Summary
Sleep timing shifts later during adolescence (second decade). This trend reverses at ~20 years and continues to shift earlier into adulthood. The current analysis examined the hypothesis that a longer free-running circadian period during late adolescence (14–17 years) compared to adulthood (30–45 years) accounts for sleep timing differences. Sex and ancestry were also examined because previous reports find that women and those with African-American ancestry have shorter free-running periods. Circadian period was measured using an ultradian dark-light protocol (2h dark/sleep, 2h dim room light (~20 lux)/wake) over 3.4 days. Dim light melatonin onsets were measured before and after the ultradian protocol, from which circadian period was derived. In contrast to our hypothesis, we found that that free-running circadian period was similar in adolescents and adults. African-American adults had shorter free-running circadian periods compared to adults of other ancestries. This ancestry difference was not seen in the adolescent group. Finally, we observed a non-significant trend for shorter free-running circadian periods in females compared to males. These data suggest that age-related changes in circadian period after late adolescence do not account for sleep timing differences. These data provide further support for ancestry-related differences in period, particularly in adults. Whether the large difference in circadian period between African American and other ancestries emerge later in development should be explored.
Keywords: tau, race, DLMO, forced desynchrony, delay
Sleep timing shifts later over the course of adolescence (second decade; age 10 to 20 years) (Roenneberg et al., 2004, Crowley et al., 2014), and older adolescents endorse more evening-type behavior compared to young adolescents (Carskadon et al., 1993, Giannotti et al., 2002). Sleep timing on non-school nights, which is more likely to be driven by internal sleep regulatory systems than on school days, delays by as much as 2.5 to 3 h (Roenneberg et al., 2004, Crowley et al., 2014) with peak “lateness” occurring at about age 18 to 20 years (Roenneberg et al., 2004, Fischer et al., 2017). This delay shift in sleep timing reverses in the third decade of life and continues to advance (shift earlier) into adulthood (Roenneberg et al., 2004, Fischer et al., 2017).
Free-running circadian period predicts how the circadian system entrains to the 24-h light-dark (LD) cycle (Duffy et al., 1999, Wright et al., 2001, Wright et al., 2005, Burgess and Eastman, 2008, Eastman et al., 2015, Eastman et al., 2016) and is correlated with morningness/eveningness (Duffy et al., 2001). Therefore, it has been suggested that a long free-running circadian period may explain the late sleep patterns of older adolescents (Carskadon et al., 2004). We compared free-running circadian period of late to post-pubertal adolescents aged 14 to 17 years and adults aged 30 to 45 years to determine whether differences in free-running circadian period could explain age-related differences in sleep and circadian rhythm timing. We chose late adolescence to control for puberty stage and because this is the time when sleep timing is late and free-running circadian period is predicted to be long. A narrow age range of adults older than 30 was chosen to ensure a developmentally distinct comparison group, who typically shows earlier sleep timing than older adolescents. Ancestry and sex differences in free-running circadian period were also examined within each age group because previous reports in adults show shorter free-running circadian periods in females compared to males (Duffy et al., 2011, Eastman et al., 2017) and in African-Americans compared to White/European-Americans (Smith et al., 2009, Eastman et al., 2012, Eastman et al., 2015, Eastman et al., 2016, Eastman et al., 2017).
Method
Participants
Forty-four participants (21 males) aged 14.3 to 17.8 years (“adolescents”) and 44 participants (23 males) aged 30.8 to 45.8 years (“adults”) completed the study protocol. Table 1 includes demographic information about the adolescent and adult samples. Participants self-reported their race/ancestry by choosing one of the following: White/Caucasian, Asian/Asian-American, Native American/Alaskan Native, Native Hawaiian/Pacific Islander, Black/African-American, multiracial, or specified another race/ancestry. They also self-reported their ethnicity by choosing either Not Hispanic/Latino or Hispanic/Latino. Adolescent reports of race and ethnicity were confirmed by a parent using the same response categories. Circadian phase preference was measured with the Morningness Questionnaire of Smith and colleagues (1989), which ranges in score from 13 (eveningness) to 55 (morningness). Mid-sleep on free days (corrected for weekend oversleep) was assessed using the Munich Chronotype Questionnaire (Roenneberg et al., 2004).
Table 1.
