SUMMARY
Background
Genetic background influences allergic immune responses to environmental stimuli. Non-obese-diabetic (NOD) mice are highly susceptible to environmental stimuli. Little is known the interaction of autoimmune genetic factors with innate immunity in allergies, especially skin hypersensitivity.
Objectives
We sought to study the interplay of innate immunity and autoimmune genetic factors in contact hypersensitivity (CHS) using various innate immune-deficient NOD mice.
Methods
TLR2-, TLR9- and MyD88-deficient NOD mice were used to investigate CHS. The cellular mechanism was determined by flow cytometry in vitro and adoptive cell transfer in vivo. To investigate the role of MyD88 in dendritic cells (DCs) in the CHS, we also used CD11cMyD88+MyD88−/−NOD mice, in which MyD88 is expressed only in CD11c+cells.
Results
We found that innate immunity negatively regulates CHS as innate immune-deficient NOD mice developed exacerbated CHS accompanied by increased skin-migrating CD11c+ DCs expressing more MHC-II and CD80. Moreover, MyD88−/−NOD mice had increased number of CD11c+CD207−CD103+ DCs and activated T effector cells in the skin-draining lymph nodes. Strikingly, re-expression of MyD88 in CD11c+ DCs (CD11cMyD88+MyD88−/−NOD) restored hyper CHS to normal level in MyD88−/−NOD mice.
Conclusion
Our results suggest that autoimmune prone NOD genetic background aggravates CHS regulated by innate immunity, through DCs and T effector cells.
Keywords: contact sensitivity, dendritic cells, non-obese diabetic (NOD) mouse, Toll-like receptor, Tregs
1. Introduction
Cutaneous delayed-type hypersensitivity and related contact hypersensitivity (CHS) reactions are classical examples of antigen-specific T cell-mediated allergic immune responses in the skin (1). CHS responses can be initiated by keratinocytes and dendritic cells (DCs) (2). Innate immunity also plays an indispensable role in the CHS response. Our previous study showed that Toll-like receptor (TLR) 4 ligand, lipopolysaccharide (LPS), a common innate immune stimulus, can stimulate iNKT cells to produce IL-4 facilitating the B-1 B cell activation and recruitment of antigen-specific T effector cells in CHS reaction (3). Jin et al found that TLR2 deficient C57BL/6 mice expressed defective CHS responses with impaired ear swelling, reduced cellular infiltration, and decreased epidermal thickening and local IFN-γ expression (4). Further study by Klekotka et al demonstrated that the downstream adaptor protein MyD88, rather than upstream TLRs, mediates the CHS response (5).
Genetic factors play an important role in skin immune responses including CHS. Recent studies suggested that autoimmunity contributes to the susceptibility to allergies including skin allergy (6, 7). The genetic background of the non-obese-diabetic (NOD) mouse carries multiple autoimmune susceptibility loci (8). In addition to the unique MHC, NOD mice have several defects in non-MHC genetic loci including impaired T regulatory function (9). NOD mice are mainly used for type 1 diabetes (T1D) studies; however, the NOD genetic background predisposes to other autoimmune disorders, such as arthritis (10), sialitis (11) and thyroiditis (12). Human studies showed that T1D patients have increased incidence of allergy (13, 14). However, little is known how the autoimmune genetic factors affect allergy. To investigate the interaction of autoimmune genetic factors with innate immunity in the CHS response, we studied CHS immune responses using NOD mice deficient in innate immunity.
2. MATERIALS AND METHODS
2.1 Mice
Wild type (WT) NOD, TLR2−/−, TLR9−/−, MyD88−/− and CD11cMyD88+MyD88−/− mice, all on the NOD genetic background, were generated at Yale University. IFN-γR−/−NOD mice were purchased from the Jackson Laboratory and maintained at Yale University. The procedures applied in this study were approved by IACUC of Yale University and 1st Local Ethics Committee of Jagiellonian University Medical College.
2.2 Reagents
Hapten picryl chloride (PCl, TNP-Cl – trinitrophenyl chloride) was from Chemical Alta (Edmonton, Canada); fluorescein isothiocyanate (FITC), dibutyl phthalate (DBP), protease inhibitor cocktails, SYBRgreen JumpStart Taq and bovine serum albumin (BSA) were from Sigma-Aldrich (St Louis, Missouri); IFN-γ ELISA Set, biotinylated anti-mouse IgG1 mAb and TMB substrate were from BD Biosciences (San Jose, California); TNP-BSA was from Biosearch Technologies (Petaluma, California); streptavidin-horseradish peroxidase was from Vector Laboratories (Burlingame, CA); fluorochrome or biotin conjugated mAbs were from BioLegend (San Diego, California); fixation and permeabilization buffer, anti-mouse CD16/CD32 mAb and PE conjugated-anti-mouse FoxP3 mAb were from eBioscience (San Diego, California). Complete RPMI medium contained 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 25 mM HEPES, 5×10−5 M 2-ME, and 10% FBS. RPMI media and supplements were from Sigma-Aldrich. Heat-inactivated fetal bovine serum (FBS) was from Gemini Bio-Products (West Sacramento, California). Anti-mouse CD4 (GK 1.5) and CD8 (TIB 105) hybridomas were generously provided by the late Dr. Charles Janeway Jr. (Yale University). Magnetic beads conjugated with goat anti-rat IgG were from Qiagen (Valencia, California). EasySep mouse CD11c positive selection kit was from STEMCELL Technologies Canada Inc. (Vancouver, BC, Canada). TRIzol Reagent was from Thermo Fisher Scientific (Waltham, Massachusetts). Isopropyl alcohol and DEPT-treated water were from AmericanBio, Inc. (Natick, Massachusetts). iScript cDNA Synthesis Kit was from (BIO-RAD, Hercules, California). Primers for qPCR were synthesized by Sigma-Aldrich Co. (St Louis, MO). Primer sequences for: IL-10 (F-CTGGACAACATACTGCTAACCG, R-GGGCATCACTTCTACCAGGTAA), TGF-β (F-AGCCCGAAGCGGACTACTAT, R-TTCCCGAATGTCTGACGTATT), IFN-γ (F-CTGGCAAAAGGATGGTGACATG, R- TGGCAATACTCATGAATGCATCCTT), and GAPDH (F- AGGTCGGTGTGAACGGATTTG, R- TGTAGACCATGTAGTTGAGGTCA).
