Abstract
This study was aimed at determining the efficacy of epinephrine, followed by chest compressions, in producing a return of spontaneous circulation (ROSC) during cyanide (CN)- or hydrogen sulfide (H2S)-induced toxic cardiac pulseless electrical activity (PEA) in the rat. Thirty-nine anesthetized rats were exposed to either intravenous KCN (n = 27) or H2S solutions (n = 12), at a rate that led to a PEA within less than 10 min. In the group intoxicated by CN, 20 rats were mechanically ventilated and received either epinephrine (0.1 mg/kg i.v. n = 10) followed by chest compressions or saline (n = 10, “control CN”) when in PEA. PEA was defined as a systolic pressure below 20 mmHg and a pulse pressure of less than 5 mmHg for 1 min. In addition, seven spontaneously breathing rats were also exposed to the same CN protocol, but infusion was stopped when a central apnea occurred; then, as soon as a PEA occurred, epinephrine (0.1 mg/kg IV) was administered while providing manual chest compressions and mechanical ventilation (CPR). Finally, 12 rats were intoxicated with H2S, while mechanically ventilated, and received either saline (n = 6, “control H2S”) or epinephrine (n = 6) with CPR when in PEA. None of the control-intoxicated animals resuscitated (10 rats in the control CN group and 6 in the control H2S group). In contrast, all the animals intoxicated with CN or H2S that received epinephrine followed by chest compressions, returned to effective circulation. In addition, half of the spontaneously breathing CN-intoxicated animals that achieved ROSC after epinephrine resumed spontaneous breathing. In all the animals achieving ROSC, blood pressure, cardiac output, peripheral blood flow and V̇O2 returned toward baseline, but remained lower than the pre-intoxication levels (p < 0.01) with a persistent lactic acidosis. Epinephrine, along with CPR maneuvers, was highly effective in resuscitating rodents intoxicated with CN or H2S. Since epinephrine is readily available in any ambulance, its place as an important countermeasure against mitochondrial poisons should be advocated. It remains critical to determine whether the systematic administration of epinephrine to any victims found hypotensive following CN or H2S intoxication could prevent PEA, decrease post-ischemic brain injury and increase the efficacy of current antidotes by improving the circulatory status.
Keywords: Cyanide, Hydrogen sulfide, Acute cardiac failure, Toxic pulseless electrical activity, Epinephrine
Introduction
The treatment of an acute cyanide (CN) or hydrogen sulfide (H2S) intoxication, two of the most emblematic mitochondrial poisons, must resolve a difficult challenge: the potential benefits of any specific antidotes effective against either poison, being a cobalt compound [7, 8, 15, 20, 22, 38] or a methemoglobin-producing agent [2, 3, 17, 21, 22, 32–34]—as well as compounds improving cyanide elimination [2, 3], are dictated by the promptness of their administration by first responders. However, not all ambulance services carry specific CN/H2S antidotes, and if they do, it is usually in small quantity. Stockpiling CN/H2S antidotes in every ambulance or hospital [16] throughout the country, in the eventuality of mass casualty (like in a fire for CN), must take into account the relative rarity of a catastrophic large-scale intoxication with CN or H2S and the variety of other potential chemical threats.
CN or H2S can both be lethal—within minutes—through a reduction in cardiac contractility leading to cardiac asystole [23, 26, 37]. Indeed, these mitochondrial poisons can cause a pulseless electrical activity (PEA) due to their direct inhibition of various ion cardiac channels, including calcium (Ca) exchange in the cardiomyocytes [12, 26]. CN and H2S can also produce an early depression in medullary respiratory neurons leading to central apnea [24] typically along with a loss of consciousness. Although a secondary hypoxic cardiac arrest can occur in the context of a prolonged apnea, breathing usually resumes in non-sedated animals preceded by the production of gasps [24], unless circulation is already severely altered. On a clinical standpoint, we have found that as soon as a marked depression in cardiac contractility occurs, whether or not associated with a depression of breathing, the evolution is usually lethal by pulseless electrical activity (PEA), much more rarely by ventricular fibrillation [23, 26, 37].
Epinephrine, along with chest compressions, is the treatment of choice of asystole by PEA [28] as recommended by the American Heart Association [13, 29] or the European Resuscitation Council [14]. Epinephrine, in contrast to specific CN or H2S antidotes, is always available to pre-hospital providers (e.g., paramedics) and can be administered using intravenous (IV), as well as non-IV routes, i.e., intramuscular or intra-osseous.
However, the toxic effects of CN or H2S, which literally prevent cardiomyocytes to contract [23, 26], are quite unique and although epinephrine has been shown to be as effective as a “cobalt” antidote in resuscitating pigs during lethal forms of CN intoxications [4], there is no comprehensive study investigating the interest of epinephrine during toxic asystole produced by H2S or CN. As the mechanism of toxic cardiac depression by both CN and H2S appears to differ from those leading to post-anoxic PEA [12, 26], there is no current evidence that epinephrine could be effective in treating CN- or H2S-induced life-threatening cardiac asystole.
In addition, the possibility that epinephrine may even be toxic for the intoxicated heart, due to the increased contractility in face of a reduced oxygen utilization, should also be considered. In this study, we were interested in the capacity of epinephrine, associated with chest compressions and ventilation (CPR), to restore spontaneous circulation (ROSC) in various rat models of toxic cardiac arrest (PEA), following CN or H2S exposure—conditions which in our experience are well beyond spontaneous reversibility [23, 26]. The effects of epinephrine and chest compressions on PEA were studied in urethane anesthetized rats in the 3 following experimental conditions: (1) “Primary” PEA following lethal KCN infusion in mechanically ventilated rats; (2) “Secondary” PEA, following a central apnea in spontaneously breathing animals, obtained by stopping KCN infusion as soon as breathing stopped; (3) PEA following H2S (NaSH) infusion in mechanically ventilated animals.
Materials and Methods
Animal Model
Adult male Sprague–Dawley rats weighing 452 ± 99.7 g were studied. The study was approved by the Pennsylvania State University, College of Medicine Institutional Animal Care and Use Committee (protocols #, 46905-B, 47198-A, and 46389-A). All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council (US) Institute for Laboratory Animal Research).
