Abstract
Objective
The Apolipoprotein E (APOE) ε4 allele is the most important genetic risk factor for late onset Alzheimer’s disease (LOAD). Many ε4 carriers, however, never develop Alzheimer’s disease. The purpose of this study is to characterize the variability in phenotypic expression of the ε4 allele, as measured by the longitudinal trajectory of cognitive test scores and MRI brain volumes, in cognitively intact elders.
Method
Healthy older adults, ages 65–85, participated in a five-year longitudinal study that included structural MRI and cognitive testing administered at baseline and at 1.5 and 5 years post-enrollment. Participants included 22 ε4 non-carriers, 15 ε4 carriers who experienced a decline in cognition over the five-year interval, and 11 ε4 carriers who remained cognitively stable.
Results
No baseline cognitive or volumetric group differences were observed. Compared to non-carriers, declining ε4 carriers had significantly greater rates of atrophy in left (p=.001, Cohen’s d=.691) and right (p=.003, d=.622) cortical gray matter, left (p=.003, d=.625) and right (p=.020, d=.492) hippocampi, and greater expansion of the right inferior lateral ventricle (p<.001, d=.751) over five years.
Conclusions
This study illustrates the variability in phenotypic expression of the ε4 allele related to neurodegeneration. Specifically, only those individuals who exhibited longitudinal declines in cognitive function experienced concomitant changes in brain volume. Future research is needed to better understand the biological and lifestyle factors that may influence the expression of the ε4 allele.
Keywords: APOE ε4, Aging, Alzheimer’s disease, MRI, Cognition
The apolipoprotein E (APOE) ε4 allele confers an increased risk for developing late onset Alzheimer’s disease (LOAD; Corder et al., 1993; Liu et al., 2015; Smith et al., 1998). APOE ε4 homozygotes experience a 13–15 fold increased chance of being diagnosed during their lifetime, whereas ε4 heterozygotes experience a threefold increase (Ashford, 2004). Even prior to the diagnosis of Mild Cognitive Impairment (MCI) or LOAD, older adults possessing an APOE ε4 allele experience greater cognitive deficits in episodic memory, executive functioning, perceptual speed, and global cognition than non-carriers (Wisdom, Callahan, & Hawkins, 2011). Longitudinal studies of cognitively intact, older ε4 carriers and non-carriers demonstrate greater cognitive decline in the former group over time (Pietrzak et al., 2015; Rao et al., 2015; Woodard et al., 2010). Likewise, longitudinal structural MRI studies indicate significantly different trajectories of neurodegeneration in the hippocampus and ventricles between cognitively intact, older ε4 carriers and non-carriers (Lu et al., 2011; Reiter et al., 2017; Roussotte et al., 2014).
Such studies tend to treat ε4 carriers as a homogeneous group. Yet, many cognitively intact elders with the ε4 allele never develop LOAD during a normal lifespan (Relkin, Kwon, Tsai, & Gandy, 1996). A lack of uniform deterioration among ε4 carriers may be explained by biological (e.g. APOE ε4 zygosity, co-morbid medical illnesses) and/or lifestyle (e.g. education, physical activity) factors that likely modulate the impact of the ε4 allele on the development of AD (Schwartz, Rapkin, & Healy, 2016; Small, Rosnick, Fratiglioni, & Backman, 2004; Smith et al., 2014).
The primary purpose of this longitudinal study is to examine the variability in phenotypic expression of the ε4 allele in elders who were cognitively intact at study entry. Over the course of the five-year interval, a subset of ε4 carriers experienced a decline in cognitive status (“ε4+ Declining”). We hypothesized that the ε4+ Declining group would experience greater volumetric changes as measured by structural MRI than ε4 carriers who experienced no meaningful changes in cognitive performance over the five-year interval (“ε4+ Stable”) and ε4 non-carriers (“ε4−”). A previous study conducted on this sample (Reiter et al., 2017) identified differences in longitudinal changes in hippocampal volume, global gray matter volume, and ventricular size between ε4 carriers and non-carriers. The present study focused on differences in the rate of volumetric change in these anatomical regions between Stable and Declining ε4 carriers and non-carriers.
