Abstract
Introduction
Epidermal growth factor receptors EGFR and ErbB2 are overexpressed in schwannomas and meningiomas. Preclinical and clinical data indicate that lapatinib, an EGFR/ErbB2 inhibitor, has antitumor activity against vestibular schwannomas in neurofibromatosis type 2 (NF2) patients. Its antitumor activity against meningiomas, however, is unknown.
Methods
We conducted a retrospective review of patients with NF2 and progressive vestibular schwannomas treated on a Phase II clinical trial with lapatinib (NCT00973739). We included patients with at least one volumetrically measurable meningioma (>0.5 cm3) who received at least five 28-day courses of treatment. Patients received lapatinib 1,500 mg daily. Meningioma response was assessed using 3-dimensional MRI volumetrics. Progressive meningioma growth and response were defined as +20% and −20% change in tumor volume from baseline, respectively. Off-treatment was defined as any period >5 months without lapatinib.
Results
Eight patients (ages: 20–58 years) who met criteria had 17 evaluable meningiomas with a combined volume of 61.35 cc at baseline, 61.17cc during treatment, and 108.86 cc (+77.44% change) off-treatment, P = 0.0033. Median time on-treatment and off-treatment was 15.5 and 16.7 months, respectively. On-treatment mean and median annualized growth rates were 10.67% and 1.32%, respectively. Off-treatment mean and median annualized growth rates were 20.05% and 10.42%, respectively. The best volumetric response was −26.1% after 23 months on lapatinib. Two tumors increased >20% volumetrically on-treatment, compared to 8 tumors off-treatment.
Conclusions
These data suggest that lapatinib may have growth-inhibitory effects on meningiomas in NF2 patients, and support prospective studies of lapatinib for NF2 patients with progressive meningiomas.
Keywords: Neurofibromatosis type 2, meningioma, epidermal growth factor receptor, lapatinib, volumetrics
Introduction
Neurofibromatosis type 2 (NF2) is an autosomal dominant disorder caused by inactivation of the NF2 gene located on chromosome 22q. Patients with NF2 are predisposed to develop multiple tumors in both the peripheral and central nervous systems (CNS), including schwannomas, meningiomas and ependymomas.[1]
The epidermal growth factor receptors EGFR and ErbB2 (also known as Her2) are transmembrane receptor tyrosine kinases that are overexpressed and/or constitutively activated across a spectrum of cancers, and overexpressed in schwannomas and in meningiomas. A number of studies have analyzed the expression of EGFR and ErbB2 in meningiomas [2–9], and EGFR expression appears to be inversely correlated with histological grade.[8] In one study, 58% of the meningiomas were positive for EGFR, with expression of ErbB2 protein predominantly localized to the capillary endothelium.[4] A preclinical study by Wang et al. demonstrated that regulation of ErbB2 affected the proliferation, apoptosis, invasion of human meningioma cells in vitro.[10] However, the precise role of EGFR and ErbB2 in the pathogenesis and progression of meningiomas remains unclear.
In addition to NF2-associated meningiomas, inactivation of the NF2 gene has also been demonstrated in approximately 60% of sporadic meningiomas, [11–16] with a strong association with tumor location, i.e. frequent NF2 loss in convexity versus skull base meningiomas. More recently, inactivation of NF2 has also been recognized as a frequent event in radiation-induced meningiomas.[17] Loss of NF2 has been associated with upregulation of receptor tyrosine kinases including EGFR through a variety of proposed mechanisms [18–20]. Preclinical and clinical data indicate that lapatinib, a dual EGFR/ErbB2 small molecule kinase inhibitor, has antitumor activity against schwannomas.[4, 21] We therefore hypothesized that lapatinib may also have growth-inhibitory effects against meningiomas in patients with NF2.
Using data from a previously published prospective phase 2 clinical trial with lapatinib for progressive vestibular schwannomas in adult and pediatric patients with NF2, [22] we retrospectively reviewed patients with at least one measurable meningioma and their response to lapatinib.