2.3 Induction of CHS in vivo
Mice were sensitized by application of 150μL of 5% picryl chloride (PCl, TNP-Cl) in acetone-ethanol mixture to the shaved abdomen. Four days later, all the mice were challenged on both ears with 10μL of 0.4% PCl in olive oil-acetone mixture. For sensitization with fluorescein isothiocyanate (FITC), 400μL of 0.5% FITC in acetone and dibutyl phthalate was applied to shaved abdominal skin. After 5 days, mice were challenged on the ears with 10μL of 0.1% FITC in the same solvent. The ear thickness was measured with a micrometer before and 24 hours after challenge.
2.4 Induction of CHS after CD11c+ DC transfer
Donor mice were sensitized with 150μL of 5% PCl (7.5mg). Three days later, CD11c+ DCs were isolated from ALNs using a CD11c isolation kit (StemCell Technology). 4×104 CD11c+ DCs were injected intradermally into two spots on the abdomen of the recipients. The experimental and control mice were challenged with 10μL of 0.4% PCl 4 days later and CHS responses were tested 24 hours after the challenge.
2.5 Lymphocyte proliferative response to trinitrophenyl (TNP) and IFN-γ concentration in cell culture supernatants
Mice were sensitized with 5% PCl (150μL). Four days after sensitization, cell suspensions from ALNs were prepared. ALNC (3×106) cells were incubated with 100μg of mouse immunoglobulins conjugated with TNP (TNP40-Ig) in 1 mL complete RPMI medium for 48 hours. IFN-γ secretion was measured in the supernatants using an ELISA Set. In proliferation assay, ALNC (3×105/well) were cultured with different concentrations of TNP40-Ig, in 200μL of the complete RPMI medium for 48 hours. After adding 0.5μCi/well [3H]-thymidine and additional 18h incubation, the cells were harvested and [3H]-thymidine incorporation was determined on a beta counter. The background (cultures without antigen) was subtracted from the cpm of the cultures with antigen.
2.6 Staining of lymphoid cells and flow cytometry analysis
To determine the phenotype and migration of skin DCs into ALNs, ALNC were isolated 3 days post FITC or PCl sensitization. Cells were washed and pre-incubated with 0.5μg of anti-mouse CD16/CD32 mAb (Fc blocker) for 15min at 4°C followed by incubation with appropriate fluorochrome- or biotin-conjugated mAbs. An additional incubation with PE-streptavidin was performed (at 4°C, for 30min) whenever biotin-conjugated mAb was used. For FoxP3 staining, ALNC and auricular ear draining lymph node cells (ELNC) were harvested from PCl-sensitized and ear challenged mice. Cells were incubated with Fc blocker and stained for surface markers. After washing, the cells were treated with fixation and permeabilization buffer followed by incubation with anti-mouse/rat FoxP3-PE mAb at 4°C for 30min before analyzing with flow cytometry. Gating information for all the flow cytometry analysis is presented in Fig. S2.
2.7 Antibody measurement
Mice were sensitized and challenged with PCl as described above. The sera were collected 24 hours after challenge. TNP-specific antibodies were measured by ELISA. Briefly, 96-well plates were coated with bovine serum albumin (BSA) conjugated with TNP hapten (TNP-BSA, 50μg/mL) or BSA (50μg/mL) alone in PBS, at 4°C overnight. After washing, the plates were blocked with PBS containing 10% FBS for 1h at room temperature (RT). The tested samples and different concentrations of standard reagent were added in the wells and incubated for 2 hours at RT. After washing, the plates were incubated with biotinylated anti-mouse IgG1 mAb for 1 hour followed by incubation with streptavidin-horseradish peroxidase for 30 minutes. The enzymatic reaction was stopped 30 minutes after adding TMB substrate. Colorimetric absorbance was determined at the wavelength 450nm.
2.8 Cytokine mRNA expression in the skin
Mice were sensitized with 5% PCl (150μL) on the shaved abdomen. Five days later two skin punches (0.6cm) were collected from each mouse and homogenized in 1 mL TRIzol Reagent. RNA was precipitated by mixing the homogenates with 0.5 mL isopropyl alcohol and washed with 75% ethanol. The air-dried RNA pellet was dissolved in DEPC-treated water. cDNA was synthesized using iScript cDNA Synthesis Kit, according to the manufacturer’s protocol. Quantification of mRNA content for all cytokine genes and housekeeping gene GAPDH was performed in Real-Time Cycler. The PCR reaction mix for each gene and GAPDH contains SYBR green JumpStart Taq, forward and reverse primers, DNase-free water, and cDNA template.
2.9 Statistical analysis
Student’s t-test or Mann Whitney’s test or ANOVA followed by Tukey’s test were used with Prism software. P<.05 was considered statistically significant.