Method
Participants
Healthy, cognitively intact older adults, ages 65–85, were recruited as part of a five-year longitudinal study (Rao et al., 2015; Seidenberg et al., 2009) via newspaper advertisements and a telephone screen. An in-person study visit involved APOE genotyping based on a polymerase chain reaction method and administration of a cognitive screening battery to determine if the potential participant was cognitively intact. This battery consisted of the Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), Dementia Rating Scale 2 (DRS-2; Jurica, Leitten, & Mattis, 2001), and Rey Auditory Verbal Learning Test (RAVLT; Rey, 1958). Criteria used to define intact cognition in this study have been previously reported (Seidenberg et al., 2009). All protocols, in full compliance with APA ethical standards, were approved by the local Institutional Review Board and participants received compensation. A total of 72 participants were tested at baseline (T1) with all measures for the current study. Only the 48 participants who returned for both neuropsychological and structural MRI follow-up sessions (1.5 years (T2) and 5 years (T3)) were included in the current study. On this basis, the study attrition rate over five years was 33%; three participants had T1 and T3 data but were missing T2 (all were ε4+) and 21 participants (13 ε4+) had T1 and T2 data but were not scanned at T3 due to: health decline (6), deceased (5), lost to follow-up (3), refused scan (3), moved away (3), and no longer safe to be scanned (1). No group differences were observed in attrition rates at each of the two follow-up scan sessions.
The 48 participants were subdivided by APOE ε4 allele inheritance: 26 ε4 carriers (ε4+) and 22 ε4 non-carriers (ε4−). The ε4 carrier group was further subdivided based on changes in cognitive status over the 5-year study interval. ε4+ participants were defined as “Declining” if a reduction from baseline performance of at least one standard deviation (SD) was observed on at least one of the three principal outcome indices (Woodard et al., 2010). Using these criteria, 15 ε4+ participants (58%) were classified as Declining and 11 ε4+ participants (42%) were classified as Stable.
No significant differences were observed between the three groups for mean age in years (ε4−: 73.1 yrs, SD = 5.2; ε4+ Stable: 71.9 yrs, SD = 4.3; ε4+ Declining: 73.0 yrs, SD = 4.3; p = .768, ; 95% CIs [71.1, 75.1], [69.0, 74.7], and [70.5, 75.4]) or for number of females (ε4−: 18, 82%; ε4+ Stable: 9, 82%; ε4+ Declining: 12, 80%; p = .989, Cramèr’s V = 0.022). However, significant group differences (F(2, 45) = 4.45, p = .017, ) were observed for mean years of education (ε4−: 14.0 yrs, SD = 1.8; ε4+ Stable: 16.8 yrs, SD = 3.2; ε4+ Declining: 14.8 yrs, SD = 3.1; 95% CIs [12.8, 15.1], [15.2, 18.4], and [13.4, 16.2]), with the ε4+ Stable group being significantly more educated than the ε4− group. No significant baseline group differences were observed on the MMSE, DRS-2, or RAVLT.
There were no significant group differences in the number of months between T1 and T2 (ε4−: 18.5 mos, SD = 1.7; ε4+ Stable: 18.2 mos, SD = 0.6; ε4+ Declining: 18.3 mos, SD = 0.8; p = .761, ; 95% CIs [18.0, 19.1], [17.4, 19.0], and [17.7, 19.0]) or between T1 and T3 (ε4−: 59.0 mos, SD = 2.6; ε4+ Stable: 58.2 mos, SD = 4.5; ε4+ Declining: 56.8 mos, SD = 4.4; p = .2091, ; 95% CIs [57.4, 60.6], [55.9, 60.4], and [54.9, 57.7]).
