Abstract
Objective
To determine the prognostic value of serum chromogranin-A (CGA) in a two-cohort study of men with metastatic castrate resistant prostate cancer (mCRPC) and to compare with circulating tumor cells (CTCs) based prognosis.
Patients and methods
A two cohort based evaluation for serum CGA for prognostication in CRPC stage was performed using a screening cohort of 256 men with mCRPC and an independent validation cohort of 92 men with mCRPC. In both cohorts, men receiving proton pump inhibitors and those with non-castrate levels of testosterone (>50 ng/dl) were excluded. Serum CGA was measured in a homogeneous automated immunofluorescent assay using time-resolved amplified cryptate emission. In the validation cohort, CTC enumeration was also performed using the FDA cleared CELLSEARCH®CTC test. Cox proportional hazard regression models were used for prognostic association of serum CGA and CTC counts with overall survival.
Results
In the screening cohort 200 men were eligible for analysis. The median serum CGA was 100.3 ng/mL (interquartile range 67–161.3) and 34/200 were above the reference range. In the subset of men with Gleason scores ≥ 8, elevated CGA was associated with shorter overall survival [hazard ratio (HR) 2.19, p = 0.017]. In the validation cohort for 71 men eligible for analysis the median serum CGA was 90 ng/mL (interquartile range 55–156) and 31/71 patients had an elevated CGA. 51% of patients had a Gleason score ≥8 and 66/71 patients had CTCs enumerated with 26/66 with a CTC count ≥ 5 per 7.5ml blood sample (unfavorable). Both elevated serum CGA (HR 1.91, p = 0.043) and unfavorable CTC counts (HR 2.97, p = 0.0012) were adversely associated with overall survival and patients with ≥ 5 CTCs and elevated serum CGA had the shortest overall survival (HR 3.76, p = 0.008).
Conclusion
Elevated serum CGA was negatively associated with OS in men with mCRPC. Serum CGA represents a prognostic biomarker that may complement CTC enumeration.
Keywords: Castrate resistant prostate cancer, serum chromogranin-A, circulating tumor cells, prognostic biomarker
Introduction
Androgen deprivation therapy is a widely used treatment in hormone sensitive prostate cancer stage and is effective for variable time periods. Disease progression after androgen deprivation inevitably develops resulting in castration resistant prostate cancer (CRPC). A subset of advanced prostate cancer tumors are non-androgen axis signaling dependent and harbor neuroendocrine features (including small-cell or large-cell subtypes) and are termed treatment-related neuroendocrine prostate cancer NEPC (tNEPC) (1). Exposure to androgen deprivation enriches neuroendocrine differentiation and a wide incidence of this has been reported, ranging from 3% to 71% in CRPC biopsy specimens (2–4). The selective pressure during androgen deprivation therapy may induce focal neuroendocrine differentiation of prostatic adenocarcinoma. tNEPC has also been associated with a more aggressive clinical course, lower response to conventional therapies for advanced prostate cancer and shorter overall survival (5). Since tNEPC is typically non-secretory for PSA, a circulatory biomarker associated with tNEPC would be advantageous in clinical practice.
Chromogranin-A (CGA), a 49 kDa protein produced in the neuroendocrine system, is a widely used marker for neuroendocrine tumors, making it an attractive potential biomarker for monitoring the course of tNEPC (6). Neuroendocrine cells in the prostate are suspected to regulate growth through autocrine and paracrine signaling and lack androgen receptor expression (7, 8). Serum CGA correlates with number of CGA-positive neuroendocrine cells present in prostatic tumor tissue (9). Small retrospective studies have shown increased serum levels of CGA in nearly 45% of CRPC patients, with conflicting results in terms of its prognostic value (10–12). However, these studies were limited by the use of different thresholds of CGA, lack of validation cohorts, and inclusion of patients on proton pump inhibitors (PPIs), which are known to elevate serum CGA levels (13), making it difficult to accurately interpret its prognostic or predictive value.
CTCs are a validated prognostic biomarker and the CELLSEARCH® test is the only FDA-cleared test for prognostication in CRPC (14). Here we present the results of a two-cohort study retrospectively analyzing a prospective sample collection to determine the prognostic value of serum CGA in CRPC stage patients and comparing the prognostic value of serum CGA to circulating tumor cells (CTCs).