Methods
Patient eligibility
This was a single institution, retrospective study performed at NYU Langone Health. Patients included in this study were previously enrolled in a phase 2 trial of lapatinib in adult and pediatric patients with NF2 and progressive vestibular schwannomas (ClinicalTrials.gov: NCT00973739); a study that was conducted under a protocol approved by the institutional review board of NYU Langone Medical Center.[22] Eligibility for the trial included age greater than 3 years with a clinical diagnosis of NF2 and progressive vestibular schwannoma, defined as tumor growth or hearing progression within the past 12 months. From this cohort, we included patients who had at least one volumetrically measurable meningioma greater than 0.5 cm3, had received at least five courses of lapatinib, and available imaging studies included at least one off-treatment evaluation time point defined as any period of greater than 5 months after discontinuation of lapatinib.
Treatment
All eligible patients were ≥18 years of age and received the protocol treatment dose of lapatinib at 1,500 mg once daily in continuous 28-day courses.[22] As previously reported, treatment was well tolerated in all trial patients, and none of the patients included in the present study required dose reductions for toxicity or other reasons. On this study, lapatinib was continued until disease progression of the primary target tumor (vestibular schwannoma).
Response evaluation
Volumetric assessments were performed using 3-dimensional tumor volumetrics with postcontrast, T1-weighted magnetization-prepared rapid acquisition with gradient echo sequences at 1-mm slice thickness, and no gap, using semi-automated segmentation software (Vitrea® platform), as previously described.[22, 23] We defined volumetric response or progression as a ≥20% decrease or increase, respectively, in meningioma volume compared to the baseline measurement. These criteria are consistent with consensus recommendation for response assessments in neurofibromatosis trials.[23] Tumor volume measurements were taken pre-treatment, on-treatment, and off-treatment. Measurements taken 1 year after the start of lapatinib were used to assess on-treatment tumor volume. Follow-up measurements to evaluate off-treatment tumor volume were collected. Patients with multiple post-therapy measurements allowed for longitudinal tumor volume assessments and were standardized as an average annual growth rate (annualized growth rate) to compare tumors at 1-year off-treatment.
Statistical methods
Data analysis was primarily descriptive. Meningioma volume was analyzed at the tumor-level. A nonparametric Wilcoxon Signed-Rank test was used to explore differences in tumor volume and growth from baseline between on- and off-treatment measurements. All analyses were performed using the R statistical program (R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
All patients were previously consented to participate in the Phase 2 trial (NCT00973739). All patients remained on 1500 mg of lapatinib daily during the duration of their therapy without any dose reductions due to toxicities. Of the 17 patients from the original Phase 2 trial, 14 patients had at least one meningioma, eight of which were eligible for this retrospective study. Reasons for exclusion were insufficient meningioma size at baseline in one patient, no volumetrically measurable meningioma on-treatment or off-treatment in four patients, and two patients with imaging studies that precluded volumetric measurements due to technical reasons. One of the eligible patients had a total of nine meningiomas, two of which were volumetrically measurable. Baseline patient characteristics, meningioma locations and relevant treatment time points are summarized in Table 1. Of the eight patients who met eligibility criteria all were adults (age range 20–58 years) with a total of 17 evaluable meningiomas. The median time on-treatment was 15.5 months (range 5–21.9 months) and the median time off-treatment was 16.7 months (range 7.8–29.5 months). All of the tumors included in this study were diagnosed on imaging, and did not undergo biopsy or resection.
Table 1.
Summary of patient characteristics, meningioma location, and study time points.
| Patient | Age [years] |
Gender | Meningioma Location | Total time on-treatment [months] |
Last measurable time point off –treatment [months] |
|---|---|---|---|---|---|
| 1 | 22 | F | Left temporal horn | 20 | 16.9 |
| Right parietal parasagittal | |||||
| 2 | 28 | M | Right lateral ventricle | 21.9 | 11.9 |
| Left inferior parasagittal | |||||
| 3 | 48 | F | Right temporal | 5 | 16.7 |
| High left parietal | |||||
| 4 | 22 | F | Right ventricle | 5.5 | 29.5 |
| Left parasagittal parietal | |||||
| Left anterior temporal | |||||
| 5 | 58 | M | Right falx | 15.6 | 10.4 |
| 6 | 20 | M | Right frontal | 15.5 | 7.8 |
| 7 | 29 | F | Left occipital falx | 16 | 18.8 |
| Right frontal convexity | |||||
| Inferior frontal | |||||
| 8 | 46 | F | Right parietal | 13.1 | 16.7 |
| Left falcine | |||||
| Left ventricular |
Volumetric responses
Meningioma volumes are summarized in Table 2. The total volume for all 17 meningiomas combined was 61.35 cm3 at baseline, 61.17 cm3 while on-treatment and 108.86 cm3 off-treatment. The calculated tumor growth by total volume was +77.4% off-treatment. Total percent change from baseline while on-treatment was −0.29% and +77.4% once off-treatment. Tumor volume differences were significant (p=0.003).