The rats were first anesthetized with inhalation of isoflurane 3.5% in O2 for few minutes, allowing the intraperitoneal injection of urethane (1.2 g/kg). All the animals were then tracheostomized (14G Surflo catheter) [23, 26] and were equipped as previously described [37]. A catheter (PE-50 tubing) was inserted into the right femoral artery for continuous monitoring of systemic arterial blood pressure and arterial blood sampling and into the left ventricle (via the right carotid artery) for the measurement of left ventricular pressure and cardiac contractility. Additional catheters were inserted into the right femoral vein for KCN or NaSH infusion, and one of the jugular veins for epinephrine administration.
Inspiratory flow was measured using a pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS) [22], and mixed-diluted expired O2 (FEO2) and CO2 fractions (FECO2) were measured continuously using O2 (Oxystar-100, CWE, Inc. Ardmore, PA) and CO2 (Model 17630, VacuMed, Ventura, CA) analyzers for determination of minute ventilation (V̇E). Oxygen uptake (V̇O2) and CO2 output (V̇CO2) were computed as previously described [11, 36].
The arterial and ventricular catheters were connected to pressure transducers (BD DTXPlus Transducer, Becton–Dickinson, Franklin Lakes, NJ). Left ventricular systolic pressure (LVSP) and heart rate (HR) were determined for the ventricular pressure signal, and LV dP/dt max was computed by derivative of the left ventricular pressure signal. Aortic, carotid and femoral blood flows were measured using transonic flow probes connected to the transit-time flowmeter (TS420, Transonic Systems Inc., Ithaca, NY). The electro-cardiogram was recorded by a bioelectric amplifier. All the analog output signals were digitized at 200 Hz [23, 26].
Protocol
Intravenous Potassium Cyanide Infusion
Cyanide solution (KCN, Sigma, St. Louis, MO) was diluted in sterile saline at a concentration of 0.5 mg/ml [23]. KCN was infused at the rate of 0.75 mg/kg/min, a dose that we found to be lethal by PEA within 10 min [23].
In a first group, animals were mechanically ventilated (frequency of breathing: 80 breaths/min, minute ventilation: ~ 350 ml/min) and infusions were maintained until PEA [23]. PEA was defined as the persistent of QRS complexes with no effective cardiac contractions, i.e., systolic pressure below 20 mmHg and a pulse pressure of less than 5 mmHg for one minute.
In a second group, KCN was infused at the same rate while the rats were spontaneously breathing. KCN infusion was stopped as soon as breathing stopped. This apnea has been shown to lead to auto-resuscitation or to a rapid reduction in cardiac contractility and subsequent PEA [23]. In this group, mechanical ventilation was initiated at the onset of cardiac arrest.
Intravenous NaSH Infusion
NaSH solution was prepared immediately prior to each experiment in airtight syringe, using sodium hydrosulfide hydrate (NaSH Sigma, St. Louis, MO) at a concentration of 0.8 mg/ml. NaSH solution was infused at the rate of 2 mg/kg/min until PEA occurred [36, 37].
Epinephrine Infusion
Epinephrine (0.1 mg/kg diluted in 1 ml of saline) was given via an IV bolus injection 1 min into PEA. The dose of 0.1 mg/kg was chosen since it has been shown to be effective during other types of PEA in rats [10] and would correspond to doses of about 0.02 mg/kg in an adult human, in keeping with the body surface area and assuming a correction factor of about 5 and according to the recommendations for conversion of doses between animals and humans [39]. Chest compressions were performed between the thumb and index at a frequency of 150 min as soon as epinephrine was administered.
Arterial blood (i-STAT System) was sampled before PEA and then 15–30 and 30–60 min later in all surviving animals.
All rats were euthanized 1 h later by i.v. injection of barbiturate (150 mg/kg), followed by aortic dissection.
Data Analysis
Assuming that no spontaneous recovery would be observed in untreated animals presenting toxic PEA, the minimum number of animals needed for adequate study power should be 10 rats in each group (control and epinephrine) if a 70% ROSC were to be expected or 6 rats in each group for an expected incidence of recovery of 90%.
The primary outcome was a return of spontaneous circulation (ROSC). ROSC was computed as the time from injection of epinephrine to the onset of the recovery of rhythmic spontaneous blood pressure signal. The incidence and time to ROSC and to return to spontaneous breathing were also determined.
Carotid blood pressure, carotid and femoral blood flow, cardiac output, maximal left ventricular dP/dt, V̇O2 and V̇CO2 were averaged and compared to baseline as secondary outcomes using repeated-measured ANOVA followed by Bonferroni’s post hoc comparisons. Chi squared test was used to compare incidence of events. The statistical analyses were conducted using GraphPad Prism 5 (Graphpad Software, La Jolla, CA). p < 0.05 was regarded as significant.
Results
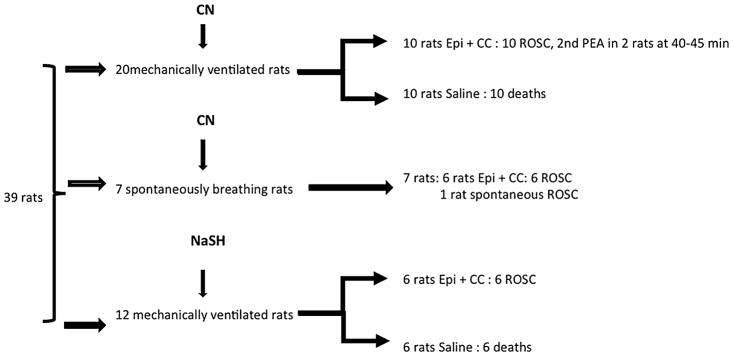
A total of 39 rats were studied (Fig. 1): twenty mechanically ventilated rats received an infusion of cyanide until PEA occurred; 10 animals received saline 1 min into PEA, while 10 animals received epinephrine and chest compressions. In addition, in seven spontaneously rats, KCN infusion was stopped when an apnea occurred. Six out of these 7 animals went into PEA and received epinephrine and chest compressions. Twelve mechanically ventilated rats received an infusion of NaSH until PEA occurred, 6 received saline while 6 animals received epinephrine, 1 min into PEA, following the same protocol.