3. RESULTS
3.1 The role of TLRs and MyD88 in CHS response in NOD mice
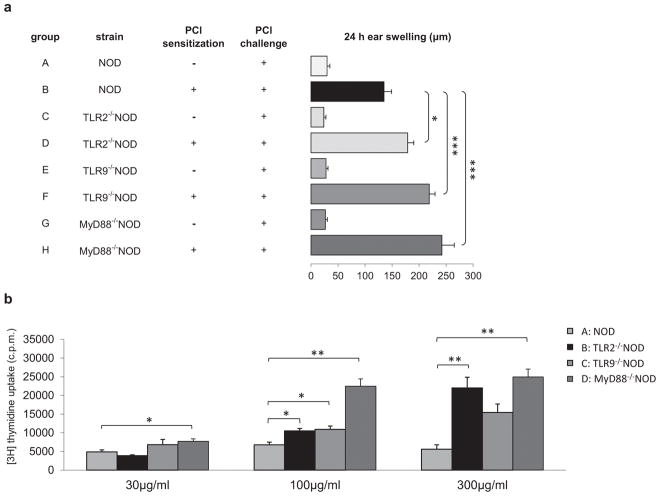
We first tested whether NOD mice, which are genetically prone to sterile inflammation, develop normal T cell-mediated CHS. Our results showed that NOD mice had normal CHS response to PCl, which was mediated by CD4+ cells (Fig. S1a) and IFN-γ-dependent (Fig. S1b). We then studied the role of innate immunity in CHS response in NOD mice. TLRs comprise a major family of innate immune receptors involved in both innate and adaptive immune responses (15). In the absence of TLR2, C57BL/6 mice resulted in significantly decreased CHS reaction compared to TLR2-sufficient C57BL/6 mice (4). To determine the role of TLRs in CHS response in NOD mice, which are prone to autoinflammation (16), we tested TLR2- and TLR9-deficient NOD mice. In contrast to the published data in TLR2−/− C57BL/6 strain, our results demonstrate that the CHS immune response was markedly increased in TLR2- or TLR9-deficient NOD mice (Fig. 1a, groups D and F vs B). To confirm the hyper-CHS response seen in vivo we examined the antigen specific response of ALNC in vitro. In line with the ear swelling results, ALNC from TLR2- or TLR9-deficient NOD mice showed significantly stronger responses to TNP-Ig compared with WT NOD mice (Fig. 1b, groups B and C vs A). As MyD88 plays a crucial role downstream of the TLR2 and TLR9 signaling pathway (15), next, we studied CHS responses in MyD88−/−NOD mice. Consistent with the augmented CHS response seen in TLR2−/− or TLR9−/− NOD mice, MyD88−/−NOD mice also showed hyper-CHS responses compared to WT NOD mice (Fig. 1a, group H vs B). The in vivo hyper-response in MyD88−/−NOD mice was further confirmed by significantly stronger antigen-specific responses of ALNC in vitro (Fig. 1b, group D vs A).
Fig. 1.
Role of TLRs and MyD88 in CHS in NOD mice. (A) TLR2 and TLR9 and MyD88 deficiency in NOD mice showed an increased CHS response to PCl. WT NOD (group B) or TLR2−/− (group D) or TLR9−/− (group F) or MyD88−/− (group H) NOD mice were sensitized with 5% PCl. Four days later all the mice were challenged with 0.4% PCl and tested for CHS response 24 hours later. N = 8–12, *P <.05 and ***P <.001 (ANOVA followed by Tukey’s test). (B) TLR2−/− and TLR9−/− and MyD88−/− NOD mice develop stronger antigen specific responses in vitro. WT NOD mice (group A) or TLR2−/− (group B) or TLR9−/− (group C) or MyD88−/− (group D) NOD mice were sensitized with 5% PCl. Four days later ALNs were isolated and ALNC were cultured in the presence of the antigen, TNP40-Ig, at different concentration for 48 hours. The mean background in cultures in the absence of the TNP-Ig for NOD, TLR2−/−NOD, TLR9−/−NOD and MyD88−/−NOD was 10744±257, 24997±774, 10387±600 and 6440±570 respectively. N = 3, *P <.05 and **P <.01 (Two-tailed Student’s t-test). All error bars represent SE.
3.2 The importance of MyD88 expression in DCs and hyper-CHS responses in NOD mice
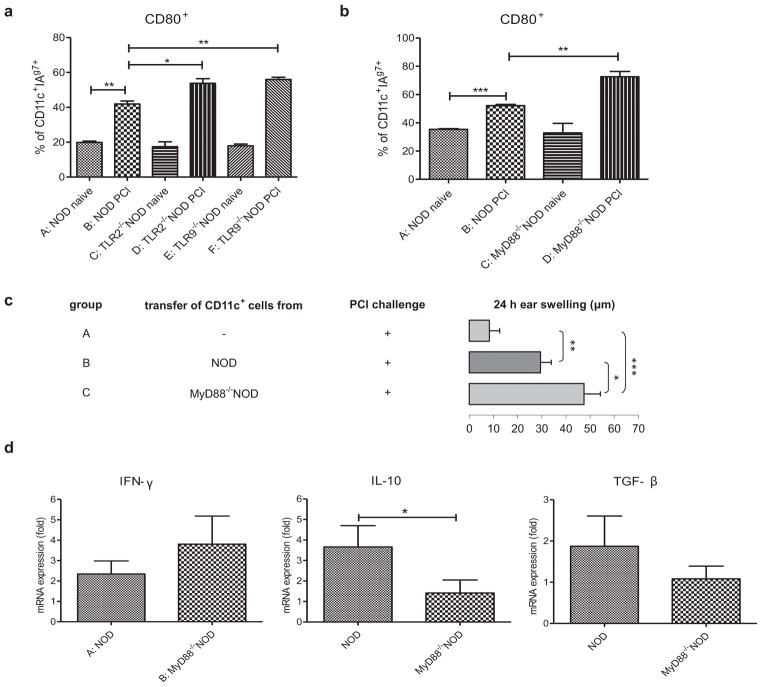
To investigate the cellular mechanism by which NOD mice develop hyper-CHS immune responses in the absence of TLRs or MyD88, we examined the phenotype of DCs, which are known to express high levels of TLRs (17) and are essential in CHS induction (18). We found a significantly higher percentage of CD11c+DCs in TLR2- or TLR9- and MyD88-deficient NOD mice that expressed the costimulatory molecule CD80 and the NOD MHC class II molecule I-Ag7 (Figs 2a and 2b). Our data suggest that DCs involved in the CHS response are more activated in innate immunity deficient NOD mice. DCs are known to have a potent ability to present antigens to T cells and induce effective immune responses (19, 20) or contribute to induction of antigen-specific FoxP3+ T regulatory cells (Tregs) (21). As MyD88 is essential signaling molecule downstream of most of the TLRs (15) therefore we studied the effect of MyD88 on the function of CD11c+ DCs and tested their ability to induce CHS responses in vivo. Fig. 2c showed that CD11c+ cells isolated from MyD88−/−NOD mice were stronger inducers of CHS response than CD11c+ cells isolated from WT NOD mice (group C vs B). Moreover, MyD88−/−NOD mice had higher IFN-γ and lower IL-10 and TGF-β mRNA expression in the skin compared to the WT NOD mice (Fig. 2d, group B vs A). Thus, our results suggest that DCs play an important role in allergenic response in the skin and the hyper-CHS response found in MyD88−/−NOD mice is most likely due to the deficiency of MyD88 in DCs.