Subjects and methods
Patient selection
Screening cohort (SC)
At a single institution between May 2002 and April 2009, blood samples were obtained from men who were failing androgen deprivation therapy for metastatic hormone sensitive prostate cancer (at time of diagnosis of mCRPC). Enrollment of the patient population was performed on an IRB-approved study and after obtaining written consent. Definition of failure of androgen deprivation for metastatic prostate cancer and progression to castrate resistance stage was defined as development of new metastatic lesions on imaging or biochemical progression (two serially rising PSAs at least one week apart) during continuous androgen deprivation.
Validation cohort (VC)
Between June 2013 and August 2015, 92 men with mCRPC were enrolled in a multi-site prospective clinical and biospecimen collection study (NCT#01953640). Eligible men had histological diagnosis of metastatic prostatic adenocarcinoma, progression on ADT [defined as 20% increase in sum of longest diameters of measurable lesions, appearance of new measurable lesions in lymph nodes (≥ 2.0 cm), viscera or soft tissue (≥ 1.0 cm), two or more new lesions on bone scan, or two consecutive increases in PSA levels, taken at least one week apart and ≥ 2.0 ng/mL]. After providing written informed consent, blood samples were collected for serum CGA and circulating tumor cell (CTC) enumeration. All patients started abiraterone acetate and prednisone (AA/P) after the first sample collection was performed and underwent a second blood sample collection after 12 weeks of treatment with AA/P.
In both cohorts, only men with sub-castrate levels of total testosterone (as defined by ≤ 50 ng/dl) were included. All CRPC patients receiving PPIs or with impaired renal and hepatic function were excluded from both cohorts, as artefactual elevations in chromogranin-A levels have been reported with these conditions previously (13).
Specimen Methods
Blood obtained from patients was processed for serum generation after collection in BD SST™ 6.0 mL vacutainers. All specimens underwent centrifugation at 3,000 rpm for 10 minutes to generate serum. For the validation cohort, after consent, blood obtained from patients was processed for serum generation after collection in BD SST™ 6.0 mL vacutainers. All specimens underwent centrifugation at 3,000 rpm for 10 minutes to generate serum fractioned into multiple aliquots and labeled with coded identifiers and stored at −80°C. This was performed within 30 to 45 minutes of sample collection from patients. After the initial centrifugation a protease inhibitor cocktail was added, the ingredients of which included 10 mL PBS (Invitrogen No. 14190300);1 tablet complete of mini, EDTA-free protease inhibitor (Roche No. 11 836 170 001); Sodium Vanadate Na3VO4 and PMSF (Sigma No. P7626-5G). One tablet of the protease inhibitor was completely dissolved in 10 mL PBS and 5 μL/mL Na3VO4 with10 μL/mL PMSF and was added for stock solution preparation (with 100 mM of PMSF) of which 50 μL was added to each serum specimen for all assays which were performed without undergoing freeze-thaw cycles
Assay Methods
Serum Chromogranin-A (CGA) was measured in a homogeneous automated immunofluorescent assay using time-resolved amplified cryptate emission (TRACE). CGA was sandwiched between a mouse monoclonal antibody against CGA (labeled with europium cryptate - TRACE donor) and a second mouse monoclonal antibody against CGA (labeled with XL665-TRACE acceptor), bringing the two antibodies into close proximity. A nitrogen laser at 337 nm was used to excite the antigen-antibody complex, resulting in a fluorescent energy emission at 620 nm (internal reference) and energy transferred by nonradiative dipole-dipole coupling to XL665, resulting in energy emission at 665 nm. The ratio of energy emitted at 665 nm to that emitted at 620 nm is calculated for each sample and the signal intensity is proportional to the number of antigen-antibody complexes formed, and therefore to CGA concentration.
Prior to 2012 (for all samples collected in the screening cohort), the TRACE immunochemiluminometric assay was performed on an internal instrument with a chromogranin reference range ≤ 225 ng/mL. On September 20, 2012, Mayo Medical Laboratories started performing TRACE assays on the KRYPTOR Compact instrument (ThemoFisher Scientific, Waltham MA) with a new chromogranin reference range ≤ 93 ng/mL. All samples collected in the VC were analyzed on this instrument. To minimize any effect from the different testing instruments and enable comparison between our screening and validation cohorts, CGA levels were considered elevated if values were above the upper limit of normal as determined for each respective testing time period.
CTC enumeration
Whole blood was collected in CellSearch® Circulating Tumor Cell Kits (Menarini Silicon Biosystems, San Diego, CA) per the manufacturer’s instructions and circulating tumor cell (CTC) enumeration was performed using the FDA cleared CellSearch® CTC test as previously described (14).