Adolescent and middle-aged adult sample demographics.
| Adolescents | Adults | ||
|---|---|---|---|
| Total N | 44 | 44 | |
| Age (years) | 16.2 ± 1.0 | 38.0 ± 4.2 | |
| (14.3 – 17.8) | (30.8 – 45.8) | ||
| Sex (N) | |||
| Females | 23 | 21 | |
| Males | 21 | 23 | |
| Ancestry/race (N) | |||
| African American | 19 | 15 | |
| White | 16 | 26 | |
| Other1 | 9 | 3 | |
| Ethnicity (N) | |||
| Non-Hispanic | 34 | 39 | |
| Hispanic2 | 10 | 5 | |
| Morningness Score | 36.8 ± 5.3 | 38.8 ± 6.4 | |
| % Morning Type | 7 | 30 | |
| Mid-sleep on free days3 | 4:27 ± 1:13 | 4:25 ± 1:14 | |
| Home Sleep Schedule4 | |||
| Lights off | 23:40 ± 1:11 | 23:03 ± 1:10 | |
| Lights on | 08:40 ± 1:11 | 8:03 ± 1:10 | |
The Other category of self-reported ancestries in the adolescent sample includes multiracial (n=7), Native American/Alaskan Native (n=1), and unknown (n=1); adult sample includes Asian (n=2) and unknown (n=1).
The Hispanic adolescent sample includes these self-reported ancestries: African American (n=1), White (n=5), Native American/Alaskan Native (n=1), multiracial (n=2), and unknown (n=1). The Hispanic adult sample includes these self-reported ancestries: White (n=4) and unknown (n=1).
N=41 for the adolescent group; n=43 for the adult group.
Home sleep schedules were tailored to each individual based on pre-study sleep logs.
Participants were healthy and without personal histories of a sleep disorder, psychotic disorder, bipolar disorder, or neurological disorder, and without a diagnosis of any chronic medical conditions or developmental disorder. Participants were medication-free, except for two adolescent and two adult females who were taking oral contraceptives. Participants did not endorse depressive symptoms (scored ≤ 16 on the Center for Epidemiologic Studies – Depression (CES-D) scale (Radloff, 1977)). Participants did not travel more than 2 time zones within the month before starting the study. Participants reported their usual sleep duration was between 6 and 10 hours and were no more than moderate caffeine users (<300 mg/day). In the adolescent sample, Body Mass Index (BMI; kg/m2), computed from measurements of weight and height taken in the laboratory, ranged from 16.5 to 32.1, which corresponds to the 6th to 97th percentiles based on age and sex according to the child and teen criteria set by the Centers for Disease Control. BMI ranged from 17.3 to 34.9 in the adult sample. Participants were not color blind or deficient as measured by the Ishihara Color Blindness test.
A board-certified pediatric physician examined adolescent participants to determine pubertal status based on the criteria of Tanner (1962). The study’s entry criteria required that adolescents be late to post-pubertal (Tanner stage 4 or 5). The same physician examined all adolescent participants in the study and was blinded to the eligibility criterion.
The study was approved by the Rush University Medical Center’s Institutional Review Board, in compliance with the Declaration of Helsinki. Adult participants provided written consent to take part in the study. A parent provided written consent for their child, and the adolescent co-signed the consent forms to acknowledge assent. All participants were paid.
Protocol
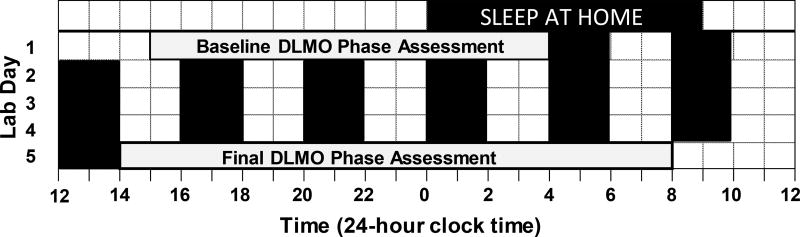
As part of a larger study constructing phase response curves (PRCs) to bright light (Crowley and Eastman, 2017), participants completed the 2-week protocol shown in Figure 1 twice in a counterbalanced order. During one laboratory protocol, participants were exposed to bright light. During the other laboratory protocol, participants remained in dim room light. We only used data from the dim light protocol for the current analysis to compute free-running circadian period. Twenty-one participants completed the dim light protocol first and 23 completed the dim light protocol second. There were 8 days between the two protocols with participants sleeping on an individualized fixed 9-h sleep schedule at home (see below). Adolescent participants participated in the 5-day laboratory protocols during their school summer vacation (June, July, and August). To limit the range in photoperiod between age groups, adults completed the study in May, June, July, August, and September.