MRI Acquisition and Processing
High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12°; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224). A scanner upgrade took place near the end of the final retest period. Two ε4+ participants and one ε4- had their third scan conducted on a GE MR750 3.0 Tesla scanner (TE = 3.9 ms; TR = 9.6 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12°; number of excitations (NEX)=1; slice thickness = 1.0 mm; FOV = 24 cm; resolution=256×224). A between-scanner comparison showed no systematic differences. Whole brain and regional volumes were derived from T1- weighted SPGR images using the longitudinal stream in Freesurfer v.5.1 software (Fischl et al., 2002). A previous study (Reiter et al., 2017) demonstrated longitudinal differences between ε4+ and ε4- participants primarily in volumes of the hippocampus, inferior lateral and lateral ventricles, and cortical gray matter. To minimize multiple comparisons, we confined the analysis to determine the effect of cognitive decline within the ε4+ group to eight bilateral brain volumes: hippocampi, inferior lateral ventricles, lateral ventricles, and cortical gray matter.
Statistical Analyses
All brain volumes were converted to residualized z-scores controlling for intracranial volume (ICV) and age at baseline. Longitudinal linear mixed effects (LME) models were used to examine the effects of group and time on anatomical brain volumes (Rao et al., 2015; Reiter et al., 2017). Time was based on the number of days from T1. For our LME models, the ε4- group served as the reference group. Although years of education differed between groups (see above), education did not significantly correlate with any of the volumetric measurements; as a result, education was not used as a covariate in the LME analyses. To control for inflated Type 1 error rate due to multiple comparisons, False Discovery Rate (FDR) correction procedures were applied to all LME analyses.
Results
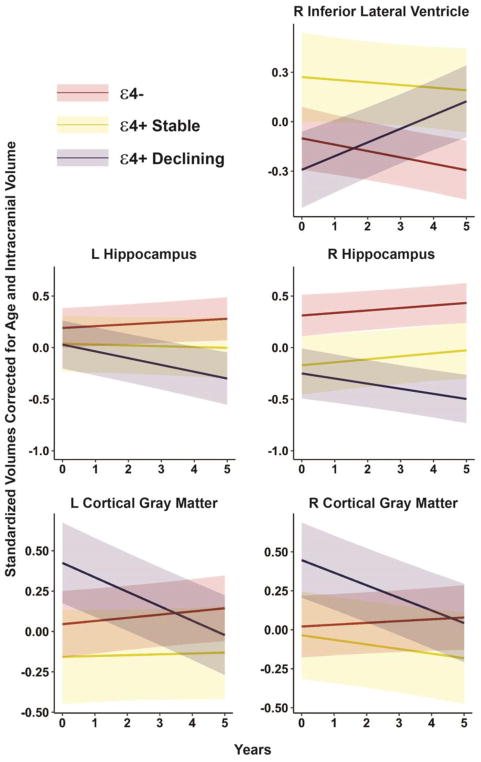
Table 1 summarizes the results of the LME analyses applied to bilateral volumes derived from the hippocampi, cortical gray matter, lateral ventricles, and inferior lateral ventricles. There were no significant baseline differences between groups for all eight brain volumes. Over the five-year follow-up interval, the ε4+ Declining group had significantly more atrophy than the ε4- group in the left (p = .001, Cohen’s d = .691) and right (p = .003, d = .622) cortical gray matter volumes and in the left (p = .003, d = .625) and right (p = .020, d = .492) hippocampi. The ε4+ Declining group also had significantly greater expansion of the right inferior lateral ventricle than the ε4- group over the five-year interval (p < .001, d = .751). These findings reflect medium to large effect sizes. Figure 1 displays regression lines depicting baseline and predicted annualized rates of change in volumes (in standard score units, adjusted for age and intracranial volume) over the five-year study period by group. The figure demonstrates that the group separation in volume change becomes more pronounced at five years post baseline.
Table 1.
Baseline Characteristics by Gene and Cognitive Decline Status
| Variable | ε4- (n = 22) | ε4+ Stable (n = 11) | ε4+ Declining (n = 15) | p - value | Effect Size |
|---|---|---|---|---|---|
| Age (years) | 73.1 (5.2) | 71.9 (4.3) | 73.0 (4.3) | 0.77 | 0.01a |
| Sex (N, % Female) | 18, 82% | 9, 82% | 12, 80% | 0.99 | 0.02b |
| Education (years) | 14.0 (1.8) | 16.8 (3.2) | 14.8 (3.1) | 0.02 | 0.17a |
| Family History (N, % Yes) | 0, 0% | 7, 64% | 12, 80% | 0.35 | 0.35b |
Note. Values are presented as means with standard deviations in parentheses unless otherwise noted. Bolded values are statistically significant at p < 0.05; ε4− = ε4 non-carriers; ε4+ Stable = ε4 carriers that remained cognitively stable over 5 years; ε4+ Declining = ε4+ carriers that cognitively declined over 5 years;
= Partial Eta Squared;
= Cramer’s V.
