Abstract
Objectives:
To model the cost-effectiveness (CE) of a biomarker-based approach to select patients for neoadjuvant chemotherapy (NAC) prior to radical cystectomy (RC) in muscle-invasive bladder cancer (MIBC).
Materials and Methods:
We obtained most recently clinical studies on locally-advanced bladder cancer treated by RC, including stage distributions, overall survival (OS) estimates, associated costs, and utilization/response to NAC. Additionally, we estimated the putative efficacy of 3 biomarkers to select patients for NAC: DNA repair gene panel [ATM, RB1, and FANCC], ERCC2, and RNA subtypes. A decision analysis model was developed to evaluate the CE of biomarker-based approaches to select patients with MIBC for NAC. Comparison of CE included RC alone, unselected NAC plus RC, and NAC based on the 3 aforementioned biomarkers.
Results:
The DNA repair gene panel-based approach to NAC was the most CE strategy (mean OS of 3.14 years, $31,482/life year). Under this approach, 38% would undergo NAC, approximately twice the number of patients who are currently receiving NAC for MIBC. Such an approach would improve mean OS by 5.2 months, 1.6 months, and 4.4 months compared to RC alone, a hypothetical scenario where all patients received NAC, and compared to current estimates of NAC utilization, respectively.
Conclusions:
A biomarker-based strategy to identify MIBC patients who should undergo NAC was more cost-effective than unselected use of NAC or RC alone. As further data becomes available, such a model may serve as a basis for incorporating biomarkers into clinical decision making.
Keywords: bladder cancer, biomarker, cost, neoadjuvant chemotherapy, response
INTRODUCTION
Despite level 1 evidence,(1–3) adoption of neoadjuvant chemotherapy (NAC) prior to radical cystectomy (RC) in patients with muscle-invasive bladder cancer (MIBC) has been slow and rates of utilization hover between 20-30% nationwide.(4) Randomized trials evaluating NAC in patients with T2-T4aN0M0 disease indicate an overall survival (OS) benefit with hazard ratio (HR) of 0.87 and absolute survival benefit of 5-10%.(2, 3) Beyond potential toxicity concerns, lack of utilization is related to a perceived relatively low benefit and concern that many patients with organ-confined disease do not need additional treatment.
A predictive biomarker for response to NAC could avoid chemotherapy in those unlikely to respond and thereby expedite surgery and reduce cost and morbidity of NAC. Alternatively, selecting patients most likely to respond to NAC will enrich the population of patients receiving and benefiting from NAC. Several possible biomarkers have been described including mutations in DNA repair genes ATM, RB1, and FANCC,(5) mutations in excision repair cross-complementation group 2 (ERCC2) gene,(6, 7) protein biomarkers,(8) and RNA subtyping of bladder cancer.(9, 10) Although difficulties exist in assessing the potential risks and benefits of incorporating molecular markers into decision-making regarding use of NAC, decision analysis modeling can improve our understanding of the efficacy and cost implications of using biomarkers to guide treatment. Furthermore, this model could serve as basis for rationale incorporation of emerging biomarkers into clinical decision making for complex oncology. In this study, we evaluated the cost-effectiveness (CE) of potential biomarkers to guide the use of NAC in patients with MIBC.
MATERIALS AND METHODS
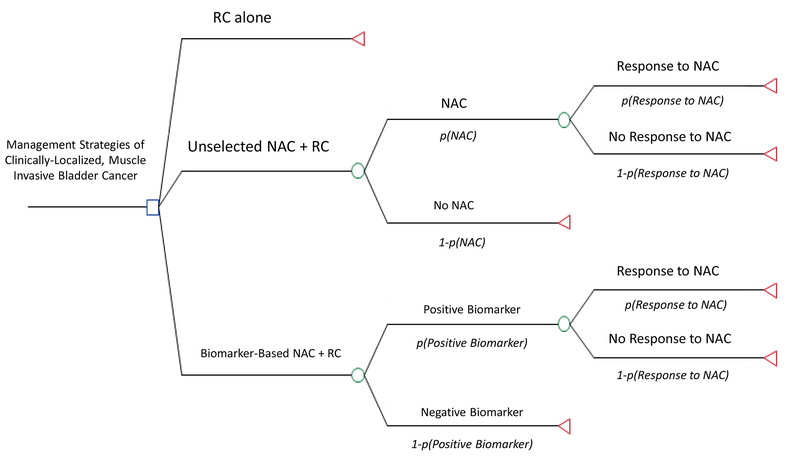
A decision analysis model was developed using TreeAge Pro 2016 to evaluate the CE of different management approaches in locally-advanced, MIBC (T2-T4aN0M0). All patients were assumed eligible for cisplatin-based NAC. There are 3 treatment arms in the model (Figure 1): 1) patients who undergo RC alone, 2) patients who undergo unselected NAC followed by RC, and 3) patients who undergo biomarker-directed NAC followed by RC. The primary endpoint was the CE of different approaches for use of NAC based on 5-year OS. Baseline assumptions in the model were varied by ±10% to generate confidence intervals. One-way and two-way sensitivity analyses were used to evaluate the impact of different assumptions on outcomes of the model.