Statistical Methods
CGA levels were considered elevated if values were above the upper limit of normal (≤ 93 ng/mL) as determined for each respective testing time period. The primary endpoint of this analysis was association of serum CGA levels with overall survival (OS), defined as time from study enrollment to death or last follow up. Cox proportional hazard regression analysis was performed on clinical parameters, pretreatment CGA and CTC counts for associations with overall survival (OS). Only factors significant in univariate analysis were included in multivariate analysis. Kaplan-Meier method was used to estimate the OS, with 95% confidence intervals using JMP-10 (SAS institute, Cary, NC). Receiver operator curves (ROC) for elevated CGA (> upper limit of normal) and unfavorable CTCs (>= 5 cells) evaluated the area under the curve (AUC) for predicting survival at 33 months. For the ROC analyses, AUCs for both markers were determined and compared alone and in various combinations. Area-proportional Venn diagrams were generated using the online web application BioVenn (15).
Results
Evaluation of chromogranin-A as a prognostic biomarker in the screening cohort
In the screening cohort of 256 men with metastatic prostate cancer, 35 were excluded for non-castrate testosterone levels ≥50 ng/dl (1.7 nmol/L) and an additional 21 men were excluded for use of a PPI at time of blood collection. Of the remaining 200 men with CRPC, the median age was 72 years, 81 (40.5%) had a Gleason score (GS) ≥ 8, and the median PSA was 17.7 ng/mL (Table 1). The median time from ADT to CRPC was 3.23 years (range 0.39 to 21.7 years). At median follow up 2.2 years, 156 (78%) were deceased. The median serum CGA was 100.3 ng/mL (interquartile range 67–161.3), the mean was 184.8 ng/mL (standard deviation 396), and 34/200 (17%) had an elevated CGA (above reference range of 225 ng/mL). Across the entire cohort, an abnormally elevated CGA had no statistically significant associations with overall survival [Hazard ratio (HR) 1.35, 95% Confidence Interval (95%CI) 0.89–1.97).
Table 1.
Patient demographics from screening and validation cohorts.
| ScreeningCohort | ValidationCohort | |
|---|---|---|
|
| ||
| # patients | 200 | 71 |
| Age (IQ range) | 72 (65, 77) | 73 (66, 78) |
| Median time (years) from initial diagnosis to CPRC (range) | 6.3 (0.2, 25.2) | 5.5 (0.45, 23.7) |
| Median time (years) from start of ADT to CRPC (range) | 3.2 (0.4, 21.7) | 2.8 (0.3 – 18.2) |
| Median duration (years) from CRPC to death or last follow up (range) | 2.2 (0.1–9.6) | 1.82 (0.11, 3.50) |
| Deceased | 156 | 31 |
|
| ||
| Gleason score | ||
| Missing | 18 | 0 |
| <8 | 101 | 35 |
| ≥8 | 81 | 36 |
|
| ||
| Laboratory Data at time of CRPC | ||
| Median PSA (ng/ml) (IQ range) | 17.7 (7.3, 64.8) | 11.8 (5.4, 36) |
| Median CGA (ng/ml) (IQ range) | 100 (67, 161) | 90 (55–156) |
| Median testosterone (ng/dl) (IQ range) | 4.5 (1.5, 9.1) | 7 (6.9, 10) |
|
| ||
| CTC count | ||
| Median (range) | 2 (0, 308) | |
| ≥5 (unfavorable) | 26 | |
| <5 (favorable) | 39 | |
| Missing | 6 | |
| Volume of metastatic disease* | ||
| High | 40 | |
| Low | 31 | |
high volume= visceral metastases or ≥4 bone lesions with ≥1 outside vertebral bodies and pelvis
However, as higher Gleason scores have been associated with an increased degree of neuroendocrine differentiation(16), we evaluated serum CGA in the subset of men with higher Gleason scores. In the subset of 81 men with a Gleason score of ≥ 8, an elevated chromogranin was associated with a shorter OS [HR 2.19, 95%CI: 1.16–3.85, p = 0.02). In the multivariate analysis, CGA remained associated with overall survival for men with GS ≥ 8 (p = 0.02) after adjusting for PSA, Gleason score, time from diagnosis to study treatment and radiographic evidence of recurrent prostate cancer.
