Abstract
Objective
Metacognitive ability, or the ability to accurately identify, appraise and monitor one’s deficits, is a commonly impaired in Alzheimer’s disease (AD). Poor metacognitive ability prevents correct appraisal of a range of physical, cognitive, and emotional symptoms and facilitates anosognosia, which has important clinical implications for individuals (e.g. diminished treatment adherence, increased engagement in high-risk situations, earlier institutionalization) and caregivers (e.g., higher burden). Indeed, preserved awareness regarding cognitive deficits appears to be essential for optimal decision-making. However, the neural correlates of metacognitive disturbance are still debated in the literature, partly because of the subjective nature of traditional awareness measures.
Method
Here, an objective Feeling of Knowing (FOK) task was used to measure metamemory capacity in a group of cognitively diverse older adults, including 14 with mild to moderate AD and 20 cognitively healthy older adults. The association between three different objective metamemory measures of the FOK task and regional cortical thickness (12 bilateral ROIs hypothesized to support self-awareness) was analyzed using partial correlations adjusted for gender and diagnostic group.
Results
Less accurate metamemory at the local and global levels was associated with reduced right posterior cingulate cortical thickness (r = −0.42, p = 0.02) and reduced right medial prefrontal (r = −0.39, p = 0.029), respectively.
Conclusions
To our knowledge, this was the first study to examine metacognition in relation to cortical thickness. Both global and local metamemory functions appear to rely on the integrity of right sided midline regions, known to be important for processing self-referential information. Findings are conceptualized with regard to the Default Mode Network, and also considered in relation to recent findings pointing to the right insula as a region critical for self-awareness.
Keywords: Awareness, Anosognosia, Metacognition, Alzheimer’s disease, Cortical thickness
1. Introduction
Metacognition, the ability self-monitor and control own thought processes, is often impaired in dementia, especially Alzheimer’s Disease (AD) (Mograbi, Ferri, et al., 2012; Morris & Hannesdottir, 2004). Anosognosia, or the lack of awareness regarding a range of deficits, including physical (i.e., motor, perceptual), cognitive, emotional, and even neuropsychiatric symptoms (Orfei et al., 2007) is a component of impaired metacognition. Within the larger umbrella term of metacognition, falls metamemory. The latter is a construct that is defined by self-monitoring and self-awareness of cognitive symptoms, more specifically memory processes (i.e., decisions on encoding and retrieval of specific information) (Metcalfe & Dunlosky, 2008). Anosognosia has been extensively studied in the realm of metacognition, and specifically in metamemory. Studies in the field of metamemory have used objective methodologies to confirm that a subset of individuals with AD present with difficulties monitoring cognitive performance, and fail to adjust their confidence levels in the face of poor memory performance or even external feedback about poor performance (Cosentino, Metcalfe, Butterfield, & Stern, 2007; Mograbi, Brown, Salas, & Morris, 2012; for a review, see Souchay, 2007). There is a growing body of evidence showing that impaired metamemory abilities have significant clinical implications for patients and caregivers. Studies suggest that anosognosic AD patients are at higher risk of engaging in unsafe behaviors and presenting impaired decision making capacities (Cosentino, Metcalfe, Cary, De Leon, & Karlawish, 2011; Starkstein, Jorge, Mizrahi, Adrian, & Robinson, 2007), as well as presenting reduced treatment compliance (Arlt, Lindner, Rösler, & Von Renteln-Kruse, 2008; Patel & Prince, 2001). Further, anosognosia has been shown to detrimentally affect individuals and caregivers throughout a large span of the disease: from being a risk factor in preclinical stages of illness (MCI) (Petersen et al., 1999; Small, Fratiglioni, Viitanen, Winblad, & Bäckman, 2000; Spalletta et al., 2014) to increased caregiver burden in later stages, when individual is diagnosed with AD (Kelleher, Tolea, & Galvin, 2016; Turró-Garriga et al., 2013).
Studies exploring the neuroanatomical correlates of unawareness in individuals with AD have highlighted associations with various regions, including inferior, medial and orbital frontal cortices, temporal and parietal regions, midline structures such as the cingulate cortex, and also the right insula (Amanzio et al., 2011; Cosentino et al., 2015; Shany-Ur et al., 2014; for review, see Zamboni & Wilcock, 2011). These results map onto previous findings from literature in healthy individuals, which consistently point to the role of the prefrontal cortex (PFC) in metamemory processes (Chua, Schacter, & Sperling, 2009; Fleming & Dolan, 2012; Schnyer, Nicholls, & Verfaellie, 2005) and highlight the insula as important for the detection of errors (Craig, 2009; Klein et al., 2007; Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010). Cortical midline regions, such as the anterior (ACC) and posterior cingulate cortex (PCC), also emerge as a highly consistent set of structures involved in self-referential processes, which are essential for successful metamemory (for a review, see Northoff et al., 2006), seemingly through a role in accurate self-monitoring (Johnson et al., 2002, 2005) and in error detection (Carter et al., 1998; Carter & van Veen, 2007). Considering the laterality, neuroimaging studies examining the basis of self-awareness deficits in AD patients have been somewhat inconsistent, with some pointing to contributions by regions in both hemispheres (Amanzio et al., 2011; Salmon et al., 2006; Shibata, Narumoto, Kitabayashi, Ushijima, & Fukui, 2008) and others pointing to a greater contribution of right sided regions (Cosentino et al., 2015; Harwood et al., 2005; Starkstein et al., 1995), the latter consistent with historical findings in stroke and traumatic brain injury that show greater deficits in self-awareness with right sided lesions (Feinberg, 2002; Prigatano & Schacter, 1991). Consistent with a ‘right-hemisphere hypothesis’, recent behavioral findings demonstrated that memory awareness in AD was correlated with performance on non-verbal cognitive tasks that have been shown to rely more strongly on right-sided networks than verbal cognitive tasks (Shaked et al., 2014).
Some of the discrepancy in results across imaging studies may be explained in part by the nature of tools used to assess memory awareness (Clare, Marková, Verhey, & Kenny, 2005). The vast majority of the studies exploring the neuroanatomical basis of awareness in dementia used subjective assessments to measure aspects of self-awareness, such as the discrepancy between a patient and caregiver’s report of symptoms, or a clinical rating of awareness (i.e., anosognosia) based on interview with the patient. While such measures can provide important information about a patient’s insight into their symptoms, these tools are also susceptible to a number of factors such as the patient’s willingness to discuss his or her own difficulties upon interview, a characteristic likely influenced by the patient/clinician relationship, as well as the patient’s personality style and/or cultural background. Additionally, the accuracy of informant ratings is also influenced by various factors, such as informant personality, motivation and knowledge regarding the patients’ disease (Ready, Ott, & Grace, 2004; Roberts, Clare, & Woods, 2009).