Fig. 1.
Summary of the experimental protocol. CN cyanide, NaSH sulfide, Epi epinephrine, ROSC return of spontaneous circulation, CC chest compressions
CN-Induced Cardiac Asystole in Mechanically Ventilated Animals
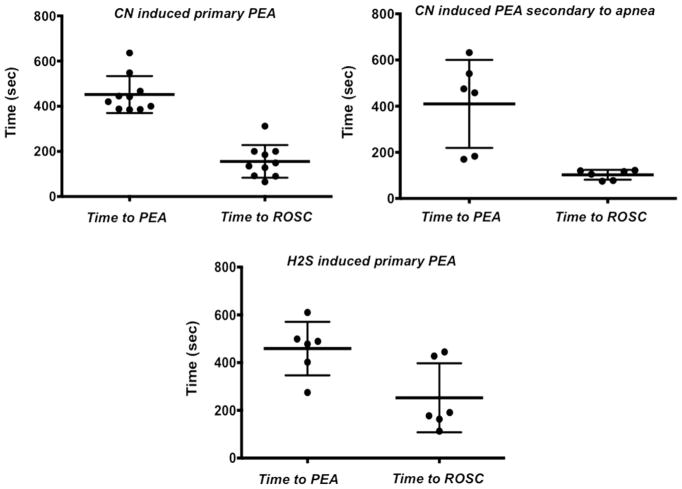
At the dose of 0.75 mg/kg/min, KCN produced a brief period of tachycardia and increased blood pressure immediately followed by a rapid and profound depression in cardiac contractility and bradycardia leading to a decrease in blood pressure and cardiac output, which resulted in a PEA within about 7 min (451 ± 81 s), as illustrated in Figs. 2 and 3. In 10 rats, epinephrine was injected and chest compressions were initiated one minute into complete PEA, which restored an effective circulation in all animals with an averaged delay of 156 ± 72 s (Fig. 3). In three animals, ROSC was produced as soon as epinephrine was administered, i.e., even before the generation of chest compressions could produce significant increase in blood pressure, so PEA duration ranged between 65 and 90 s in these 3 rats. Of note, manual chest compressions only produced a systolic blood pressure ranging at best between 40 and 60 mmHg.
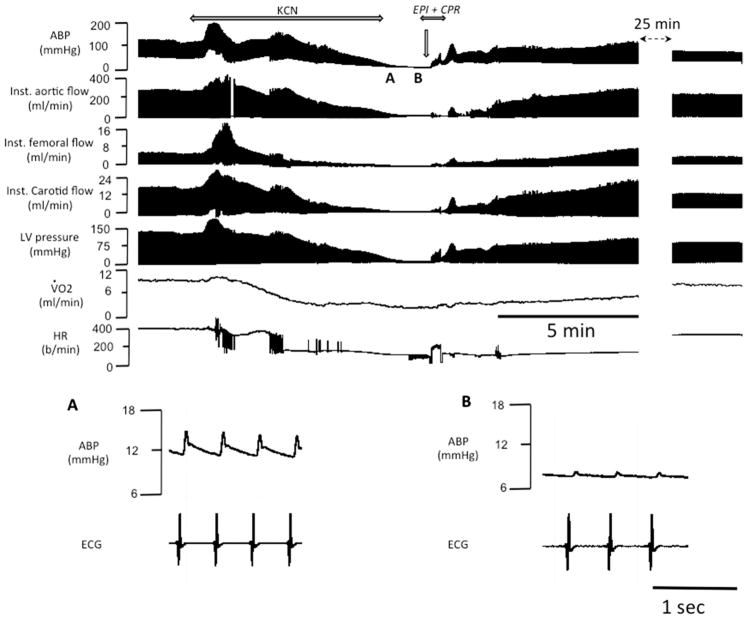
Fig. 2.
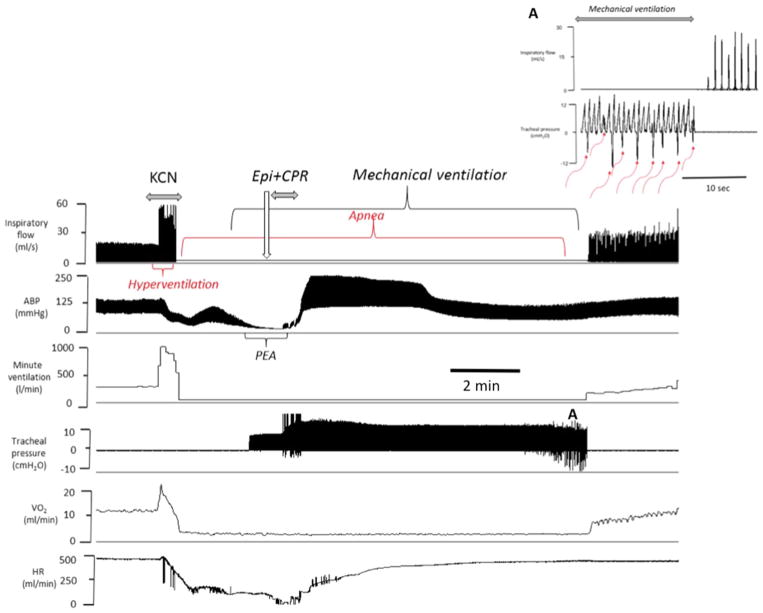
Example of the effects of epinephrine administered following KCN infusion (0.75 mg/kg/min)-induced PEA (inset A and B) in one rat. From top to bottom, carotid blood pressure (ABP), instantaneous aortic flow, carotid and femoral flow, left ventricular pressure, V̇O2 and HR are displayed. Note that KCN led to a PEA within 6 min; while the rat was in asystole, a bolus of injection of epinephrine allowed ROSC
Fig. 3.