Figure 1.
Regression lines depicting baseline and predicted annualized rates of change in volumes (in standard score units, adjusted for age and intracranial volume) over the five-year study period by group. ε4- = ε4 non-carrier; ε4+ Stable = Cognitively stable ε4 carrier; ε4+ Declining = Cognitively declining ε4 carrier.
In a direct comparison of the two ε4+ groups, the ε4+ Declining group exhibited significantly greater atrophy of the right hippocampus (p = .030, d = .457) and left cortical gray matter (p = .017, d = .506) and expansion of the right inferior lateral ventricle (p = .011, d = .541) than the ε4+ Stable group over the five-year interval. These findings reflect medium effect sizes. However, these three interaction effects did not survive FDR correction likely due to the small sample size.
Discussion
Results confirmed our hypothesis that the ε4 group that exhibited a decline in verbal episodic memory and global cognitive function over the five-year retest interval exhibited greater brain atrophy than ε4 non-carriers. In contrast, ε4 carriers who experienced no significant five-year changes in memory and cognitive test performance exhibited atrophy rates comparable to ε4 non-carriers. The greater atrophy rates in the ε4+ Declining group occurred bilaterally in the hippocampi and global gray matter, and in the right inferior lateral ventricle. Importantly, the three groups showed no differences in brain volumes at the baseline examination. Furthermore, all participants were cognitively intact at enrollment as an entry criterion, and did not differ from each other on measures of episodic memory or global cognition at baseline.
ε4 carriers exhibit significantly greater rates of hippocampal and cortical gray matter atrophy and ventricular enlargement than ε4 non-carriers (Reiter et al., 2017). Results from the current study indicate that these group differences may be driven by a subset of ε4 carriers who exhibited cognitive decline over the five-year retest interval. This study demonstrates the correspondence between longitudinal changes in cognition and brain volumes in a subgroup of ε4 carriers, a finding that has not previously received significant attention in the literature.
Whereas possession of the ε4 allele increases risk of AD, not all ε4 carriers demonstrate AD-related neurodegeneration during their lifespan (Relkin et al., 1996). Given the increasing risk of AD with increasing age (Hebert et al., 1995), it is conceivable that a longer follow-up interval would be needed to see such changes in our Stable ε4 carriers. However, our ε4+ Declining and Stable groups did not differ in mean age.
There are also data to suggest that the increased risk of developing AD in the presence of the ε4 allele diminishes after the age of 80 (Breitner et al., 1999). Thus, it is unlikely that the group differences are simply related to the length of the follow-up interval. There are several physiological pathways whereby the ε4 allele may exert deleterious effects that lead to neurodegeneration and contribute to loss of cognitive function. The apoE4 isoform expressed by the ε4 allele preferentially binds to low-density lipoprotein receptors, resulting in wide ranging effects on lipid and cholesterol metabolism, including increased production of very low density lipoproteins, the release of pro-inflammatory cytokines, and atherosclerosis (Zhang, Wu, & Wu, 2011). In nervous tissue, apoE4 is associated with decreased lipoprotein lipase activity and decreased delivery of free-fatty acids to brain cells leading to altered lipid membrane homeostasis, decreased cerebral glucose metabolism, and increased glial activation and inflammation; it impairs the processing and removal of extracellular amyloid beta peptide, increases the phosphorylization of tau, and impairs cholinergic function (Lane & Farlow, 2005). Collectively, these processes (among others) may lead to neuronal cell dysfunction and apoptotic processes leading to cell death. However, the susceptibility to the effects of the APOE ε4 allele on peripheral and brain lipid metabolism, and ultimately on brain structure and function, vary considerably in the population.
