
| 6 to <7 hours/night | 1.2 (1.0–1.4) | 1.1 (0.7–1.8) | 0.8 (0.6–1.2) | |||
| 7 to <9 hours/night [ref.] | 1.0 | 1.0 | 1.0 | |||
| ≥9 hours/night | 1.4 (1.0–1.8) | <0.001 | 1.8 (0.7– 4.9) | 0.40 | 1.2 (0.5–3.2) | 0.11 |
| Psychological Factors | ||||||
| Depression | n=6317 | n=444 (222 pairs) | n=616 (308 pairs) | |||
| No depression (PHQ<5.0) [ref.] | 1.0 | 1.0 | 1.0 | |||
| Mild Depression (PHQ ≥5.0 and <10.0) | 2.7 (2.3–3.1) | 2.4 (1.4–3.9) | 2.5 (1.5–4.1) | |||
| Moderate and Severe (PHQ ≥10.0) | 4.2 (3.6–4.9) | <0.001† | 2.9 (1.4–6.0) | <0.001† | 4.6 (2.4–8.7) | <0.001† |
| PTSD | n=6457 | n=456 (228 pairs) | n=632 (316 pairs) | |||
| No PTSD (PCL<30) [ref.] | 1.0 | 1.0 | 1.0 | |||
| Mild/Moderate PTSD symptoms (PCL ≥30 and <50) | 2.5 (2.1–2.9) | 1.9 (1.2–3.2) | 2.6 (1.6–4.1) | |||
| Severe PTSD symptoms (PCL ≥50) | 4.8 (4.0–5.7) | <0.001† | 1.2 (0.5–2.8) | 0.07† | 3.2 (1.6–6.5) | <0.001† |
Items in bold are statistically significant at p<0.05, ref: reference category, BMI: body mass index, PA= physical activity in moderate-intensity equivalent minutes per week (weekly moderate activity minutes) + (2 × weekly vigorous activity minutes), min=minutes, wk=week
All odds ratios are adjusted for educational attainment and family income. The individual-level analyses are further adjusted for race and age explicitly, and the within-pair analyses intrinsically adjust for age, race, and other factors due to the co-twin control design. The sample sizes indicate # of individuals with complete data for these variables, and within-pair analyses are restricted to pairs with complete data for all variables.
p-for trend
Smoking was significantly associated with lifetime chronic back pain in the individual-level analyses, with current smokers (OR 1.4 [95% CI 1.2–1.6]) and former smokers (OR 1.2 [95% CI 1.1–1.4]) more likely to report lifetime chronic back pain compared to never smokers. However, smoking was not associated with lifetime chronic back pain in within-pair analyses adjusting for familial factors (Table 2). Alcohol consumption was significantly protective of lifetime chronic back pain in the individual-level analysis, with a modestly lower odds of lifetime chronic back pain (OR 0.8 [95% CI 0.7–0.9]) for moderate (≤2 drinks/day) or more than moderate (≥3 drinks/day) drinking. However, these associations were no longer significant in the within-pair analyses, although the magnitude of associations were similar.
PA was significantly associated with lifetime chronic back pain in the individual-level analysis, with PA levels above the recommendations from the 2008 Guidelines (≥150 min/wk) associated with a lower odds of lifetime chronic back pain (OR 0.8 [95% CI 0.7–0.9]) irrespective of the activity category. However, physical activity was not significantly associated with lifetime chronic back pain in the within-pair analyses. In individual-level analyses, typical sleep duration was significantly associated with lifetime chronic back pain, with individuals sleeping <6 hours/night being 1.9 times more likely to have lifetime chronic back pain. Sleep duration, however, was not significantly associated with lifetime chronic back pain after adjustment for familial factors in the within-pair analyses.
Co-twin control analysis of potentially modifiable psychological factors and lifetime chronic back pain
In contrast to the results for lifestyle risk factors, the psychological risk factors of depression and PTSD were consistently associated with chronic back pain in the individual-level (p < 0.001) and within-pair analyses (p < 0.001 for both MZs and DZs) (Table 2). Depression and PTSD showed significant and large magnitude associations with lifetime chronic back pain in the within-MZ-pair analysis, including for moderate/severe depression (PHQ ≥10.0: OR 4.6 [95% CI 2.4–8.7]) and severe PTSD (PCL ≥50.0: OR 3.2 [95% CI 1.6–6.5]) symptoms.
