Abstract
Several clinically successful tumor-targeting mAbs induce NK cell effector functions. Human NK cells exclusively recognize tumor-bound IgG by the FcR CD16A (FcγRIIIA). Unlike other NK cell activating receptors, the cell surface density of CD16A can be rapidly downregulated in a cis manner by the metalloproteinase ADAM17 following NK cell stimulation in various manners. CD16A downregulation takes place in cancer patients and this may affect the efficacy of tumor-targeting mAbs. We examined the effects of MEDI3622, a human mAb and potent ADAM17 inhibitor, on NK cell activation by antibody-bound tumor cells. MEDI3622 effectively blocked ADAM17 function in NK cells and caused a marked increase in their production of IFNγ. This was observed for NK cells exposed to different tumor cell lines and therapeutic antibodies, and over a range of effector/target ratios. The augmented release of IFNγ by NK cells was reversed by a function-blocking CD16A mAb. In addition, NK92 cells, a human NK cell line that lacks endogenous FcγRs, expressing a recombinant non-cleavable version of CD16A released significantly higher levels of IFNγ than NK92 cells expressing equivalent levels of wildtype CD16A. Taken together, our data show that MEDI3622 enhances the release of IFNγ by NK cells engaging antibody-bound tumor cells by blocking the shedding of CD16A. These findings support ADAM17 as a dynamic inhibitory checkpoint of the potent activating receptor CD16A, which can be targeted by MEDI3622 to potentially increase the efficacy of anti-tumor therapeutic antibodies.
Keywords: Immunotherapy, NK cell, Cancer, Antibody, Cytokine, Cytotoxicity
Introduction
CD16A (FcγRIIIA) recognizes IgG1 and IgG3 antibodies attached to target cells [1]. This FcR associates with Fcγ and/or CD3ζ chains and is one of the NK cell’s most potent activating receptors [2]. Unlike other NK cell activating receptors, CD16A’s cell surface density is regulated by a proteolytic process that results in its rapid and efficient downregulation in expression upon antibody engagement and by various other stimuli [3–7]. This process is referred to as ectodomain shedding and is primarily mediated by a disintegrin and metalloproteinase-17 (ADAM17) [4, 5, 7, 8]. ADAM17 is a membrane-associated protease that cleaves CD16A in a cis manner at a specific location proximal to the cell membrane [7, 8].
Therapeutic antibodies have been generated against a variety of tumor antigens and tested in clinical trials for assorted malignancies [9]. Several clinically successful tumor-targeting antibodies, such as trastuzumab (anti-HER2) and rituximab (anti-CD20), utilize FcR recognition as a mechanism of action [2, 10]. A limitation of therapeutic antibodies is the development of resistance in patients and the non-responsiveness of some malignancies [11, 12]. Modifying the Fc region of these antibodies to improve their therapeutic efficacy has been a major focus [9, 13]; however, if CD16A is downregulated in expression, this strategy may have limited effectiveness. Indeed, CD16A downregulation has been reported to occur in the tumor environment of patients, in individuals receiving therapeutics antibodies, and during the ex vivo expansion of NK cells for adoptive transfer into cancer patients [14–18].
There have been extensive efforts to develop ADAM17 inhibitors [19]. A primary focus has been on targeting its activity in tumor cells where ADAM17 facilitates the release of various growth factors and adhesion molecules [20–23]. Initial pharmacological inhibitors of ADAM17 were small-molecule antagonists [19]. However, to overcome issues of specificity and in vivo half-life, recent efforts have focused on function-blocking antibodies of ADAM17 [24–29]. MEDI3622 is a human mAb generated through screening scFv phage libraries using ADAM17. Its epitope is distinct from other ADAM17 mAbs and has been mapped to a surface loop unique to the metalloprotease catalytic domain of ADAM17, resulting in high specificity and a potent inhibitory activity [30]. MEDI3622 has been reported to directly inhibit the growth of human head and neck as well as colorectal tumor cells in vitro and in a mouse xenograft model [28, 29].