Fig. 2.
Role of DCs in CHS in TLR- or MyD88-deficient NOD mice. (A) Increased percentage of CD11c+I-Ag7+ DCs expressing CD80 in TLR2 and TLR9 deficient NOD mice. WT NOD (group B) or TLR2−/− (group D) or TLR9−/− (group F) NOD mice were sensitized with 5% PCl. Mice in negative controls (groups A, C and E) were not treated with PCl. ALNs were isolated 3 days later and ALNC were stained with different monoclonal antibodies (anti-CD11c-APC, anti-CD80-PE, anti-I-Ag7-FITC). Gating information for the flow analysis is presented in Fig. S2. N=3, *P <.05 and **P <.01 (Two-tailed Student’s t-test). (B) Increased percentage of CD11c+ I-Ag7+ DCs expressing CD80 in MyD88−/−NOD mice. WT NOD (group B) or MyD88−/−NOD (group D) mice were sensitized with 5% PCl. Mice in negative controls (groups A and C) were not treated with PCl. ALNs were isolated 3 days later and ALNC were stained with different monoclonal antibodies (anti-CD11c-APC, anti-CD80-PE, anti-I-Ag7-FITC). Gating information for the flow analysis is presented in Fig. S2. N=3, **P <.01 and ***P <.001 (Two-tailed Student’s t-test). (C) CD11c+ cells isolated from MyD88−/−NOD mice are stronger inducers of CHS response than CD11c+ cells isolated from WT NOD mice. Donor mice were immunized with PCl three days before the isolation of CD11c+ cells from ALN. Purified CD11c+ cells isolated from NOD (group B) or MyD88−/−NOD (group C) mice were transferred s.c. into naïve NOD recipients, respectively. The recipients were ear-challenged with 0.4% PCl after receiving the cells and tested for CHS responses 24 hours later. N=6–10, *P <.05 and **P <.01 and ***P <.001 (Two-tailed Student’s t-test). (D) Cytokine mRNA expression in skin homogenates. NOD (group A) and MyD88−/−NOD (group B) mice were sensitized with PCl. Five days later mRNA expression was measured in skin homogenates. N=10–12, *P <.05 (Mann Whitney’s test). All error bars represent SE.
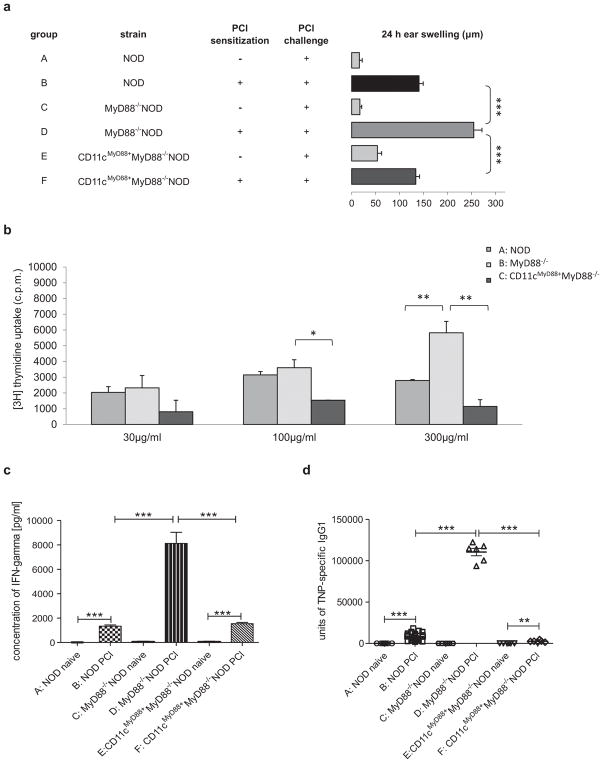
To further investigate the role of MyD88 in DCs in the CHS response, we generated CD11cMyD88+MyD88−/−NOD mice, in which MyD88 is only expressed in CD11c+cells (22). It is intriguing that “rescuing” MyD88 expression in CD11c+DCs completely restored the hyper-CHS responses in MyD88−/−NOD mice to normal CHS responses seen in WT NOD mice (Fig. 3a, group F vs D and B). The in vivo skin inflammatory response was further confirmed by the results of antigen-specific ALNC proliferation in vitro (Fig. 3b) and their IFN-γ production (Fig. 3c). Moreover, we found that the hyper-CHS response to PCl in MyD88−/−NOD mice was accompanied by a highly significant increase of TNP-specific IgG1 antibodies compared to WT NOD mice (Fig. 3d, group D vs B). Importantly, expression of MyD88 in CD11c+ DCs also restored the level of TNP-specific IgG1 antibody to that found in the WT mice (Fig. 3d, group F vs B).
Fig. 3.