Figure 1.
Protocol to measure the free-running circadian period in adults and adolescents. After 8 or 9 days of sleeping on an individualized fixed 9-h sleep schedule at home, participants completed a 5-day laboratory protocol. On days 1 and 5, the salivary dim light melatonin onset (DLMO) was measured in < 5 lux. An ultradian light-dark (LD) cycle occurred in the intervening 3.4 days; 2-h sleep/dark (black rectangles) alternated with 2 h of required wake in dim room light (~20 lux). The ultradian LD cycle produced forced desynchrony, which allowed the circadian system to free run. DLMO phase shift from baseline to final phase assessments was used to calculate the free-running circadian period.
Participants were required to keep a fixed 9-h sleep schedule at home for 8 or 9 days before they were admitted to the laboratory for 5 consecutive days (Figure 1). Sleep schedules at home were individualized and based on their reported pre-study sleep times. Bedtimes (lights out) ranged from 21:00 to 02:00 in the adolescents and 21:30 to 01:30 in the adults. Thus, wake-up time (lights on) ranged from 06:00 to 11:00 for the adolescents and 06:30 to 10:30 for the adults. Participants wore an actigraph (Actiwatch Spectrum, Philips Respironics, Inc., Bend OR) on their non-dominant wrist, completed daily sleep logs, and telephoned daily to a time-stamped voicemail messaging system at bedtime and wake time to measure compliance to the home sleep/wake schedule. When sleeping at home, participants visited the lab every 2 to 3 days to download the actigraphy data and review sleep logs with them; participants were questioned about inconsistencies between the actogram and sleep logs.
Following the stable 9-h sleep schedule at home, participants arrived at noon to the laboratory and stayed for 5 consecutive days (Figure 1). On days 1 and 5, participants completed a circadian phase assessment to determine the dim light melatonin onset (DLMO). Salivary melatonin concentration was measured from approximately 2 mL of saliva collected every 30 minutes using Salivettes (Sarstedt, Nümbrecht, Germany). Baseline saliva sampling began at 15:30 and ended at 03:30. Final saliva sampling began at 14:30 and ended at 08:00 the next day. Participants remained awake in dim light (< 5 lux) sitting in comfortable recliners, except when they needed to use the attached washroom (also < 5 lux). They were not allowed to eat or drink in the 10 mins before each sample and washroom trips were discouraged during this time. Saliva samples were immediately centrifuged and frozen. Melatonin concentrations were measured using commercially available radioimmunoassay (RIA) kits (Alpco, Salem NH, USA) by SolidPhase, Inc. (Portland, ME). An individual’s samples were analyzed in the same batch. Intra-assay coefficients of variation for low and high levels of salivary melatonin were 4.1% and 4.8%, respectively. Inter-assay coefficients of variation for low and high levels of salivary melatonin were 6.6% and 8.4%, respectively.
An ultradian light-dark (LD) cycle (2h light:2h dark) began immediately after the baseline phase assessment and continued for 3.4 days. All participants were on the same schedule shown in Figure 1. This LD cycle creates forced desynchrony; a 4-h day is beyond the limits of entrainment, and therefore the circadian clock free runs (Kripke et al., 2007, Burgess and Eastman, 2008, Smith et al., 2009, Eastman et al., 2012, Eastman et al., 2015, Eastman et al., 2016) even when the ratio of light to dark is equal (Borbely and Huston, 1974). During the 2-h sleep/dark episodes, all participants laid in bed in the dark and were instructed to try to sleep. Dividers were set up between the beds. During the intervening wake/light episodes, participants engaged in quiet activities (e.g., board games, reading, watched pre-recorded television shows or movies). Uncaffeinated food and drink were available ad libitum. Participants were unaware of the time of day and the length of the sleep episodes during the ultradian LD protocol. They did not have access to cell phones, clocks, or anything that displayed time. The windowless experimental room was illuminated by three fluorescent (4100 K) ceiling fixtures controlled by dimmer switches locked to the lowest position. Light levels averaged 23.9 ± 7.1 lux at the angle of gaze during the wake episodes.
Participants were instructed to abstain from medications that influence endogenous melatonin (e.g., non-steroidal anti-inflammatory drugs) or sleep (e.g., antihistamines) throughout the study. Recreational drugs and nicotine were prohibited throughout the study. Alcohol was not allowed in the 5 days before the laboratory session. Urine toxicology screens for common drugs of abuse, including nicotine, and breath alcohol tests confirmed compliance. Caffeine was prohibited in the 72 h before the laboratory session.