We investigated for the first time the effects of blocking ADAM17 with MEDI3622 on NK cell activation induced by therapeutic antibody-bound tumor cells. Cytokine production by NK cells is a key effector function and in particular they are major producers of IFNγ, which has broad anti-cancer activity. This includes crosstalk with leukocytes of the innate and adaptive immunity, induction of ICAM-1 and MHC surface expression on tumor cells that promote leukocyte attachment and stimulation, and inhibition of cell proliferation and angiogenesis in developing and established tumors [31–34]. We show that combining MEDI3622 with a tumor antigen-targeting antibody greatly augments the production of IFNγ by NK cells and that this is due to blocking CD16A shedding.
Materials and methods
Antibodies
The anti-human mAbs PE-conjugated anti-CD107a (LAMP-1), unconjugated and allophycocyanin-(APC) conjugated anti-CD16 (3G8), PE/Cy7-conjugated anti-CD56 (HCD56), PerCP-conjugated anti-CD3 (UCHT1), and isotype-matched negative control mAbs were purchased from BioLegend (San Diego, CA). APC-conjugated anti-CD62L (L-selectin) was purchased from Ancell (Bayport, MN). APC-conjugated F(ab′)2 donkey anti-human IgG (H + L) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The anti-ADAM17 mAb MEDI3622 was generated from a human phage display library displaying scFv and converted into an IgG1, as previously described [28]. Trastuzumab and rituximab, human IgG1 mAbs, were manufactured by Genentech (South San Francisco, CA). An isotype-matched negative control human IgG1 antibody was obtained from Sigma (Saint Louis, MO).
Cells
Peripheral blood was obtained from mice housed in a specified pathogen free facility. Mice used in this study were Adam17flox/flox (Adam17tm1.2Bbl/J) mice and Vav1-Cre mice (B6.Cg-Tg(Vav1-cre)A2Kio/J). The Adam17flox/flox and Vav1-Cre mice were crossed to the C57BL/6J genetic background (both ≥ 98.4%) and then crossed together to generate Adam17flox/flox/Vav-Cre mice and littermate Adam17flox/flox mice, as we have previously described [35, 36]. Adam17flox/flox/Vav-Cre mice and Adam17flox/flox mice are referred to below as conditional ADAM17 knockout and control mice, respectively. Total leukocytes were obtained from peripheral blood by red blood cell lysis using 0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2 solution.
Fresh human peripheral blood leukocytes from plateletpheresis were obtained from Innovative Blood Resources (St. Paul, MN). PBMCs were further enriched on a Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient and then NK cells were purified by negative depletion using an EasySep human NK cell kit (StemCell Technologies, Cambridge, MA), as per the manufacturer’s instructions, with > 95% viability and > 90% enrichment of CD56+ CD3− lymphocytes. Viable cell counting was performed using a Countess II automated cell counter (Life Technologies Corporation, Bothell, WA).
The human NK cell line NK92 was obtained from ATCC (Manassas, VA) and cultured per the company’s instructions. NK92 cells lack endogenous FcγRs [37], and these cells were stably transduced with pBMN-IRES-EGFP with or without recombinant CD16A or non-cleavable CD16A (also referred to as CD16A-S197P) by retrovirus generation and infection procedures described previously [8]. The Burkitt’s lymphoma cell line Raji and ovarian cancer cell SKOV-3 were obtained from ATCC and grown per the company’s instructions. The ovarian cancer cell line MA-148 (University of Minnesota, USA) was maintained in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 100 U/ml penicillin, and 100 U/ml streptomycin (Gibco).
