Abstract
Recent studies report that a single subtype of α1-adrenergic receptor (α1-AR), the α1A-subtype, mediates robust cardioprotective effects in multiple experimental models of heart failure, suggesting that the α1A-subype is a potential therapeutic target for an agonist to treat heart failure. Moreover, we recently found that the α1A-subtype is present in human heart. The goal of this study was to assess the inotropic response mediated by the α1A-subtype in human myocardium, and to determine if the response is down-regulated in myocardium from failing human heart. We measured in-vitro contractile responses of cardiac muscle preparations (trabeculae) isolated from the right ventricle from non-failing and failing human hearts. Addition of the α1A-subtype agonist A61603 (100 nM) resulted in a large positive inotropic response (force increased ≈ 2-fold). This response represented ≈ 70% of the response mediated by the β-adrenergic receptor agonist isoproterenol (1 μM). Moreover, in myocardium from failing hearts, α1A-subtype responses remained robust, and only slightly reduced relative to non-failing hearts. We conclude that α1A-subtype-mediated inotropy could represent a significant source of inotropic support in the human heart. Furthermore, the α1A-subtype remains functional in myocardium from failing human hearts and thus, might be a therapeutic target to support cardioprotective effects in heart failure patients.
Keywords: alpha-1-adrenergic, contraction, inotropic, right ventricle, human
INTRODUCTION
The myocardial contractile response to endogenous catecholamines is driven by cardiomyocyte beta-1 adrenergic receptors (β1-ARs) and alpha-1-ARs (α1-ARs). β1-adrenergic responses have been extensively studied and found to have consistent effects among studies. These effects include a robust positive inotropic effect (PIE).
In contrast, α1-AR responses have been less studied and show considerable variation among studies (11). For example, there is marked species-dependent variation in the magnitude and direction of α1-AR inotropic responses, ranging from a robust positive inotropic effect in rabbit and rat, to no response in dog (12). Moreover, in studies of mice, a marked regional variation was found, with a positive inotropic effect (PIE) in left ventricular (LV) myocardium, but a negative inotropic effect (NIE) in right ventricular (RV) myocardium (33). The presence of disease altered the α1-AR response; the NIE of non-failing RV mouse myocardium was switched to a PIE in mouse models of RV failure induced by pulmonary fibrosis (6), or secondary to LV failure post-infarction (32).
Cardiac myocytes have two predominant α1-AR subtypes (α1A and α1B) and each subtype can mediate markedly different inotropic effects. For example, in rabbit the α1A-subtype mediates a NIE, but the α1B- subtype mediates a PIE (29), whereas in human the α1A-subtype mediates a PIE, but the α1B- subtype mediates a NIE (30).
Recently, α1-ARs have gained new significance by the suggestion that α1-ARs can mediate powerful cardioprotective effects in heart failure (4, 16, 23). Specifically, chronic stimulation of the α1A-subtype protects against cell death and improves function in mouse heart failure models based on doxorubicin toxicity (5, 22), trans-aortic constriction (21), or RV failure due to pulmonary fibrosis (7). These findings raise the possibility that α1A-subtype signaling in humans might be a novel therapeutic target to treat heart failure.
The goal of this study was to assess the inotropic effect of α1A-subtype stimulation in human myocardium and determine how the inotropic effect is changed in human myocardium from failing hearts. We used the α1A-subtype-selective agonist A61603 that is highly potent and selective for the α1A-subtype (22). We found a robust positive inotropic response to A61603 in myocardium from both non-failing and failing human hearts. The α1A-subtype inotropic response represented ≈ 70% of the response elicited by stimulation of the β1-AR using isoproterenol.
We conclude that α1A-subtype-mediated inotropy could represent a significant source of inotropic support in the human heart. Furthermore, the α1A-subtype remains functional in myocardium from failing human hearts. As the α1A-subtype can mediate cardioprotective effects in animal heart failure models, the presence of a functional α1A-subtype in myocardium from failing human hearts suggests that the α1A-subtype, might be a therapeutic target to support cardioprotective effects in heart failure patients.
METHODS
Human Hearts
All human tissues were experimented on with approval from the Institutional Review Board (IRB) of The Ohio State University and conform to the declaration of Helsinki. Informed consents were acquired from cardiac transplant patients. Explanted hearts from patients undergoing cardiac transplantation at The Ohio State University Wexner Medical Center were obtained directly in the operating room and immediately (within seconds) flushed with cardioplegic solution after removal from donors/patients as described previously (10, 19). The hearts were transferred to the laboratory (within 10–30 minutes) in cold cardioplegic solution containing (in mM): 110 NaCl, 16 KCL, 16 MgCl2, 10 NaHCO3, and 0.5 CaCl2. All hearts were procured and treated with identical protocols, personnel, solutions and timing regardless of the source. All end-stage failing hearts (n = 6) were acquired from patients in the operating room. Non-transplantable donor hearts (n = 6) were acquired in the operating room in collaboration with Lifeline of Ohio Organ Procurement. The biometric characteristics of these hearts are provided in Table 1.