What other factors might influence the variability in the phenotypic expression of the ε4 allele? Physically active ε4 carriers experience significantly less hippocampal atrophy over time compared to inactive ε4 carriers (Smith et al., 2014). Similarly, physically active ε4 carriers experience increased memory-related brain activation (Smith et al., 2011) and reduced neurodegeneration of white matter fiber populations within projection and association tracts (Smith et al., 2016) compared to sedentary ε4 carriers. Importantly, these brain imaging differences were not observed in non-carriers, suggesting that physical activity has a specific neuroprotective effect in ε4 carriers. Moreover, we previously reported that physically inactive ε4 carriers have a greater probability of cognitive decline over 18 months compared to physically active ε4 carriers (Woodard et al., 2012). In addition, Schreiber and colleagues (2016) found that low vascular risk and lifetime engagement in stimulating cognitive activities served to preserve cognitive abilities in healthy ε4 carriers. Schwartz and colleagues (2016) speculate that greater brain structural integrity, high socioeconomic status, lack of brain injury, high occupational achievement, and perseverance, all indicators of enhanced brain and cognitive reserve, may moderate the effect of the ε4 allele on cognitive decline. Of note, level of educational attainment, an important indicator of cognitive reserve, was 2.0 years greater in the ε4+ Stable group than the ε4+ Declining group, although this difference was not significant.
Other possible explanations involve ε4 allele dose and family history of AD. Martins, Oulhaj, de Jager, and Williams (2005) found that ε4 homozygotes displayed a greater rate of cognitive decline than ε4 heterozygotes over a three year interval. Our results are unlikely to be influenced by this factor because only two of our ε4 carriers were homozygotes (ε4/ε4) and both were in the cognitively stable group. Family history of AD has also been examined as an additional risk factor for cognitive decline and neurodegeneration in ε4 carriers. Donix and colleagues (2010) found that family history of AD explained more variance in cortical thickness than APOE ε4 status. Family history is unlikely to have influenced our results because there were no statistically significant group differences in family history of AD between the ε4+ Stable (64%) and ε4+ Declining (80%) groups (p = 0.35).
The ε4+ Declining group had significantly greater expansion of the right inferior lateral ventricle than the ε4- group. In contrast, there were no significant group differences in the volume of the main body of the lateral ventricles. In the absence of hydrocephalus, increased ventricular volume is an indirect estimate of atrophy within periventricular structures (Tang, Holland, Dale, Younes, & Miller, 2015). Not surprisingly, as the hippocampus and other medial temporal lobe structures decrease in volume, the inferior horns of the lateral ventricles expand (Thompson et al., 2004), which our results appear to typify.
A limitation of this study is the small sample size. Nevertheless, we found medium to large effect sizes in the right inferior lateral ventricle, hippocampi, and cortical grey matter regions. A further limitation, relevant to all longitudinal studies, was attrition. The participants who were missing data at one of the three sessions were excluded from the study. There were no group differences between included and excluded participants at either time-point in age, education, or family history, and no baseline test score differences between those included or excluded at T2. However, despite all having intact scores at baseline, those unable to be scanned at T3 (year five) were more likely to be male (p = 0.036) and had lower baseline scores on the DRS-2 total (p = 0.003) and RAVLT total trials (p = 0.012). Notably, all three participants who missed T2 were ε4+, and 13 of 21 who missed T3 were ε4+. Thus, inclusion of these missing participants would likely have strengthened the present findings.
Another limitation of this study is the absence of data on AD specific biomarkers, such as can be derived from cerebrospinal fluid analyses of tau and amyloid proteins or positron emission tomography imaging of amyloid deposition. Lim and colleagues (2013) found that Aβ amyloid burden explained 14% of the variance in episodic memory performance in ε4 carriers, but had no relationship with memory performance in ε4 non-carriers. Another study found that ε4 carriers with high Aβ levels had greater declines in verbal memory and global cognition over 1.5 years than ε4 carriers with low Aβ levels (Mormino et al., 2014). If these data were available in the current study, we would hypothesize that AD biomarkers would be more abnormal in ε4 Declining than in ε4 Stable carriers.