Statistical Analysis
DLMO was determined by linear interpolation across the time points before and after the melatonin concentration increased to and stayed above 4 pg/mL (Carskadon et al., 1997, Crowley et al., 2016). Three adolescent participants showed a clear rise of melatonin, but were high melatonin secretors (n=2) or low melatonin secretors (n=1). The thresholds to compute DLMO were therefore adjusted to 10 pg/mL and 1 pg/mL, respectively. Phase shifts were defined as baseline DLMO – final DLMO. The DLMO phase shift from the baseline to final phase assessments was divided by 4 days to derive the daily phase shift because there were 4 days between phase assessments. Circadian period was computed using the following: |daily phase shift – 24 h|.
An analysis of variance with factors age, sex, and ancestry tested our hypotheses: 1) adolescents have longer free-running circadian period compared to adults (main effect of age); 2) females have shorter free-running circadian period than males (main effect of sex); and 3) African-American participants have shorter free-running periods than other ancestries (main effect of ancestry). Age-by-sex and age-by-ancestry interactions were also examined to test whether the hypothesized main effects differed by age group. Effect sizes for each comparison were computed using Cohen’s d (mean difference/pooled variance) and described with the criterion proposed by Cohen (0.2=small; 0.5=medium; 0.8=large) (Cohen, 1988).
Results
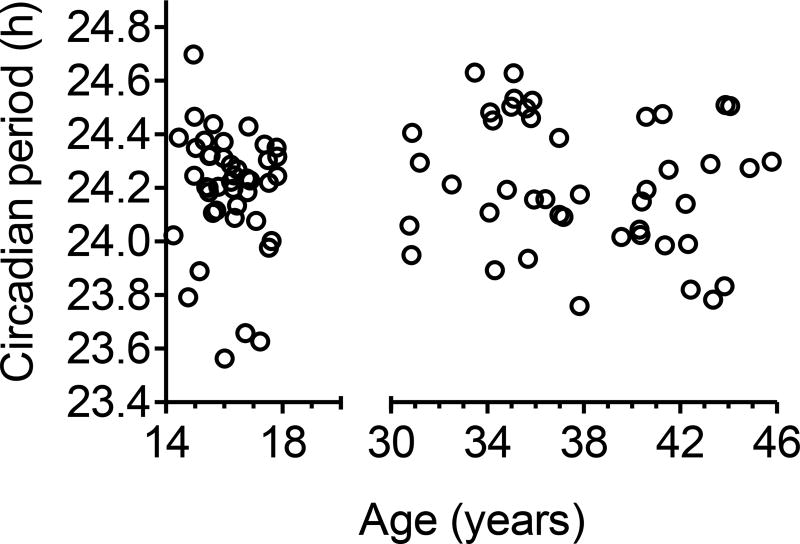
In the adolescents, free-running circadian period ranged from 23.56 to 24.70 h, and 16% had a circadian period ≤C; 24 h. In the adults, circadian period ranged from 23.76 to 24.63 h, and 20% had a circadian period ≤ 24 h. Circadian period did not differ between adolescents (24.19 ± 0.22 h) and adults (24.22 ± 0.24 h) [F(1,80)=0.06, p=0.82], and was not correlated with age within either age group (Figure 2).
Figure 2.
Free-running circadian period measured in 44 adolescents (14.3–17.8 years) and 44 middle-aged adults (30.8 to 45.8 years) in an ultradian LD protocol plotted with respect to their age.