Table 1.
Patient Characteristics
| Non-Failing | Race | Sex | Age | Heart Weight (g) | Cause of Death | LVEF (%) |
|---|---|---|---|---|---|---|
| 380071 | Caucasian | Female | 43 | 603 | Anoxia, respiratory arrest | 55 |
| 712301 | Caucasian | Male | 67 | 527 | Blunt injury, ICH | Not measured |
| 632941 | Caucasian | Female | 68 | 402 | Natural causes, CVA/Stroke | Not measured |
| 313956 | Caucasian | Female | 38 | 406 | Drug overdose | 60 |
| 574165 | Caucasian | Male | 62 | 584 | Blunt injury/MVA | Not measured |
| 394176 | Caucasian | Male | 57 | 748 | Blunt injury/Non-MVA | Not measured |
| Failing | Race | Sex | Age | Heart Weight (g) | Etiology | LVEF (%) |
|---|---|---|---|---|---|---|
| 774694 | Caucasian | Female | 50 | 486 | Ischemic | < 25 |
| 514955 | Caucasian | Female | 67 | 556 | Ischemic | < 25 |
| 911614 | Caucasian | Male | 65 | 716 | Non-ischemic | < 25 |
| 956256 | Caucasian | Male | 64 | 714 | Non-ischemic | < 25 |
| 203056 | African American | Male | 51 | 704 | Non-ischemic | < 25 |
| 724569 | Caucasian | Male | 64 | 636 | Non-ischemic | < 25 |
LVEF = left ventricular ejection fraction. ICH = intracerebral hemorrhage
MVA = motor vehicle accident
Trabecula preparation
The right ventricle of the heart was transferred from the cardioplegic solution to a cold modified Krebs-Henseleit solution (K-H) bubbled with 95% O2-5% CO2 containing (in mM): 137 NaCl, 5 KCl, 0.25 CaCl2, 20 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 10 dextrose, and 20 BDM (2,3-butanedione monoxime) and pH of 7.4. A linear, small, and free-running trabecula was isolated under a stereo dissection microscope, and kept in this solution at 0–4 °C until the time of the experiment, which was typically 2 hours or less. Approximately 10 trabeculae from each heart were harvested for use in separate parallel studies. One muscle per heart was used for this study. For both non-failing and failing groups, data from 6 hearts were averaged per group. Muscles were transferred into custom-made setups as previously described for animal models (15) and the perfusion solution was changed to a modified K-H but without BDM. This solution was maintained at 37 °C and continuously bubbled with 95% O2-5% CO2 resulting in pH of 7.4. Continuous electrical stimulation was initiated (frequency of 0.5 Hz, and stimulation voltage ~20% above threshold) and CaCl2 concentration of the solution was slowly raised to 2 mM over ~15 minutes. Muscles were gradually stretched (over a few minutes) until an increase in developed force was exceeded by an increase in resting tension. This length (optimal length) roughly corresponds to sarcomere length of ~2.2 μm, which is near or at the in vivo sarcomere length at end-diastole (24). Dimensions of the muscles from the non-failing hearts used were 347 ± 81 μm wide, 232 ± 55 μm thick, and 2.7 ± 0.7 mm long. Dimensions of the muscles from the failing hearts were not significantly different; 329 ± 35 μm wide, 247 ± 56 μm thick, and 2.4 ± 0.5 mm long.
Inotropic stimulation
After assessment of baseline contractility, we used supramaximal doses of both agonists. First, 100 nM of A61603 (Sigma) was added, and data was recorded when contractions had stabilized. Thereafter, the drug was washed out, and muscle contractile parameters returned back to baseline. Next, 1 μM isoproterenol was added, and data were again collected.
Statistical analysis
Data are presented as mean ± SE. Statistical tests (ANOVA with post hoc analysis using Tukey correction for multiple comparisons, and paired and unpaired t-tests) were performed using Prism 7 software (GraphPad Software, Inc., La Jolla, CA) with a significance level set at P<0.05.