Cell stimulation
Enriched human NK cells and NK92 cells were stimulated with antibody-bound tumor cells as described [5, 8]. Briefly, cells were incubated with the CD20+ Burkitt’s lymphoma cell line Raji ± anti-CD20 mAb rituximab (1 µg/ml), or the HER2+ ovarian cancer cell lines SKOV-3 or MA-148 ± the anti-HER2 mAb trastuzumab (1 µg/ml) at an effector:target ratio of 10:1 (320,000 cells: 32,000 cells), unless otherwise specified, and for the indicated time points at 37 °C plus 5% CO2. In some experiments, MEDI3622 (1 µg/ml), the selective ADAM17 inhibitor BMS566394 (5 µM) [4, 8], or the function-blocking anti-CD16 mAb 3G8 (1 µg/ml) was added.
Flow cytometry and ELISA
For cell staining, nonspecific antibody binding sites were first blocked and then the cells were stained with the indicated antibodies and examined by flow cytometry, as previously described [5, 8]. For CD107a detection, PE-conjugated anti-CD107a was added prior to NK cell stimulation and brefeldin and monensin (BD Biosciences, San Jose, CA) were added 1 h after NK cell stimulation. The cells were then incubated for an additional 1 h. For controls, fluorescence minus one was used as well as appropriate isotype-matched antibodies since the cells of interest expressed FcRs. A FSC-A/SSC-A plot was used to set an electronic gate on leukocyte populations, and FSC-A/FSC-H and SSC-A/SSC-H plots were used to set an electronic gate on single cells. A Zombie viability kit was used to assess live vs. dead cells, as per the manufacturer’s instructions (BioLegend). ELISA was performed by a cytometric bead-based Flex Set assay for human IFNγ (BD Biosciences) and a LEGENDplex assay for granzyme A and granzyme B (BioLegend), as per the manufacturer’s instructions. All flow cytometric analyses were performed on FACSCanto and FACSCelesta instruments using FACSDIVA v8.0.1 (BD Biosciences).
Statistical analyses
Statistical analyses were performed by use of GraphPad Prism (GraphPad Software, La Jolla, CA, USA). After assessing for approximate normal distribution, all variables were summarized as mean ± SD. Comparison between two groups was done with Student’s t test, with p < 0.05 taken as statistically significant.
Results
MEDI3622 stains NK cells and blocks ADAM17 function
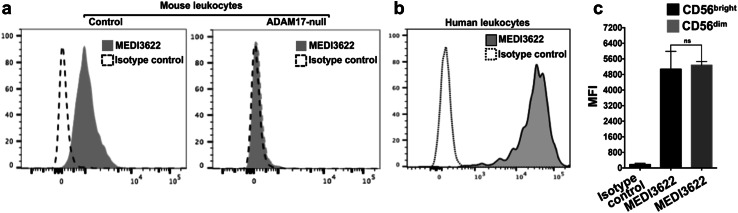
The anti-ADAM17 mAb MEDI3622 has been reported to have both human and mouse reactivity [28]. We investigated its capacity to recognize human and mouse leukocytes. To verify its specificity for ADAM17, we stained mouse peripheral blood leukocytes from control and conditional ADAM17 knockout mice. The latter mice lacked ADAM17 in their leukocytes. In Fig. 1a, we show that the MEDI3622 mAb stained control mouse leukocytes but not ADAM17-null leukocytes above an isotype control antibody. MEDI3622 also stained peripheral blood leukocytes from healthy human donors (Fig. 1b), and the staining was typically brighter than mouse leukocytes, which is consistent with its higher affinity for human ADAM17 [28]. In human peripheral blood, CD56+ CD3− NK cells consist of CD56bright and CD56dim subsets, representing immature and mature NK cells, respectively [38]. We found that MEDI3622 stained both NK cell populations (Fig. 1c).
Fig. 1.