Re-expression of MyD88 in CD11c+ DCs restores normal CHS responses in MyD88−/−NOD mice. (A) Presence of MyD88 in CD11c+ DCs restores normal CHS response in vivo. WT NOD (group B) or MyD88−/− (group D) or CD11cMyD88+MyD88−/− (group F) NOD mice were sensitized with 5% PCl or not (group A, C and E, respectively). Four days later all the mice were challenged with 0.4% PCl and tested for CHS 24 hours after challenge. N = 6–14, ***P <.001 (Two-tailed Student’s t-test). (B) CD11cMyD88+MyD88−/−NOD mice develop normal hapten specific response in vitro. WT NOD (group A) or MyD88−/− (group B) or CD11cMyD88+MyD88−/− (group C) NOD mice were sensitized with 5% PCl. ALNs were isolated 4 days later and proliferation assay was performed as described in Fig. 2B. N = 3, *P <.05 and **P <.01 (Two-tailed Student’s t-test). (C) Presence of MyD88 in CD11c+ DCs restores normal IFN-γ production. WT NOD (group B) or MyD88−/− (group D) or CD11cMyD88+MyD88−/− (group F) NOD mice were sensitized with 5% PCl or not (group A, C and E, respectively). ALNs were isolated 4 days later and cultured in the presence of antigen, TNP40-Ig for 48 hours. Culture supernatants were harvested and tested for IFN-γ production by ELISA kit. N = 6–12, ***P <.001 (Two-tailed Student’s t-test or Mann Whitney’s test). (D) Presence of MyD88 in CD11c+ DCs restores normal level of TNP-specific IgG1 in serum. WT NOD (group B) or MyD88−/− (group D) or CD11cMyD88+MyD88−/− (group F) NOD mice were sensitized with 5% PCl or not (group A, C and E, respectively). Four days later, all the mice were challenged with 0.4% PCl. The serum was collected 24 hours after challenge. Anti-TNP specific IgG1 antibody was measured by ELISA. N = 6–24. ***P <.001 (Two-tailed Student’s t-test or Mann Whitney’s test). All error bars represent SE.
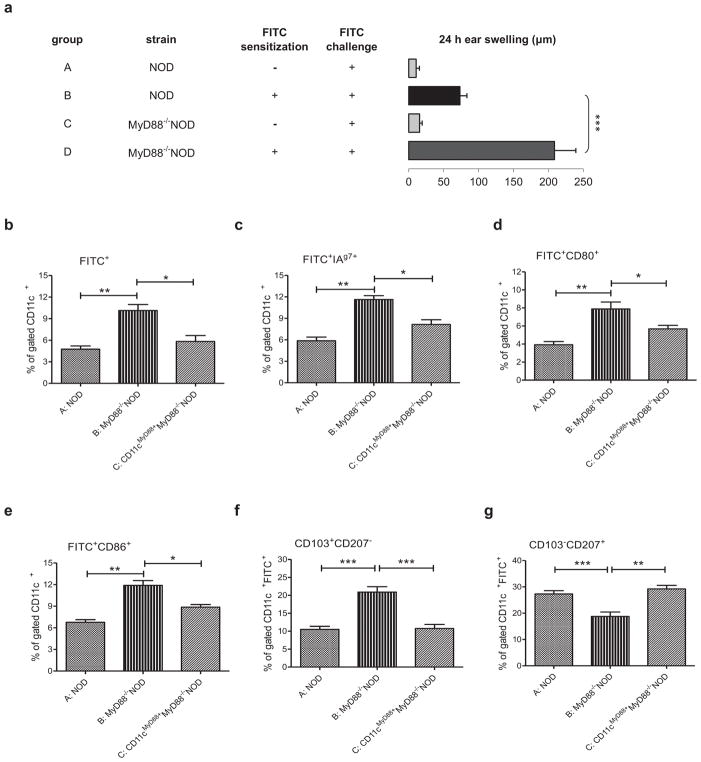
It is known that both Langerhans cells (LCs) and dermal dendritic cells (DDCs) migrate into skin-draining lymph nodes (LNs) to present antigen to naïve T lymphocytes in the initiation phase of skin immune responses (23). To test whether hyper-CHS seen in MyD88−/−NOD mice is associated with alterations in DC migration, we tested DC migration in vivo in FITC-induced CHS reactions in order to trace the DC migration. Similar to the results using PCl, MyD88−/−NOD mice also expressed hyper-reactivity to FITC (Fig. 4a, group D vs B). To examine DC migration, we analyzed FITC+DCs from ALNs by flow cytometry. There was a significantly higher frequency of migrating DCs (FITC+CD11c+) co-expressing NOD MHC class II molecule I-Ag7, co-stimulatory molecules CD80 and CD86 in ALNs of MyD88−/−NOD mice when compared to control NOD mice (Figs 4b–e, group B vs A). However, the restoration of MyD88 expression in CD11c+DCs led to a similar DC migration and phenotype in MyD88−/−NOD to that seen in WT mice (Figs 4b–e, group C vs A). We also investigated the expression of langerin (CD207) and integrin (CD103), in migrating DCs. Our results showed a significantly higher frequency of FITC+CD11c+CD103+CD207− DCs but lower percentage of FITC+CD11c+CD103−CD207+ cells in MyD88−/−NOD mice compared to WT or CD11cMyD88+MyD88−/−NOD mice (Figs 4f and 4g, group B vs A), whereas other DC subsets, such as FITC+CD11c+CD103+CD207+ and FITC+CD11c+CD103−CD207−, were similar (data not shown).
Fig. 4.
Characterization and tracking of skin DCs in MyD88−/− NOD mice. (A) MyD88-deficient mice on the NOD background develop exaggerated CHS response to FITC. WT NOD and MyD88−/−NOD mice were sensitized with 0.5% FITC (groups B and D) or not (groups A and C). The animals were challenged, 5days later, with FITC and tested for CHS response. N = 4–10, ***P <.001 (ANOVA followed by Tukey’s test). (B–G) Stronger migration and more activated phenotype of skin CD11c+ DCs in MyD88 deficient NOD mice. WT NOD mice (group A), MyD88−/− (group B) and CD11cMyD88+MyD88−/− (group C) NOD mice were sensitized with 0.5% FITC. Three days later, ALNs were isolated and stained with appropriate monoclonal antibodies: anti-I-Ag7-biotin-strep-PE, anti-CD11c-PerCP-Cy5.5, anti-CD11c-APC, anti-CD80-APC, anti-CD86-APC, anti-CD103-PerCP-Cy5.5, anti-CD207-PE. Gating information for the flow analysis is presented in Fig. S2. N = 3, *P <.05 and **P <.01 and ***P <.001 (Two-tailed Student’s t-test). All error bars represent SE.