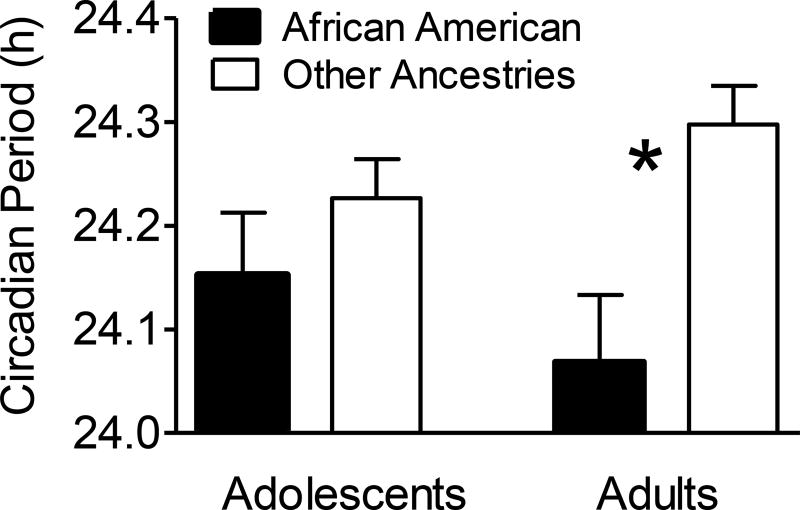
African Americans had shorter free-running circadian periods compared to all other ancestry groups combined [24.12 ± 0.20 h vs. 24.26 ± 0.20 h; F(1,80)=10.21, p=.002], but this difference was greater for the adult group compared to the adolescent group (Figure 3). As shown in Table 2, circadian period for adults differed by 13.8 minutes, on average, between African-American and other ancestries [t(42)=3.30, p=.002], whereas free-running circadian period did not differ between African-American and other ancestries in the adolescent sample [t(42)=1.09, p=.34]. A similar pattern emerged when only non-Hispanic African American and non-Hispanic White participants were included in the analysis.
Figure 3.
Mean free-running circadian period (+ SEM) for African Americans versus all other ancestries in the sample of adolescents (N=19 and N=25, respectively) and adults (N=15 and N=29, respectively). Other ancestries included mostly White (N=42), but also Native American/Alaskan Native, Asian, more than one ancestry, and unknown (see Table 1). *p<.01
Table 2.
Mean ± SD, mean difference, and effect sizes for free-running circadian period comparisons between sex and ancestry within and between age groups.
| Adolescents | Adults | Mean Age Difference | ||
|---|---|---|---|---|
| 24.19 ± 0.22 h | 24.22 ± 0.24 h | 1.8 mins (d=0.13) | ||
| Ancestry: | ||||
| African American | 24.15 ± 0.26 h | 24.07 ± 0.25 h | 4.8 mins (d=0.31) | |
| Non-African American1 | 24.23 ± 0.19 h | 24.30 ± 0.20 h | 4.2 mins (d=0.36) | |
| Mean Ancestry difference | 4.8 mins (d=0.36) | 13.8 mins* (d=1.05) | ||
| Sex: | ||||
| Male | 24.23 ± 0.22 h | 24.26 ± 0.24 h | 1.8 mins (d=0.13) | |
| Female | 24.16 ± 0.23 h | 24.17 ± 0.24 h | 0.6 mins (d=0.04) | |
| Mean Sex difference | 4.2 mins (d=0.31) | 5.4 mins (d=0.37) | ||
p < .01; Cohen’s d effect size: 0.2 = small; 0.5 = medium; 0.8 = large
White + Other ancestry categories (see Table 1).
Note: Age did not differ between African American and non-African American adolescents or adults. Age did not differ between male and female adolescents or adults.
Descriptively, African-American adolescents had a longer average free-running circadian period compared to African-American adults (4.8-min difference), whereas the opposite pattern emerged in the other ancestry categories (4.2-min difference). These differences showed small effects sizes (Table 2), however, and did not reach statistical significance (p’s > 0.19).
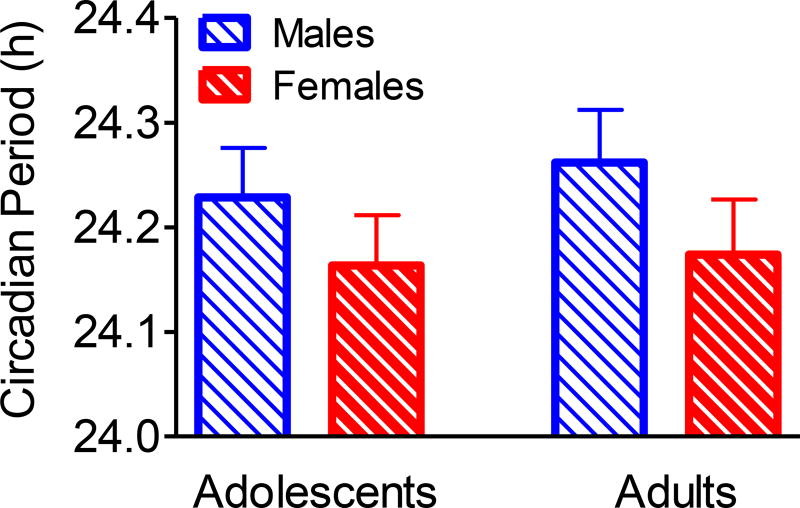
Circadian period did not differ between males and females (Figure 4 and Table 2), though means trended in the expected direction with females having a shorter circadian period compared to males [F(1,80)=2.75, p=0.10]. The difference was 4.7 mins, on average. This pattern was similar in both adults and adolescents as there was no age-by-sex interaction [F(1,80)=0.09, p = 0.77]. Also, there were no sex differences when examined within age groups (Table 2) or within ancestry categories (African American and all other races combined; data not shown).