DISCUSSION
This study found that most lifestyle-related risk factors were associated with lifetime chronic back pain but that these associations were primarily due to confounding by familial and genetic factors. This suggests that the comorbidity of adverse lifestyle and chronic back pain may in part be a result of shared familial predispositions, rather than to a causal link. An exception to this pattern was the association of severe obesity (BMI ≥35 kg/m2) with lifetime chronic back pain, which persisted even after adjustment for familial factors. In contrast to lifestyle factors, the potentially modifiable psychological factors of PTSD and depression were strongly associated with chronic back pain even after accounting for familial factors. This indicates that the co-occurrence of PTSD or depression with chronic back pain is likely not explained by underlying genetic or early environmental factors predisposing individuals to both mental health problems and chronic pain.
Obesity predicts back pain in some but not all population-based longitudinal studies, but a causal link has been called into question[8, 41]. A non-causal relationship between obesity and back pain is supported by the results of several prior co-twin control studies, which have yielded non-significant results in both cross-sectional [8, 41–43] and longitudinal[43] within-MZ-pair analyses. Perhaps our findings are due to the relatively large proportion of individuals (10%) who were classified as severely obese (BMI ≥35 kg/m2), a category not included in previous studies of European twins. It is possible that there is a threshold of BMI beyond which the development or maintenance of chronic back pain is substantially facilitated. A link between severe obesity and back pain could be explained by a variety of causes including mechanical stresses from excess weight[44], systemic effects from low-grade inflammation from adipose tissue [45], or mediation by other factors such as depression or other chronic conditions. Additional longitudinal studies are still needed to ascertain what component of the obesity-back pain association is due to obesity occurring as a consequence, rather than a cause, of chronic back pain.
Our results showing that the smoking-back pain association disappears after adjustment for familial factors is in accordance with prior co-twin control analyses,[46–49] and argues against a causal link between smoking and back pain. Our individual-level analyses of alcohol consumption suggested a small protective effect of alcohol consumption, as has occasionally been noted previously [43, 50], but this association was no longer present after adjustment for familial factors. Overall, co-twin control results for the association of smoking and alcohol consumption with chronic back pain are consistent with a recent report suggesting no protective effect of healthy lifestyle behaviors on the prevention of chronic back pain in men[16]. To our knowledge, this is the first co-twin control study examining the association of typical sleep duration with chronic back pain. However, our results do not suggest sleep as an important lifestyle risk factor for chronic back pain.
Studies of singletons and co-twin control studies have yielded conflicting findings on whether PA increases or decreases the likelihood of back pain outside of occupational settings, and U-shaped associations have also been suggested [26, 39, 47, 51–55]. Furthermore, evidence from randomized controlled trials indicates strong but short-term (<1 year) protective effects of exercise alone[56]. Our individual-level analyses found a lower likelihood of chronic back pain amongst those reporting sufficient weekly PA levels according to the 2008 Guidelines, but these associations were not significant after adjustment for familial factors. Various factors may explain the conflicting PA-back pain relationships described in the literature. First, differential effects on back pain may exist for work-related and leisure-time activity. One possible example of differential effects would be if greater repetitive heavy labor at work was to increase back pain risk (depending on job-related characteristics), but greater leisure-time PA was protective against back pain (depending on the activities in question). This explanation is supported by the tendency for greater activity to be associated with back pain risk in occupational settings, in contrast with occasional protective associations in community-based settings[54]. It is important to note that prior randomized trials indicating beneficial effects of exercise included various types of exercise interventions, but all would have been classified as leisure-time PA, not work-related PA [56]. Alternatively, whether activities during work or leisure-time have positive or detrimental effects may have more to do with the nature of the specific actions (such as lifting and twisting), positions (such as sitting or standing), cumulative loads, etc. Distinctions between domestic and recreational leisure-time activities may also need to be considered[52]. Finally, individuals with high levels of PA may be more prone to a major physically traumatic injury. Major back injury might confound the relationship between PA and back pain risk in a manner analogous to what is seen with knee injury as a confounder of the effect of PA on knee osteoarthritis risk[57]. Unfortunately, the self-reported BRFSS PA items we used in the current study did not permit separation of work-related activities from domestic and recreational leisure-time activities, or other subtleties related to activity type. A second major hurdle in ascertaining PA is the inaccuracy of self-report. To our knowledge, all prior back pain co-twin control studies of PA and back pain have used self-reported activity. Current technology such as accelerometry and the use of ‘wearable tech’ offer the potential for continuous and objective monitoring, with great improvements over self-report[58]. However, even so, these technologies have limitations yet to be solved, such as discrimination of sitting from standing, distinguishing work from leisure activity, identifying exercise on stationary machines, and others. A greater understanding of the effects of PA on back pain is a vital goal for future research, given the many known health benefits of PA aside from possible effects on pain[59].