MEDI3622 recognizes ADAM17 on mouse and human leukocytes. a Mouse peripheral blood leukocytes from control and ADAM17 conditional knockout mice (ADAM17-null) were stained with MEDI3622 or an isotype control antibody and examined by flow cytometry. y-axis = cell number. Representative data from at least three independent experiments using separate mice are shown. b Human peripheral blood leukocytes were isolated from healthy donors and stained by MEDI3622 or an isotype control antibody. Data are representative of three independent experiments using separate blood donors. c Staining by MEDI3622 was measured on CD56bright CD3− and CD56dim CD3− cells, as indicated. Isotype control antibody staining was measured on all CD56+ CD3− cells. Bar graphs show mean fluorescence intensity (MFI) ± SD of at least three independent experiments using separate donors. ns not significant
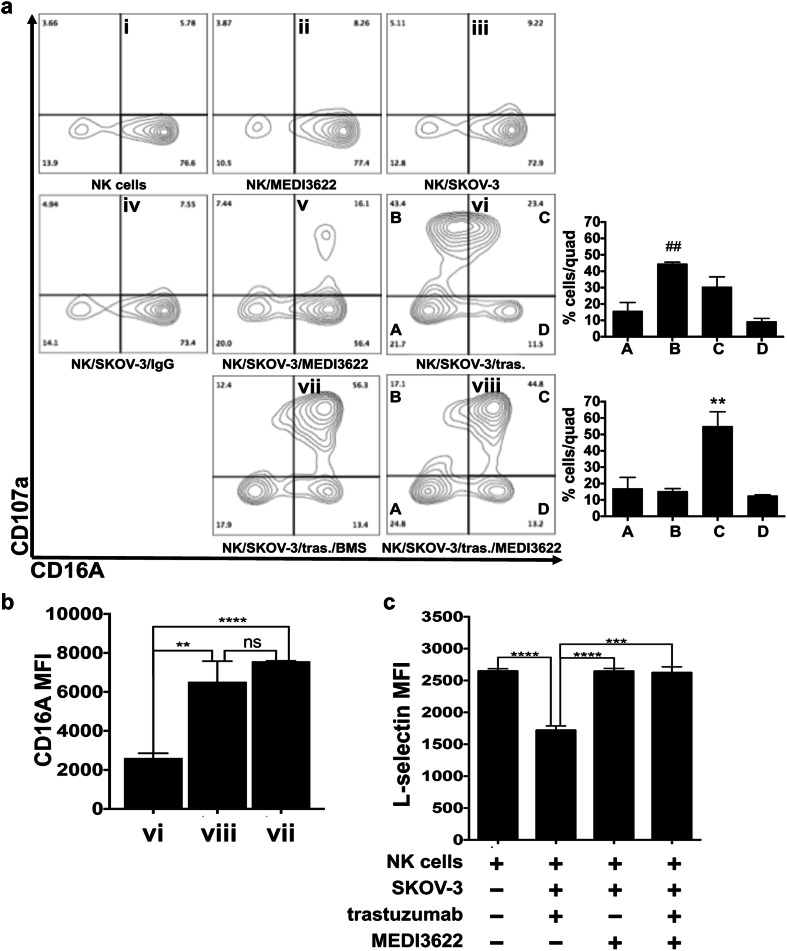
CD16A in human NK cells undergoes rapid ectodomain shedding by ADAM17 upon their activation with various stimuli [4, 5, 7, 8]. We examined the effects of MEDI3622 on CD16A downregulation by NK cells upon their stimulation with antibody-bound tumor cells. Enriched peripheral blood NK cells from healthy donors when exposed to MEDI3622, control IgG, and/or SKOV-3 cells, an ovarian cancer cell line that expresses HER2, demonstrated little to no upregulation in expression of the activation marker CD107a (Fig. 2a, panels ii–v). NK cells exposed to SKOV-3 cells and the anti-HER2 therapeutic mAb trastuzumab underwent a distinct upregulation of CD107a, and these activated cells downregulated their CD16A expression (Fig. 2a, panel vi). MEDI3622-treated NK cells exposed to SKOV-3 cells and trastuzumab also demonstrated a similar upregulation of CD107a, but their downregulation of CD16A was greatly diminished (Fig. 2a, panels viii vs. vi). This occurred as well for NK cells exposed to trastuzumab-bound SKOV-3 cells in the presence of the small molecule ADAM17 inhibitor BMS566394 (Fig. 2a, panel vii). Hence, in the absence of MEDI3622 we observed a significantly greater percentage of CD107a+ CD16Alow NK cells and conversely in the presence of MEDI3622 there was a significantly greater percentage of CD107a+ CD16Ahigh NK cells following their exposure to trastuzumab-bound tumor cells (Fig. 2a, bar graphs). The higher surface levels of CD16A retained on NK cells activated by trastuzumab-bound tumor cells when blocking ADAM17 activity are also represented as mean fluorescence intensity (MFI) in Fig. 2b. CD62L is another ADAM17 substrate expressed by NK cells [39], and its downregulation was also blocked when NK cells were exposed to trastuzumab-bound tumor cells in the presence of MEDI3622 (Fig. 2c). Taken together, these findings demonstrate that MEDI3622 effectively blocked ADAM17 activity in NK cells, but not their activation.