The current study aimed to measure memory awareness using a previously developed objective metamemory test (Cosentino et al., 2007) less likely to be influenced by the above factors, and which enables examination of a number of different metamemory processes and metrics. This 20-item task, based on a Feeling of Knowing (FOK) framework, instructs individuals to learn five new pieces of information over four trials and to make global judgments regarding their overall memory performance on the test, as well as local judgments about the likelihood of getting individual items correct (Cosentino et al., 2015, 2007; Cosentino, Metcalfe, Cary, et al., 2011). The enhanced precision offered by this objective metamemory assessment may provide a more reliable and therefore powerful means of examining the neuroanatomic substrates of memory awareness, or metamemory. Moreover, it allows for the examination of multiple types of metamemory accuracy including relative accuracy (i.e., resolution; do judgments shift in accord with actual memory performance?) and absolute accuracy (i.e., calibration; do judgments reveal under or over-confidence?), the latter of which can be measured at both the global and local levels. Beside evidences highlighting that these different metacognitive metrics represent distinct aspects of metacognition (Schraw, 2009), they are frequently and interchangeably used in experimental paradigms to characterize the integrity of memory awareness in patient populations such as AD, but little is known about the extent to which they represent distinct constructs and/or rely on distinct anatomic substrates. Indeed, we are unaware of any studies that have examined separately the neuroanatomic correlates of these metrics.
Cortical thickness represents a novel indicator of brain health and a useful tool for the study of healthy and clinical populations. In fact, it has been found to be more sensitive to age-associated decline in grey matter, in comparison to volumetric measurements based on magnetic resonance images (MRI) (Hutton, Draganski, Ashburner, & Weiskopf, 2009) and to be useful in improving the clinical diagnosis of probable AD (Lerch et al., 2008). Recent studies exploring the anatomic substrates of self-awareness have employed cortical thickness measures when studying healthy subjects (Buchy & Lepage, 2015) and individuals with schizophrenia and psychosis (Buchy, Stowkowy, MacMaster, Nyman, & Addington, 2015; Emami, Guimond, Mallar Chakravarty, & Lepage, 2016), with results pointing to a role for prefrontal, temporal and insular cortices in metacognitive processes. However, to our knowledge, cortical thickness measures have not yet been used to explore metacognitive impairment in AD.
As an extension of previous work from Cosentino et al. (2015), the aim of the present study was to further knowledge of the anatomic substrates of memory awareness by examining the extent to which different objective metamemory measures are associated with regional cortical thickness, in a group of cognitively diverse older adults, including AD patients. The objective metamemory task used in the current study also allows us to examine the neuroanatomic associations of multiple metamemory processes (Cosentino et al., 2016, 2007). Taking together findings of neural correlates of self-awareness in clinical and healthy populations, we hypothesized that metamemory in this group would be correlated with the thickness of the ventromedial PFC and insula, as well as with midline structures including the cingulate and precuneus. Finally, we expected to find a stronger association between metamemory and right hemisphere structures compared to left sided regions.
2. Methods
Please note that the present study is an extension of a previous work, therefore, for a full description of the methodology, see Cosentino et al. (2015).
2.1. Participants
Fourteen individuals with mild to moderate probable AD were recruited through the Columbia University Medical Center (CUMC) Department of Neurology Memory Disorders Clinic. Diagnoses were made according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al., 2011). AD stages (mild to moderate) were defined as a score of 19 or greater on the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975). Individuals with a history of neurologic illness or injury, or ongoing psychiatric illness were excluded. All participants provided informed consent and were given monetary compensation.
Twenty control elders without dementia (HE) were recruited through the Alzheimer’s Disease Research Center at CUMC, local senior centers, and market mailing procedures that target a diverse group of elders in New York City. Controls were thoroughly screened by interview to exclude individuals with neurologic, psychiatric, or severe medical disorders, and were eligible for the study if they were age 55 or above, and scored at least 27 on the MMSE.
2.2. Procedures
All participants completed the metamemory test prior to any cognitive testing to ensure that experience with cognitive tests did not alter their expectations for performance in the context of the metamemory test. Several cognitive tests were administered to characterize differences in primary cognition across the AD and HE groups. The majority of participants underwent structural magnetic resonance imaging (MRI) of the brain within two months of their cognitive assessment, with an average duration of 0.77 (SD=2.46) months. Three participants with AD were scanned outside the two months window at 3.2, 3.9, and 4.7 months intervals, and two healthy controls had intervals of 4.1 and 9.4 months. Analyses were conducted with and without the individual with the 9.4 months interval to be sure that exclusion of this individual did not affect the results. This study was approved by the CUMC Institutional Review Board and all individuals provided informed consent prior to participation.
2.3. Measures
2.3.1. Objective metamemory test
The metamemory test was a modified episodic Feeling of Knowing (FOK) task (Hart, 1965) consisting of four trials with five items in each trial, yielding a total of 20 metamemory items. Stimuli were selected after several rounds of piloting aimed at eliminating floor or ceiling effects in both AD and HE groups. The final stimuli for the AD participants consisted of five pieces of “pseudo trivia” regarding an individual from history and fake information about his background (e.g., Cole Porter attended law school in Chicago). Such stimuli performed better than actual facts, or than entirely fictional names and information. A potential risk of using these stimuli is that they are falsifiable; however, they are not easily falsifiable. That is, one would have to be very knowledgeable about these particular individuals to know that the stimuli were untrue (i.e., even someone who is an expert in Cole Porter’s music is likely to be unsure of his past educational experience). We assume that if it were to occur, the overall effect on the metamemory score would be relatively small. More difficult items consisting of entirely fictional names and background information were used in the non-demented elders to prevent ceiling effects. All participants with AD completed the standard version of the task described below. As the current HE sample partook in a larger study regarding the cognitive basis of metamemory deficits in AD, healthy controls received one of three versions of the FOK task (i.e., standard, query, or feedback) (for a full description of the different metamemory task conditions see Cosentino et al., 2015). Previous work has established that there are no differences in performance across these three conditions in healthy adults; different conditions were originally administered in this group solely for the purpose of collecting normative data (Cosentino, Metcalfe, & Stern, 2011).
Dependent Variables
For this study, three dependent variables (i.e., metacognitive metrics) were used for all conditions of the metamemory task including resolution, local calibration, and global calibration.