Figure 1.
Decision Analysis Model.
Abbreviation Key: RC = radical cystectomy; NAC = neoadjuvant chemotherapy; lower case p = probability.
Defining the Patient Population
The National Cancer Database (NCDB) was used to estimate proportion of clinical T2 (78.9%), T3 (13.7%), and T4 (7.7%).(4) Survival data depends primarily on pathologic stage and there is considerable discrepancy between clinical and pathologic staging at the time of RC.(11) A multicenter study of 788 patients undergoing RC found pathologic upstaging and downstaging occurred in 42% and 22%, respectively.(11) The stage distribution of patients undergoing RC for non-metastatic, MIBC was corrected for pathologic staging (Table 1).
Table 1.
Calculation of Pathologic Stage and 5-Year Overall Survival Outcomes Based on Established Clinical Stage Distribution in Muscle-Invasive Bladder Cancer
| Pathological Stage Correction (%) | ||||||
|---|---|---|---|---|---|---|
| Clinical Stage (%) | <T2N0 | T2N0 | T3N0 | T4N0 | TanyN+ | 5-year OS (%) |
| cT2 (78.9) | 22.7 | 22.2 | 29.5 | 3.5 | 22.1 | 56.1 |
| cT3 (13.7) | 16.3 | 6.3 | 50.1 | 3.3 | 24 | 51.3 |
| cT4 (7.7) | 0 | 4.1 | 10.1 | 32.8 | 53 | 39.6 |
| 5-year OS (%) | 81 | 65 | 50 | 47 | 31 | |
Estimating Patient Survival
The 5-year OS of patients undergoing RC alone was obtained from several sources. The SWOG 8710 study found a 5-year OS for pT0 patients of 85%(1) and a cohort of >1,000 patients who underwent RC at the University of Southern California (USC) found 85% 5-year OS for node-negative patients with pT0,pTa,pTis.(12) In the same USC cohort, the 5-year OS for patients with pT1N0 was 76%, yielding an average 5-year OS of 81% for <pT2N0.(12) The 5-year OS for stages pT2-T4 and pTanyN1-2 were obtained from a multicenter study of >2,700 RC cases.(13)
In order to determine the 5-year OS for each clinical stage, we used the expected distribution of pathologic stages for each clinical stage and multiplied by the expected 5-year OS for each pathologic stage (Table 1). Using this weighted-average approach, the 5-year OS for the entire cohort (cT2-T4) was 54.2%.
The survival benefit of NAC was estimated by a meta-analysis of 15 randomized clinical trials which indicated a decreased risk of all-cause mortality associated with cisplatin-based NAC (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.79-0.96).(3) This translates into 13% fewer deaths with a 5-year OS for NAC of 60.2% (if 100% of eligible patients receive NAC).
Treatment Assumptions
In the unselected NAC arm, the model was tested assuming all or just a portion of patients accept NAC. An NCDB analysis of MIBC patients who underwent RC found that 20.9% received NAC in 2010.(4) In some centers, NAC is only given in high-risk patients and the observed patterns of NAC utilization likely reflects this risk-adopted approach.(14) We modeled a scenario of 20% of eligible patients receiving NAC. In the biomarker-directed NAC arm, all patients underwent biomarker testing. Those with a positive marker receive NAC and those with a negative marker receive RC alone. Since current markers are not perfect in their prediction of response, not every patient with marker alterations (positive marker) benefits from chemotherapy. The percent of responders based on prior clinical studies was incorporated into the model.