Validation of serum chromogranin-A as a prognostic biomarker
In the validation cohort, of the 92 men enrolled with CRPC, 21 were excluded for PPI use. The median age was 71 years, 36 (50.7%) had a Gleason score ≥ 8, and the median PSA was 11.8 ng/dl (Table 1). The median time from initiation of ADT to development of castration resistance was 2.8 years (range 0.29 – 18.22). After a median follow up of 1.82 years, 31 (43.7%) were deceased. The median CGA was 90 ng/mL (IQ range 55–156), the mean was 174.7 ng/mL (standard deviation 280.2), and 31 (44%) had a CGA above the reference range (>93 ng/mL).
Including all 71 patients, elevated serum CGA levels were associated with shorter OS (HR 1.91, 95%CI 1.02 – 3.67, p 0.043, Table 2). In the sub-cohort of 36 patients with high Gleason scores, the median OS in men with an elevated serum CGA was 6.9 months shorter compared to men with a normal serum CGA (12.0 months vs 18.9, log-rank p = 0.043). In univariate analysis, age, Gleason score, volume of metastatic disease had no associations with OS.
Table 2.
Cox regression univariate analysis for associations of clinical and laboratory parameters with overall survival. Parameters that reach statistical significance (p<0.05) are emboldened.
| Overall survival | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariate Analysis | |||||||
|
| |||||||
| No pts. | HR | 95% CI | p value | ||||
| Screening Cohort | CGA (entire cohort) | Elevated | 34 | 1.35 | 0.89 | 1.97 | 0.154 |
| Normal | 166 | ||||||
|
| |||||||
| CGA (subset GS ≥ 8) | Elevated | 15 | 2.19 | 1.16 | 3.85 | 0.0169 | |
| Normal | 66 | ||||||
|
| |||||||
| Entire Validation Cohort (VC) | Age | >73 | 34 | 1.64 | 0.88 | 3.12 | 0.880 |
| ≤ 73 | 37 | ||||||
|
| |||||||
| Gleason score at diagnosis | ≥ 8 | 36 | 1.21 | 0.64 | 2.26 | 0.549 | |
| ≤ 7 | 35 | ||||||
|
| |||||||
| CTCs | Unfavorable ≥5 | 26 | 2.97 | 1.55 | 5.81 | 0.0012 | |
| Favorable <5 | 39 | ||||||
|
| |||||||
| Chromogranin | Elevated | 31 | 1.91 | 1.02 | 3.67 | 0.043 | |
| Normal | 40 | ||||||
|
| |||||||
| VC Subset with Gleason ≥8 | CTCs | Unfavorable ≥5 | 14 | 3.96 | 1.50 | 11.70 | 0.006 |
| Favorable <5 | 19 | ||||||
|
| |||||||
| Chromogranin | Elevated | 14 | 2.49 | 1.01 | 6.47 | 0.0487 | |
| Normal | 22 | ||||||
CTC enumeration was available for 66/71 (93%) men and were unfavorable (≥ 5) in 26 (39%). In 14/71 men (20%), no circulating tumor cells were identified. In all 71 patients, unfavorable CTC counts were associated with a poorer OS (HR 2.97, 95%CI 1.55–2.81, p 0.0012, Table 2). In the subset of 36 men with GS ≥ 8, CTCs were evaluated in 33/36 (92%) and were unfavorable in 14/33 (42.4%), and no circulating tumor cells were detected in 8/33 (24.2%). In men with GS ≥ 8, unfavorable CTC counts were adversely associated with OS (HR 3.96, 95%CI 1.5 – 11.7, p 0.0059).