First, the relative accuracy of judgments (i.e., resolution) was calculated, reflecting the extent to which judgments shift in accord with performance, that is, predictions for performance are raised when memory accuracy increases, and predictions for performance are lowered, when accuracy decreased. The nonparametric Goodman-Kruskal gamma statistic, a rank order correlation (Nelson & Narens, 1984), was employed as a measure of resolution. Gamma compares the relative number of concordant and discordant judgment/accuracy pairs, discarding “ties”, or instances in which either the rating or accuracy in one pair is equal to that in another pair. A score of 1 indicates perfect concordance between all of the 20 judgment/accuracy pairs within the test. Given the limitations of gamma, including a tendency to be pulled to an extreme value on the basis of only one concordance or discordance, or a possibility that a score is undefined in the event of all ties, gamma scores were corrected in line with previous suggestions (Souchay, Moulin, Clarys, Taconnat, & Isingrini, 2007). This correction has been applied in the context of signal detection theory to address instances in which hit rates are equal to one or false alarm rates are equal to zero, resulting in undefined signal detection measures (Snodgrass & Corwin, 1988). When applied to the calculation of gamma, this correction entails adding 1 to the overall number of concordances as well as the overall number of discordances. This adjustment draws scores slightly away from extreme values and assigns a score of zero when a score would otherwise be undefined in the case of all ties. For example, if an individual made judgments of “Yes” for every item, regardless of performance, each item would be tied with every other item and a score would thus be incalculable. By adjusting the calculation, this person’s performance is considered equal to 0, representing a random association between predictions and performance. The correlation between calculable gamma scores and the adjusted scores in this sample was 0.99 (p < .01).
Second, the absolute accuracy of judgments (i.e., calibration) was used to evaluate the degree and direction of discrepancies between predicted and actual performance, revealing the extent to which individuals are over or under confident in their estimations. Two different calibration scores were calculated:
Local Calibration: In order to calculate local calibration scores, ordinal ratings (Yes-Maybe-No) were translated into interval data (1, 0.5, and 0). Average accuracy was then subtracted from the average rating to determine the extent to which individuals were over or under confident on an item-by-item basis. A score of zero indicates perfect calibration, positive scores indicate overconfidence, and negative scores indicate underconfidence.
Global Calibration: We also calculated global calibration at each learning trial to evaluate individuals’ ability to make pre-test predictions regarding the overall number of items that they would correctly remember out of the five studied. Global calibration scores were determined by subtracting the actual accuracy at each trial from the predicted global accuracy, and dividing by the total number of items.
2.3.2. Immediate auditory attention
2.3.2.1. Forward digit span
This subtest from the Wechsler Memory Scales-Third edition WMS-III (Wechsler, 1997) required participants to repeat a series of digits beginning with only two and increasing until the participant failed two consecutive items at a given series length. The dependent variable was the total raw score.
2.3.3. Memory
2.3.3.1. Philadelphia repeatable verbal learning test (PVLT)
The PVLT is a list learning task in which participants are required to learn 9 words (comprising three different categories: fruit, tools, and furniture) over the course of five trials. The dependent variables were delayed recall and recognition (Price et al., 2009).
2.3.3.2. Biber figure learning test
This nonverbal list learning task consists of 9 black and white geometric designs presented over five trials. Designs were presented one at a time in a fixed order, for three seconds each. During the test phase, participants were asked to draw as many designs as they could remember. After a 30 min delay, participants were again asked to recall as many designs as possible, and subsequently to copy each of the stimuli to ensure that constructional abilities required for intact performance did not affect memory performance. Each drawing was scored according to strict guidelines on a scale of zero to three. The dependent variables were delayed recall and recognition (Glosser, Goodglass, & Biber, 1989).
2.3.4. Executive functioning
2.3.4.1. Graphic pattern generation (GPG)
The GPG test requires participants to generate multiple unique designs among arrays of dots. The short form of the test is characterized by a row of stimuli, consisting of 20 identical 5-dot arrays (Sunderaraman et al., 2015). The test requires participants to generate as many novel designs as they can using exactly four lines to join the dots in each array. The first instance of a perseveration and the first instance of a rule violation were corrected. The dependent variable was total number of perseverations (Glosser & Goodglass, 1990).
2.4. Structural neuroimaging
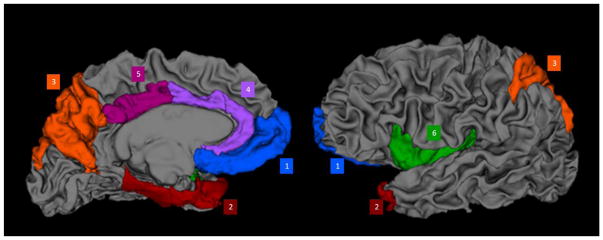
FreeSurfer (v. 5.3) was used to measure regional cortical thickness. Cortical thickness is derived by the reconstruction of the grey/white matter boundary and the cortical surface, and then the calculation of the distance between these surfaces at each point across the cortical mantel. For each region, the average distance between the grey/white matter surface and the grey/cerebrospoinal surface is calculated to reflect the cortical thickness of that region. To limit the number of analyses, twelve bilateral ROIs previously implicated in studies of awareness were derived, including right and left sided: (1) Medial Prefrontal: medial orbital and frontal pole; (2) Temporal: entorhinal, parahippocampal, temporal pole; (3) Parietal: superior parietal, precuneus; (4) Anterior Cingulate Cortex (ACC): caudal and rostral ACC; (5) Posterior Cingulate Cortex (PCC); and (6) Insula (see Figure 1).
Figure 1.
A 3-dimensional representation of the brain’s white matter derived in FreeSurfer with the cortical areas used in the study superimposed and labeled by color. Blue = medial prefrontal lobe (medial orbital and frontal pole); Red = temporal (entorhinal cortex, parahippocampus, temporal pole); Orange = parietal (superior parietal lobule, precuneus); purple = anterior cingulate cortex (caudal and rostral anterior cingulate cortex); magenta = posterior cingulate cortex; green = insula.
2.5. Data analysis
Independent samples t-tests were first used to characterize differences in demographic variables. Then, one-way ANCOVAs were conducted to determine statistically difference between cognitive and metacognitive variables across the HE and AD groups controlling for age. A general linear model adjusted for gender based on recommendations for cortical thickness analyses (Barnes et al., 2010) and age was used to compare ROI cortical thickness across HE and AD groups.
For the primary analyses, partial correlations examined the association between metamemory scores and the 12 ROIs, adjusting for metamemory test condition (which differed across healthy elders), gender, and any other demographic variables correlated with metamemory in preliminary bivariate analyses. Partial correlations were conducted separately for each metamemory score (resolution, global calibration and local calibration). Given the relatively small size of the HE and AD samples, correlations were conducted in the entire sample, adjusting for diagnostic group. The results of such analyses therefore are interpreted to reflect the regions of the brain related to metamemory in both groups. As the regions examined were all hypothesis-driven, and the sample size was relatively small, corrections for multiple comparisons were not applied.