Time to PEA and ROSC produced by the CN and H2S and epinephrine, respectively (individual data and mean ± SD)
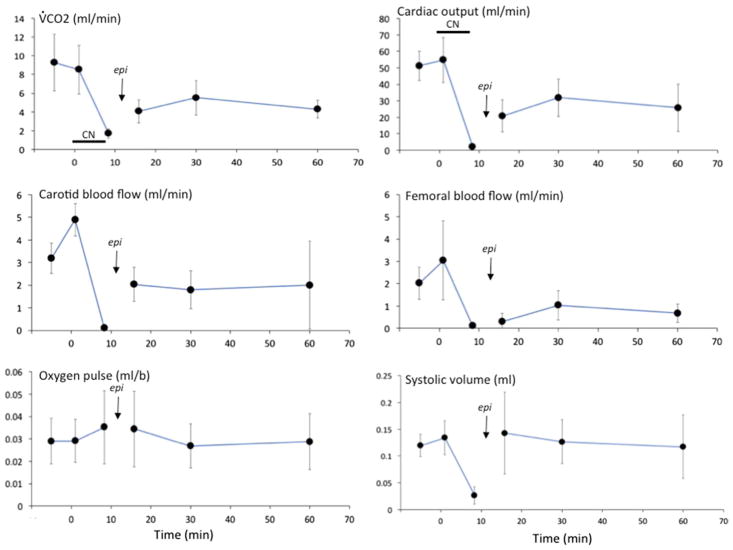
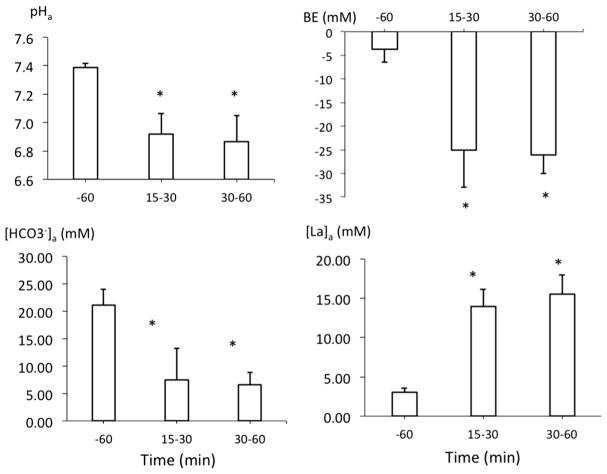
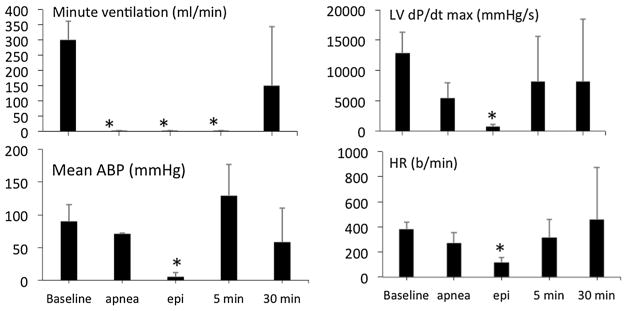
As shown in Figs. 2, 3 and 4, after ROSC was obtained, arterial blood pressure (ABP) transiently rose above baseline (p < 0.05), before decreasing below baseline. Maximal LVdP/dt, cardiac output, HR, V̇O2, V̇CO2, femoral and carotid blood flow also remained lower than baseline throughout the 60-min period of recovery (Figs. 4 and 5). Lactate averaged 14.0 ± 2.2 mM and 15.5 ± 2.4 mM at 30 and 60 min (p < 0.01), respectively (Fig. 6).
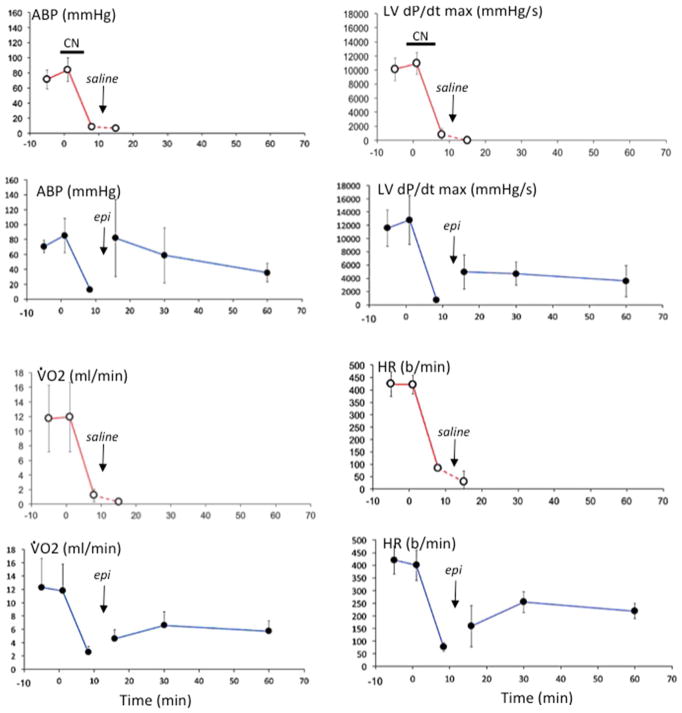
Fig. 4.
Mean (± SD) arterial blood pressure (ABP), left ventricular maximal dP/dt, pulmonary oxygen uptake (O2) and heart rate (HR) versus time. Time 0 is the onset of infusion of KCN. Data obtained in animals receiving saline at the time of PEA are displayed in red, while data from the animals treated with epinephrine are shown in blue. Data are shown then 5, 30 and 60 min following administration of saline or epinephrine
Fig. 5.
Mean (± SD) V̇CO2, cardiac output, carotid blood flow, femoral blood flow, O2 pulse and systolic volume versus time in response to KCN infusion and epinephrine. Data are shown at the time of PEA, then 5, 30 and 60 min following administration of epinephrine
Fig. 6.
Mean (± SD) arterial pH, base excess (BE), bicarbonate (HCO3−) and lactate (La) concentrations in the blood before KCN exposure then 15–30 and 30–60 min following ROSC
Of note, two epinephrine-treated rats, presented a new episode of PEA at 40 and 45 min into recovery, respectively. They both responded to a new administration of epinephrine; the 60-min data obtained in these 2 animals were not included in the analysis. The 10 animals that received saline did not resuscitate (Chi squared test, p < 0.001).