In contrast to lifestyle-related factors, the psychological factors of depression and PTSD remained associated with chronic back pain even after adjustment for familial factors. These results are therefore in accordance with the considerable weight placed on psychological factors in risk stratification tools such as StarT Back, where 4 out of 9 items pertain to psychological factors, but none target modifiable lifestyle factors[60]. Ours is the first co-twin control study to demonstrate a significant depression-back pain association after full adjustment for genetic and environmental factors, in contrast to an earlier co-twin control study of middle-aged Spanish twins. The current study may have been better able to detect a depression-back pain association due to our larger sample size, or the discrepant results across studies may have been due to the different study populations. Our results also demonstrate that associations with back pain are of similar magnitude for PTSD and depression. This is not surprising given the known overlap between many of the psychological constructs considered to be predictors of poor back pain outcomes[61], but this overlap may have particular importance when considering the close comorbidity of PTSD and depression. Two major hypotheses have been proposed as possible explanations for this comorbidity, including 1) latent factors common to both conditions (for example, close links between PTSD’s ‘dysphoria’ factor and somatic aspects of depression), or 2) overlapping symptoms between the two conditions (such as impaired sleep or anhedonia) [62]. Given that the scarce prior longitudinal studies of PTSD and back pain suggest an effect on back pain at least as strong as depression[63, 64], an important goal for future research should be greater understanding of the similarities and differences between depression and PTSD as predictors of chronic back pain, especially in populations where PTSD may be prevalent, such as combat exposed veterans.
The co-twin control design is a unique and powerful epidemiologic model for isolating the associations between risk factors and outcomes that exist independent of unknown and known confounders, including familial factors[10]. The vast majority of prior twin studies of back pain have been conducted in European samples, and the current study is one of the first co-twin control studies from the United States. Limitations of our study include its cross-sectional design, which does not allow us to infer temporality. Therefore, where cross-sectional associations are observed, it cannot be known whether a putative risk factor causes back pain, or vice versa, or whether both directions of effect contribute. This shortcoming is most relevant for the putative risk factors studied other than obesity and smoking history, since alcohol consumption, PA, sleep duration, depression, and PTSD were ascertained with respect to current habits or symptoms. Our outcome of lifetime chronic back pain (rather than current back pain) may have increased the likelihood that back pain preceded the risk factor under study, rather than followed it. In addition, this definition did not require any specific minimum duration of pain needed to constitute ‘chronic’, so that some individuals with acute pain (<3–6 months duration) may have self-identified as having had chronic symptoms. Our outcome also did not specify the particular location in the back where pain was experienced (lumbar vs. thoracic). However, based on the generally low frequency of thoracic pain without concurrent lumbar pain[65], and the high percentage of agreement between general back pain questions and lumbar-specific questions[66], we expect that the associations seen in the current study primarily reflect lumbar pain. This is supported by the fact that the individual-level associations seen were consistent with that expected from the low back pain literature. Another possible limitation of our study may be the generalizability of our conclusions, given our sample comprised of older male military Veterans. However, it should be noted that only a quarter of our sample had ever been users of VA health care services or had ever applied for disability compensation for any health condition, and household income levels and rates of college completion for our sample were similar to current statistics for US older adults in the general population [67]. For historical reasons related to military service at the time of the Vietnam conflict (and the military records with which the VET Registry was originally assembled), our study sample includes few non-whites, and does not include females.
CONCLUSIONS
In summary, this is the first US co-twin control study to examine multiple putative risk factors for back pain. Our results show that severe obesity (BMI ≥35 kg/m2) is associated with lifetime chronic back pain independent of familial factors, but that other potentially modifiable lifestyle factors are not. In contrast, PTSD and depression were strongly associated with chronic back pain even after adjustment for familial factors.
ACKNOWLEDGEMENTS
The authors would like to thank the members of the VET Registry and the VET Registry study staff.