Table 2.
Summary of meningioma tumor volumes [cm3].
| Patient | Meningioma Number |
Baseline Combined Volumes |
On-treatment Meningioma Volumes |
Off-treatment Meningioma Volumes |
|---|---|---|---|---|
| 1 | 1 | 1.79 | 4.72 | 2.62 |
| 2 | 1.27 | 1.30 | 1.67 | |
| 2 | 3 | 1.76 | 1.30 | 1.19 |
| 4 | 0.61 | 0.63 | 0.51 | |
| 3 | 5 | 10.05 | 9.63 | 10.30 |
| 6 | 0.71 | 0.68 | 0.80 | |
| 4 | 7 | 5.73 | 5.55 | 7.30 |
| 8 | 17.79 | 14.18 | 52.03 | |
| 9 | 2.55 | 3.40 | 8.94 | |
| 5 | 10 | 0.72 | 0.85 | 0.87 |
| 6 | 11 | 2.23 | 2.40 | 3.80 |
| 7 | 12 | 4.63 | 5.21 | 5.32 |
| 13 | 2.76 | 2.67 | 3.41 | |
| 14 | 1.51 | 1.53 | 1.75 | |
| 8 | 15 | 3.29 | 3.17 | 3.85 |
| 16 | 2.39 | 2.21 | 2.38 | |
| 17 | 1.56 | 1.74 | 2.12 | |
| TOTAL | 61.35 | 61.17 | 108.86 |
Therapy received before study
None of the patients had undergone any therapy or medication for their meningiomas prior to study enrollment.
On-treatment response evaluation
There were 8 (47%) tumors that demonstrated volumetric decrease while on-treatment with 26.1% as the best volumetric response after 23 months on-treatment compared to baseline measurement. There were 7/17 (41%) tumors that had <20% volumetric increase and only 2/17(12%) tumors that increased >20% volumetrically (PD) while on-treatment. Concurrent therapies received by these patients while on the original trial were surgical or radio-therapeutic strategies (ie: focal radiation or gamma-knife) directed towards their vestibular schwannomas only.
Off-treatment response evaluation
The tumor volumes measured off-treatment demonstrated that 3/17 (18%) tumors had volumetric decrease, 6/17 (35%) tumors had volumetric increase of ≤20%, and 8/17 (47%) had volumetric increase >20% (PD) compared to their baseline measurements.
Therapy received off-study
There were two patients who received therapy during their off-treatment response evaluations. One patient received bevacizumab until everolimus was added 4 months later, accounting for the only patient whose meningiomas demonstrated a continued response off-treatment at −16.4% and −32.4%, 12-months off-treatment. The other patient received bevacizumab for 6 weeks , subsequently transferred care to a different institution, and was lost to follow up. His tumor demonstrated a 20.8% increase in tumor size on his last follow-up, 15 months off-treatment.