3. Results
We evaluated 34 participants including 14 individuals with mild to moderate AD (mean age=77.81 ± 11.56 years, mean education=17.29 ± 2.56 years) and 20 cognitively healthy elders (mean age=68.26 ± 9.04 years, mean education=16.25 ± 2.22 years). We previously reported results from volumetric analyses in these same individuals (Cosentino et al. (2015). As expected, global cognition (F(1, 31)=62.54, p<.001), verbal memory – delayed recall (F(1, 31)=169.88, p<.001), verbal memory – recognition (F(1, 31)=59.91, p<.001), nonverbal memory – delayed recall (F(1, 29)=67.51, p<.001), nonverbal memory – recognition (F(1, 28)=7.96, p=.009) and executive functioning scores (F(1, 27)=7.25, p=.012) were significantly lower in the AD group as compared to the healthy older adults (see Table 1). With regard to the metacognitive scores, none of them differed significantly across groups.
Table 1.
Demographic, cognitive and metacognitive variables by group.
| HE (n=20) | AD (n=14) | |
|---|---|---|
| Age | 68.26 (9.04) | 77.81 (11.56)* |
| Education | 16.25 (2.22) | 17.29 (2.56) |
| MMSE | 29.25 (1.45) | 23.29 (2.34)** |
| Verbal memory | ||
| • Delayed recall | 7.60 (1.31) | 0.79 (1.31)** |
| • Recognition | .96 (.07) | .67 (.12)** |
| Nonverbal memory | ||
| • Delayed recall | 22.00 (5.53) | 4.83 (4.06)** |
| • Recognition | .93 (.15) | .74 (.14)* |
| Auditory attention | 11.00 (2.15) | 10.46 (2.05) |
| Executive functioning | 3.47 (1.81) | 5.73 (2.41)* |
| Metamemory | ||
| • Resolution | .53 (.47) | .30 (.58) |
| • Local calibration | .02 (.15) | .07 (.23) |
| • Global calibration | −.09 (.18) | .11 (.21) |
HE indicates Healthy Elders; AD indicates Alzheimer’s Disease, MMSE indicates Mini-Mental State Exam total score.
p < .05.
p < .001.
Table 2 shows the results of cortical thickness by group in each of the 12 ROIs. Compared to the HE group, AD participants had significantly thinner cortex in the following right sided regions: posterior cingulate (F (1,31)=7.23, p=.011), insula (F (1,31)=6.56, p=.016) and parietal cortex (F (1,31)=10.60, p=.003); and the following left sided regions: insula (F (1,31)=8.39, p=.007) and parietal cortex (F (1,31)=15.77, p<.001).
Table 2.
Cortical thickness
| Right | Left | |||
|---|---|---|---|---|
|
| ||||
| HE | AD | HE | AD | |
| Prefrontal | 2.57 (.32) | 2.45 (.22) | 2.52 (.23) | 2.46 (.27) |
| Temporal | 2.73 (.37) | 2.43 (.41) | 2.58 (.29) | 2.46 (.40) |
| Parietal | 2.24 (.12)** | 2.10 (.14) | 2.28 (.12)** | 2.07 (.17) |
| Anterior cingulate | 2.76 (.32) | 2.75 (.29) | 2.85 (.37) | 2.80 (.32) |
| Posterior cingulate | 2.53 (.14)* | 2.39 (.16) | 2.58 (.18) | 2.51 (.16) |
| Insula | 2.88 (.29)* | 2.52 (.43) | 2.88 (.25)** | 2.57 (.32) |
HE indicates Healthy Elders; AD indicates Alzheimer’s Disease, MMSE indicates Mini-Mental State Exam total score.
Thickness values are presented in millimeters. Analyses compare gender adjusted ROI cortical thickness across HE and AD
p<.05.
p<.01.
3.1. Correlates of metamemory
3.1.1. Resolution
Gamma was not significantly associated with education (r=.27, p=.124), verbal memory (r=−.28, p=.105), or global cognition (r=.26, p=.132). While gamma was related to age (r=−.35, p=.041), age was related to diagnostic group (r=−.43, p=.011), and the associations of age with gamma became non-significant when controlling for group. Results of the partial correlation between gamma and each ROI, adjusted for diagnostic group, gender, and metamemory task condition, revealed no significant associations. See Table 3 for all correlational results.
Table 3.
Correlation between metamemory scores and cortical thickness
| Metamemory Scores | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Resolution | Local Calibration | Global Calibration | ||||
|
| ||||||
| Right | Left | Right | Left | Right | Left | |
| Prefrontal | .03 | .16 | −.30 | −.05 | −.39* | −.09 |
| Temporal | .17 | .28 | .05 | .31 | −.05 | .07 |
| Parietal | .09 | .01 | −.15 | .26 | −.13 | .16 |
| Anterior cingulate | .09 | .10 | −.15 | −.14 | −.19 | −.19 |
| Posterior cingulate | −.03 | −.15 | −.42* | −.15 | −.33 | −.17 |
| Insula | .15 | .18 | .06 | .08 | −.12 | −.03 |
HE indicates Healthy Elders; AD indicates Alzheimer’s Disease, MMSE indicates Mini-Mental State Exam total score.
Bivariate analysis in the entire group were adjusted for diagnostic group, gender and metamemory test condition
p<.05.
3.1.2. Local calibration
Local calibration was not significantly associated with education (r=.24, p=.181), verbal memory (r=−.15, p=.389), global cognition (r=−.04, p=.838), or age (r=.22, p=.222). Results of the partial correlation between local calibration and each ROI, adjusted for diagnostic group, gender, and metamemory task condition, revealed an association between calibration and the right posterior cingulate cortical thickness (r=−.42, p=.020).
3.1.3 Global calibration
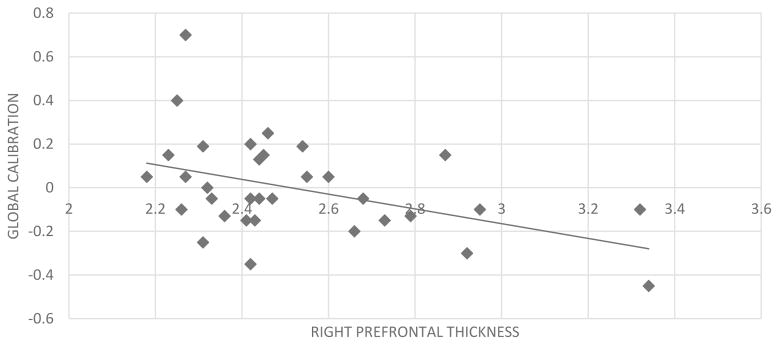
Global calibration was not significantly associated with education (r=.26, p=.137), or global cognition (r=−.29, p=.093). Although performance was related to verbal memory (r=−.49, p=.004) and age (r=.45, p=.007), verbal memory and age were related to diagnostic group (respectively, r=.94, p<.001; r=−.43, p=.011) and the associations of verbal memory and age with global calibration became non-significant when controlling for group. Results of the partial correlation between global calibration and each ROI, adjusted for diagnostic group, gender, and metamemory task condition, revealed an association between calibration and right medial prefrontal cortical thickness (r=−.39, p=.029) (see Figure 2).