RESULTS
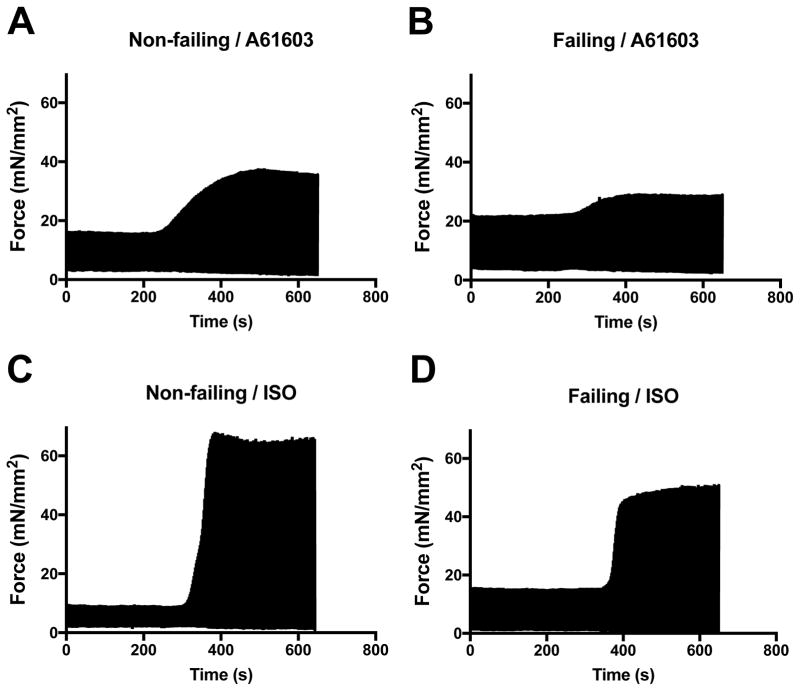
Figure 1 shows typical example records of the contractile responses to A61603 for a single muscle from a non-failing heart (A), and for a single muscle from a failing heart (B). After washout of A61603, Figure 1 also shows the time course of the contractile response to isoproterenol (ISO) for these same muscles (in panels C and D respectively).
Figure 1. Representative slow time base recordings of contraction force.
Force developed by electrically paced trabeculae showing the inotropic responses to addition of the agonists A61603 (100 nM) or Isoproterenol (ISO, 1 μM). Individual contractions are not evident on this time scale.
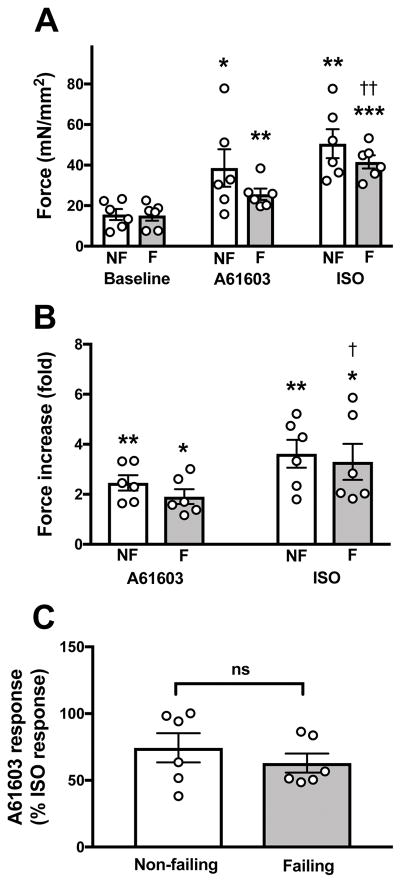
For all muscles studied, the effects of A61603 and ISO on force development are summarized in Figure 2. Shown are the individual experimental values (one muscle per heart) and the grouped data (mean ± SE, n= 6 hearts per group). Baseline absolute contraction force (mN/mm2) was similar in myocardium from non-failing (16 ± 3 mN/mm2) vs. failing hearts (15 ± 3 mN/mm2) (Figure 2A). Both A61603 and ISO elicited a robust positive inotropic effect (PIE) in non-failing and failing myocardium. However, the inotropic responses showed considerable variability between subjects. After A61603 addition, there was a trend for greater absolute force level in non-failing myocardium (39 ± 9 mN/mm2) versus failing myocardium (26 ± 3 mN/mm2), but this trend did not reach statistical significance (P = 0.21). Likewise, there was a trend for greater force level after ISO in non-failing (51 ± 7 mN/mm2) vs failing (42 ± 3 mN/mm2) myocardium which did not reach statistical significance (P = 0.27). Basal contractions and responses to ISO were similar to those observed in our recent studies (10, 18–20).