CN-Induced Central Apnea
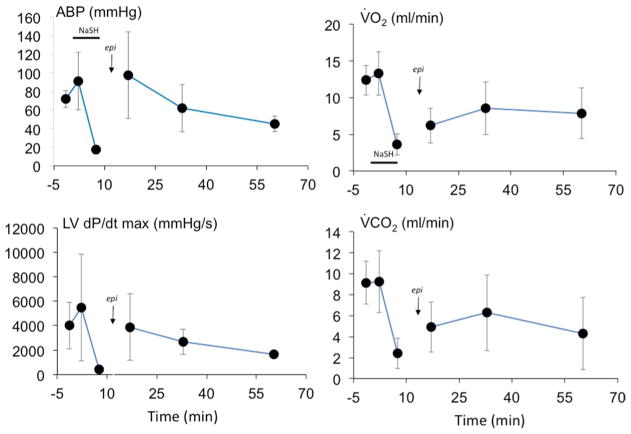
In seven spontaneously breathing rats, KCN infusion was discontinued as soon as the animal stopped breathing, following a short period of hyperventilation (Figs. 7 and 8). Since the time to produce an apnea (243 ± 171 s, Fig. 3) was much shorter than the time required to develop a PEA, the quantity of CN that was administered was lower than the dose delivered in mechanically ventilated animals. One animal produced gasps, spontaneously auto-resuscitated and never presented a PEA; this rat was excluded from the analysis. The other 6 rats presented a rapid decrease in cardiac contractility leading to PEA within 409 ± 190 s after the end apnea. Mechanical ventilation was initiated as soon as PEA occurred, followed by one dose of epinephrine (0.1 mg/kg) and chest compressions (Figs. 7 and 8). ROSC was obtained in 102 ± 21 s, followed by recovery of spontaneous breathing between 683 and 940 s in 3 animals (Fig. 3). Of interest, the animals that did not recover their spontaneous breathing had a lower blood pressure and circulatory parameters than baseline levels after 15-min recovery (Fig. 9).
Fig. 7.
Example of the effects of epinephrine following KCN infusion (0.75 mg/kg/min) induced PEA in one spontaneously breathing rat. From top to bottom, inspiratory flow, arterial blood pressure, minute ventilation, tracheal pressure, O2 and heart rate are displayed. Following a brief period of hyperventilation, an apnea occurred followed by a rapid decrease in blood pressure, O2 and heart rate leading to a PEA. Epinephrine was administered while mechanical ventilation was initiated, as shown on the tracheal pressure signal. Circulation was restored within 30 s after the injection of epinephrine. Note that spontaneous breathing movements started to be generated within 10 min (red arrows) (Color figure online)
Fig. 8.
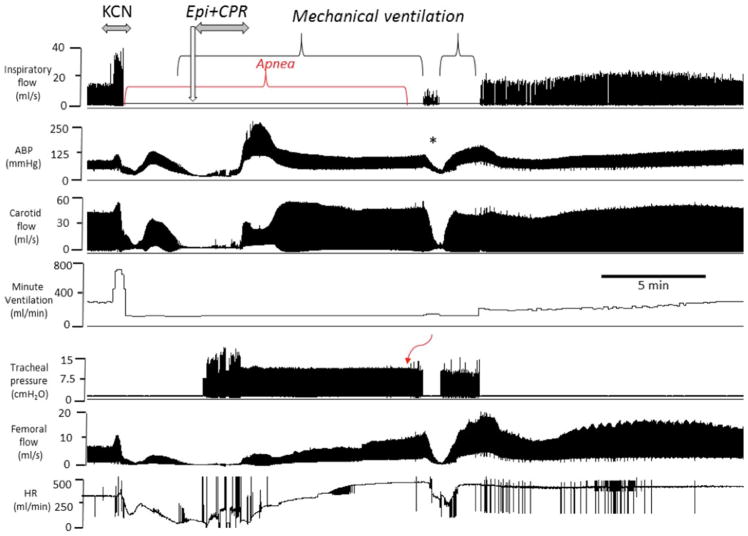
Example of the effects of epinephrine following KCN infusion (0.75 mg/kg/min) induced PEA in another spontaneously breathing rat. From top to bottom, inspiratory flow, arterial blood pressure, carotid blood flow, minute ventilation, tracheal pressure, femoral flow and hear rate are displayed. Like in Fig. 7, following a brief period of hyperventilation, an apnea occurred followed by a rapid decrease in blood pressure, blood flow and heart rate leading to a PEA. Epinephrine was administered while mechanical ventilation was initiated. Circulation was restored after the injection of epinephrine with chest compressions. The asterisk (*) shows a period where mechanical ventilation was briefly interrupted while breathing was present but still depressed, which was associated with a drop in blood pressure and blood flow. Note that spontaneous breathing movements started to be generated one minute later in a stable manner
Fig. 9.
Mean (± SD) minute ventilation, arterial blood pressure (ABP), left ventricular maximal dP/dt and heart rate (HR) in breathing spontaneously rats before and at different time after CN-induced apnea
H2S-Induced Primary Cardiac Arrest
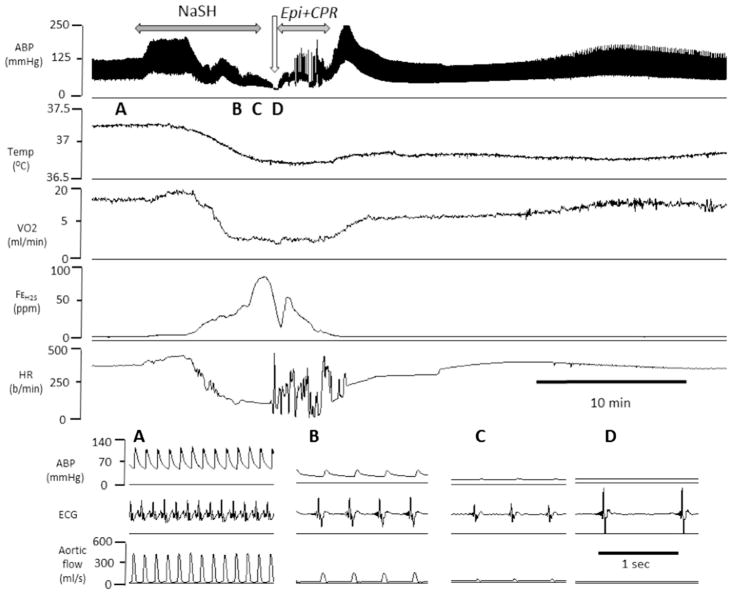
Twelve rats were exposed to a dose of H2S that would lead to a PEA within less than 10 min [37]. Six rats received saline and 6 rats received epinephrine and chest compressions one minute into PEA. The responses to H2S, as well as the effects of epinephrine, replicated those of CN (Figs. 10 and 11). Times to PEA and to ROSC are displayed in Fig. 3. In contrast to the intoxicated animals that received saline, all the animals survived after epinephrine. Of note, expired H2S, a marker of soluble sulfide in the arterial blood, re-increased during the period of chest compressions (Fig. 10), but dropped to zero within < 3 min, reflecting the short-lasting persistence of free H2S in the arterial blood after sulfide exposure. Lactate concentration was high at the end of the recovery period (16.98 ± 0.02 mM at 30 min).