Figure 2.
Association between global calibration scores and right prefrontal cortex thickness
3.1.4. Comparison of Correlation Values
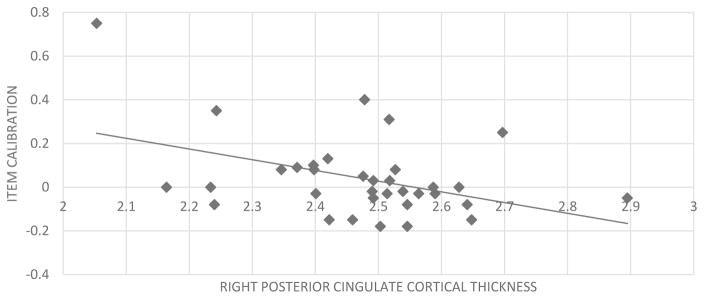
We then implemented Steiger’s z tests to determine more carefully whether the unique anatomic correlates of each calibration score reflected a formal double dissociation (i.e., local calibration with PCC versus global calibration with mPFC). Results indicated that the relationship between local calibration and the right mPFC (r=−.30) was not statistically different from its significant correlation with the right PCC (r=−.39; Steiger’s z=−.65, p=.514). Similarly, the relationship between global calibration and the right PCC (r=−.33, p=.068), was not statistically different from its significant correlation with the right mPFC (Steiger’s z= −0.35, p=.726) (see Figure 3).
Figure 3.
Association between item calibration scores and right posterior cingulate cortex thickness
4. Discussion
The aim of the current study was to explore the neural basis of three different components of metamemory in cognitively diverse older adults through the use of objective metamemory testing and cortical thickness measures. In fact, studies exploring metacognitive processes in healthy subjects highlighted that relative and absolute accuracy are two distinct metacognitive monitoring processes (Schraw, 2009; Koriat, 1997; Maki, Shields, Wheeler, & Zacchilli, 2005). However, studies exploring these different components of metamemory in aging are scarce and, to the best of our knowledge, the present study is the first comparing directly relative and absolute accuracy and their neural correlates.
Consistent with previous findings using different neuroimaging techniques, we found an global association between metamemory and right sided midline regions, specifically the medial PFC and the PCC, regions previously linked to the processing of self-referential information in healthy adults and individuals with MCI (for review see Northoff et al., 2006 and van der Meer, Costafreda, Aleman, & David, 2010). Indeed, studies with healthy adults showed that the medial PFC and the PCC are co-activated during self-referential tasks (Johnson et al., 2002; Morel et al., 2014). Similar results have been highlighted in studies with MCI patients. For example, Ries et al. (2007) showed an involvement of cortical midline structures, specifically the mPFC and the PCC, during self-referential processing and this association is mediated by the level of awareness MCI patients had regarding their cognitive difficulties. Additionally, studies highlighted that the pattern of activation is different during self-reflective processing than during other referential processes (Feyers, Collette, D’Argembeau, Majerus, & Salmon, 2010; for review see, van der Meer et al., 2010). These studies, along with the current findings, suggest that processing self-related information about one’s memory may rely on brain regions crucial for processing other types of information about the self as well.
These neuroimaging associations support behavioral data suggesting that people with anosognosia for cognitive and/or motor deficits are not impaired at perspective taking in general, but seem to have a specific deficit in self-perception. Ramachandran & Rogers-Ramachandran (1996) showed that patients suffering from anosognosia for hemiplegia were able to acknowledge the paralysis of others, even when they were unaware of their own paralysis. In the context of dementia, a few studies have explored the issue of perspective taking with regard to metacognitive abilities by asking people with dementia to evaluate the performance of their relatives or of a fictional person suffering from dementia (Clare et al., 2012; Duke, Seltzer, Seltzer, & Vasterling, 2002; Mograbi, Brown, Landeira-Fernandez, & Morris, 2014). The results indicate that people with dementia were generally accurate in predicting the performance of someone else (e.g., caregiver or fictional person), despite difficulties in evaluating their own performance, especially regarding memory. Compelling new findings in the context of anosognosia for hemiplegia suggest that patients with anosognosia may improve their awareness for cognitive and/or motor deficits by adopting a third-person perspective. Besharati, Kopelman, Avesani, Moro, & Fotopoulou (2015) recently described two case-studies of patients who presented a reinstatement of motor awareness after seeing themselves in a video, suggesting that self-observation through a third-person perspective positively influences metacognitive abilities.
Studies exploring the neural basis of self-awareness, including the current study highlighting the mPFC and the PCC in metamemory, point to an overlap between regions implicated in self-awareness and those that comprise the default mode network (DMN). This neural network is activated when participants are not engaged in a specific cognitive task and is thought to be involved in self-referential thinking (Berlingeri et al., 2015; Gerretsen et al., 2014; Le Berre & Sullivan, 2016; Perrotin et al., 2015). The DMN includes principally the mPFC and the PCC, spreading to parietal and temporal regions (for review see Buckner, Andrews-Hanna, & Schacter, 2008).
As suggested by studies showing an association between the right hemisphere and anosognosia in AD (Cosentino et al., 2015; Harwood et al., 2005; Starkstein et al., 1995), our results highlight correlations between right sided regions (mPFC and PCC) and absolute accuracy scores (both global and local). However, there is no correlation with the relative accuracy score. This difference provides the first anatomic evidence that these two components of metacognition assess different aspects of metacognitive monitoring (Schraw, 2009). Indeed, absolute accuracy measures the precision of judgments, whereas relative accuracy explores the correspondence between judgments versus performance. For the same task, an individual can have good absolute accuracy on average (i.e., accurately predict that he will get 2 out of five right) but poor relative accuracy (predicts that he will get the correct answers incorrect and vice versa). Supporting this idea, a number of studies showed discrepant results when comparing the two measures. For example, absolute accuracy improves with practice and seems influenced by task difficulty and participants ability, while these factors do not influence relative accuracy (Koriat, 1997; Maki et al., 2005). Specifically in aging, previous findings point to a preservation of relative accuracy but absolute accuracy seems to decline with age (for review see Castel, Middlebrooks, & McGillivray, 2015). Despite these findings, little is known regarding the relationships between these different metacognitive measures. As suggested in the literature, our results shed light on future research evaluating both components of metacognitive monitoring, and raise interesting questions about whether selectively preserved monitoring systems might be harnessed to improve deficits in a separate system.