Biomarker Assumptions
For demonstrative purposes, we examined 3 potential biomarkers including genomic alterations in the DNA repair genes ATM, RB1, and FANCC,(5) missense mutations in ERCC2,(6) and RNA subtypes of bladder cancer.(9)
In the study by Plimack et al., 22 of 58 patients (38%) had an alteration in ATM, RB1 or FANCC (biomarker-positive).(5) The rate of these mutations in The Cancer Genome Atlas (TCGA) was identical at 38.1% (ATM 15.4%, RB1 21.1% and FANCC 1.6%).(15–17) Of the 22 patients, 20 patients (91%) were ≤pT1N0 at time of RC.
Data on performance of ERCC2 was derived from a validation study by Liu et al. which revealed an ERCC2 mutation in 10 of 48 patients (21%).(6) The rate in the TCGA for ERCC2 mutations was lower at 10.5% in a larger cohort so this rate was used in the model.(15–17) Of the biomarker-positive patients, 80% were ≤pT1N0 at time of RC.
The impact of RNA subtyping on outcomes was based on an analysis by Choi et al. Basal, luminal, and p53-like subtypes constituted 32%, 33%, and 35% of the cohort and had a response to NAC (defined as ≤pT1N0) of 47%, 62%, and 6%, respectively.(9) In the model we assumed that patients with basal and luminal subtypes would receive NAC for an average response of 55.6% and those with p53-like tumors would proceed to RC alone. Based on a recent analysis suggesting limited benefit of NAC for luminal subtypes, we also performed a separate model wherein only patients with basal subtype tumors undergo NAC.(10)
A recent study found that patients who underwent NAC and were found to still have residual disease at RC had significantly worse 5-yr recurrence-free survival (50% vs 63%; p=0.01), cancer specific survival (40% vs 59%; p=0.003), and OS (33% vs 48%; p=0.02) than pathologic stage-matched controls who underwent RC alone.(18) In the model, we similarly lowered OS in non-responders to biomarker-directed NAC by 31.25%.
Cost Assumptions
All patients were assumed to undergo RC. The average cost for cystectomy and complications within 90 days was obtained from >7,000 cystectomies using Surveillance, Epidemiology, and End Results Program (SEER)-Medicare linked data and estimated at $33,000, including all Medicare and co-insurance reimbursement and patient-liability costs.(19)
The costs for NAC and adjuvant chemotherapy were estimated at $10,500 for cisplatin-based regimens (gemcitabine/cisplatin or dose-dense methotrexate, vincristine, Adriamycin, cisplatin).(20) The cost of salvage therapy was complicated due to the recent approval of immune checkpoint inhibitors, for which there is insufficient cost data. A cost of $77,000 was estimated based on other biologic regimens.(21) Patients receiving NAC and those with ≤pT2N0 were assumed to not receive adjuvant chemotherapy, whereas 30% of those with chemo-naïve, non-organ confined disease were assumed to accept adjuvant chemotherapy. Similarly, 30% of those with non-organ confined disease were assumed to receive salvage chemotherapy. While a recent report found that use of adjuvant chemotherapy was around 20%, we used a higher rate as our model limited the use of adjuvant chemotherapy to patients with non-organ confined disease.(22) These assumptions were subsequently tested in the model to determine the impact of varying the rate of acceptance of adjuvant and salvage therapies.
Post-RC surveillance was modeled according to guidelines on imaging, lab, and urine studies from the National Comprehensive Cancer Network.(23) Cost of imaging studies was based on Medicare national average allowable fees.(24–26) Cost for laboratory tests were based on the Centers for Medicare & Medicaid Services Clinical Laboratory Fee Schedule.(27) A level 3 established outpatient physician visit was assumed at surveillance time-points. Based on these calculations, the cost (minimum-maximum) of surveillance was $1,746.92 ($1,164.64-$2,329.28) for year 1, $1,652.06 ($1,114.92-$2,189.20) for year 2, and $543.08 per year for years 3-5.
Estimated costs of dying from bladder cancer were abstracted from an analysis of SEER-Medicare linkage data(28) which included all inpatient and outpatient claims among beneficiaries dying of bladder cancer made in the last-year of life.(29) Adjusted for inflation, the cost of dying from bladder cancer was estimated at $93,775.51 in 2017 dollars.
The cost of the marker was estimated at $2,000. While the exact cost of sequencing varies between genetic tests, this was approximated between costs of commercially-available tests and the current cost of whole exome sequencing at our sequencing Core. This was modeled at varying costs and this cost will likely decrease over time.