Figure 4.
Mean free-running circadian period (+ SEM) for males and females in the sample of adolescents (N=21 and N=23) and middle-aged adults (N=21 and N=23). Differences between males and females did not reach statistical significance (p=0.1).
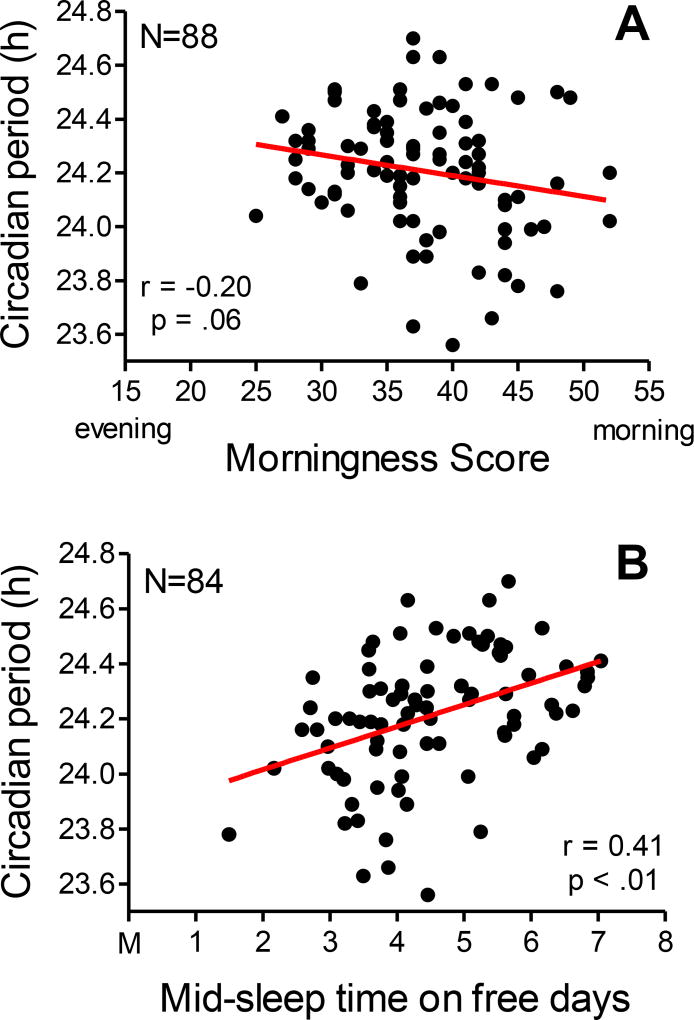
Adolescent morningness scores ranged from 28 to 52 and adult scores ranged from 25 to 52. Means did not differ between age groups (p=0.12), but there were more morning types (score ≥ 44) in the adult group (N=13) than the adolescent group (N=3) [χ2 = 7.64, p < .01]. There were no evening types (scores ≤ 22) in either group. Mid-sleep times on free days ranged from 02:43 to 06:51 in adolescents and 01:29 to 07:02 in adults; means did not differ between age groups (p=0.58). We found a trend for an association between morningness and circadian period when age groups were combined (Figure 5A); morningness was associated with shorter free-running circadian periods. This trend did not persist when the age groups were examined separately (p’s >.15). Later mid-sleep times on free days were associated with longer free-running circadian periods in the adolescent group (r=0.35, p=.03), the adult group (r=0.47, p<.01), and when age groups were combined (Figure 5B). There were no sex or ancestry differences in morningness score or mid-sleep time on free days.
Figure 5.
Scatter plots showing associations between free-running circadian period and morningness score (A) and mid-sleep time on free days (B). Age groups are combined.
Discussion
Free-running circadian period was similar in adolescents aged 14 to 17 years and adults aged 30–45 years. African-American adults had a shorter free-running circadian period compared to adults of the other ancestries studied. This large ancestry difference in circadian period was not seen in the adolescent group. Finally, we observed a trend of shorter free-running circadian periods in females compared to males similar to previous studies in adults (Duffy et al., 2011, Eastman et al., 2017), though this difference was not statistically significant.