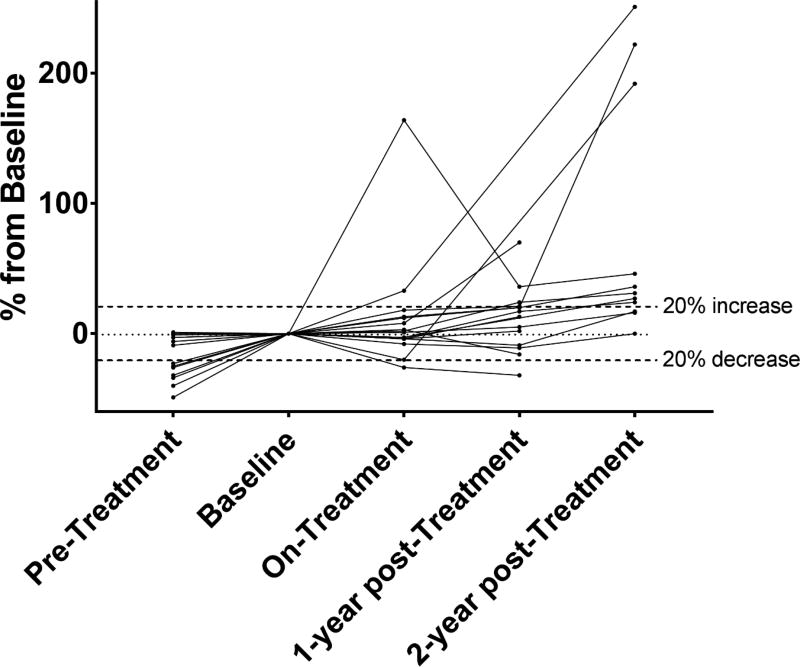
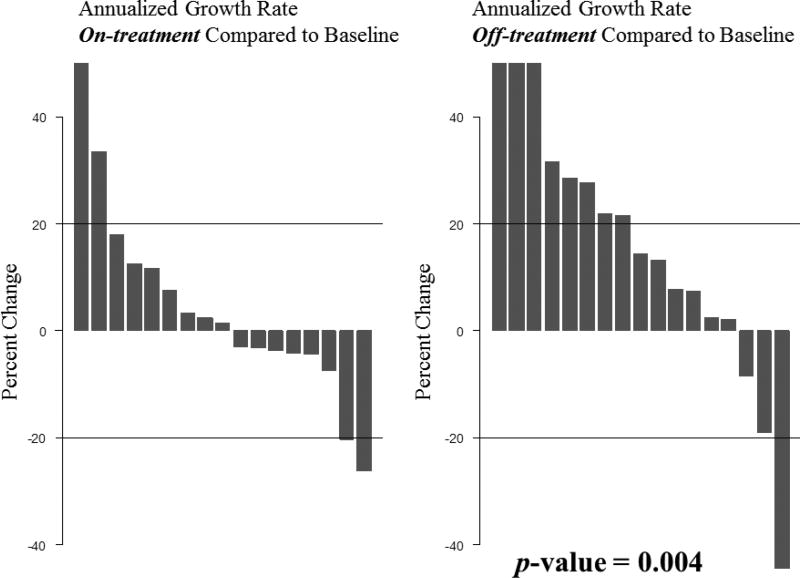
We created two figures to demonstrate changes in individual tumor size over time. Figure 1 demonstrates individual tumor size percent changes over time relative to their baseline. Figure 2 shows waterfall plots demonstrating the annualized growth rates while on-treatment versus off-treatment. The on-treatment data shows that the majority of meningiomas sustained annualized growth rates of <20% change in volume, one meningioma >20% annualized tumor shrinkage, and only two meningiomas with a >20% annualized increase in volume. In contrast, the off-treatment data shows eight meningiomas with >20% annualized increase in volume, three of which with a >40% increase. One meningioma showed >20% annualized tumor shrinkage. The on-treatment mean and median annualized growth rates were 10.67% and 1.32%, respectively. In contrast, the off-treatment mean and median annualized growth rates were 20.05% and 10.42%, respectively. The difference was statistically significant with a p-value of 0.004.
Figure 1.
Changes in individual tumors over time relative to their baseline.
Fig. 2.
Waterfall plot depicting the annualized meningioma growths rate on-treatment with lapatinib compared to off-treatment. Tumor annualized growth rate differences were statistically significant (p=0.004).
Conclusions
Meningiomas represent a major source of morbidity and mortality in NF2 patients, and effective, non-surgical treatment options are urgently needed, especially in patients with a large tumor burden including multiple meningiomas in surgically challenging or inaccessible locations. While bevacizumab has emerged as an effective treatment option for a subset of NF2 patients with progressive vestibular schwannomas, meningiomas generally do not respond to this therapy.[24] Everolimus, a mammalian target of rapamycin complex 1 (mTORC1) inhibitor, has also been studied in NF2 patients,[25, 26] and one study analyzed six meningiomas from NF2 patients treated with everolimus. In this cohort, everolimus treatment did not induce meningioma shrinkage, but appeared to delay the volumetric growth, with median time-to-progression for mengiomas increasing on treatment. Annualized growths rates, however, were not calculated and statistical significance was not determined in this small sample.