3.3 MyD88−/−NOD mice have increased numbers of CD4+CD25+FoxP3− activated T cells in ALNs
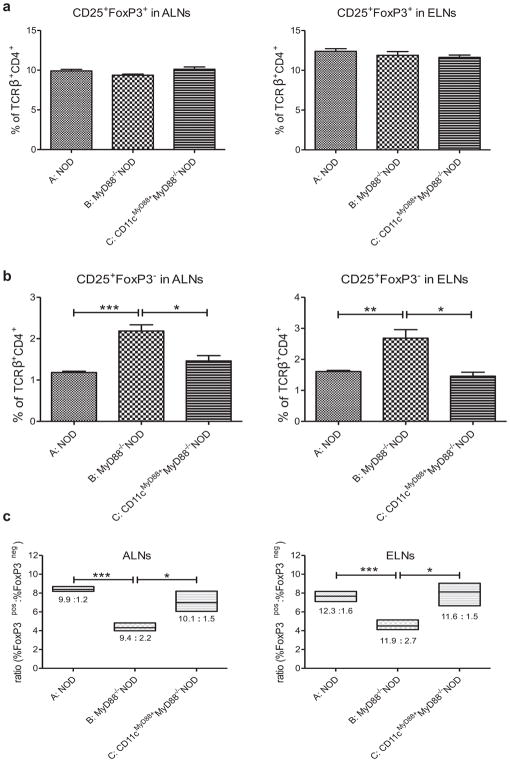
It has been suggested that CD207+ DCs could induce FoxP3+ Tregs (24). We hypothesized that a lower frequency of CD207+ DCs might indicate fewer FoxP3+ Tregs in MyD88−/−NOD mice. Interestingly, the frequencies of CD4+CD25+FoxP3+ Tregs were similar in WT, MyD88−/− and CD11cMyD88+MyD88−/−NOD mice in ALNs and auricular ear draining lymph nodes (ELNs) (Fig. 5a). In contract, there was a higher percentage of CD4+CD25+Foxp3− activated effector T cells in both ALNs and ELNs cells from MyD88−/−NOD mice after CHS induction (Fig. 5b, group B vs A), leading a decreased ratio of Tregs to T effector cells in LNs (Fig. 5c). However, the re-expression of MyD88 in CD11c+ cells restored the frequency of CD4+ T effector cells in LNs to those seen in the control mice (Fig. 5b, group C vs A) as well as the ratio of Tregs to T-effector-cells (Fig. 5c, group C vs group A).
Fig. 5.
Decreased ratio of CD4+CD25+FoxP3+ Tregs/CD4+CD25+ activated T cells in ALNs and ELNs in MyD88−/−NOD mice. (A) Frequencies of Tregs. (B) Frequencies of T effector cells. (C) Ratio of CD4+CD25+FoxP3+ Tregs/CD4+CD25+ activated T cells. WT NOD mice (group A), MyD88−/− (group B) and CD11cMyD88+MyD88−/− (group C) NOD mice were sensitized with 5% PCl. Four days later, mice were challenged on both sides of the ears with PCl. The ALNs and ELNs were collected 24 hours after challenge. ALNC and ELNC were stained with monoclonal antibodies: anti-TCRβ-FITC, anti-CD4-PerCP-Cy5.5, anti-CD25-APC and anti-FoxP3-PE. Gating information for the flow analysis is presented in Fig. S2. N = 3–4, *P <.05; **P <.01; *** P <.001 (Two-tailed Student’s t-test). All error bars represent SE.
4. DISCUSSION
Our novel findings in this study are i) innate immunity mediated by TLRs and MyD88 plays a protective role in CHS responses in autoimmune prone NOD genetic background, which is in contrast to the previously reported in C57BL/6 strain; ii) DCs are important innate cells in CHS responses and intriguingly, iii) the expression of MyD88 in DCs is essential in regulating CHS responses.
The NOD mouse has been an important model of T1D for many years (25). The NOD mice are genetically prone to immune dysregulation, which promotes multi-organ auto-inflammation (16) and allergy (26, 27). Studies have shown that human subjects with autoimmune disorders are more susceptible to allergy (28, 29, 30). Additionally, the association of the innate immunity with genetic makeup, such as C57BL/6 vs NOD, is not known and there has not been published study about CHS response in NOD mice and to our knowledge, our current work is the first investigation in the literature. Thus far, most of CHS studies in the literature used mice on C57BL/6 background. It is important to investigate different genetic backgrounds in order to get closer to translational research as the genetic makeup in human subjects is very different and highly divers. Thus, we believe that our study will provide important information to the scientific community and the public that contact allergy can be very different in different genetic background. The aim of our study was to characterize innate immune mechanisms involved in CHS responses in NOD mice. It is clear that TLRs play an important role in CHS reactions in non-autoimmune prone mouse strains (31, 4). What was not clear is the role of TLRs in the CHS response in NOD strain, considering the multiple autoimmune prone loci of the NOD genetic background. Our study demonstrated that TLRs play a protective role in CHS response in NOD mice, as in the absence of TLR2 or TLR9, NOD mice showed significantly exacerbated CHS response to PCl in vivo and in vitro. Importantly, the hyper-CHS response was even greater in MyD88-deficient NOD mice, which suggested that the enhanced CHS response seen in TLR2- or TLR9-deficient NOD mice is MyD88 dependent. This is in sharp contrast to the published reports in MyD88-deficient C57BL/6 mice, which cannot mount CHS responses (31, 5). Our results support a recent study that MyD88−/−NOD mice developed more severe asthma symptoms than WT mice after airway immunization with ovalbumin (32). Thus, our data in CHS responses and the data from others in airway allergy strongly suggest that MyD88 and its dependent TLR2 and TLR9 molecules function as negative regulators in allergic responses in auto-inflammation prone NOD mice.