Our finding that free-running circadian period was similar in adolescents and adults was unexpected. We hypothesized that circadian period would be longer in adolescents than adults, and would partly explain late sleep times during this developmental stage. Morningness score and mid-sleep time on free days also did not differ between age groups (Table 1) and may contribute to the lack of difference seen in circadian period between the adolescents and adults. Mid-sleep times on free days measured from the Munich Chronotype Questionnaire averaged ~04:30 in both age groups, which is close to previous estimates in adolescents, but is descriptively later than previous estimates for adults aged 30–45 years, which range between ~03:15 and 03:45 (Fischer et al., 2017). It is possible that some adults were late because they required flexible schedules or were unemployed to participate in our study, which required living in our laboratory for 5 consecutive days twice. On the other hand, about 30% of the adults were morning types compared to only 7% of adolescents. Moreover, mid-sleep times at home in the 8 or 9 days before the laboratory session were about 40 mins later in the adolescents (04:10) compared to the adults (03:33), on average (calculated from Home Sleep Schedule, Table 1), which is consistent with previous reports (Fischer et al., 2017). Therefore, similarities in circadian phase preference and chronotype may not completely explain these unexpected results.
Our estimate of free-running circadian period in this group of late to post-pubertal 14 to 17 year olds averaged 24.19 h, which is descriptively shorter than other estimates of circadian period in adolescents. Carskadon and colleagues (1999) provided the first estimate of circadian period averaging 24.33 h (SD=0.21 h; range: 24.08 to 24.60 h) in mostly Tanner 4 and 5 adolescents (N=10, 5 girls, 13.2–15.2 years) run in a 28-h forced desynchrony protocol. Average circadian period was 24.27 h in a larger group of adolescents (N=27, 14 girls; Tanner 1–5, 9–15 years) they studied in the same 28-h forced desynchrony protocol (Carskadon et al., 2004). Ancestry may account for differences between adolescent estimates of circadian period. The Carskadon samples (1999, 2004) were recruited from Providence, Rhode Island and the surrounding areas and the current sample was recruited from Chicago, Illinois and the surrounding areas. Our previous report (Eastman et al., 2017) showed that circadian period of African-American adults (24.07 h, N=32) was shorter than European-Americans (24.33 h, N=31). Forty-three percent of the current group of older adolescents were self-described African Americans. The percent of African-Americans in the Carskadon sample is unknown, but there were likely fewer African-American adolescents based on the racial composition of Rhode Island. We did not find a statistical difference in free-running circadian period between African-American adolescents and adolescents of other ancestries; however, means were in the expected direction. Our sample with more African-Americans may have decreased the mean estimate of free-running circadian period and more adolescents of other ancestries may have increased circadian period estimates reported by Carskadon and colleagues. Further support for this reasoning comes from the similarity between free-running circadian periods of the non-African-American adolescents in the current sample (24.23 h) and that of the Carskadon sample (24.27 h).
The sample of adolescents studied by Carskadon and colleagues also included younger pre- to mid-pubertal adolescents, whereas the current sample only included late and post-pubertal adolescents. Our older group of adolescents had descriptively shorter average free-running circadian periods than the group with younger adolescents, however, which is the opposite of what is hypothesized if circadian period predicts the later sleep timing of late adolescence. When compared to adults (Czeisler et al., 1999, Wright et al., 2001), Carskadon and colleagues (2004) found free-running circadian period of the pre- to post-pubertal adolescents was about 9 minutes longer than adults aged 21 to 41 years. By contrast, our estimate of circadian period in late and post-pubertal adolescents was similar to adults in the current study, and close to estimates (24.15 ± 0.2 h) from adults aged 18 to 74 years (Duffy et al., 2011). These data indicate that late and post-pubertal adolescents have adult-like circadian periods. It is possible that circadian period at earlier stages of puberty in humans may be altered.