Our retrospective analysis shows significantly lower annualized growth rates of meningiomas on-treatment with lapatinib compared to off-treatment with lapatinib, suggesting that lapatinib may have growth-inhibitory effects on meningiomas in NF2 patients.
In reporting our original Phase II study for vestibular schwannomas and lapatinib; it was stated that we had not observed any imaging responses in meningiomas, and that these tumors continued to progress in many of our patients during the study period.[22] However, this observation was based on routine clinical measurements, but not on a systematic or volumetric analysis.
Although prior clinical trials with EGFR inhibitors for patients with sporadic meningiomas have been unsuccessful,[27] lapatinib, a combined EGFR/ErbB2 inhibitors has not been previously tested in patients with meningiomas. In adult and pediatric patients with malignant, intra-axial brain tumors, conventional daily dosing of lapatinib is generally not sufficient to achieve therapeutic concentrations or target inhibition.[28, 29] However, extra-axial brain tumors including meningiomas and vestibular schwannomas are considered to be outside of the blood brain barrier. Correspondingly, intratumoral concentrations of lapatinib were found to be significantly higher in vestibular schwannoma tissue from NF2 patients, with mean intratumoral lapatinib concentrations >4-fold of mean plasma levels,[30] although target inhibition of EGFR and ErbB2 was incomplete. As we do not have on-treatment meningioma tissue samples available from our patient cohort, it is unknown what drug levels were achieved in tumor tissue and whether target inhibition was complete. Accumulating clinical and pre-clinical data indicate that pulse high-dose administration of EGFR and/or ErbB2 inhibitors may be superior to standard dose administration,[28, 31, 32] and pulse high-dose lapatinib administration at doses up to 5,250 mg per day for two consecutive days per week is feasible in cancer patients.[32–34] Therefore, alternative dosing regimens using lapatinib could also be explored in NF2 patients.
Our present study has several limitations, including, but not limited to the retrospective design. We do not have information about the EGFR or ErbB2 status of the meningiomas observed on this study, and we had a limited number of time points available for analysis, especially prior to lapatinib therapy, restricting our ability to assess the pre-treatment growth velocity in all meningiomas. The evaluation of off-treatment growth could have led to a bias in interpretation of growth kinetics, and the reasons for discontinuing lapatinib were not random, but driven by progression in a different tumor type.
In summary, we observed differences in kinetics of meningioma growth during and after therapy with lapatinib, suggesting that treatment with lapatinib may have the potential to arrest or reduce the growth of NF2 related meningiomas in a subset of NF2 patients. Lapatinib at the standard dosing schedule is well-tolerated in NF2 patients[22], and alternative dosing regimens such as pulse-dosing could be considered. Based on our data, we believe that prospective clinical trials with lapatinib for NF2 patients with progressive meningiomas, are warranted, ideally using a randomized design. If successful, lapatinib could also be explored for the treatment of patients with progressive and inoperable NF2 mutant sporadic meningiomas.[35]
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center
Footnotes
Conflicts of Interest: None
References
- 1.Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. doi: 10.1186/1750-1172-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chozick BS, Benzil DL, Stopa EG, Pezzullo JC, Knuckey NW, Epstein MH, Finkelstein SD, Finch PW. Immunohistochemical evaluation of erbB-2 and p53 protein expression in benign and atypical human meningiomas. Journal of neuro-oncology. 1996;27:117–126. doi: 10.1007/BF00177474. [DOI] [PubMed] [Google Scholar]