Figure 2. Inotropic effects of A61603 and ISO on myocardium from non-failing (NF) and failing (F) hearts.
Individual experimental data (one muscle per heart) and pooled data (mean ± SE, n = 6 hearts per group) for: (A) baseline forces and forces after addition of A61603 or ISO; (B) data for each muscle scaled relative to its pre-drug (baseline) contraction force; (C) responses to A61603 scaled relative to the response to ISO. * P<0.05; ** P<0.01; *** P<0.001 for comparisons relative to baseline; † P<0.05; †† P<0.01 for comparisons of ISO relative to A61603.
To minimize inter-subject variability, the contractile responses to A61603 and ISO were expressed relative to the baseline force before agonist (Figure 2B). After addition of A61603, force was increased ≈ 2-fold relative to the baseline force (Figure 2B) (2.4-fold for non-failing vs. 1.9-fold for failing). Following washout of A61603, after addition of isoproterenol, force was increased to a higher level (≈ 3-fold relative to the baseline) (3.6-fold for non-failing vs. 3.2-fold for failing). However, the higher level of force after ISO compared to after A61603 only reached statistical significance for the failing group, but not for the non-failing group.
Isoproterenol is known to induce a high level of activation of contraction. Here, we compared the activation of contraction due to A61603 with that due to ISO (Figure 2C). The increase of force elicited by A61603 was ≈ 70% relative to the increase of force elicited by isoproterenol (74% for non-failing vs. 63% for failing). There was no statistical difference between the non-failing and failing group. These data suggest that for both non-failing and failing groups the α1A-subtype is potentially a significant source of inotropic support.
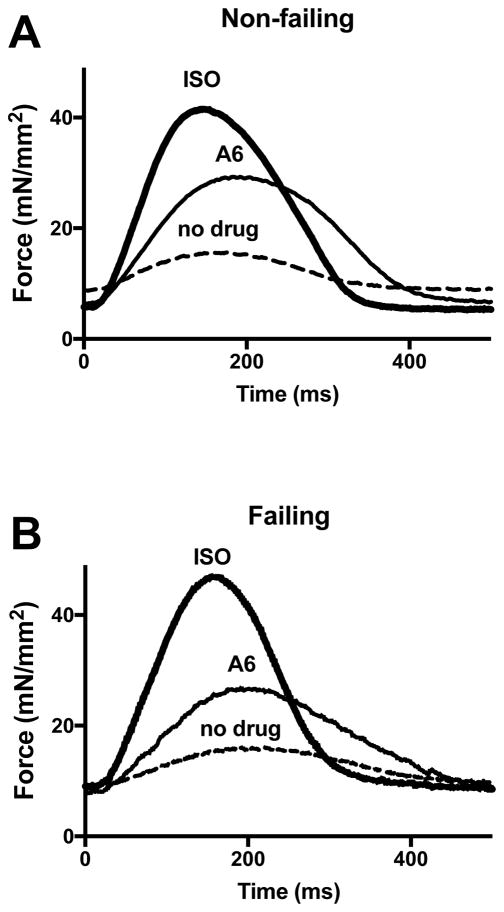
Figure 3 shows representative records of single electrically-evoked contractions both before and after addition of agonists for two trabeculae, one from a non-failing heart and one from a failing heart. For both non-failing and failing heart, A61603 increased the contraction amplitude without affecting the time course of contraction or relaxation. In contrast, as expected, ISO increased the contraction amplitude and appreciably accelerated the timing of the phases of contraction and relaxation. Both agonists caused small reductions in diastolic force level. For non-failing muscles, the drop of diastolic force after addition of A6 or ISO (1.4 ± 0.4 and 0.9 ± 0.3 mN/mm2 respectively) did not differ versus failing muscles (2.3 ± 0.6 and 1.6 ± 0.6 mN/mm2 respectively).
Figure 3. Representative fast time base recordings of twitch contractions.
(A) electrically-evoked twitch contraction records of trabecula from non-failing heart at baseline (no drug) and after addition of 100 nM A61603. Following washout of A61603, the effect after addition of 1 μM ISO is shown; (B) contraction records of trabecula from failing heart.