The most important finding of our study is the role of DCs in the hyper-CHS response via MyD88. It is known that DCs express a broad repertoire of innate receptors (33) and skin DCs carry dermal antigens including haptens migrate to the local LNs where they prime antigen-specific T cells and induce the expression of skin-specific homing receptors on the primed T cells (34). It is conceivable that the hyper-CHS response in NOD mice, in the absence of TLR2, TLR9 or MyD88, is due to altered function of DCs. To test this hypothesis we examined the DC phenotype and their migration in these mice. We found a higher percentage of CD80+CD11c+IAg7+ DCs in ALNs of PCl sensitized TLR- and MyD88-deficient NOD mice compared with NOD controls. Moreover, we found an increased percentage of migrating FITC+CD11c+ DCs in ALNC of MyD88−/−NOD mice, which had hyper-CHS responses. It is remarkable that re-expression of MyD88 in DCs restored not only the percentage of migrating DCs but also normal CHS responses in vivo and in vitro to the level seen in WT mice. Our results provide direct evidence that DCs play an important role in CHS response and that MyD88 is critical in modulating the migration and function of DCs in CHS reactions.
It was previously reported that during inflammation, DDCs are rapidly mobilized from the skin and home to draining LNs within 48 hours, preceding the arrival of LCs (35). Recent studies in C57BL/6 mice have revealed the role of MyD88 in DC migration and priming of naive CD4+ T-cell responses (36, 37). However, Langerhans cells do not require MyD88-dependent signals for migration in CD8-mediated CHS to DNFB in C57BL/6 mice (38). In our model of CD4-mediated CHS to PCl we found that sensitized MyD88−/−NOD mice had a decreased ratio of Tregs (CD4+CD25+FoxP3+) to activated T cells (CD4+CD25+FoxP3−) in ALNs and ELNs. Studies using ovalbumin as antigen showed that either Langerin+ DDC (39) or CD103− DCs (40) induced Tregs in C57BL/6 mice. In NOD mice, we found an increased frequency of migrating CD11c+CD103+CD207− DDCs but decreased frequency of migrating CD11c+CD103−CD207+ LCs in ALNs, in the absence of MyD88. Interestingly, re-expression of MyD88 in CD11c+ DCs reversed the above migration phenotype to resemble that seen in WT NOD mice. It is thought that in the skin-draining LNs, CD103+ DDCs are responsible for cross-priming naïve T cells and likely induce Th1-type immune responses (41). In contrast, epidermal CD207+CD103− LCs promote Th17 responses to extracellular pathogens, which may ultimately lead to tolerance/anergy (42). In addition, bacteria-primed LCs can drive the development of CD4+Foxp3+ Tregs (43). Thus, it is possible that the enhanced CHS response in TLR- and MyD88-deficient NOD mice is associated with enriched percentages of Th1-inducing CD103+ DDCs in the draining LNs. Decreased percentages of tolerizing CD103−CD207+ DDCs in the draining LNs in TLR- and MyD88-deficient NOD mice do not seem to play a role in upregulation of CHS as the level of Treg cells in LNs is similar in knock out and WT NOD mice. This is also consistent with our results of stronger CHS response in NOD recipients after transfer of CD11c+ cells from MyD88−/−NOD donors. Higher percentage of CD4+ T effector cells also results in a decreased ratio of CD4+CD25+FoxP3+ Treg/CD4+CD25+FoxP3− activated T cells in LNs of MyD88-deficient NOD mice. It is conceivable that the hyper-CHS response and the increase of T effector CD4+ cells found in MyD88-deficient NOD mice is due to lack of MyD88 in CD11c+ DCs and re-expression of MyD88 in CD11c+ DCs restores the phenotype to that observed in WT NOD mice. Increasing evidence suggests that IL-10 is an important link in the regulatory network of DCs, Treg and T effector cells, to prevent excessive immune response (44). Using DC specific deletion of IL-10 receptor (IL-10R) mouse, Girard-Madoux M.J.H. et al demonstrated that DCs are essential to prevent exacerbated T effector cell reactivation in the skin (44). Our finding of the enhanced CHS but decreased IL-10 expression in the skin in MyD88−/−NOD mice comparing to WT NOD mice is in line with Girard-Madoux’s results in DC specific deletion of IL-10R mouse. In summary, our current study provides novel insight into the role of genetic background in CHS responses and the protective role of innate immunity in the auto-inflammation prone NOD mouse strain. More importantly, we revealed direct evidence that MyD88 is critical in modulating CHS reaction via DCs.
Supplementary Material
Acknowledgments
STATEMENT OF FUNDING
Supported by grant from National Science Center UMO-2012/05/B/NZ6/00997 to MS and NIH (DK092882, DK100500 and Diabetes Mouse Core of DK P30-11-015) to LW.