A third possible reason for the differences in free-running circadian period between the current study’s adolescent group and the group studied by Carskadon and colleagues (1999, 2004) could be the different T cycles of the forced desynchrony (FD) (4-h day vs. 28-h day). These protocols produced different light exposures before and during the FD. A dim light history can increase sensitivity to the non-photic effects of light (Hebert et al., 2002), and may have affected circadian period. It is difficult, however, to determine how light sensitivity may have differed between our ultradian FD and the infradian FD. Not only is dark distributed differently, but the duration of days in the laboratory also differed. In the current study, participants were exposed to a total of 12 h of dim room light (~24 lux) per day for 3.4 days (40.8 h total), whereas in the 28-h FD, adolescents were exposed to 16.33 h of dim room light (15–20 lux) for 14 days (229 h total). Before the FD cycles began, our participants were in very dim light (< 5 lux) for 12 h and the Carskadon sample was in dim room light (15–20 lux) for 36 h. Conceivably, exposure to dim light for a longer duration before and during the 28-h FD protocol may have increased sensitivity to light in the Carskadon study and may explain the longer free-running circadian period in their sample compared to the current sample. To determine whether T cycles influence circadian period estimates, however, sex and ancestry would need to be controlled.
It is unclear why our adolescent sample did not show the large differences in free-running circadian period between ancestry groups as the adults. We did not prospectively aim to examine ancestry differences in this sample, and therefore only used a self-report item to identify race/ancestry. A more comprehensive assessment of ancestry, such as collecting biological parents’ ancestry and perhaps including genetic ancestry profiles, may be needed to determine whether this difference also manifests during adolescence. Alternatively, the difference between ancestries occurs later than the adolescent years.
The current study found a trend for a sex difference, with females having a circadian period that was about 4 to 5 mins shorter than males. Duffy and colleagues (2011) found that adult women (N=52) are more likely to have a short circadian period compared to adult men (N=105) aged 18 to 74 years. The average difference between men and women was about 6 minutes. Previously, we found circadian period was 10 mins shorter in non-Hispanic European-American adult women (N=16) compared to non-Hispanic European-American adult men (N=15), but this sex difference did not appear in African-American adults (Eastman et al., 2017). When we tested for sex differences in circadian period (age groups combined) in those who were not African-American (N=26 males and N=28 females) and for those who were non-Hispanic White (N=18 males and 15 females), the difference in free-running circadian period between males and females was about 6 mins. This average sex differences is similar to that of Duffy and colleagues, but a non-significant trend continued to emerge. It is possible that our sample was too small to detect a statistical difference.
The current analysis suggests that late sleep timing of late to post-pubertal adolescents is not related to free-running circadian period, and that other factors are likely driving this delay shift in sleep timing. These data provide further support for ancestry-related differences in circadian period in adults. Whether the difference in circadian period between African American and other ancestries emerge later in development could be explored.
Acknowledgments
Research reported in this publication was supported by NHLBI/NIH grant R01 HL105395 to SJC.
Footnotes
Conflicts of Interest: Stephanie J. Crowley, PhD has no actual or potential conflicts of interest to disclose; Charmane I. Eastman, PhD has no actual or potential conflicts of interest to disclose.
Author contributions: SJC conceived and designed the experiment, coordinated the study, analyzed the data, created the figures and tables, and wrote the manuscript. CIE designed the experiment and edited the figures, tables, and manuscript.
References
- Borbely AA, Huston JP. Effects of two-hour light-dark cycles on feeding, drinking and motor activity of the rat. Physiol Behav. 1974;13:795–802. doi: 10.1016/0031-9384(74)90264-9. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–76. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann NY Acad Sci. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–32. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed) Lawrence Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- Crowley SJ, Eastman CI. Human adolescent phase response curves to bright white light. J Biol Rhythms. 2017;32:334–44. doi: 10.1177/0748730417713423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Suh C, Molina TA, Fogg LF, Sharkey KM, Carskadon MA. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Med. 2016;20:59–66. doi: 10.1016/j.sleep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, Lebourgeois MK, et al. A Longitudinal Assessment of Sleep Timing, Circadian Phase, and Phase Angle of Entrainment across Human Adolescence. PLoS One. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Invest Med. 1999;47:141–50. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–99. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans) Chronobiol Int. 2012;29:1072–77. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Suh C, Tomaka VA, Crowley SJ. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. 2015;5:8381. doi: 10.1038/srep08381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci Rep. 2016;6:36716. doi: 10.1038/srep36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. Sex and ancestry determine the free-running circadian period. J Sleep Res. 2017;26:547–50. doi: 10.1111/jsr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US - Influence of age and sex. PLoS One. 2017;12:1–17. doi: 10.1371/journal.pone.0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psych. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. Growth at Adolescence. Blackwell; Oxford: 1962. [Google Scholar]
- Wright KP, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98:14027–32. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]