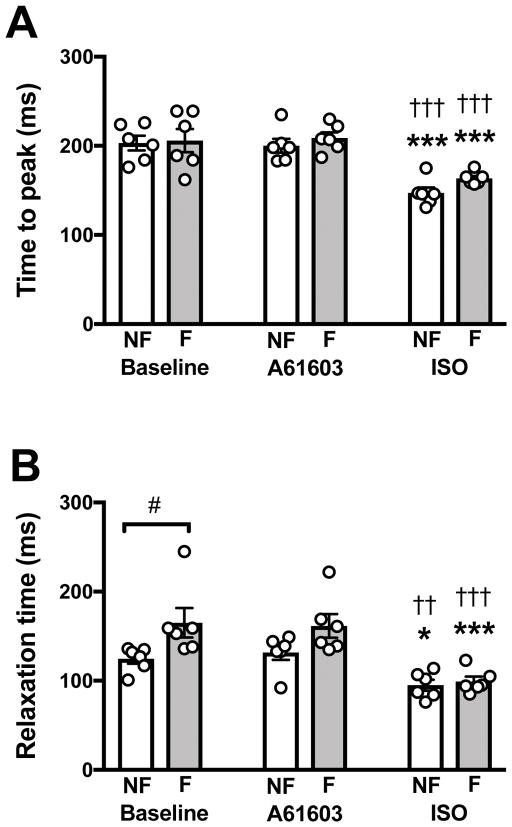
The contraction and relaxation phases were quantitated from the time between the start and peak of the contraction (Figure 4 A), and from the time from the peak of contraction to 50% relaxation (Figure 4B). Figure 4 shows that the pooled data for the contraction/relaxation timing parameters had less inter-subject variability compared to the inotropic responses (Figure 2). The time to peak contraction (Fig. 4A) was not different between myocardium from non-failing vs. failing hearts. For each muscle, a change in timing parameters from before versus after agonist were evaluated using a paired t-test. For both non-failing and failing myocardium, the time to peak was not changed by addition of A61603 (P > 0.7, paired t-test). However, the time to peak was significantly decreased after addition of isoproterenol (P < 0.05, paired t-test). The time to 50% relaxation (Fig. 4B) was higher in myocardium from failing vs. non-failing hearts indicating impaired relaxation. Relaxation time was not changed by addition of A61603 (P > 0.4, paired t-test). After addition of isoproterenol, relaxation time was appreciably reduced (P < 0.05, paired t-test), and there was no difference in relaxation time between non-failing and failing groups.
Figure 4. Effect of A61603 and ISO on the timing of contraction and relaxation.
Individual experimental data and pooled (mean ± SE) data: (A) contraction time quantitated from the time interval from the start of contraction to peak of contraction; (B) relaxation time quantitated from the time interval from the peak of contraction to the time at 50% decrease of force. * P<0.05; *** P<0.001 for comparisons relative to baseline; †† P<0.01; ††† P<0.001 for comparisons of ISO relative to A61603; # for comparison between non-failing and failing.
DISCUSSION
We found that the α1A-subtype was functional in human myocardium from both non-failing and failing hearts. The α1A-subtype produced a robust positive inotropic response representing about ≈ 70% of the response produced by the β1-AR. The α1A-subtype response was not appreciably downregulated in myocardium from failing hearts. These findings suggest that in the human heart the α1A-subtype might represent a source of significant inotropic support.
Previous studies in experimental animals suggest that the α1A-subtype might be a novel therapeutic target to treat heart failure. In this context, we found that for human heart the α1A-subtype remains functional in myocardium from failing hearts and thus might be a therapeutic target to treat patients with heart failure.
α1A-subtype inotropy in human myocardium
Previously, α1-AR mediated inotropic responses involving increases of contraction in the range of 30–60% were reported for rat and rabbit myocardium (2, 3, 26). Similar α1-AR mediated inotropic responses were reported for human myocardium (3). For myocardium from failing rat hearts and from failing human hearts, the inotropic response to α1-AR stimulation was reported to be relatively increased compared to that evoked by β-AR stimulation (3, 28). Moreover, human allografts explanted 10 years after transplantation showed α1-AR mediated positive inotropic responses of similar size as native explanted hearts (≈ 50–60%) demonstrating that α1-AR inotropic responses survived long-term immune-suppressant therapy (27).
Previously, we found that for myocardium dissected from cultured human cardiac tissue slices, the a1A-subtype elicited a positive inotropic effect (PIE) whereas the a1B subtype elicited a negative inotropic effect (NIE). In the current study using trabeculae from explanted hearts we confirmed the presence of a PIE elicited by stimulation of the a1A-subtype. Moreover, we found that the inotropic response mediated by the α1A-subtype was appreciable, representing about ≈ 70% of the response produced by the β1-AR. We also found that a robust a1A-subype inotropic response was present in myocardium from failing hearts.