Fig. 10.
Example of the effects of epinephrine after H2S infusion (0.8 mg/min)-induced PEA in one rat. From top to bottom, carotid blood pressure (ABP), temperature, V̇O2, expired fraction of H2S and HR are displayed. During sulfide infusion, blood pressure as well as V̇O2 decreased leading to a typical PEA as shown in the insets A–D. Injection of epinephrine during complete cardiac asystole followed by chest compressions allowed ROSC
Fig. 11.
Mean (± SD) arterial blood pressure (ABP), left ventricular maximal dP/dt, pulmonary oxygen uptake (V̇O2) and CO2 output (V̇CO2) versus time. Time 0 is the onset of infusion of NaHS. Data are shown 5, 30 and 60 min following administration epinephrine. All rats presented a ROSC
Discussion
A bolus injection of one dose of epinephrine (0.1 mg/kg) with chest compressions resuscitated rats presenting a cardiac arrest from PEA produced by either CN or H2S intoxication. A restoration of eupneic breathing was also observed in half of the animals that were studied during spontaneous breathing.
Epinephrine, in contrast to specific CN or H2S antidotes, is accessible to most first responders in relatively large quantities and could therefore be used as an effective countermeasure against CN and sulfide intoxication and should be considered as part of the strategy of emergency treatment against mitochondrial poisons as discussed in the following paragraphs.
Current Countermeasures Against CN and H2S Intoxication
Medications producing methemoglobinemia (such as nitrite) or cobalt compounds (vitamin B12 analogs) can be used against either poison as they can trap free CN [3, 8, 18] and free/soluble H2S [21]. To be effective, antidotes must be administered as soon as possible following CN exposure and, even more critically, within minutes following H2S exposure [21]. As mentioned in our introduction, not all ambulances are currently equipped with CN/H2S antidotes. In addition, 911 dispatchers are not necessarily aware of the toxic cause of an acute event, involving coma and shock. It is therefore essential to offer a realistic strategy that can be implemented by first responders when treating life-threatening CN or H2S intoxications. The use of epinephrine appears to be very effective in restoring circulation but also to provide and maintain sufficient cerebral blood flow and gas exchange increasing immediate survival and allowing specific antidotes to be administered, at least for CN.
Epinephrine Following CN and H2S Intoxication
CN intoxication can be lethal by producing a PEA either via primary direct cardiac toxicity and/or when PaO2 drops to critical levels following a respiratory arrest [23]. In the latter, as soon as blood pressure dropped following apnea, the evolution was always a rapid cardiac arrest by terminal PEA.
One dose of epinephrine, associated with mechanical ventilation and chest compressions, did allow ROSC in all animals. Compared to cyanide, sulfide intoxication presents an important difference [22]: the very rapid disappearance of the free form of H2S after exposure. Indeed, the capacity of many molecules, e.g., proteins and metallo-compounds in blood and cells to trap free H2S [22], and of the mitochondria to oxidize H2S into sulfite, sulfate and thiosulfate [9, 27], is responsible for the quick spontaneous disappearance of free/diffusible H2S from the blood and tissues, i.e., within few minutes, a capacity only overwhelmed during “supra-lethal” forms of sulfide intoxication [35]. In keeping with the fast off-kinetics of free H2S in the blood, antidotes interacting with the H2S/HS− should be administered within a time window not always compatible with a clinically relevant scenario, which has made it difficult to design an effective strategy based on specific H2S antidotes [21]. The present study confirms that expired H2S, which reflects the level of free H2S in the arterial blood—although re-increasing briefly during chest compressions—virtually disappeared within < 3 min. In contrast, the pools of combined H2S interacting with cardiac ion channels for instance [26] have been shown to persist for at least 30 min [22] and are likely to be responsible for some of the persisting symptoms. Epinephrine was clearly able to reverse the depression in cardiac contractility produced by H2S, at least within the range of intoxication of the present study, to restore an effective circulation, akin to what we observed with CN. Since the toxicity of H2S on the cardiomyocytes is primarily related to the inhibition of LCa channels [26], the site and nature of H2S and epinephrine interactions remain to be explored.
Four important methodical aspects should be discussed here. First, intoxications by CN and H2S were produced though IV infusion, a scenario very different from the typical modalities of exposure, which is inhaled for CN and H2S, or via oral ingestion for CN. For instance, as both CN [24] and H2S [19] intoxication produce a central apnea, exposure to these toxic agents should cease when inhaled and only resume during gasping, creating a fundamentally different pattern of exposure than during IV administration.
Second, we have previously established that the potassium contained in KCN solution had no measurable effects on respiration or circulation, while no relevant changes in potassium concentration in the blood were found during KCN infusion at the dose used in the present study [23].
Third, epinephrine was injected as soon as witnessing the development of a PEA, conditions that are rarely met in a clinically relevant scenario.