FUNDING SOURCES: This work was supported by VA Career Development Award # 1IK2RX001515 from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service. Funding for this study was also provided by Cooperative Studies Program #569. Drs. Suri and Boyko are Staff Physicians at the VA Puget Sound Health Care System in Seattle Washington. Dr. Goldberg and Smith are Research Scientists at the VA Puget Sound Health Care System. The participation of Drs. Suri, Boyko, Smith and Goldberg in this study was funded by the VA Puget Sound Health Care System. The contents of this work do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Annals of the rheumatic diseases. 2014;73(6):968–74. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. The Journal of bone and joint surgery. 2006;88 Suppl 2:21–4. [DOI] [PubMed] [Google Scholar]
- 3.Atchison JW, Vincent HK. Obesity and low back pain: relationships and treatment. Pain Manag. 2012;2(1):79–86. [DOI] [PubMed] [Google Scholar]
- 4.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol. 2010;171(2):135–54. [DOI] [PubMed] [Google Scholar]
- 5.Lake JK, Power C, Cole TJ. Back pain and obesity in the 1958 British birth cohort. cause or effect? Journal of clinical epidemiology. 2000;53(3):245–50. [DOI] [PubMed] [Google Scholar]
- 6.Andersen JH, Haahr JP, Frost P. Risk factors for more severe regional musculoskeletal symptoms: a two-year prospective study of a general working population. Arthritis and rheumatism. 2007;56(4):1355–64. [DOI] [PubMed] [Google Scholar]
- 7.Hemingway H, Shipley M, Stansfeld S, et al. Are risk factors for atherothrombotic disease associated with back pain sickness absence? The Whitehall II Study. J Epidemiol Community Health. 1999;53(4):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordonana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. The spine journal : official journal of the North American Spine Society. 2015;15(5):1106–17. [DOI] [PubMed] [Google Scholar]
- 9.Von Korff M, Shortreed SM, Saunders KW, et al. Comparison of back pain prognostic risk stratification item sets. The journal of pain : official journal of the American Pain Society. 2014;15(1):81–9. [DOI] [PubMed] [Google Scholar]
- 10.McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5(5):546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Co-twin Control Methods [database on the Internet]. Wiley; 2014. Available from: http://onlinelibrary.wiley.com/book/10.1002/9781118445112. [Google Scholar]
- 12.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta geneticae medicae et gemellologiae. 1987;36(1):61–6. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin research : the official journal of the International Society for Twin Studies. 2002;5(5):476–81. [DOI] [PubMed] [Google Scholar]
- 14.Tsai M, Mori AM, Forsberg CW, et al. The Vietnam Era Twin Registry: a quarter century of progress. Twin Res Hum Genet. 2013;16(1):429–36. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg CW, Goldberg J, Sporleder J, Smith NL. Determining zygosity in the Vietnam era twin registry: an update. Twin Res Hum Genet. 2010;13(5):461–4. [DOI] [PubMed] [Google Scholar]
- 16.Bohman T, Alfredsson L, Jensen I, Hallqvist J, Vingard E, Skillgate E. Does a healthy lifestyle behaviour influence the prognosis of low back pain among men and women in a general population? A population-based cohort study. BMJ Open. 2014;4(12):e005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? Jama. 2010;303(13):1295–302. [DOI] [PubMed] [Google Scholar]
- 18.NHLBI. Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. National Heart Lung and Blood Institute; 2009. [cited 2009 10/20/2009]; Available from: http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm.