Comparing the prognostic value of circulating tumor cells to and serum chromogranin-A in the validation cohort
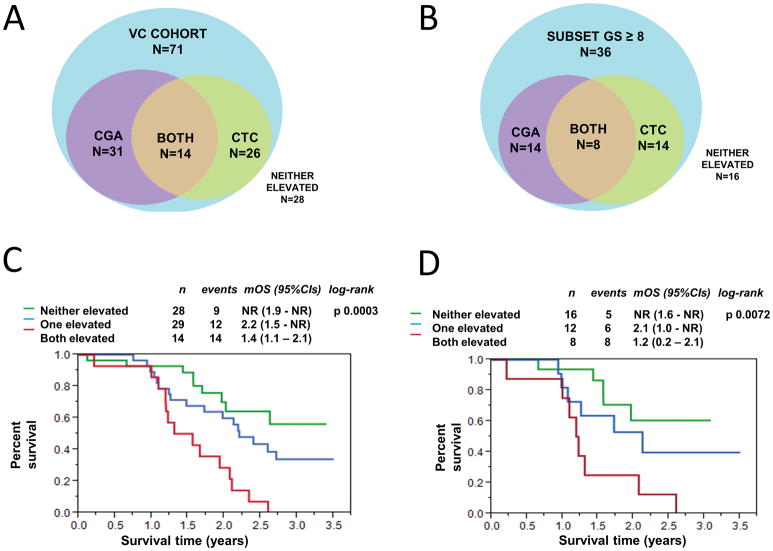
We subsequently analyzed the validation cohort to determine the frequency of abnormal CGA elevation and/or unfavorable CTCs in the entire cohort (Figure 1A), and the subset with GS ≥ 8 (Figure 1B). Of the 71 patients in the validation cohort, 17 patients had elevated CGA only, 12 had unfavorable CTCs only, and 14 patients had both an elevated CGA and unfavorable CTCs. In multivariate analysis in the entire VC, after correcting for CTCs, an elevated serum CGA no longer remained statistically significant (HR 1.88, 95%CI 0.97 – 3.78, p = 0.061). In the subset of patients with GS ≥ 8, 6 patients had elevated serum CGA only, an additional 6 had unfavorable CTC counts only, and 8 had both an elevated serum CGA and unfavorable CTC enumeration. In the high Gleason group, after adjusting for CTCs, elevation of serum CGA was not statistically significant (HR 2.24, 95%CI 0.82 – 6.71, p = 0.11).
Figure 1.
Overlap of CTCs and serum CGA. Area proportional Venn diagrams describe elevation of serum CGA (purple), unfavorable CTC (green), both elevated serum CGA and unfavorable CTC (yellow), or neither elevated (blue) in the VC cohort (1A) and the subset with a Gleason score ≥ 8 (1B). Kaplan-Meier overall survival analysis grouped by elevated CGA and/or unfavorable CTC (neither elevated, either one elevated, or both elevated) for the entire cohort (1C) and the subset of men with GS ≥ 8 (1D).
In the Kaplan-Meier overall survival analysis, patients with both an elevated serum CGA and unfavorable CTC enumeration had a median OS of 1.4 years (95%CI 1.1–2.1) (Figure 1C). This was significantly shorter than patients with only one elevated marker [median OS 2.2 years (95%CI 1.5-not reached)], and patients with no elevation in either serum CGA or CTC (median OS not reached, 95%CI 1.9 – not reached). Similar observations were observed in the subset of patients with GS ≥ 8 (Figure 1D).
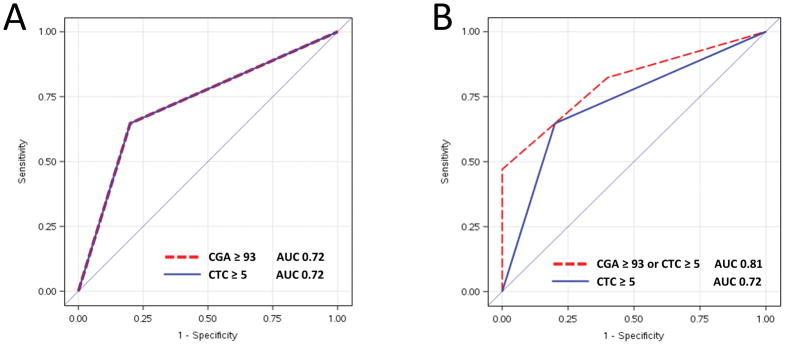
Finally, we calculated the area under the curve (AUC) in a prognostic model of the high Gleason group using CTC enumeration only, serum CGA only, or CTC + serum CGA. In the high GS group, both CTCs and serum CGA had the same AUC of 0.72 (Figure 2A). A prognostic model including either elevation in CTC or CGA demonstrated an incremental benefit over CTC count alone, though this was not statistically significant (0.81 vs 0.72, p = 0.36; Figure 2B).
Figure 2.