Contrary to what might be expected based on previous findings from Cosentino et al. (2015), we did not find an association between resolution score, measured by gamma, and the cortical thickness of the right insula. It is interesting that the structural correlates of metamemory diverge depending on whether the structural integrity of the brain is defined by volume or thickness. This divergence is consistent with previous studies comparing grey matter volume and thickness measures, which have reported differences in the results of the two methods. Such discrepant findings have been attributed to methodological (spatial registration and conversion methods of the targets) and/or biological variables (changes in surface area, grey/white matter intensity contrast and gyrification) (Blankstein, Chen, Mincic, McGrath, & Davis, 2009; Hutton et al., 2009; Kong et al., 2015). Additionally, Kong et al. (2015) suggested that, contrary to cortical thickness measures, grey matter volume analysis reflects morphometric changes in both cortical and subcortical regions. In line with this reasoning, it might be argued that the association between relative accuracy and insular volume observed in Cosentino et al. (2015) may have in part reflected subcortical changes. Future work in larger samples is needed to more comprehensively characterize the extent to which different forms of metacognitive monitoring may map onto different brain regions, as well as specific markers of structural integrity.
The current study was limited by a relatively small sample size. Ideally, correlations would have been conducted both across and within diagnostic groups to determine differences in the strength or regions of association within each group. It is important to note that the current analyses, adjusted for diagnostic group, speak only to the regions that relate to metamemory in both groups, and it is certainly possible that other regions may be more or less relevant for one group in particular. However, to the best of our knowledge, this is the first study to explore the neural correlates of metamemory in older adults using cortical thickness as a neuroimaging measure, and to examine the correlates of three distinct metamemory processes. Future studies should explore the differences between healthy and pathological populations regarding the neural correlates of metamemory using an objective assessment of this ability.
Research aiming to better understand metamemory is particularly relevant for the treatment and care of individuals with impaired awareness. Metamemory and impaired awareness of memory deficits were shown to be closely related and based on metacognitive theories, lack of awareness can be seen as a deficit of metacognitive monitoring (Bertrand, Landeira-Fernandez, & Mograbi, 2016; Ernst, Moulin, Souchay, Mograbi, & Morris, 2015). In fact, studies highlighted an association between metamemory performance and clinically rated memory awareness (Cosentino et al., 2007; Reed, Seab, & Jagust, 1992). Moreover, the negative impact of anosognosia on AD patients and their caregivers is well established in the literature, with numerous studies demonstrating a link between unawareness of cognitive deficits and increased engagement in high-risk situations (Arlt, Lindner, Rösler, & Von Renteln-Kruse, 2008; Starkstein, Jorge, Mizrahi, Adrian, & Robinson, 2007) and caregiver burden (Rymer et al., 2002; Seltzer, Vasterling, Yoder, & Thompson, 1997; Turró-Garriga et al., 2013). Additionally, previous findings have highlighted the importance of preserved memory awareness for everyday decision-making capacity, in particular, for the management of medications (Cosentino, Metcalfe, Cary, et al., 2011). As the diagnosis of AD moves closer and closer to preclinical stages, optimizing metamemory and broader aspects of self-awareness through tailored interventions will be critical for extending an individual’s independence and allowing them to better navigate cognitive and functional loss. To the extent that the various facets of metamemory (namely relative and absolute accuracy) and their respective neurocognitive substrates can be more clearly articulated, intervention strategies can be more precisely and effectively tailored to the individual.
Public Significance Statements.
The present study explores the neural correlates of the capacity to monitor memory performance (or metamemory), which is frequently impaired in patients with Alzheimer’s disease. The results suggest that, in cognitively diverse older adults, this capacity relies on the integrity of right sided brain regions. These findings help to better understand the mechanisms underlying metamemory and might be used to effectively tailor intervention strategies to the individual.
Acknowledgments
This study was supported by NIH Grants K23AG032899, R01AG026114, R00NS060766, R01NS076837. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR000040, formerly the National Center for Research Resources, Grant Number UL1RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Elodie Bertrand acknowledges financial support from the Capes Foundation, Ministry of Education of Brazil (Grant BEX 3489/15-9).
References
- Amanzio M, Torta DME, Sacco K, Cauda F, D’Agata F, Duca S, … Geminiani GC. Unawareness of deficits in Alzheimer’s disease: role of the cingulate cortex. Brain: A Journal of Neurology. 2011;134(Pt 4):1061–76. doi: 10.1093/brain/awr020. [DOI] [PubMed] [Google Scholar]
- Arlt S, Lindner R, Rösler A, Von Renteln-Kruse W. Adherence to medication in patients with dementia: Predictors and strategies for improvement. Drugs and Aging. 2008;25(12):1033–1047. doi: 10.2165/0002512-200825120-00005. [DOI] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, … Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53(4):1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Berlingeri M, Ravasio A, Cranna S, Basilico S, Sberna M, Bottini G, Paulesu E. Unrealistic representations of “the self”: A cognitive neuroscience assessment of anosognosia for memory deficit. Consciousness and Cognition. 2015;37:160–177. doi: 10.1016/j.concog.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Landeira-Fernandez J, Mograbi DC. Metacognition and perspective-taking in Alzheimer’s disease: a mini-review. Metacognition and Perspective-Taking in Alzheimer’s Disease: a mini-review. Frontiers in Psychology. 2016 Nov;7:1–7. doi: 10.3389/fpsyg.2016.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharati S, Kopelman M, Avesani R, Moro V, Fotopoulou AK. Another perspective on anosognosia: Self-observation in video replay improves motor awareness. Neuropsychological Rehabilitation. 2015;25(3):319–52. doi: 10.1080/09602011.2014.923319. [DOI] [PubMed] [Google Scholar]
- Blankstein U, Chen JYW, Mincic AM, McGrath PA, Davis KD. The complex minds of teenagers: neuroanatomy of personality differs between sexes. Neuropsychologia. 2009;47(2):599–603. doi: 10.1016/j.neuropsychologia.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Buchy L, Lepage M. Modeling the Neuroanatomical and Neurocognitive Mechanisms of Cognitive Insight in Non-clinical Subjects. Cognitive Therapy and Research. 2015;39(4):415–423. doi: 10.1007/s10608-015-9674-8. [DOI] [Google Scholar]