- 3.Carroll RS, Black PM, Zhang J, Kirsch M, Percec I, Lau N, Guha A. Expression and activation of epidermal growth factor receptors in meningiomas. J Neurosurg. 1997;87:315–323. doi: 10.3171/jns.1997.87.2.0315. [DOI] [PubMed] [Google Scholar]
- 4.Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol. 2004;108:135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 5.Potti A, Panwalkar A, Langness E, Sholes K, Tendulkar K, Chittajalu S, Koch M. Role of her-2/neu overexpression and clinical features at presentation as predictive factors in meningiomas. American journal of clinical oncology. 2004;27:452–456. doi: 10.1097/01.coc.0000128724.63600.c7. [DOI] [PubMed] [Google Scholar]
- 6.Loussouarn D, Brunon J, Avet-Loiseau H, Campone M, Mosnier JF. Prognostic value of HER2 expression in meningiomas: an immunohistochemical and fluorescence in situ hybridization study. Human pathology. 2006;37:415–421. doi: 10.1016/j.humpath.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Laurendeau I, Ferrer M, Garrido D, D'Haene N, Ciavarelli P, Basso A, Vidaud M, Bieche I, Salmon I, Szijan I. Gene expression profiling of ErbB receptors and ligands in human meningiomas. Cancer investigation. 2009;27:691–698. doi: 10.1080/07357900802709175. [DOI] [PubMed] [Google Scholar]
- 8.Wernicke AG, Dicker AP, Whiton M, Ivanidze J, Hyslop T, Hammond EH, Perry A, Andrews DW, Kenyon L. Assessment of Epidermal Growth Factor Receptor (EGFR) expression in human meningioma. Radiat Oncol. 2010;5:46. doi: 10.1186/1748-717X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahzouni P, Movahedipour M. An immunohistochemical study of HER2 expression in meningioma and its correlation with tumor grade. Pathology, research and practice. 2012;208:221–224. doi: 10.1016/j.prp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Tu Y, Wang S, Xu S, Xu L, Xiong Y, Mei J, Wang C. Role of HER-2 activity in the regulation of malignant meningioma cell proliferation and motility. Mol Med Rep. 2015;12:3575–3582. doi: 10.3892/mmr.2015.3805. [DOI] [PubMed] [Google Scholar]
- 11.Papi L, De Vitis LR, Vitelli F, Ammannati F, Mennonna P, Montali E, Bigozzi U. Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum Genet. 1995;95:347–351. doi: 10.1007/BF00225206. [DOI] [PubMed] [Google Scholar]
- 12.Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN, Ramesh V, Gusella JF, Wiestler OD, von Deimling A. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146:827–832. [PMC free article] [PubMed] [Google Scholar]
- 13.De Vitis LR, Tedde A, Vitelli F, Ammannati F, Mennonna P, Bigozzi U, Montali E, Papi L. Screening for mutations in the neurofibromatosis type 2 (NF2) gene in sporadic meningiomas. Hum Genet. 1996;97:632–637. doi: 10.1007/BF02281874. [DOI] [PubMed] [Google Scholar]
- 14.Leone PE, Bello MJ, de Campos JM, Vaquero J, Sarasa JL, Pestana A, Rey JA. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18:2231–2239. doi: 10.1038/sj.onc.1202531. [DOI] [PubMed] [Google Scholar]
- 15.Kros J, de Greve K, van Tilborg A, Hop W, Pieterman H, Avezaat C, Lekanne Dit Deprez R, Zwarthoff E. NF2 status of meningiomas is associated with tumour localization and histology. J Pathol. 2001;194:367–372. doi: 10.1002/path.909. [pii]. 10.1002/path.909. [DOI] [PubMed] [Google Scholar]
- 16.Lomas J, Bello MJ, Arjona D, Alonso ME, Martinez-Glez V, Lopez-Marin I, Aminoso C, de Campos JM, Isla A, Vaquero J, Rey JA. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42:314–319. doi: 10.1002/gcc.20141. [DOI] [PubMed] [Google Scholar]