Receiver Operating Characteristic Curve (ROC) comparing circulating tumor cells (CTCs) to serum chromogranin (CGA) in patients with high Gleason Score
2A: The ROC curves for CTCs ≥ 5 (blue line) and serum CGA >93 ng/mL (red line) overlap with an identical area under the curve (AUC) of 0.72
2B: A prognostic model including either CTCs ≥ 5 or serum CGA >93 ng/mL is compared to CTCs ≥ 5 demonstrated an incremental, non-statistically significant improvement
Discussion
In this two cohort study, which included a prospective validation cohort in which CTCs were being collected, we evaluated the prognostic value of serum CGA in men with CRPC and compared it to CTC count which is an FDA-cleared prognostic biomarker in this stage. Our results from the screening cohort identified an elevated serum CGA level was adversely associated with overall survival, limited to the subset of men with CRPC with GS ≥8. Prostatic adenocarcinomas with higher Gleason scores have a greater likelihood of neuroendocrine differentiation (9, 17), providing rationale for our hypothesis that elevated serum CGA might be a useful marker in this subset of patients based on correlation with poor survival especially. We validated the prognostic value of serum CGA in men diagnosed initially with GS ≥8 in an independent cohort of men with CRPC. Interestingly, in our validation cohort serum CGA was associated with shorter survival across the entire cohort, probably due to the higher relative proportion of patients with GS ≥8 in this cohort. On multivariate analysis in the sub-cohort of patients with GS≥8 after adjusting for CTCs, serum CGA was no longer statistically significant, as an independent prognostic biomarker in CRPC, but this observation is limited by a relatively small sample size.
We directly compared the prognostic value of CTCs to serum CGA in men with CRPC with all samples collected at the same time. We found that CGA identifies an additional subset of patients of higher risk CRPC in an additional subset of patients with favorable or even undetected CTCs. The CellSearch® selection method is dependent on expression of the epithelial cell adhesion molecule (EpCAM) or cytokeratins, however the CTC profile for NEPC has lower cytokeratin expression, attributed to epithelial to mesenchymal transition (18). The cellular changes associated with tNEPC may result in circulating malignant cells remaining undetected by CTC enumeration systems reliant on epithelial markers. Thus the heterogeneity of prostate cancer may be better captured by assessing multiple complementary biomarkers, including both CTCs and serum CGA. We compared the prognostic value of CTC and CGA in the subset of men with high GS, with both biomarkers having an identical AUC. The clinical value of CGA may be attributed to the biological differences of NE cells and adenocarcinoma cells. NE cells are resistant to hormonal deprivation and do not secrete prostate-specific antigen (PSA)(1). Several studies have evaluated the prognostic value of serum CGA in men with CRPC, with conflicting results. Serum CGA was reported both as adversely correlated with OS (12, 19–21) and having no associations with OS (22, 23).
Compared to previous evaluations, our study has several strengths, including being the only study with an independent validation cohort to validate a specific CGA assay. Additionally, unlike several of the previous reports on CGA (12, 19, 23), we excluded patients taking proton pump inhibitors to limit the influence of non-malignant CGA elevation. We also followed the REMARK criteria for tumor prognostic biomarker studies (24). Limitations of our study include a difference in the patient cohorts and the small sample size of our prospective cohort. While our screening cohort was retrospective and did not have CTC enumeration our validation cohort was prospectively obtained and retrospectively analyzed. Another potential limitation could be on account of the instrument used in the screening cohort to perform the TRACE immunochemiluminometric assay to quantify serum chromogranin A levels, which was changed in 2012 from an internal instrument to the Kryptor Compact instrument. We aimed to limit any influence by using the reference ranges to determine chromogranin A elevation as these were extensively internally validated.
While our sample size was too small to identify a statistically significant improvement in AUC using a model incorporating both CTC and CGA, we were able to further risk stratify patients based on the elevation of one or more biomarker. Next steps will include evaluating serum CGA as a prognostic biomarker in larger castration resistant prostate cancer cohorts.
Conclusions
Elevated serum CGA is adversely associated with overall survival in the subset of men with CRPC and initially diagnosed with high Gleason scores. The prognostic value of serum CGA is comparable to CTCs, and combining both biomarkers improves the ability to stratify survival outcomes. Further evaluation of CGA as a marker for neuroendocrine differentiation in earlier stages of disease and evaluating CGA as a predictive biomarker in men with high Gleason tumors in multi-variable analysis is warranted.
Acknowledgments
This study is supported in part by R01 CA21209 to M.K; Joseph and Gail Gassner support to M.K; and for the prospective clinical trial that performed the CTC collections by the Mayo Clinic Center for Individualized Medicine with support for M.K. D.H, R.C.