The response to α1A-subtype stimulation did not involve a change in the timing of contraction or relaxation. This is consistent with the known lack of an effect of α1-AR stimulation on heart rate in-vivo. In contrast, stimulation of β1-ARs markedly accelerated the timing of contraction and relaxation. Faster twitch kinetics are compatible with the known increase in heart rate that occurs during β1-AR stimulation in-vivo.
Relation to previous studies
In studies of mouse myocardium, we previously reported that α1-AR stimulation elicits contrasting inotropic responses in LV versus RV myocardium (33). Specifically, α1-AR agonist elicited a PIE in LV myocardium but a NIE in RV myocardium. Moreover, stimulation of the single α1A-subtype also resulted in contrasting responses, again with a PIE in LV, but a NIE in RV (33). These responses in mouse differ from those observed in human. We recently reported that for human LV myocardium α1A-subtype stimulation elicited a PIE (30). In the current study, we found that for human RV myocardium, α1A-subtype stimulation also elicited a robust PIE. Thus, contrasting inotropic responses of LV versus RV myocardium following α1A-subtype stimulation were not observed in human.
For mouse RV myocardium, we found that the NIE of α1-AR stimulation was switched to a PIE in myocardium from failing hearts (32). Moreover, we found that that this switch was due to the α1A-subtype which elicited a NIE in non-failing RV myocardium but a PIE in RV myocardium from failing hearts (6). For human myocardium, in this study we did not observe appreciable difference in the α1A-subtype inotropic response in RV myocardium from non-failing versus failing human hearts.
Collectively, these findings of different effects of α1A-subtype stimulation in mouse versus human suggests that caution is needed when extrapolating to human from studies using mice.
Previous studies suggest that the 3 cardiac α1-AR subtypes have different roles in cardiac inotropy. The α1A-AR subtype mediates a PIE in this study, consistent with previous studies (6, 17). In contrast, the α1B-AR subtype generally mediates a NIE (6, 13, 25) including in human myocardium (30). The α1D-AR subtype mediates coronary vasoconstriction (31). Thus, the inotropic response of human myocardium to non-subtype-selective α1-AR agonist stimulation is likely complicated by both a PIE mediated by the α1A-AR subtype and a NIE mediated by the α1B-AR subtype.
α1A-subtype mediates cardioprotective effects
In studies of mouse models of heart failure, several lines of evidence suggest that the α1A-subtype can mediate powerful cardioprotective effects. Overexpression of the α1A-subtype increases contractility in-vivo (17), protects against pressure-overload-induced dysfunction (8) and limits post-infarct cardiomyopathy (9). Moreover, α1A-subtype stimulation protects cardiac myocytes against necrosis induced by oxidative-stress, or toxicity from norepinephrine or doxorubicin (14). Chronic administration of an α1A-subtype agonist protects against cardiomyopathy due to doxorubicin toxicity (5, 22), trans-aortic constriction (21), or RV failure due to pulmonary fibrosis (7). Notably, these chronic administration studies used a low dose of agonist that did not raise blood pressure through vascular α1-AR activation.
In human, a large clinical trial (ALLHAT) of an α1-AR antagonist was stopped prematurely because of an increase in heart failure in the α1-AR-antagonist-treated group (1). This result suggests that a reduced level of α1-AR activation is injurious, and is consistent with a cardioprotective effected mediated by α1-ARs.
Together, these findings suggest the possibility that cardioprotective α1A-subtype signaling in humans might be a novel therapeutic target to treat heart failure. In view of this possibility, the current study suggests that the α1A-subtype is active in failing human myocardium.
Limitations
We studied RV endocardial trabeculae which might have different inotropic properties compared to other RV regions and might also differ from LV myocardium. Moreover, for the heart failure patients in this study, the extent of pathological involvement of the RV is unclear.
Some heart failure patients were implanted with an LVAD which might lessen the extent of RV injury. However, there were no RV functional differences noted between heart failure patients with or without LVAD.
Downregulation of β-ARs occurs in heart failure. However, the β-AR inotropic response was not reduced in myocardium from heat failure patients. Nevertheless, the relaxation time was increased in the heart failure group, consistent with injury to RV myocardium.
The agonist ISO was always evaluated after A61603. In pilot studies, we found that the effects of ISO took a long time to wash out and were not completely reversed. A long wash out time would result in considerable time-dependent loss of function (run-down) that occurs due to progressive impairment of excitation-contraction coupling (18). Therefore, in this study, ISO was used after A61603. We used a single maximal dose of each agonist. Our recent study found that A61603 is highly potent in both non-failing and failing human myocardium with an EC50 of 5 nM for activation of cell signaling that is well below the 100 nM dose used in the current study (30).