In conclusion, our study demonstrates that the degree of long-term longitudinal changes in episodic memory and global cognition are linked with the severity of changes in brain atrophy in persons who possess the APOE ε4 allele. Thus, this study illustrates the variability in phenotypic expression of the ε4 allele, which may be associated with potential lifestyle factors, such as physical activity, and cognitive reserve. Larger scale studies are required to examine these potential influences as a means for understanding modifiable risk and protective factors associated with genetic risk.
Table 2.
Cognitive Performances by Group Across Time
| Variable | ε4- | ε4+ Stable | ε4+ Declining | p–value | Effect Size | |
|---|---|---|---|---|---|---|
| Baseline | MMSE | 29.3 (0.8) | 29.6 (0.8) | 28.9 (1.0) | 0.23 | 0.06 |
| DRS-2 Total | 141.1 (2.0) | 140.6 (1.6) | 140.1 (4.4) | 0.55 | 0.03 | |
| RAVLT Total Trials | 49.2 (8.2) | 51.7 (7.7) | 46.9 (8.0) | 0.32 | 0.05 | |
| RAVLT DR | 9.8 (2.1) | 10.7 (3.1) | 9.1 (2.8) | 0.31 | 0.05 | |
| 18 Months | MMSE | 29.7 (0.5) | 29.3 (0.9) | 29.5 (0.8) | 0.35 | 0.05 |
| DRS-2 Total | 139.0 (2.8) | 138.9 (2.1) | 138.3 (3.5) | 0.81 | 0.01 | |
| RAVLT Total Trials | 47.5 (7.6) | 54.6 (6.0) | 40.4 (7.6) | < 0.01 | 0.34 | |
| RAVLT DR | 9.4 (2.6) | 11.8 (2.7) | 5.8 (2.0) | < 0.01 | 0.47 | |
| 5 Years | MMSE | 29.7 (0.7) | 29.4 (0.7) | 28.3 (2.7) | 0.07 | 0.13 |
| DRS-2 Total | 141.7 (2.2) | 141.3 (1.9) | 138.9 (6.1) | 0.11 | 0.11 | |
| RAVLT Total Trials | 48.5 (7.5) | 50.6 (6.4) | 42.7 (13.5) | 0.13 | 0.10 | |
| RAVLT DR | 10.0 (2.5) | 11.1 (2.0) | 6.2 (4.2) | < 0.01 | 0.31 |
Note. Values are presented as means with standard deviations in parentheses. Bolded values are statistically significant at p < 0.05; Sample sizes at baseline: ε4- = 22, ε4+ Stable = 11, ε4+ Declining = 15; Sample sizes at 18 Months: ε4− = 22, ε4+ Stable = 11, ε4+ Declining = 15; Sample sizes at 5 Years: ε4− = 20, ε4+ Stable = 9, ε4+ Declining = 13. ε4− = ε4 non−carriers; ε4+ Stable = ε4 carriers that remained cognitively stable over 5 years; ε4+ Declining = ε4+ carriers that cognitively declined over 5 years; DRS-2 = Dementia Rating Scale-2nd Edition, RAVLT = Rey Auditory Verbal Learning Test, DR = Delayed Recall; Effect sizes are partial eta squared values.
Table 3.