- 19.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. The American journal of medicine. 2010;123(1):87 e7–35. [DOI] [PubMed] [Google Scholar]
- 20.Strath SJ, Bassett DR Jr., Ham SA, Swartz AM. Assessment of physical activity by telephone interview versus objective monitoring. Medicine and science in sports and exercise. 2003;35(12):2112–8. [DOI] [PubMed] [Google Scholar]
- 21.Yore MM, Ham SA, Ainsworth BE, et al. Reliability and validity of the instrument used in BRFSS to assess physical activity. Medicine and science in sports and exercise. 2007;39(8):1267–74. [DOI] [PubMed] [Google Scholar]
- 22.Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39(4):305–13. [DOI] [PubMed] [Google Scholar]
- 23.Loustalot F, Carlson SA, Fulton JE, Kruger J, Galuska DA, Lobelo F. Prevalence of self-reported aerobic physical activity among U.S. States and territories--Behavioral Risk Factor Surveillance System, 2007. Journal of physical activity & health. 2009;6 Suppl 1:S9–17. [DOI] [PubMed] [Google Scholar]
- 24.Brown DR, Carroll DD, Workman LM, Carlson SA, Brown DW. Physical activity and health-related quality of life: US adults with and without limitations. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(10):2673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans. Hyattsville, MD: U.S. Department of Health and Human Services; 2008. [12/29/2015]; Available from: http://health.gov/paguidelines/guidelines/summary.aspx. [Google Scholar]
- 26.Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143(1–2):21–5. [DOI] [PubMed] [Google Scholar]
- 27.National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. 2015. [cited 2015 12/29/2015]; Available from: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 28.Watson NF, Badr MS, Belenky G, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J Clin Sleep Med. 2015;11(8):931–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General hospital psychiatry. 2010;32(4):345–59. [DOI] [PubMed] [Google Scholar]
- 30.Norris FH, Hamblen JL. Standardized self-report measures of civilian trauma and PTSD In: Wilson JP, Keane TM, Martin T, eds. Assessing psychological trauma and PTSD. New York: Guilford Press; 2004. p. 63–102. [Google Scholar]
- 31.Campbell KA, Rohlman DS, Storzbach D, et al. Test-retest reliability of psychological and neurobehavioral tests self-administered by computer. Assessment. 1999;6(1):21–32. [DOI] [PubMed] [Google Scholar]
- 32.Keen SM, Kutter CJ, Niles BL, Krinsley KE. Psychometric properties of PTSD Checklist in sample of male veterans. Journal of rehabilitation research and development. 2008;45(3):465–74. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behaviour research and therapy. 1996;34(8):669–73. [DOI] [PubMed] [Google Scholar]
- 34.Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC. Performance characteristics of the posttraumatic stress disorder checklist and SPAN in Veterans Affairs primary care settings. General hospital psychiatry. 2007;29(4):294–301. [DOI] [PubMed] [Google Scholar]
- 35.Magruder K, Yeager D, Goldberg J, et al. Diagnostic performance of the PTSD checklist and the Vietnam Era Twin Registry PTSD scale. Epidemiol Psychiatr Sci. 2015;24(5):415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VA. Using the PTSD Checklist for DSM-IV (PCL) National Center for PTSD; 2013. [cited 2015 1/7/2015]; Available from: http://www.ptsd.va.gov/professional/pages/assessments/assessment-pdf/pcl-handout.pdf. [Google Scholar]
- 37.Noss A Household Income for States: 2010 and 2011. U.S. Census Bureau; 2012. [cited 2016 1/7/2016]; Available from: https://www.census.gov/prod/2012pubs/acsbr11-02.pdf.
- 38.Bergen SE, Gardner CO, Aggen SH, Kendler KS. Socioeconomic status and social support following illicit drug use: causal pathways or common liability? Twin Res Hum Genet. 2008;11(3):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubscher M, Ferreira ML, Junqueira DR, et al. Heavy domestic, but not recreational, physical activity is associated with low back pain: Australian Twin low BACK pain (AUTBACK) study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;23(10):2083–9. [DOI] [PubMed] [Google Scholar]
- 40.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. International journal of epidemiology. 2005;34(5):1089–99. [DOI] [PubMed] [Google Scholar]
- 41.Leboeuf-Yde C, Kyvik KO, Bruun NH. Low back pain and lifestyle. Part II--Obesity. Information from a population-based sample of 29,424 twin subjects. Spine. 1999;24(8):779–83; discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 42.Dario AB, Ferreira ML, Refshauge K, et al. Are obesity and body fat distribution associated with low back pain in women? A population-based study of 1128 Spanish twins. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2015. [DOI] [PubMed] [Google Scholar]
- 43.Hestbaek L, Leboeuf-Yde C, Kyvik KO. Are lifestyle-factors in adolescence predictors for adult low back pain? A cross-sectional and prospective study of young twins. BMC musculoskeletal disorders. 2006;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh D, Park W, Hwang D, Levy MS. Severe obesity effect on low back biomechanical stress of manual load lifting. Work. 2015;51(2):337–48. [DOI] [PubMed] [Google Scholar]