Fig. 2.
MEDI3622 blocks CD16A downregulation by NK cells stimulated by antibody-bound tumor cells. a Human peripheral blood leukocytes were isolated from healthy donors, NK cells were enriched, as described in “Materials and methods”, and then incubated with SKOV-3 cells, trastuzumab (tras.), control human IgG, BMS566394 (BMS), and/or MEDI3622, as indicated, for 2 h at 37 °C (effector:target ratio = 10:1). NK cells were then stained for CD56, CD3, CD107a, and CD16A and examined by flow cytometry (CD56+ CD3−-gated cells are shown). Data are representative of three independent experiments using separate blood donors. For the bar graphs, the letters correspond with the lettered quadrants in the adjacent contour plot (i.e., panel vi or viii) and indicate the percentage of cells in each quadrant (quad). Mean ± SD of three independent experiments using separate donors is shown. Statistical significance is indicated as ##p < 0.01 vs. quadrant B in panel viii; **p < 0.01 vs. quadrant C in panel vi. b CD16A levels on CD107a+ NK cells in the indicated contour panels is shown. c Enriched NK cells were treated as described above. CD62L staining levels by CD56+ CD3− cells are shown. b, c The bar graphs show MFI ± SD of three independent experiments using separate donors. Statistical significance is indicated as **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
MEDI3622 enhances IFNγ production by NK cells in the presence of antibody-bound tumor cells
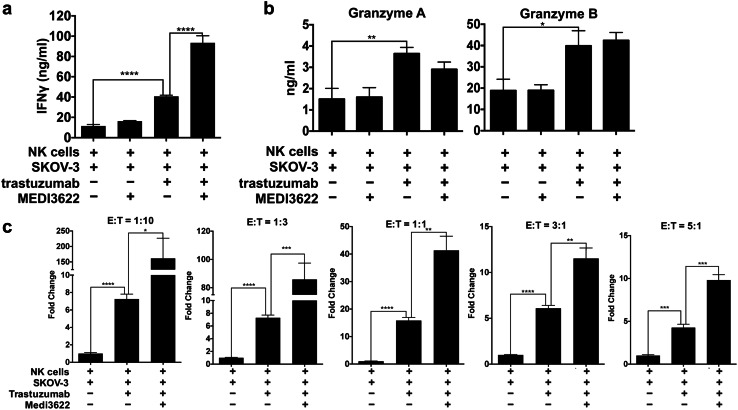
NK cells produce high levels of IFNγ when stimulated by antibody-bound tumor cells [40]. For instance, NK cells exposed to SKOV-3 cells and trastuzumab released significantly higher levels of IFNγ than NK cells when exposed to SKOV-3 cells alone (Fig. 3a). Remarkably, IFNγ release was further enhanced by MEDI3622-treated NK cells exposed to SKOV-3 cells and trastuzumab, whereas this was not observed by MEDI3622-treated NK cells exposed to SKOV-3 cells alone (Fig. 3a). We also examined the NK cell cytotoxic granule components granzyme A and granzyme B. We found that the levels released by NK cells exposed to SKOV-3 cells and trastuzumab were significantly higher than NK cells exposed to SKOV-3 cells alone (Fig. 3b); however, MEDI3622 treatment of the NK cells did not further increase their release (Fig. 3b), which is consistent with our earlier observation that blocking ADAM17 activity did not significantly increase ADCC by human NK cells [5].