Predicted Annual Brain Volume Change Over 5 Years by APOE ε4 and Cognitive Decline Status
| Region | Intercept (baseline) | Slope (time) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| ε4− a | v. ε4+ Stable b | v. ε4+Declining b | ε4− c | v. ε4+ Stable d | v. ε4+Declining d | |
| Left Hemisphere | ||||||
| Lateral Ventricle | −0.101 (0.199) | 0.494 (0.344) | −0.003 (0.312) | −0.025 (0.010) | 0.008 (0.017) | 0.022 (0.016) |
| Inferior Lateral Ventricle | 0.060 (0.174) | 0.149 (0.302) | −0.315 (0.274) | −0.044 (0.019) | 0.025 (0.033) | 0.062 (0.030) |
| Hippocampus | 0.190 (0.204) | −0.154 (0.353) | −0.160 (0.321) | 0.018 (0.017) | −0.025 (0.031) | −0.085 (0.028) |
| Cortical Gray Matter Volume | 0.045 (0.210) | −0.202 (0.364) | 0.380 (0.330) | 0.020 (0.020) | −0.014 (0.036) | −0.109 (0.033) |
| Right Hemisphere | ||||||
| Lateral Ventricle | −0.135 (0.209) | 0.645 (0.362) | 0.035 (0.328) | −0.018 (0.009) | −0.003 (0.016) | 0.028 (0.015) |
| Inferior Lateral Ventricle | −0.099 (0.186) | 0.378 (0.322) | −0.190 (0.292) | −0.039 (0.021) | 0.018 (0.036) | 0.121 (0.033) |
| Hippocampus | 0.311 (0.200) | −0.486 (0.346) | −0.565 (0.314) | 0.025 (0.019) | 0.007 (0.033) | −0.072 (0.030) |
| Cortical Gray Matter Volume | 0.022 (0.207) | −0.057 (0.359) | 0.424 (0.325) | 0.011 (0.019) | −0.041 (0.033) | −0.091 (0.030) |
Note. All values are mean z-scores residualized by baseline age and intracranial volume (ICV) with standard errors of the mean in parentheses; Bolded values are statistically significant after controlling for multiple comparisons using false discovery rate; ε4− = ε4 allele non-carrier; ε4+ Stable = ε4 allele carriers that remained cognitively stable over 5 years; APOE = Apolipoprotein E; ε4+ Declining = ε4 allele carriers that cognitively declined over 5 years. Intercepts reflect the mean volume at baseline for each region for the ε4− group alonea, or for each of the ε4+ groups relative to the ε4− groupb. Slopes reflect the estimated mean annual rate of change from baseline for the ε4− group alonec, or for each of the ε4+ groups relative to the ε4− groupd.
Public Significance Statement.
Possession of an APOE ε4 allele significantly increases the risk of developing late onset Alzheimer’s disease (LOAD). This longitudinal study illustrates that cognitively intact ε4 elders exhibit varying rates of brain atrophy as a function of declines in cognition function. This variability in phenotypic expression of the ε4 allele may ultimately lead to the identification of modifiable biological and/or lifestyle factors that mitigate the risk of developing LOAD.
Acknowledgments
We thank Piero Antuono, Alissa M. Butts, Kelli L. Douville, Christina M. Figueroa, Malgorzata Franczak, Amelia Gander, Evan Gross, Leslie M. Guidotti-Breting, Nathan C. Hantke, Kathleen E. Hazlett, Emily Hoida, Cassandra Kandah, Christina D. Kay, Melissa A. Lancaster, Monica Matthews, Sarah K. Miller, Andria L. Norman, Michael A. Sugarman, and Qi Zhang for their assistance. This work was supported by the National Institutes of Health Grant R01 AG022304. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Contributor Information
Dana A. Kelly, Department of Psychology, Rosalind Franklin University of Medicine and Science
Michael Seidenberg, Department of Psychology, Rosalind Franklin University of Medicine and Science.
Katherine Reiter, Department of Psychology, Marquette University.
Kristy A. Nielson, Department of Psychology, Marquette University
John L. Woodard, Department of Psychology, Wayne State University
J. Carson Smith, Department of Kinesiology, School of Public Health, University of Maryland.
Sally Durgerian, BrainDataDriven LLC.