RESULTS
The results of the model are shown in Table 2. The least effective strategy is RC alone with an average 5-year OS of 54.2% and mean survival of 2.71 years. For strategies of NAC prior to cystectomy without a biomarker, 5-year OS is 60.2% if all get NAC and 55.4% if only 20% accept NAC. In the biomarker-based approaches, the 5-year OS was 56%, 59% and 62.8% for arms using ERCC2, RNA subtyping, and mutations in the DNA repair genes ATM, RB1, and FANCC, respectively.
Table 2.
Cost-Effectiveness According to Treatment Strategy
| 5-year OS (%) | Mean OS (Years) | % Patients Undergoing NAC | Cost ($) (Range*) | Cost-Effectiveness (cost [$] per life year)(Range*) | |
|---|---|---|---|---|---|
| Traditional Approaches | |||||
| Cystectomy Alone | 54.2 | 2.71 | 0 | 95,552 (84,727-106,659) | 35,259 (31,265-39,358) |
| Cystectomy + NAC^ | 60.2 | 3.01 | 100 | 96,708 (85,933-107,729) | 32,129 (28,549-35,790) |
| Cystectomy + NAC** | 55.4 | 2.77 | 20 | 95,783 (84,968-106,873) | 34,579 (30,674-38,582) |
| Biomarker-Based Approaches | |||||
| DNA Repair Genes (ATM, RB1, FANCC) | 62.8 | 3.14 | 38 | 98,855 (87,720-110,216) | 31,482 (27,936-35,101) |
| ERCC2 | 56 | 2.80 | 10.5 | 98,204 (86,964-109.380) | 35,072 (31,059-39,064) |
| RNA Subtyping (basal and luminal get NAC) | 59 | 2.95 | 65 | 105,592 (93,648-117,696) | 35,794 (31,745-39,897) |
Model was analyzed with all costs and probabilities of adjuvant and salvage +/− 10%
Assuming 100% of eligible patients receive NAC
Only 20% of eligible patients actually receive neoadjuvant chemotherapy
Abbreviation Key: OS = overall survival; NAC = neoadjuvant chemotherapy
The overall cost of care was higher in arms that received NAC in large part based on the proportion of patients who received NAC. The most expensive strategy was based on subtyping since all had the cost of the marker and 65% had the added cost of chemotherapy. The most cost-effective strategy was based on DNA repair genes ($31,482/life year). The least cost-effective strategy was based on RNA subtyping ($35,794/life year). If NAC is only used in patients with basal subtype tumors then the overall cost is $102,205 with an expected mean OS of 2.77 years and CE of $36,897/life year.
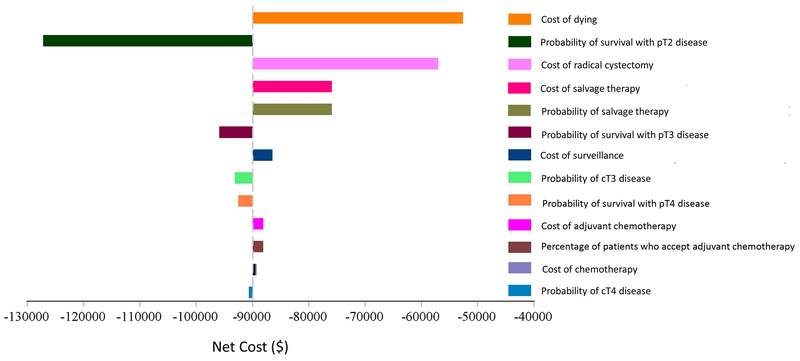
A tornado diagram was created to evaluate the impact of different factors on the cost of bladder cancer care (Figure 2). The cost of dying has the highest impact on the model due to the high cost of the last year of life and the significant mortality rate. Unsurprisingly, the cost of RC had a high impact. Finally, the probability of receiving and cost of salvage treatment were next most influential on overall cost since salvage therapy ($77,000) is much more expensive than adjuvant chemotherapy ($10,500). Since there is insufficient data on the likelihood of receiving salvage treatments such as immune checkpoint blockade, we modeled the impact of 10%, 30%, 50% and 70% of patients receiving such therapies (Table 3). The cost can vary by as much as $29,437 per patient. This has a greater impact on patients who do not receive NAC as they are more likely to have non-organ confined disease and require salvage therapy.
Figure 2.