- Buchy L, Stowkowy J, MacMaster FP, Nyman K, Addington J. Meta-cognition is associated with cortical thickness in youth at clinical high risk of psychosis. Psychiatry Research. 2015;233(3):418–23. doi: 10.1016/j.pscychresns.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Castel AD, Middlebrooks CD, McGillivray S. Monitoring Memory in Old Age: Impaired, Spared, and Aware. In: Dunlosky J, Tauber SK (Uma), editors. The Oxford Handbook of Metamemory. Vol. 1. New York, NY: Oxford University Press; 2015. [DOI] [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. Journal of Cognitive Neuroscience. 2009;21(9):1751–65. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L, Marková I, Verhey F, Kenny G. Awareness in dementia: A review of assessment methods and measures. Aging & Mental Health. 2005;9(5):394–413. doi: 10.1080/13607860500142903. [DOI] [PubMed] [Google Scholar]
- Clare L, Nelis SM, Martyr A, Whitaker CJ, Marková IS, Roth I, … Morris RG. “She might have what I have got”: the potential utility of vignettes as an indirect measure of awareness in early-stage dementia. Aging & Mental Health. 2012;16(5):566–75. doi: 10.1080/13607863.2011.652594. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, … Stern Y. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia. 2015;75:163–9. doi: 10.1016/j.neuropsychologia.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43(7):1004–1019. doi: 10.1016/S0010-9452(08)70697-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Cary MS, De Leon J, Karlawish J. Memory Awareness Influences Everyday Decision Making Capacity about Medication Management in Alzheimer’s Disease. International Journal of Alzheimer’s Disease. 2011;2011:483897. doi: 10.4061/2011/483897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Stern Y. Impaired metamemory in Alzheimer’s Disease: A failure to attend, detect, or integrate? Journal of the International Neuropsychological Society: JINS. 2011;17:72. [Google Scholar]
- Cosentino S, Zhu C, Bertrand E, Metcalfe J, Janicki S, Cines S. Examination of the metacognitive errors that contribute to anosognosia in Alzheimer’s disease. Cortex. 2016 doi: 10.1016/j.cortex.2016.08.003. [DOI] [PubMed]
- Craig ADB. How do you feel--now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Duke LM, Seltzer B, Seltzer JE, Vasterling JJ. Cognitive components of deficit awareness in Alzheimer’s disease. Neuropsychology. 2002;16(3):359–69. doi: 10.1037/0894-4105.16.3.359. [DOI] [PubMed] [Google Scholar]
- Emami S, Guimond S, Mallar Chakravarty M, Lepage M. Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophrenia Research. 2016;170(1):66–72. doi: 10.1016/j.schres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Ernst A, Moulin CJA, Souchay C, Mograbi DC, Morris R. Anosognosia and Metacognition in Alzheimer’s Disease. In: Dunlosky J, Tauber SUK, editors. The Oxford Handbook of Metamemory. New York, NY: Oxford University Press; 2015. [DOI] [Google Scholar]
- Feinberg TE. Altered Egos: How the Brain Creates the Self. Oxford University Press; 2002. Retrieved from https://books.google.com/books/about/Altered_Egos.html?id=TDw_BKkxeKIC&pgis=1. [Google Scholar]
- Feyers D, Collette F, D’Argembeau A, Majerus S, Salmon E. Neural networks involved in self-judgement in young and elderly adults. NeuroImage. 2010;53(1):341–347. doi: 10.1016/j.neuroimage.2010.05.071. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ. The neural basis of metacognitive ability. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2012;367(1594):1338–49. doi: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: Resting state functional connectivity. Schizophrenia Research. 2014;160(1):43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosser G, Goodglass H. Disorders in executive control functions among aphasic and other brain-damaged patients. Journal of Clinical and Experimental Neuropsychology. 1990;12(4):485–501. doi: 10.1080/01688639008400995. [DOI] [PubMed] [Google Scholar]
- Glosser G, Goodglass H, Biber C. Assessing visual memory disorders. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1989;1(2):89–91. Retrieved from http://psycnet.apa.orgjournals/pas/1/2/82. [Google Scholar]
- Harwood DG, Sultzer DL, Feil D, Monserratt L, Freedman E, Mandelkern MA. Frontal lobe hypometabolism and impaired insight in Alzheimer disease. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2005;13(11):934–941. doi: 10.1176/appi.ajgp.13.11.934. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48(2):371–80. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, Lee J, Davidson RJ. The cerebral response during subjective choice with and without self-reference. Journal of Cognitive Neuroscience. 2005;17(12):1897–906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher M, Tolea MI, Galvin JE. Anosognosia increases caregiver burden in mild cognitive impairment. International Journal of Geriatric Psychiatry. 2016;31(7):799–808. doi: 10.1002/gps.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. NeuroImage. 2007;34(4):1774–81. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kong L, Herold CJ, Zöllner F, Salat DH, Lässer MM, Schmid LA, … Schröder J. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: A matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Research: Neuroimaging. 2015;231(2):176–183. doi: 10.1016/j.pscychresns.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Koriat A. Monitoring one’s own knowledge during study: A cue-utilization approach to judgments of learning. Journal of Experimental Psychology: General. 1997;126(4):349–370. doi: 10.1037/0096-3445.126.4.349. [DOI] [Google Scholar]
- Le Berre A-P, Sullivan EV. Anosognosia for Memory Impairment in Addiction: Insights from Neuroimaging and Neuropsychological Assessment of Metamemory. Neuropsychology Review. 2016:1–12. doi: 10.1007/s11065-016-9323-3. [DOI] [PMC free article] [PubMed]
- Lerch JP, Pruessner J, Zijdenbos AP, Collins DL, Teipel SJ, Hampel H, Evans AC. Automated cortical thickness measurements from MRI can accurately separate Alzheimer’s patients from normal elderly controls. Neurobiology of Aging. 2008;29(1):23–30. doi: 10.1016/j.neurobiolaging.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Maki RH, Shields M, Wheeler AE, Zacchilli TL. Individual Differences in Absolute and Relative Metacomprehension Accuracy. Journal of Educational Psychology. 2005;97(4):723–731. doi: 10.1037/0022-0663.97.4.723. [DOI] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, … Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Dunlosky J. Metamemory. In: Roediger HL, editor. Learning and Memory: A Comprehensive Reference. 3. Oxford: Elsevier; 2008. pp. 349–362. [DOI] [Google Scholar]
- Mograbi DC, Brown RG, Landeira-Fernandez J, Morris RG. Metacognition and attribution of difficulty for self and others in Alzheimer’s disease. Psychology & Neuroscience. 2014;7(3):417–424. doi: 10.3922/j.psns.2014.036. [DOI] [Google Scholar]