Conclusion
The α1A-subtype mediates a robust positive inotropic effect in human myocardium. Thus, α1A-subtype-mediated inotropy could represent a significant source of inotropic support in the human heart. Furthermore, the α1A-subtype remains functional in myocardium from failing human hearts. As the α1A-subtype mediates cardioprotective effects in animal heart failure models, the presence of a functional α1A-subtype in myocardium from failing human hearts suggests that the α1A-subtype, might be a therapeutic target to treat patients with heart failure.
Acknowledgments
This work was supported by Funding for this project was supported by NIH RC1HL099538 (to PMLJ), NIH R01HL113084 (to PMLJ), Department of Veterans Affairs Merit Review Award I01BX000740 (to AJB), American Heart Association Grant in Aid 15GRNT25550041 (to AJB).
Footnotes
Disclosures
None
Literature Cited
- 1.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) ALLHAT Collaborative Research Group. Jama. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 2.Aass H, Skomedal T, Osnes JB. Demonstration of an alpha adrenoceptor-mediated inotropic effect of norepinephrine in rabbit papillary muscle. J Pharmacol Exp Ther. 1983;226:572–578. [PubMed] [Google Scholar]
- 3.Andersen GG, Qvigstad E, Schiander I, Aass H, Osnes JB, Skomedal T. Alpha(1)-AR-induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am J Physiol Heart Circ Physiol. 2002;283:H1471–1480. doi: 10.1152/ajpheart.00232.2002. [DOI] [PubMed] [Google Scholar]
- 4.Baker AJ. Adrenergic signaling in heart failure: a balance of toxic and protective effects. Pflugers Arch. 2014;466:1139–1150. doi: 10.1007/s00424-014-1491-5. [DOI] [PubMed] [Google Scholar]
- 5.Beak J, Huang W, Parker JS, Hicks ST, Patterson C, Simpson PC, Ma A, Jin J, Jensen BC. An Oral Selective Alpha-1A Adrenergic Receptor Agonist Prevents Doxorubicin Cardiotoxicity. JACC Basic Transl Sci. 2017;2:39–53. doi: 10.1016/j.jacbts.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley PM, Wang G, Chang AN, Makwana O, Swigart PM, Lovett DH, Stull JT, Simpson PC, Baker AJ. The alpha1A-adrenergic receptor subtype mediates increased contraction of failing right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2015;309:H888–896. doi: 10.1152/ajpheart.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowley PM, Wang G, Joshi S, Swigart PM, Lovett DH, Simpson PC, Baker AJ. alpha1A-Subtype adrenergic agonist therapy for the failing right ventricle. Am J Physiol Heart Circ Physiol. 2017;313:H1109–H1118. doi: 10.1152/ajpheart.00153.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du XJ, Fang L, Gao XM, Kiriazis H, Feng X, Hotchkin E, Finch AM, Chaulet H, Graham RM. Genetic enhancement of ventricular contractility protects against pressure-overload-induced cardiac dysfunction. J Mol Cell Cardiol. 2004;37:979–987. doi: 10.1016/j.yjmcc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, Finch AM, Hannan RA, Dart AM, Graham RM. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006;71:735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Elnakish MT, Canan BD, Kilic A, Mohler PJ, Janssen PM. Effects of zacopride, a moderate IK1 channel agonist, on triggered arrhythmia and contractility in human ventricular myocardium. Pharmacol Res. 2017;115:309–318. doi: 10.1016/j.phrs.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endoh M. Cardiac alpha1-Adrenoceptors and Inotropy: Myofilament Ca2+ Sensitivity, Intracellular Ca2+ Mobilization, Signaling Pathway, and Pathophysiological Relevance. Circ Res. 2016;119:587–590. doi: 10.1161/CIRCRESAHA.116.309502. [DOI] [PubMed] [Google Scholar]
- 12.Endoh M, Hiramoto T, Ishihata A, Takanashi M, Inui J. Myocardial alpha 1-adrenoceptors mediate positive inotropic effect and changes in phosphatidylinositol metabolism. Species differences in receptor distribution and the intracellular coupling process in mammalian ventricular myocardium. Circ Res. 1991;68:1179–1190. doi: 10.1161/01.res.68.5.1179. [DOI] [PubMed] [Google Scholar]