Fig. 3.
MEDI3622 treatment increases IFNγ production by NK cells stimulated by antibody-bound tumor cells over a range of ratios. a Enriched human NK cells were incubated with SKOV-3 cells, trastuzumab, and/or MEDI3622, as indicated, for 4 h at 37 °C (effector:target ratio = 10:1). Secreted IFNγ levels were quantified by ELISA. b NK cells were treated as described above and their release of granzyme A and granzyme B were quantified by ELISA. c NK and SKOV-3 cells were incubated over a range of effector:target (E:T) ratios, as indicated. Secreted IFNγ levels are shown as fold change normalized to NK cells exposed to SKOV-3 cells alone. The bar graphs show mean ± SD of 3 independent experiments using separate donors. Statistical significance is indicated as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
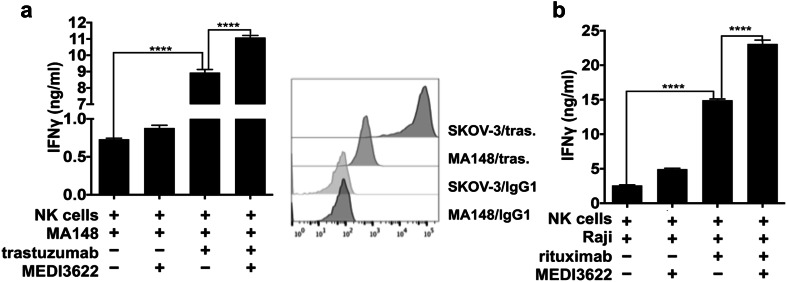
The experiments above were performed at an NK cell:tumor cell ratio of 10:1. In patients with solid tumors, NK cells are typically outnumbered by tumor cells [41]. Therefore, we examined the effects of MEDI3622 treatment on IFNγ production by NK cells exposed to trastuzumab-bound SKOV-3 cells over a range of effector:target ratios down to 1:10. At all ratios examined, IFNγ production was significantly higher by MEDI3622-treated NK cells (Fig. 3c). We also examined whether MEDI3622 treatment enhanced IFNγ production by NK cells exposed to other tumor cell lines and therapeutic antibodies. The ovarian cancer cell line MA-148 expresses HER2 at considerably lower levels than SKOV-3 cells (Fig. 4a, histogram plot). Despite these lower levels of HER2 expression, NK cells again secreted significantly higher levels of IFNγ when exposed to trastuzumab-bound MA-148 cells in the presence of MEDI3622 (Fig. 4a). Raji is a Burkitt’s lymphoma cell line that expresses the tumor-associated antigen CD20, recognized by the therapeutic antibody rituximab. Again, NK cells released the highest levels of IFNγ when exposed to rituximab-bound Raji cells and MEDI3622 (Fig. 4b).
Fig. 4.