- Mograbi DC, Brown RG, Salas C, Morris RG. Emotional reactivity and awareness of task performance in Alzheimer’s disease. Neuropsychologia. 2012;50(8):2075–84. doi: 10.1016/j.neuropsychologia.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Ferri CP, Sosa AL, Stewart R, Laks J, Brown R, Morris RG. Unawareness of memory impairment in dementia: a population-based study. International Psychogeriatrics. 2012;24(6):931–939. doi: 10.1017/S1041610211002730. [DOI] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau B, … Volkow N. Brain Activity and Functional Coupling Changes Associated with Self-Reference Effect during Both Encoding and Retrieval. PLoS ONE. 2014;9(3):e90488. doi: 10.1371/journal.pone.0090488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Hannesdottir K. Loss of “awareness” in Alzheimer’s Disease. In: Morris RG, Becker JT, editors. The Cognitive Neuropsychology of Alzheimer’s Disease. Oxford: Oxford University Press; 2004. pp. 275–296. [Google Scholar]
- Nelson TO, Narens L. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychological Bulletin. 1984;95(1):109–133. Retrieved from http://psycnet.apa.orgjournals/bul/95/1/109. [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Prigatano GP, Starkstein S, Rüsch N, Bria P, … Spalletta G. Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a systematic review of the literature. Brain: A Journal of Neurology. 2007;130(Pt 12):3075–90. doi: 10.1093/brain/awm106. [DOI] [PubMed] [Google Scholar]
- Patel V, Prince M. Ageing and mental health in a developing country: who cares? Qualitative studies from Goa, India. Psychological Medicine. 2001;31(1):29–38. doi: 10.1017/S0033291799003098. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Desgranges B, Landeau B, Mézenge F, La Joie R, Egret S, … Chételat G. Anosognosia in Alzheimer disease: Disconnection between memory and self-related brain networks. Annals of Neurology. 2015;78(3):477–486. doi: 10.1002/ana.24462. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild Cognitive Impairment. Archives of Neurology. 1999;56(3):303. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Price CC, Garrett KD, Jefferson AL, Cosentino S, Tanner JJ, Penney DL, … Libon DJ. Leukoaraiosis severity and list-learning in dementia. The Clinical Neuropsychologist. 2009;23(6):944–61. doi: 10.1080/13854040802681664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigatano GP, Schacter DL. Awareness of Deficit after Brain Injury. Oxford University Press; USA: 1991. Retrieved from https://books.google.com/books?id=xze89PCLaWMC&pgis=1. [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in Phantom Limbs Induced with Mirrors. Proceedings of the Royal Society B: Biological Sciences. 1996;263(1369):377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J. Validity of informant reports about AD and MCI patients’ memory. Alzheimer Disease and Associated Disorders. 2004;18(1):11–6. doi: 10.1097/00002093-200401000-00003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15195458. [DOI] [PubMed] [Google Scholar]
- Reed B, Seab J, Jagust W. Dementia severity, memory impairment, and awareness of memory loss in alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1992;14(21) [Google Scholar]
- Ries ML, Jabbar BM, Schmitz TW, Trivedi MA, Gleason CE, Carlsson CM, … Johnson SC. Anosognosia in mild cognitive impairment: Relationship to activation of cortical midline structures involved in self-appraisal. Journal of the International Neuropsychological Society: JINS. 2007;13(3):450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dementia and Geriatric Cognitive Disorders. 2009;28(2):95–109. doi: 10.1159/000234911. [DOI] [PubMed] [Google Scholar]
- Rymer S, Salloway S, Norton L, Malloy P, Correia S, Monast D. Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2002;16(4):248–253. doi: 10.1097/00002093-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Salmon E, Perani D, Herholz K, Marique P, Kalbe E, Holthoff V, … Garraux G. Neural correlates of anosognosia for cognitive impairment in Alzheimer’s disease. Human Brain Mapping. 2006;27(7):588–597. doi: 10.1002/hbm.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of Cognitive Neuroscience. 2005;17(5):832–46. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraw G. A conceptual analysis of five measures of metacognitive monitoring. Metacognition and Learning. 2009;4(1):33–45. doi: 10.1007/s11409-008-9031-3. [DOI] [Google Scholar]
- Seltzer B, Vasterling JJ, Yoder JA, Thompson KA. Awareness of deficit in Alzheimer’s disease: relation to caregiver burden. The Gerontologist. 1997;37(1):20–24. doi: 10.1093/geront/37.1.20. [DOI] [PubMed] [Google Scholar]
- Shaked D, Farrell M, Huey E, Metcalfe J, Cines S, Karlawish J, … Cosentino S. Cognitive correlates of metamemory in Alzheimer’s disease. Neuropsychology. 2014;28(5):695–705. doi: 10.1037/neu0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain: A Journal of Neurology. 2014;137(Pt 8):2368–81. doi: 10.1093/brain/awu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Narumoto J, Kitabayashi Y, Ushijima Y, Fukui K. Correlation between anosognosia and regional cerebral blood flow in Alzheimer’s disease. Neuroscience Letters. 2008;435(1):7–10. doi: 10.1016/j.neulet.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The Course of Cognitive Impairment in Preclinical Alzheimer Disease. Archives of Neurology. 2000;57(6):839. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology. General. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2966230. [DOI] [PubMed] [Google Scholar]
- Souchay C. Metamemory in Alzheimer’s disease. Cortex. 2007;43(7):987–1003. doi: 10.1016/S0010-9452(08)70696-8. [DOI] [PubMed] [Google Scholar]
- Souchay C, Moulin CJA, Clarys D, Taconnat L, Isingrini M. Diminished episodic memory awareness in older adults: evidence from feeling-of-knowing and recollection. Consciousness and Cognition. 2007;16(4):769–84. doi: 10.1016/j.concog.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Piras F, Sancesario G, Iorio M, Fratangeli C, … Orfei MD. Neuroanatomical correlates of awareness of illness in patients with amnestic mild cognitive impairment who will or will not convert to Alzheimer’s disease. Cortex. 2014;61:183–195. doi: 10.1016/j.cortex.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Adrian J, Robinson RG. Insight and danger in Alzheimer’s disease. European Journal of Neurology. 2007;14(4):455–460. doi: 10.1111/j.1468-1331.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Vazquez S, Migliorelli R, Teson A, Sabe L, Leiguarda R. A Single-Photon Emission Computed Tomographic Study of Anosognosia in Alzheimer’s Disease. Archives of Neurology. 1995;52(4):415–420. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- Sunderaraman P, Sokolov E, Cines S, Sullo E, Orly A, Lerer B, … Cosentino S. Untimed Design Fluency in Aging and Alzheimer’s Disease: Psychometrics and Normative Data. Applied Neuropsychology. Adult. 2015;22(5):363–72. doi: 10.1080/23279095.2014.940419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turró-Garriga O, Garre-Olmo J, Vilalta-Franch J, Conde-Sala JL, de Gracia Blanco M, López-Pousa S. Burden associated with the presence of anosognosia in Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2013;28(3):291–297. doi: 10.1002/gps.3824. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Structure & Function. 2010;214(5–6):629–43. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience & Biobehavioral Reviews. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III/WMS-III Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Zamboni G, Wilcock G. Lack of awareness of symptoms in people with dementia: the structural and functional basis. International Journal of Geriatric Psychiatry. 2011;26(8):783–92. doi: 10.1002/gps.2620. [DOI] [PubMed] [Google Scholar]