- 13.Grupp IL, Lorenz JN, Walsh RA, Boivin GP, Rindt H. Overexpression of alpha1B-adrenergic receptor induces left ventricular dysfunction in the absence of hypertrophy. Am J Physiol. 1998;275:H1338–1350. doi: 10.1152/ajpheart.1998.275.4.H1338. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O’Connell TD. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 15.Janssen PM, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- 16.Jensen BC, O’Connell TD, Simpson PC. Alpha-1-adrenergic receptors in heart failure: the adaptive arm of the cardiac response to chronic catecholamine stimulation. J Cardiovasc Pharmacol. 2014;63:291–301. doi: 10.1097/FJC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, Woodcock EA, Feneley MP, Graham RM. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 18.Milani-Nejad N, Brunello L, Gyorke S, Janssen PM. Decrease in sarcoplasmic reticulum calcium content, not myofilament function, contributes to muscle twitch force decline in isolated cardiac trabeculae. J Muscle Res Cell Motil. 2014;35:225–234. doi: 10.1007/s10974-014-9386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milani-Nejad N, Canan BD, Elnakish MT, Davis JP, Chung JH, Fedorov VV, Binkley PF, Higgins RS, Kilic A, Mohler PJ, Janssen PM. The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2015;309:H2077–2086. doi: 10.1152/ajpheart.00685.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milani-Nejad N, Chung JH, Canan BD, Davis JP, Fedorov VV, Higgins RS, Kilic A, Mohler PJ, Janssen PM. Insights into length-dependent regulation of cardiac cross-bridge cycling kinetics in human myocardium. Arch Biochem Biophys. 2016;601:48–55. doi: 10.1016/j.abb.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery MD, Chan T, Dash R, Swigart PM, Myagmar BE, Baker AJ, Simpson PC. AHA Scientific Sessions 2014. Chicago, Ill: American Heart Association; 2014. An Alpha-1A Adrenergic Receptor Agonist Prevents and Treats Heart Failure. [Google Scholar]
- 22.Montgomery MD, Chan T, Swigart PM, Myagmar BE, Dash R, Simpson PC. An Alpha-1A Adrenergic Receptor Agonist Prevents Acute Doxorubicin Cardiomyopathy in Male Mice. PLoS One. 2017;12:e0168409. doi: 10.1371/journal.pone.0168409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell TD, Jensen BC, Baker AJ, Simpson PC. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol Rev. 2014;66:308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol. 1992;263:H293–306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- 25.Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, Piascik MT, Perez DM. The alpha(1B)-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc Res. 2003;60:598–607. doi: 10.1016/j.cardiores.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Sjaastad I, Schiander I, Sjetnan A, Qvigstad E, Bokenes J, Sandnes D, Osnes JB, Sejersted OM, Skomedal T. Increased contribution of alpha 1- vs. beta-adrenoceptor-mediated inotropic response in rats with congestive heart failure. Acta Physiol Scand. 2003;177:449–458. doi: 10.1046/j.1365-201X.2003.01063.x. [DOI] [PubMed] [Google Scholar]
- 27.Skomedal T, Aass H, Geiran O, Osnes JB. Differential effects of cocaine on the positive inotropic effect of noradrenaline mediated by alpha1- and beta-adrenoceptors in failing human myocardium. Eur J Pharmacol. 2001;419:223–230. doi: 10.1016/s0014-2999(01)00980-3. [DOI] [PubMed] [Google Scholar]
- 28.Skomedal T, Borthne K, Aass H, Geiran O, Osnes JB. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. J Pharmacol Exp Ther. 1997;280:721–729. [PubMed] [Google Scholar]
- 29.Thomas RC, Jr, Cowley PM, Singh A, Myagmar BE, Swigart PM, Baker AJ, Simpson PC. The Alpha-1A Adrenergic Receptor in the Rabbit Heart. PLoS One. 2016;11:e0155238. doi: 10.1371/journal.pone.0155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RC, Singh A, Cowley P, Myagmar BE, Montgomery MD, Swigart PM, De Marco T, Baker AJ, Simpson PC. A Myocardial Slice Culture Model Reveals Alpha-1A-Adrenergic Receptor Signaling in the Human Heart. JACC Basic Transl Sci. 2016;1:155–167. doi: 10.1016/j.jacbts.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turnbull L, McCloskey DT, O’Connell TD, Simpson PC, Baker AJ. Alpha 1-adrenergic receptor responses in alpha 1AB-AR knockout mouse hearts suggest the presence of alpha 1D-AR. Am J Physiol Heart Circ Physiol. 2003;284:H1104–1109. doi: 10.1152/ajpheart.00441.2002. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the RV {alpha}1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol. 2010;298:H913–920. doi: 10.1152/ajpheart.00259.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to {alpha}1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H2013–2017. doi: 10.1152/ajpheart.00167.2006. [DOI] [PubMed] [Google Scholar]