Tornado Analysis of Variables Affecting Cost in the Model
Abbreviation Key: NAC = neoadjuvant chemotherapy
- Variables with a wide bar indicate value sensitive variables, suggesting they have a higher impact on cost in the model. For example, the cost of dying has the highest impact on the model. This is also why survival for pT2 has such a large impact since higher mortality rated result in more cost.
- The cost of radical cystectomy had a high impact since it impacts all patients.
- The probability of getting and cost of salvage were next most influential on overall cost since salvage therapy at ($77,000) is much more expensive than adjuvant chemotherapy ($10,500).
- Variables that did not have a significant impact on cost in the model (not shown) included the probability of receipt of NAC (in the unselected NAC arm), probability of response to NAC, probability of biomarker positivity, accuracy of the biomarker, cost of the biomarker, probability of survival in patients who respond to NAC, and cost of surveillance following radical cystectomy.
Table 3.
Modeling the Impact of Different Rates of Salvage Therapy on 5-Year Costs of Muscle-Invasive Bladder Cancer Management
| % Patients Undergoing Salvage Therapy for Non-Organ Confined Disease | ||||
|---|---|---|---|---|
| 10% salvage | 30% salvage | 50% salvage | 70% salvage | |
| Traditional Approaches | ||||
| Cystectomy Alone | $86,146 | $95,552 | $104,958 | $114,364 |
| Cystectomy + NAC^ | $88,526 | $96,708 | $104,892 | $113,075 |
| Cystectomy + NAC* | $86,622 | $95,783 | $104,945 | $114,106 |
| Biomarker-Based Approaches | ||||
| DNA Repair Genes (ATM, RB1, FANCC) | $89,772 | $98,855 | $107,939 | $117,022 |
| ERCC2 | $88,839 | $98,204 | $107,569 | $116,934 |
| RNA Subtyping | $95,780 | $105,592 | $115,405 | $125,217 |
Assuming 100% of eligible patients receive NAC
Only 20% of eligible patients actually receive neoadjuvant chemotherapy
Abbreviation Key: NAC = neoadjuvant chemotherapy
A two-way sensitivity analysis was conducted over a wide range of biomarker-positivity rates and ability of the biomarker to predict response to chemotherapy (Table 4). If no patients have an abnormal marker (0%) then no NAC is given and outcomes are similar to RC alone. However, costs are $2,000 higher due to the cost of the marker. If 60% of patients have a positive marker and 60% of these patients have a response to NAC, the mean survival would be 2.99 years and the cost per patient would be $104,307. On the other hand, a marker that is positive in 20% of patients and predicts a response rate of 80% would have a mean survival of 2.89 years and cost of $98,793.
Table 4.
Two-Way Sensitivity Analysis of Cost and Median Overall Survival Based on Different Probability of Biomarker Positivity and Predictive Ability
| Probability of Response to Chemotherapy if Biomarker is Positive | Probability of Biomarker Positivity | ||||
|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | |
| 40% | $97,552 | $100,814 | $104,075 | $107,338 | $110,599 |
| 2.71 years | 2.71 years | 2.72 years | 2.73 years | 2.73 years | |
| 60% | $97,552 | $99,803 | $102,055 | $104,307 | $106,559 |
| 2.71 years | 2.80 years | 2.90 years | 2.99 years | 3.08 years | |
| 80% | $97,552 | $98,793 | $100,035 | $101,277 | $102,518 |
| 2.71 years | 2.89 years | 3.07 years | 3.25 years | 3.43 years | |
| 100% | $97,552 | $97,783 | $98,015 | $98,246 | $98,478 |
| 2.71 years | 2.98 years | 3.25 years | 3.51 years | 3.78 years | |
Top row in each cell represents estimated costs at 5-years, bottom row of each cell represents median overall survival.
Assumes marker cost of $2000 for all patients
DISCUSSION
Despite level 1 evidence of a survival benefit of NAC in patients with MIBC, (1) multiple studies have demonstrated low utilization rates. There are concerns among physicians and patients about toxicity of therapy and possible delay in cystectomy. Recent studies have identified potential biomarkers such as DNA repair genes and RNA subtyping as predictive of response to NAC.(5, 6, 9) Utilizing biomarkers to enrich the response of patients undergoing NAC has the potential to increase the utilization of NAC in those patients most likely to respond and spare those unlikely to respond from potential toxicities associated with treatment.