MEDI3622 treatment increases IFNγ production by NK cells stimulated by different tumor cells and therapeutic antibodies. a Enriched NK cells were incubated with MA-148 cells, which express lower levels of HER2, trastuzumab, and/or MEDI3622, as indicated, for 4 h at 37 °C. The flow cytometric histograms show SKOV-3 or MA-148 cell staining levels by trastuzumab (tras.) or an isotype control antibody. b Enriched NK cells were incubated with Raji cells, rituximab, and/or MEDI3622, as indicated, for 4 h at 37 °C. a, b Secreted IFNγ levels were quantified by ELISA. The bar graphs show mean ± SD of at least three independent experiments using separate donors. Statistical significance is indicated as ****p < 0.0001
Role of CD16A shedding in the increased production of IFNγ by MEDI3622-treated NK cells
The mAb 3G8 recognizes the second Ig domain of CD16A and prevents its binding to the Fc region of IgG [42]. We show that 3G8 essentially normalized IFNγ production by NK cells stimulated with trastuzumab-bound SKOV-3 cells in the presence or absence of MEDI3622 (Fig. 5). Next, we sought to directly address the effects of CD16A shedding on NK cell production of IFNγ when stimulated by antibody-bound tumor cells. To do this, we used the immortalized human NK cell line NK92. These cells lack expression of endogenous FcγR yet functional recombinant CD16A can be expressed in these cells [8, 37]. We have reported that substituting the serine at position P2′ adjacent to the ADAM17 cleavage site in CD16A with a proline residue completely disrupts the shedding of this receptor in transduced NK92 cells upon their activation in various manners [8]. We show in Fig. 6a that NK92 cells expressing non-cleavable CD16A (ncCD16A) released significantly higher levels of IFNγ when exposed to SKOV-3 cells in the presence of trastuzumab, establishing that the amino acid substitution did not uncouple CD16A from Fcγ and/or CD3ζ-mediated signaling. We then compared IFNγ release by NK92 cells expressing wildtype CD16A or ncCD16A at equivalent levels (Fig. 6b). Similar to primary NK cells, NK92 cells expressing wildtype CD16A produced the highest levels of IFNγ when exposed to trastuzumab-bound SKOV-3 cells in the presence of MEDI3622 (Fig. 6c, panel i), whereas MEDI3622 treatment had no effect on IFNγ production by NK92-ncCD16A cells when exposed to trastuzumab-bound SKOV-3 (Fig. 6c, panel ii). NK92-ncCD16A cells, however, produced significantly higher levels of IFNγ than NK92-CD16A cells when exposed to trastuzumab-bound SKOV-3 cells (Fig. 6c, panel iii). Taken together, these findings indicate that MEDI3622 increased IFNγ production by NK cells exposed to antibody-bound tumor cells primarily by blocking the shedding of CD16A and not other ADAM17 substrates.
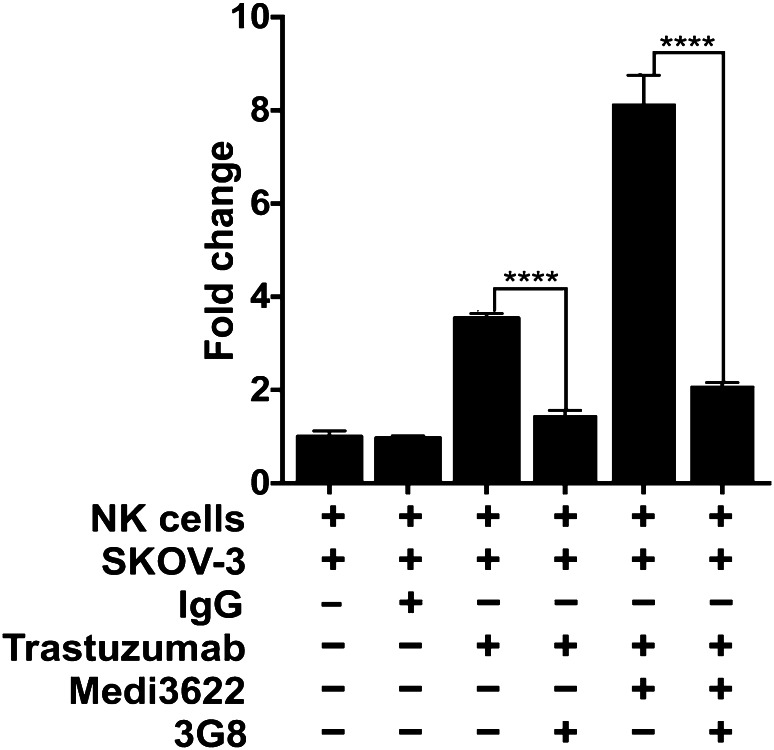
Fig. 5.