References
- 1.Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol. 2014;32(30):3383–90. doi: 10.1200/JCO.2013.54.3553. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiborn T, Bjartell A, Abrahamsson P-A. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51(4):585–9. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- 4.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. European urology. 2004;45(5):586–92. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer discovery. 2011;1(6):487–95. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gkolfinopoulos S, Tsapakidis K, Papadimitriou K, Papamichael D, Kountourakis P. Chromogranin A as a valid marker in oncology: Clinical application or false hopes? World J Methodol. 2017;7(1):9–15. doi: 10.5662/wjm.v7.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krijnen J, Janssen P, Ruizeveld de Winter J, Krimpen H, Schröder F, Kwast T. Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochemistry and Cell Biology. 1993;100(5):393–8. doi: 10.1007/BF00268938. [DOI] [PubMed] [Google Scholar]
- 8.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Annals of Oncology. 2001;12(suppl_2):S141–S4. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 9.Angelsen A, Syversen U, Haugen OA, Stridsberg M, Mjølnerød OK, Waldum HL. Neuroendocrine differentiation in carcinomas of the prostate: do neuroendocrine serum markers reflect immunohistochemical findings? The Prostate. 1997;30(1):1–6. doi: 10.1002/(sici)1097-0045(19970101)30:1<1::aid-pros1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Cabrespine A, Guy L, Gachon F, Cure H, Chollet P, Bay JO. Circulating chromogranin a and hormone refractory prostate cancer chemotherapy. J Urol. 2006;175(4):1347–52. doi: 10.1016/S0022-5347(05)00640-3. [DOI] [PubMed] [Google Scholar]
- 11.Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R, et al. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer. 2005;12(1):109–17. doi: 10.1677/erc.1.00876. [DOI] [PubMed] [Google Scholar]
- 12.Heck MM, Thaler MA, Schmid SC, Seitz AK, Tauber R, Kubler H, et al. Chromogranin A and neurone-specific enolase serum levels as predictors of treatment outcome in patients with metastatic castration-resistant prostate cancer undergoing abiraterone therapy. BJU Int. 2017;119(1):30–7. doi: 10.1111/bju.13493. [DOI] [PubMed] [Google Scholar]
- 13.Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. British journal of cancer. 2011;105(8):1173–5. doi: 10.1038/bjc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research. 2008;14(19):6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 15.Hulsen T, de Vlieg J, Alkema W. BioVenn–a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC genomics. 2008;9(1):488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Karaoğlan Ü, Celik B, Ataoğlu Ö, Biri H, Bozkirli I. Prostate cancer and neuroendocrine differentiation. International urology and nephrology. 1999;31(1):75–82. doi: 10.1023/a:1007175924082. [DOI] [PubMed] [Google Scholar]
- 17.Bollito E, Berruti A, Bellina M, Mosca A, Leonardo E, Tarabuzzi R, et al. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Annals of oncology. 2001;12(suppl_2):S159–S64. doi: 10.1093/annonc/12.suppl_2.s159. [DOI] [PubMed] [Google Scholar]
- 18.Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, Louw J, et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clinical Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taplin M-E, George DJ, Halabi S, Sanford B, Febbo PG, Hennessy KT, et al. Prognostic significance of plasma chromogranin a levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology. 2005;66(2):386–91. doi: 10.1016/j.urology.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Conteduca V, Aieta M, Amadori D, De Giorgi U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Critical reviews in oncology/hematology. 2014;92(1):11–24. doi: 10.1016/j.critrevonc.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Conteduca V, Burgio SL, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin A is a potential prognostic marker in prostate cancer patients treated with enzalutamide. The Prostate. 2014;74(16):1691–6. doi: 10.1002/pros.22890. [DOI] [PubMed] [Google Scholar]
- 22.Burgio SL, Conteduca V, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin A predicts outcome in prostate cancer patients treated with abiraterone. Endocrine-related cancer. 2014;21(3):487–93. doi: 10.1530/ERC-14-0071. [DOI] [PubMed] [Google Scholar]
- 23.Hvamstad T, Jordal A, Hekmat N, Paus E, Fosså SD. Neuroendocrine serum tumour markers in hormone-resistant prostate cancer. European urology. 2003;44(2):215–21. doi: 10.1016/s0302-2838(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) European journal of cancer. 2005;41(12):1690–6. doi: 10.1016/j.ejca.2005.03.032. [DOI] [PubMed] [Google Scholar]