In this study, a decision analysis model was utilized to compare the cost-effectiveness of strategies using a biomarker to determine which patients should undergo NAC versus unselected use of NAC or cystectomy alone. The most cost-effective strategy involved using DNA-repair genes which provided NAC to 38% of patients with 91% response rate. While we recognize that further studies are necessary to define the optimal marker for selecting patients for NAC, there are many efforts underway such as the SWOG 1314 trial. Our model is limited by the fact that the current literature still consists of small patient cohorts, however the 2-way sensitivity analysis we designed can predict the cost-effectiveness of any marker based on its performance (Table 4). One should also note that there are no direct comparisons of markers in the literature so the best marker combination cannot be extrapolated from current literature and will likely evolve over time. More recent studies suggest that the basal subtype benefits more from NAC than the luminal subtype with a suggestion that patients with luminal subtype tumors should undergo cystectomy without NAC.(10) Further data is necessary to adequately model this type of information since it is possible that NAC in some patients leads to worse outcomes due to a minimal benefit and delay in cystectomy. Refinement in clinical staging may better define the optimal patient to treat with NAC since it is known that patients with more advanced stage are more likely to benefit from NAC.(10) It is also possible that biomarkers will be beneficial in improving staging but this was beyond the scope of this model.(30)
The major cost that impacted the model was cost of dying which represents a significant proportion of total healthcare costs in the US.(31) Strategies that improve OS benefit significantly by reducing the cost of dying and avoiding salvage therapies, which more than compensate for the upfront cost of NAC. Furthermore, this study preceded the introduction of expensive treatments like immune checkpoint inhibitors that will likely raise costs. The proportion of patients who required salvage therapies significantly impacted the cost of care with as much as a $29,437 difference in scenarios where 10% vs. 70% of the patients with non-organ confined disease received salvage treatments.
The implications of this study are significant. The use of NAC was found to be cost-superior to RC alone and strategies to enrich a population for responders is cost-effective. While the best biomarker for response to NAC is not established, this type of modeling demonstrates that even an imperfect biomarker can significantly improve cost-effectiveness over unselected use of NAC. Furthermore, it is known that many eligible patients are not receiving NAC and that a biomarker for response may improve utilization by selecting patients most likely to respond.
The model created is based on assumptions which are limited by the strength of evidence. Some evidence is strong, including the benefits of NAC and outcomes of patients with bladder cancer, which are rooted in large population data or randomized trials. Other assumptions like the proportion of patients who will undergo adjuvant or salvage therapies are not as well established. Furthermore, it is difficult to model the potential benefits on survival of salvage therapies but these benefits would be similar between different arms of the study and are unlikely to impact results significantly. Finally, cost data is based on best available literature but can vary geographically and based on type of insurance. For the most part Medicare-based reimbursement was used since it is the most widely used cost metric nationally even though some patients will be younger than 65 years and will have private insurance. Despite these limitations, we feel that the proposed model should be considered an example of how to estimate the value of novel biomarkers in oncologic care.
CONCLUSIONS
A biomarker-based strategy using DNA repair genes (ATM, RB1, FANCC) to identify which patients with MIBC should undergo NAC was more cost-effective than unselected use of NAC or cystectomy alone.
Acknowledgments
Funding: This work was supported by the National Institute of Heath (T32 CA136515 Ruth L. Kirschstein Institutional National Research Award to S.L.W.)