Finally, chest compressions were only applied in the epinephrine groups and not in the control groups that received saline. Manual chest compressions produced a modest increase in systolic blood pressure and were performed with the main objective of providing some blood circulation, ensuring the delivery of epinephrine to the heart as the drug was administered in animals in circulatory arrest. In other words, the capacity of chest compressions and mechanical ventilation to restore by themselves effective cardiac contractions in case of lethal toxic cardiac arrest was not evaluated in our study, since as in any form of PEA epinephrine will be never administered without chest compressions. Conversely, the current treatment of a PEA relies on epinephrine and it would be unthinkable to use chest compressions only to treat non-shockable forms of asystole. Of note, in many instances, chest compressions were unable to meaningfully increase blood pressure levels yet epinephrine was very effective. In addition, in several rats, cardiac contractions resumed as soon as epinephrine was administered. As we did not anticipate a recovery of cardiac contractions by chest compression alone, we did not study the effects of chest compressions only or epinephrine without chest compressions. Although it will be hard to defend the view that chest compressions alone could, within sometimes seconds after epinephrine injections, explain by themselves the restoration of cardiac contractions, yet they certainly play a significant role in resuscitation, which, along with the mechanical ventilation, are part of an effective strategy of cardio-pulmonary resuscitation.
Epinephrine as a New Paradigm for the Treatment of CN- or Sulfide-Induced Acute Cardiac Failure?
Epinephrine, following chest compressions, is a fundamental component of the American Heart Association Guidelines for cardio-pulmonary resuscitation and emergency cardiovascular care [29]. It has become the standard of care for the treatment of non-shockable forms of asystole [13, 14], while the benefit of epinephrine in “shockable” rhythms remains very controversial [1]. In the adult, a dose of 1 mg of epinephrine IV every 3–5 min, until a pulse reappears, is currently recommended [29]. Higher doses have not been shown to provide significant additional benefit [5, 6], at least in non-toxic PEA, and are even considered to be potentially deleterious [25, 31]. In rats, ROSC following post-anoxic asystole is largely improved using dose ranging from 0.04 to 0.4 mg/kg [10]. Lower doses (0.01 mg/kg) appear to be as effective, if not better than higher dosage [30], to restore circulation. Whether these notions apply to toxic PEA remains to be shown. As mentioned in the method section, the dose of 0.1 mg/kg in a 500 g rat would correspond to doses of about 0.02 mg/kg in an adult human, according to the recommendations for conversion of doses between animals and humans [39].
A persistent reduced V̇O2 and cardiac output were always present after CN- or H2S intoxication-induced cardiac arrest. Whether this effect could have been prevented by an additional infusion of epinephrine [4] or by a delayed administration of antidotes [2, 3] was not tested. Also, in a clinically relevant scenario, the correction of the metabolic acidosis following PEA could play a significant role in improving these parameters.
Conclusion
Injection of epinephrine, followed by chest compressions, after CN- and H2S- induced cardiac PEA in rats allows a return to spontaneous circulation. Also, in spontaneously breathing animals, apnea induced by CN can be reversible after epinephrine. The interest of epinephrine as an effective countermeasure against CN and H2S severe intoxications should therefore be seriously considered. The fundamental question that certainly remains to be clarified is whether epinephrine could be systematically offered as an effective countermeasure in victims found in coma or presenting with low blood pressure, before presenting PEA. In conditions wherein cardiac function is already altered, the interest of using epinephrine would be to not only prevent PEA and limit the consequence of ischemic and post-ischemic insults but to also restore circulatory status with the objective of improving the efficacy of specific antidotes.
Acknowledgments
This work was in part supported by the National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Numbers R21 NS090017 and R21 NS098991.
Footnotes
Author Contributions A. J-H contributed to the design of the experiments, the analysis of the data and the writing and edition of the manuscript, T.S. performed some of the experiments and contributed to the design of experiments, V.B. contributed to the analysis of the data and the writing and editing of the manuscript, P.H. provided the original idea for the study, performed some of the experiments, supervised the study, contributed to the design of the experiments, to the analysis of the data, to the writing of the first and last draft and editing of the manuscript.
Compliance with Ethical Standards
Conflict of interest All authors declare that they have no conflict of interest.
References
- 1.Andersen LW, Kurth T, Chase M, Berg KM, Cocchi MN, Callaway C, et al. Early administration of epinephrine (adrenaline) in patients with cardiac arrest with initial shockable rhythm in hospital: propensity score matched analysis. BMJ. 2016;353:i1577. doi: 10.1136/bmj.i1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. Journal of Clinical Pharmacology. 1992;32:368–375. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 3.Bebarta VS. Antidotes for cyanide poisoning. European Journal of Emergency Medicine. 2013;20:65–66. doi: 10.1097/MEJ.0b013e3283591730. [DOI] [PubMed] [Google Scholar]
- 4.Bebarta VS, Pitotti RL, Dixon PS, Valtier S, Esquivel L, Bush A, et al. Hydroxocobalamin and epinephrine both improve survival in a swine model of cyanide-induced cardiac arrest. Annals of Emergency Medicine. 2012;60:415–422. doi: 10.1016/j.annemergmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Berg RA, Otto CW, Kern KB, Hilwig RW, Sanders AB, Henry CP, et al. A randomized, blinded trial of high-dose epinephrine versus standard-dose epinephrine in a swine model of pediatric asphyxial cardiac arrest. Critical Care Medicine. 1996;24:1695–1700. doi: 10.1097/00003246-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: A prospective, randomized study. Critical Care Medicine. 1994;22:282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Bismuth C, Baud FJ, Pontal PG. Hydroxocobalamin in chronic cyanide poisoning. Journal de Toxicologie Clinique et Experimentale. 1988;8:35–38. [PubMed] [Google Scholar]