- 17.Agnihotri S, Suppiah S, Tonge PD, Jalali S, Danesh A, Bruce JP, Mamatjan Y, Klironomos G, Gonen L, Au K, Mansouri S, Karimi S, Sahm F, von Deimling A, Taylor MD, Laperriere NJ, Pugh TJ, Aldape KD, Zadeh G. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nature communications. 2017;8:186. doi: 10.1038/s41467-017-00174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammoun S, Cunliffe CH, Allen JC, Chiriboga L, Giancotti FG, Zagzag D, Hanemann CO, Karajannis MA. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12:834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karajannis MA, Legault G, Hagiwara M, Ballas MS, Brown K, Nusbaum AO, Hochman T, Goldberg JD, Koch KM, Golfinos JG, Roland JT, Allen JC. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14:1163–1170. doi: 10.1093/neuonc/nos146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin SR, Halpin C, Blakeley JO, Slattery WH, 3rd, Welling DB, Chang SM, Loeffler JS, Harris GJ, Sorensen AG, McKenna MJ, Barker FG., 2nd Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol. 2009;93:61–77. doi: 10.1007/s11060-009-9867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes FP, Merker VL, Jennings D, Caruso PA, di Tomaso E, Muzikansky A, Barker FG, 2nd, Stemmer-Rachamimov A, Plotkin SR. Bevacizumab treatment for meningiomas in NF2: a retrospective analysis of 15 patients. PLoS One. 2013;8:e59941. doi: 10.1371/journal.pone.0059941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karajannis MA, Legault G, Hagiwara M, Giancotti FG, Filatov A, Derman A, Hochman T, Goldberg JD, Vega E, Wisoff JH, Golfinos JG, Merkelson A, Roland JT, Allen JC. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2014;16:292–297. doi: 10.1093/neuonc/not150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goutagny S, Raymond E, Esposito-Farese M, Trunet S, Mawrin C, Bernardeschi D, Larroque B, Sterkers O, Giovannini M, Kalamarides M. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol. 2015;122:313–320. doi: 10.1007/s11060-014-1710-0. [DOI] [PubMed] [Google Scholar]
- 27.Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM, Yung WK, Gilbert MR, Fine HA, Mehta M, Deangelis LM, Cloughesy TF, Robins HI, Aldape K, Dancey J, Prados MD, Lieberman F, Wen PY. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96:211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, Kubek S, Oldrini B, Chheda MG, Yannuzzi N, Tao H, Zhu S, Iwanami A, Kuga D, Dang J, Pedraza A, Brennan CW, Heguy A, Liau LM, Lieberman F, Yung WK, Gilbert MR, Reardon DA, Drappatz J, Wen PY, Lamborn KR, Chang SM, Prados MD, Fine HA, Horvath S, Wu N, Lassman AB, DeAngelis LM, Yong WH, Kuhn JG, Mischel PS, Mehta MP, Cloughesy TF, Mellinghoff IK. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2:458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Goldman S, Geyer JR, Gajjar A, Kun LE, Boyett JM, Gilbertson RJ. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: a pediatric brain tumor consortium study. Journal of neuro-oncology. 2013;114:173–179. doi: 10.1007/s11060-013-1166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karajannis M, Bergner A, Giovannini M, Welling DB, Niparko J, Slattery W, Roland JT, Golfinos J, Allen J, Blakeley J. Intratumoral Concentration and Molecular Pharmacodynamics of Lapatinib in Vivo in Sporadic and Nf2-Related Vestibular Schwannomas. Neuro-oncology. 2012;14:19–19. [Google Scholar]
- 31.Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, Clarke JL, Lassman AB. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavaud P, Rousseau B, Ajgal Z, Arrondeau J, Huillard O, Alexandre J, Hulin A, Goldwasser F. Bi-weekly very-high-dose lapatinib: an easy-to-use active option in HER-2-positive breast cancer patients with meningeal carcinomatosis. Breast Cancer Res Treat. 2016;157:191–192. doi: 10.1007/s10549-016-3798-8. [DOI] [PubMed] [Google Scholar]
- 33.Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM, Kashani-Sabet M, Ryan CJ, Rosenberg JE, Dubey S, Small EJ, Jahan TM, Hylton NM, Yeh BM, Huang Y, Koch KM, Moasser MM. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569–5575. doi: 10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu A, Faiq N, Green S, Lai A, Green R, Hu J, Cloughesy TF, Mellinghoff I, Nghiemphu PL. Report of safety of pulse dosing of lapatinib with temozolomide and radiation therapy for newly-diagnosed glioblastoma in a pilot phase II study. J Neurooncol. 2017 doi: 10.1007/s11060-017-2533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]