REFERENCES
- 1.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England journal of medicine. 2003;349(9):859–66. [DOI] [PubMed] [Google Scholar]
- 2.Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. European urology. 2005;48(2):202–5; discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 3.Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. The oncologist. 2016;21(6):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaid HB, Patel SG, Stimson CJ, Resnick MJ, Cookson MS, Barocas DA, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology. 2014;83(1):75–80. [DOI] [PubMed] [Google Scholar]
- 5.Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. European urology. 2015;68(6):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Plimack ER, Hoffman-Censits J, Garraway LA, Bellmunt J, Van Allen E, et al. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA oncology. 2016;2(8):1094–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4(10):1140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baras AS, Gandhi N, Munari E, Faraj S, Shultz L, Marchionni L, et al. Identification and Validation of Protein Biomarkers of Response to Neoadjuvant Platinum Chemotherapy in Muscle Invasive Urothelial Carcinoma. PloS one. 2015;10(7):e0131245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer cell. 2014;25(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler R, Ashab HA, Erho N, van Rhijn BW, Winters B, Douglas J, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. European urology. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. European urology. 2007;51(1):137–49; discussion 49-51. [DOI] [PubMed] [Google Scholar]
- 12.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(3):666–75. [DOI] [PubMed] [Google Scholar]
- 13.Sonpavde G, Khan MM, Lerner SP, Svatek RS, Novara G, Karakiewicz PI, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. The Journal of urology. 2011;185(2):456–61. [DOI] [PubMed] [Google Scholar]
- 14.Culp SH, Dickstein RJ, Grossman HB, Pretzsch SM, Porten S, Daneshmand S, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. The Journal of urology. 2014;191(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerner SP, Robertson G, Kim J, Cherniack A, Guo G,, editor Comprehensive molecular characterization and analysis of muscle-invasive urothelial carcinomas. American Society of Clinical Oncology; 2017: J Clin Oncol [Google Scholar]
- 16.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhindi B, Frank I, Mason RJ, Tarrell RF, Thapa P, Cheville JC, et al. Oncologic Outcomes for Patients with Residual Cancer at Cystectomy Following Neoadjuvant Chemotherapy: A Pathologic Stage-matched Analysis. European urology. 2017. [DOI] [PubMed] [Google Scholar]
- 19.Hu JC, Chughtai B, O’Malley P, Halpern JA, Mao J, Scherr DS, et al. Perioperative Outcomes, Health Care Costs, and Survival After Robotic-assisted Versus Open Radical Cystectomy: A National Comparative Effectiveness Study. European urology. 2016;70(1):195–202. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson SM, Danzig MR, Ghandour RA, Deibert CM, Decastro GJ, Benson MC, et al. Cost-effectiveness of neoadjuvant chemotherapy before radical cystectomy for muscle-invasive bladder cancer. Urologic oncology. 2014;32(8):1172–7. [DOI] [PubMed] [Google Scholar]
- 21.Nadeem H, Jayakrishnan TT, Rajeev R, Johnston FM, Gamblin TC, Turaga KK. Cost Differential of Chemotherapy for Solid Tumors. Journal of oncology practice. 2016;12(3):e299–307, 251. [DOI] [PubMed] [Google Scholar]
- 22.Reardon ZD, Patel SG, Zaid HB, Stimson CJ, Resnick MJ, Keegan KA, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. European urology. 2015;67(1):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark P, SPiess PR, Agarway N, Bangs R, Boorjian SA et al. NCCN Clinical Practice Guidelines in Oncology Bladder Cancer Version 2.2016 National Comprehensive Cancer Network, Inc.; 2016. [Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. [Google Scholar]
- 24.Vijayasarathi A, Duszak R Jr, Gelbard RB, Mullins ME. Knowledge of the Costs of Diagnostic Imaging: A Survey of Physician Trainees at a Large Academic Medical Center. Journal of the American College of Radiology. 2016;13(11):1304–10. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Hassan C, Laghi A, Kim DH. CT colonography to screen for colorectal cancer and aortic aneurysm in the Medicare population: cost-effectiveness analysis. AJR American journal of roentgenology. 2009;192(5):1332–40. [DOI] [PubMed] [Google Scholar]
- 26.Carlos RC, Axelrod DA, Ellis JH, Abrahamse PH, Fendrick AM. Incorporating patient-centered outcomes in the analysis of cost-effectiveness: imaging strategies for renovascular hypertension. AJR American journal of roentgenology. 2003;181(6):1653–61. [DOI] [PubMed] [Google Scholar]
- 27.Services CfMM. Clinical Laboratory Fee Schedule 2016. [Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/.
- 28.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010-2020. JNCI Journal of the National Cancer Institute. 2011;103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konety BR, Joslyn SA. Factors Influencing Aggressive Therapy for Bladder Cancer: An Analysis of Data From the SEER Program. The Journal of urology. 2003;170(5):1765–71. [DOI] [PubMed] [Google Scholar]
- 30.Shariat SF, Passoni N, Bagrodia A, Rachakonda V, Xylinas E, Robinson B, et al. Prospective evaluation of a preoperative biomarker panel for prediction of upstaging at radical cystectomy. BJU Int 2014;113(1):70–6. [DOI] [PubMed] [Google Scholar]
- 31.Aldridge MD, Kelley AS. The Myth Regarding the High Cost of End-of-Life Care. Am J Public Health. 2015;105(12):2411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]