The authors thank Lucy Zhang for taking care of the mouse colonies and the members in LW’s lab for technical help and scientific suggestions.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Majewska-Szczepanik M, Strzepa A, Marcińska K, Wen L, Szczepanik M. Epicutaneous immunization with TNP-Ig and zymosan induces TCRαβ+ CD4+ contrasuppressor cells that reverse skin-induced suppression via IL-17A. Int Arch Allergy Immunol. 2014;164:122–136. doi: 10.1159/000363446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan DH, Igyártó BZ, Gaspari AA. Early events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;13:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askenase PW, Itakura A, Leite-de-Moraes MC, Lisbonne M, Roongapinun S, Goldstein DR, et al. TLR-dependent IL-4 production by invariant Vα14+Jα18+ NKT cells to initiate contact sensitivity in vivo. J Immunol. 2005;175:6390–6401. doi: 10.4049/jimmunol.175.10.6390. [DOI] [PubMed] [Google Scholar]
- 4.Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, et al. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. J Allergy Clin Immunol. 2009;123:875–882. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klekotka PA, Yang L, Yokoyama WM. Contrasting roles of the IL-1 and IL-18 receptors in MyD88-dependent contact hypersensitivity. J Invest Dermatol. 2010;130:184–91. doi: 10.1038/jid.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erkes DA, Selvan S. Hapten-induced contact hypersensitivity, autoimmune reaction, and tumor regression: plausibility of mediating antitumor immunity. J Immunol Res. 2014;2014:175265. doi: 10.1155/2014/175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Kasprick A, Petersen F. Revisiting the role of mast cells in autoimmunity. Autoimmun Rev. 2015;14:751–759. doi: 10.1016/j.autrev.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths MM, Encinas JA, Remmers EF, Kuchroo VK, Wilder RL. Mapping autoimmunity genes. Curr Opin Immunol. 1999;11:689–700. doi: 10.1016/s0952-7915(99)00038-2. [DOI] [PubMed] [Google Scholar]
- 9.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 10.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 11.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Pérez Leirós C. Defective signaling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142:411–418. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis JS, Wan X, Braley-Mullen H. Transient depletion of CD4+ CD25+ regulatory T cells results in multiple autoimmune diseases in wild-type and B-cell-deficient NOD mice. Immunology. 2013;139:179–186. doi: 10.1111/imm.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao YT, Cheng WH, Liao WC, Lin CL, Shen TC, Chen WC, et al. Type 1 diabetes and increased risk of subsequent asthma. Medicine (Baltimore) 2015;94:e1466. doi: 10.1097/MD.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzedine K, Barbarot S. Atopic dermatitis and type-1 diabetes mellitus: a true positive association? Br J Dematol. 2016;174:88–94. doi: 10.1111/bjd.14350. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 16.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmun Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 18.Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–315. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- 19.Klechevsky E. Human dendritic cells – stars in the skin. Eur J Immunol. 2013;43:3147–3155. doi: 10.1002/eji.201343790. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. Decisions about dendritic cells: past, present and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DH. In vivo function of Langerhans cells and dermal DC. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idoyaga J, Florese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson JA, Wang S, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham PT, Elliot CE, Lenzo JC, Jarnicki AG, Larcombe AN, Zosky GR, et al. Sensitizing and Th2 adjuvant activity of cysteine protease allergens. Int Arch Allergy Immunol. 2012;158:347–358. doi: 10.1159/000334280. [DOI] [PubMed] [Google Scholar]
- 27.Araujo LM, Lefort J, Nahori MA, Diem S, Zhu R, Dy M, et al. Exacerbated Th2-mediated airway inflammation and hyperresponsiveness in autoimmune diabetes-prone NOD mice: a critical role for CD1d-dependent NKT cells. Eur J Immunol. 2004;34:327–335. doi: 10.1002/eji.200324151. [DOI] [PubMed] [Google Scholar]
- 28.Caffarelli C, Cavagni G, Pierdomenico R, Chiari G, Spattini A, Vanelli M. Coexistence of IgE-Mediated Allergy and Type 1 Diabetes in Childhood. Int Arch Allergy Immunol. 2004;134:288–294. doi: 10.1159/000079166. [DOI] [PubMed] [Google Scholar]
- 29.Shen TC, Chen HJ, Wei CC, Chen CH, Tu CY, Hsia TC, et al. Risk of asthma in patients with primary Sjögren’s syndrome: a retrospective cohort study. BMC Pulm Med. 2016;16:152. doi: 10.1186/s12890-016-0312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maas T, Nieuwhof C, Passos VL, Robertson C, Boonen A, Landewé RB, et al. Transgenerational occurrence of allergic disease and autoimmunity: general practice-based epidemiological research. Prim Care Respir J. 2014;23:14–21. doi: 10.4104/pcrj.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin SF, Dudda JC, Bachtanian EA, Lembo A, Liller S, Dürr C, et al. Toll-like receptor and IL-12 signaling control susceptibility contact hypersensitivity. J Exp Med. 2008;205:2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aumeunier A, Grela F, Ramadan A, Pham Van L, Bardel E, Gomez Alcala A, et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS One. 2010;5:e11484. doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edele F, Molenaar R, Gütle D, Dudda JC, Jakob T, Homey B, et al. Cutting edge: Instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 35.Tay SS, Roediger B, Tong PL, Tikoo S, Weninger W. The skin-resident immune network. Curr Dermatol Rep. 2013;3:13–22. doi: 10.1007/s13671-013-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollampalli VP, Yamashiro LH, Feng X, Bierschenk D, Gao Y, Blom H, et al. BCG skin infection triggers IL-1R-MyD88-Dependent migration of EpCAMlow CD11bhigh skin dendritic cells to draining lymph node during CD4+ T-cell priming. PLoS Pathog. 2015;11:e1005206. doi: 10.1371/journal.ppat.1005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKee AS, Mack DG, Crawford F, Fontenot AP. MyD88 dependence of beryllium-induced dendritic cell trafficking and CD4+ T-cell priming. Mucosal Immunol. 2015;8:1237–1247. doi: 10.1038/mi.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haley K, Igyártó BZ, Ortner D, Bobr A, Kashem S, Schenten D, et al. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012;188:4334–4339. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azukizawa H, Döhler A, Kanazawa N, Nayak A, Lipp M, Malissen B, et al. Steady state migratory RelB1+ langerin1+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:1420–1434. doi: 10.1002/eji.201040930. [DOI] [PubMed] [Google Scholar]
- 40.Guilliams A, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 41.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207:953–961. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igyártó BZ, Kaplan DH. Antigen presentation by Langerhans cells. Curr Opin Immunol. 2013;25:115–119. doi: 10.1016/j.coi.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Aar AM, Picavet DI, Muller FJ, de Boer L, van Capel TM, Zaat SA, et al. Langerhans cells favor skin flora tolerance through limited presentation of bacterial antigens and induction of regulatory T cells. J Invest Dermatol. 2013;133:1240–1249. doi: 10.1038/jid.2012.500. [DOI] [PubMed] [Google Scholar]
- 44.Girard-Madoux MJH, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell–induced T-cell reactivation in the skin to limit contact hypersensitivity. J Allergy Clin Immunol. 2012;129:143–150. doi: 10.1016/j.jaci.2011.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.