- 8.Borron SW, Baud FJ. Antidotes for acute cyanide poisoning. Current Pharmaceutical Biotechnology. 2012;13:1940–1948. doi: 10.2174/138920112802273182. [DOI] [PubMed] [Google Scholar]
- 9.Bouillaud F, Blachier F. Mitochondria and sulfide: A very old story of poisoning, feeding, and signaling? Antioxidants & Redox Signaling. 2011;15:379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 10.Chen MH, Lu JY, Xie L, Zheng JH, Song FQ. What is the optimal dose of epinephrine during cardiopulmonary resuscitation in a rat model? American Journal of Emergency Medicine. 2010;28:284–290. doi: 10.1016/j.ajem.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Chenuel B, Sonobe T, Haouzi P. Effects of infusion of human methemoglobin solution following hydrogen sulfide poisoning. Clinical Toxicology (Philadelphia, PA) 2015;53:93–101. doi: 10.3109/15563650.2014.996570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung JY, Wang J, Zhang XQ, Song J, Tomar D, Madesh M, Judenherc-Haouzi A, Haouzi P. Methylene blue counteracts cyanide cardiotoxicity: cellular mechanisms. Journal of Applied Physiology. 2018 doi: 10.1152/japplphysiol.00967.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: Pediatric advanced life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S526–S542. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European resuscitation council guidelines for resuscitation 2010 section 4. Adult Advanced Life Support Resuscitation. 2010;81:1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, et al. Hydroxocobalamin as a cyanide antidote: Safety, efficacy and pharmacokinetics in heavily smoking normal volunteers. Journal of Toxicology - Clinical Toxicology. 1993;31:277–294. doi: 10.3109/15563659309000395. [DOI] [PubMed] [Google Scholar]
- 16.Gasco L, Rosbolt MB, Bebarta VS. Insufficient stocking of cyanide antidotes in US hospitals that provide emergency care. Journal of Pharmacology & Pharmacotherapeutics. 2013;4:95–102. doi: 10.4103/0976-500X.110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall AH, Rumack BH. Hydrogen sulfide poisoning: an antidotal role for sodium nitrite? Veterinary and Human Toxicology. 1997;39:152–154. [PubMed] [Google Scholar]
- 18.Hall AH, Saiers J, Baud F. Which cyanide antidote? Critical Reviews in Toxicology. 2009;39:541–552. doi: 10.1080/10408440802304944. [DOI] [PubMed] [Google Scholar]
- 19.Haouzi P. Ventilatory and metabolic effects of exogenous hydrogen sulfide. Respiratory Physiology & Neurobiology. 2012;184:170–177. doi: 10.1016/j.resp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clinical Toxicology (Philadelphia, PA) 2015;53:28–36. doi: 10.3109/15563650.2014.990976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haouzi P, Sonobe T, Judenherc-Haouzi A. Developing effective countermeasures against acute hydrogen sulfide intoxication: Challenges and limitations. Annals of the New York Academy of Sciences. 2016 doi: 10.1111/nyas.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In vivo interactions between cobalt or ferric compounds and the pools of sulphide in the blood during and after H2S poisoning. Toxicological Sciences. 2014;141:493–504. doi: 10.1093/toxsci/kfu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haouzi P, Tubbs N, Rannals MD, Judenherc-Haouzi A, Cabell LA, McDonough JA, et al. Circulatory failure during noninhaled forms of cyanide intoxication. Shock. 2017;47:352–362. doi: 10.1097/SHK.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haxhiu MA, Erokwu B, van Lunteren E, Cherniack NS, Strohl KP. Central and spinal effects of sodium cyanide on respiratory activity. Journal of Applied Physiology. 1993;74:574–579. doi: 10.1152/jappl.1993.74.2.574. [DOI] [PubMed] [Google Scholar]
- 25.Hoekstra JW, Van Ligten P, Neumar R, Werman HA, Anderson J, Brown CG. Effect of high dose norepinephrine versus epinephrine on cerebral and myocardial blood flow during CPR. Resuscitation. 1990;19:227–240. doi: 10.1016/0300-9572(90)90104-m. [DOI] [PubMed] [Google Scholar]
- 26.Judenherc-Haouzi A, Zhang XQ, Sonobe T, Song J, Rannals MD, Wang J, et al. Methylene blue counteracts H2S toxicity-induced cardiac depression by restoring L-type Ca channel activity. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2016;310:R1030–R1044. doi: 10.1152/ajpregu.00527.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochimica et Biophysica Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Lin S, Callaway CW, Shah PS, Wagner JD, Beyene J, Ziegler CP, et al. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation. 2014;85:732–740. doi: 10.1016/j.resuscitation.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 30.Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, et al. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249–263. doi: 10.1016/0300-9572(94)00827-3. [DOI] [PubMed] [Google Scholar]
- 31.Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. New England Journal of Medicine. 2004;350:1722–1730. doi: 10.1056/NEJMoa032440. [DOI] [PubMed] [Google Scholar]
- 32.Scharf BA, Fricke RF, Baskin SI. Comparison of methemoglobin formers in protection against the toxic effects of cyanide. General Pharmacology. 1992;23:19–25. doi: 10.1016/0306-3623(92)90041-h. [DOI] [PubMed] [Google Scholar]
- 33.Smith L, Kruszyna H, Smith RP. The effect of methemoglobin on the inhibition of cytochrome c oxidase by cyanide, sulfide or azide. Biochemical Pharmacology. 1977;26:2247–2250. doi: 10.1016/0006-2952(77)90287-8. [DOI] [PubMed] [Google Scholar]
- 34.Smith RP, Kruszyna R, Kruszyna H. Management of acute sulfide poisoning: Effects of oxygen, thiosulfate, and nitrite. Archives of Environmental Health. 1976;31:166–169. doi: 10.1080/00039896.1976.10667212. [DOI] [PubMed] [Google Scholar]
- 35.Sonobe T, Haouzi P. H2S concentrations in the heart after acute H2S administration: methodological and physiological considerations. American Journal of Physiology Heart and Circulatory Physiology. 2016;311:H1445–H1458. doi: 10.1152/ajpheart.00464.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clinical Toxicology (Philadelphia, PA) 2015;53:525–539. doi: 10.3109/15563650.2015.1043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonobe T, Haouzi P. Sulfide intoxication-induced circulatory failure is mediated by a depression in cardiac contractility. Cardiovascular Toxicology. 2016;16(1):67–78. doi: 10.1007/s12012-015-9309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O’Brien PJ. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)) Toxicology. 2007;242:16–22. doi: 10.1016/j.tox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 39.US Department of Health and Human Services FaDA. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers edited by (CDER) CfDEaR. 2005 [Google Scholar]