- 45.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity. 2012;20(7):1491–5. [DOI] [PubMed] [Google Scholar]
- 46.Battie MC, Videman T, Gill K, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976). 1991;16(9):1015–21. [PubMed] [Google Scholar]
- 47.Junqueira DR, Ferreira ML, Refshauge K, et al. Heritability and lifestyle factors in chronic low back pain: results of the Australian twin low back pain study (The AUTBACK study). European journal of pain. 2014;18(10):1410–8. [DOI] [PubMed] [Google Scholar]
- 48.Leboeuf-Yde C, Kyvik KO, Bruun NH. Low back pain and lifestyle. Part I: Smoking. Information from a population-based sample of 29,424 twins. Spine. 1998;23(20):2207–13; discussion 14. [DOI] [PubMed] [Google Scholar]
- 49.Cederlof R, Friberg L, Jonsson E, Kaij L. Morbidity among Monozygotic Twins. Arch Environ Health. 1965;10:346–50. [DOI] [PubMed] [Google Scholar]
- 50.Bae YH, Shin JS, Lee J, et al. Association between Hypertension and the Prevalence of Low Back Pain and Osteoarthritis in Koreans: A Cross-Sectional Study. PLoS One. 2015;10(9):e0138790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartvigsen J, Christensen K. Active lifestyle protects against incident low back pain in seniors: a population-based 2-year prospective study of 1387 Danish twins aged 70–100 years. Spine. 2007;32(1):76–81. [DOI] [PubMed] [Google Scholar]
- 52.Hubscher M, Hartvigsen J, Fernandez M, Christensen K, Ferreira P. Does physical activity moderate the relationship between depression symptomatology and low back pain? Cohort and co-twin control analyses nested in the longitudinal study of aging Danish twins (LSADT). European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2015. [DOI] [PubMed] [Google Scholar]
- 53.Suri P, Hunter DJ, Rainville J, Boyko E, Guermazi A, Katz JN. Associations between Physical Activity and Facet Joint Osteoarthritis: the Framingham Study. 2013.
- 54.Taylor JB, Goode AP, George SZ, Cook CE. Incidence and risk factors for first-time incident low back pain: a systematic review and meta-analysis. The spine journal : official journal of the North American Spine Society. 2014;14(10):2299–319. [DOI] [PubMed] [Google Scholar]
- 55.Heneweer H, Staes F, Aufdemkampe G, van Rijn M, Vanhees L. Physical activity and low back pain: a systematic review of recent literature. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2011;20(6):826–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffens D, Maher CG, Pereira LS, et al. Prevention of Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med. 2016:1–10. [DOI] [PubMed] [Google Scholar]
- 57.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM & R : the journal of injury, function, and rehabilitation. 2012;4(5 Suppl):S10–9. [DOI] [PubMed] [Google Scholar]
- 58.Smuck M, Kao MC, Brar N, Martinez-Ith A, Choi J, Tomkins-Lane CC. Does physical activity influence the relationship between low back pain and obesity? The spine journal : official journal of the North American Spine Society. 2014;14(2):209–16. [DOI] [PubMed] [Google Scholar]
- 59.Shortreed SM, Peeters A, Forbes AB. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart. 2013;99(9):649–54. [DOI] [PubMed] [Google Scholar]
- 60.Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell P, Bishop A, Dunn KM, Main CJ, Thomas E, Foster NE. Conceptual overlap of psychological constructs in low back pain. Pain. 2013;154(9):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biehn TL, Contractor A, Elhai JD, et al. Relations between the underlying dimensions of PTSD and major depression using an epidemiological survey of deployed Ohio National Guard soldiers. J Affect Disord. 2013;144(1–2):106–11. [DOI] [PubMed] [Google Scholar]
- 63.Dersh J, Mayer T, Theodore BR, Polatin P, Gatchel RJ. Do psychiatric disorders first appear preinjury or postinjury in chronic disabling occupational spinal disorders? Spine. 2007;32(9):1045–51. [DOI] [PubMed] [Google Scholar]
- 64.Shaw WS, Means-Christensen AJ, Slater MA, et al. Psychiatric disorders and risk of transition to chronicity in men with first onset low back pain. Pain Med. 2010;11(9):1391–400. [DOI] [PubMed] [Google Scholar]
- 65.Hartvigsen J, Nielsen J, Kyvik KO, et al. Heritability of spinal pain and consequences of spinal pain: a comprehensive genetic epidemiologic analysis using a population-based sample of 15,328 twins ages 20–71 years. Arthritis Rheum. 2009;61(10):1343–51. [DOI] [PubMed] [Google Scholar]
- 66.Denard PJ, Holton KF, Miller J, et al. Back pain, neurogenic symptoms, and physical function in relation to spondylolisthesis among elderly men. The spine journal : official journal of the North American Spine Society. 2010;10(10):865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. Department of Health and Human Services. A Profile of Older Americans: 2014. Washington, D.C.: Administration for Community Living; 2014. [cited 2016 1/17/2016]; Available from: http://www.aoa.acl.gov/aging_statistics/profile/2014/docs/2014-Profile.pdf. [Google Scholar]