Stephen M. Rao, Schey Center for Cognitive Neuroimaging, Cleveland Clinic
References
- Ashford JW. APOE genotype effects on Alzheimer's disease onset and epidemiology. Journal of Molecular Neuroscience. 2004;23(3):157–165. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, … Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/WNL.53.2.321. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, … Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, … Bookheimer SY. Family history of Alzheimer's disease and hippocampal structure in healthy people. American Journal of Psychiatry. 2010;167(11):1399–1406. doi: 10.1176/appi.ajp.2010.09111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Albert MS, Pilgrim DM, Chown MJ, … Evans DA. Age-specific incidence of Alzheimer's disease in a community population. JAMA. 1995;273(17):1354–1359. doi: 10.1001/jama.1995.03520410048025. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2: DRS-2: Professional Manual. Psychological Assessment Resources; 2001. [Google Scholar]
- Lane RM, Farlow MR. Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer's disease. The Journal of Lipid Research. 2005;46(5):949–968. doi: 10.1194/jlr.M400486-JLR200. [DOI] [PubMed] [Google Scholar]
- Lim YY, Ellis KA, Ames D, Darby D, Harrington K, Martins RN, … Maruff P. Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimer’s & Dementia. 2013;9(5):538–545. doi: 10.1016/j.jalz.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu JT, Wang HF, Han PR, Tan CC, Wang C, … Tan L. APOE genotype and neuroimaging markers of Alzheimer's disease: systematic review and meta-analysis. Journal of Neurolology, Neurosurgery, and Psychiatry. 2015;86(2):127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, … Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. Journal of Alzheimer’s Disease. 2011;23(3):433–442. doi: 10.3233/jad-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65(12):1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, … Sperling RA. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760–1767. doi: 10.1212/wnl.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, … Maruff P. Trajectories of memory decline in preclinical Alzheimer's disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiology of Aging. 2015;36(3):1231–1238. doi: 10.1016/j.neurobiolaging.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bonner-Jackson A, Nielson KA, Seidenberg M, Smith JC, Woodard JL, Durgerian S. Genetic risk for Alzheimer's disease alters the five-year trajectory of semantic memory activation in cognitively intact elders. NeuroImage. 2015;111:136–146. doi: 10.1016/j.neuroimage.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter K, Nielson KA, Durgerian S, Woodard JL, Smith JC, Seidenberg M, … Rao SM. Five-Year Longitudinal Brain Volume Change in Healthy Elders at Genetic Risk for Alzheimer's Disease. Journal of Alzheimer’s Disease. 2017;55(4):1363–1377. doi: 10.3233/jad-160504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relkin NR, Kwon YJ, Tsai J, Gandy S. The National Institute on Aging/Alzheimer's Association recommendations on the application of apolipoprotein E genotyping to Alzheimer's disease. Annals of the New York Academy of Science. 1996;802:149–176. doi: 10.1111/j.1749-6632.1996.tb32608.x. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. 1958. [Google Scholar]
- Roussotte FF, Gutman BA, Madsen SK, Colby JB, Narr KL, Thompson PM. Apolipoprotein E epsilon 4 allele is associated with ventricular expansion rate and surface morphology in dementia and normal aging. Neurobiology of Aging. 2014;35(6):1309–1317. doi: 10.1016/j.neurobiolaging.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, Jagust W. Impact of lifestyle dimensions on brain pathology and cognition. Neurobiology of Aging. 2016;40:164–172. doi: 10.1016/j.neurobiolaging.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Rapkin BD, Healy BC. Reserve and Reserve-building activities research: key challenges and future directions. BMC Neuroscience. 2016;17(1):62. doi: 10.1186/s12868-016-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, … Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73(8):612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac D, Waring S, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50(2):355–362. doi: 10.1212/WNL.50.2.355. [DOI] [PubMed] [Google Scholar]
- Smith JC, Lancaster MA, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, … Rao SM. Interactive effects of physical activity and APOE-ε4 on white matter tract diffusivity in healthy elders. NeuroImage. 2016;131:102–112. doi: 10.1016/j.neuroimage.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, … Rao SM. Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. NeuroImage. 2011;54(1):635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, … Rao SM. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Frontiers in Aging Neuroscience. 2014;6:61. doi: 10.3389/fnagi.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Holland D, Dale AM, Younes L, Miller MI. The diffeomorphometry of regional shape change rates and its relevance to cognitive deterioration in mild cognitive impairment and Alzheimer's disease. Human Brain Mapping. 2015;36(6):2093–2117. doi: 10.1002/hbm.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, … Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. NeuroImage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiology of Aging. 2011;32(1):63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, … Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. Journal of Alzheimer’s Disease. 2010;21(3):871–885. doi: 10.3233/jad-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Sugarman MA, Nielson KA, Smith JC, Seidenberg M, Durgerian S, … Rao SM. Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Current Alzheimer Research. 2012;9(4):436–446. doi: 10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu LM, Wu J. Cross-talk between apolipoprotein E and cytokines. Mediators of Inflammation. 2011;2011:949072. doi: 10.1155/2011/949072. [DOI] [PMC free article] [PubMed] [Google Scholar]