CD16A regulates IFNγ production by NK cells stimulated by antibody-bound tumor cells. Enriched NK cells were incubated with SKOV-3 cells, trastuzumab, control human IgG1, MEDI3622, and/or the CD16A function-blocking mAb 3G8, as indicated, for 4 h at 37 °C. Secreted IFNγ levels were quantified by ELISA and shown as fold change normalized to NK cells exposed to SKOV-3 cells alone. The bar graph shows mean ± SD of at least three independent experiments using separate donors. Statistical significance is indicated as ****p < 0.0001
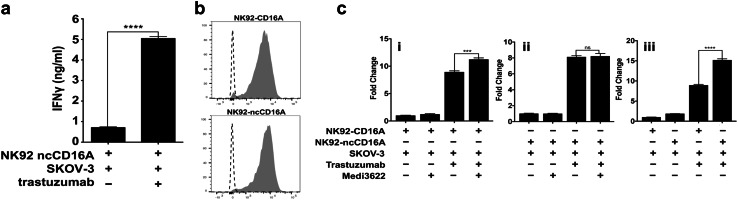
Fig. 6.
CD16A shedding regulates IFNγ production by NK cells stimulated by antibody-bound tumor cells. a NK92 cells expressing non-cleavable CD16A (ncCD16A) were incubated with SKOV-3 cells ± trastuzumab, as indicated, for 4 h at 37 °C. Secreted IFNγ levels were quantified by ELISA. b The flow cytometric histograms show staining levels by an anti-CD16 mAb or an isotype control antibody for NK92-CD16A and NK92-ncCD16A cells, as indicated. Representative data from at least three independent experiments are shown. c NK92 cells expressing wildtype CD16A or ncCD16A were incubated with SKOV-3 cells, trastuzumab, and/or MEDI3622, as indicated, for 4 h at 37 °C. Secreted IFNγ levels were quantified by ELISA and shown as fold change normalized to NK cells exposed to SKOV-3 cells alone. a, c The bar graphs show mean ± SD of at least three independent experiments. Statistical significance is indicated as ***p < 0.001;****p < 0.0001; ns not significant
Discussion
ADAM17 is a membrane-associated metalloprotease that undergoes a rapid increase in its catalytic activity upon leukocyte stimulation and cleaves various cell surface receptors [43]. We report here that the highly specific ADAM17 mAb MEDI3622 binds to human NK cells and blocks the downregulation of ADAM17 substrates upon their activation by antibody-bound tumor cells. These NK cells also demonstrated a marked enhancement in their production of IFNγ. Increased IFNγ release occurred for MEDI3622-treated NK cells exposed to trastuzumab-bound ovarian cancer cell lines, both MA-148 and SKOV-3 expressing low and high levels of HER2, respectively, as well as rituximab-bound Raji cells, a Burkitt’s lymphoma cancer cell line. The ratio of NK cells to cancer cells in the tumor environment varies widely due to many circumstances, and in patients with solid tumors, NK cells are typically outnumbered by tumor cells [41]. We found that over a range of effector:target ratios, MEDI3622-treated NK cells consistently produced higher levels of IFNγ in the presence of antibody-bound tumor cells, indicating that blocking ADAM17 activity in NK cells could result in their increased production of IFNγ in the tumor environment at all levels of infiltration. Our data demonstrate that an anti-CD16A function-blocking mAb neutralized this augmented production of IFNγ. In addition, NK92 cells expressing a non-cleavable version of CD16A released a significantly higher amount of IFNγ than NK92 cells expressing equivalent levels of wildtype CD16A when exposed to antibody-bound tumors cells. These findings provide the first direct evidence that CD16A shedding modulates IFNγ production by NK cells and underlies the effects of MEDI3622 on NK cells.
CD16A signaling in NK cells also results in their exocytosis of cytotoxic granules [2]. We have reported that ADAM17 inhibition did not significantly enhance tumor cell killing by an ADCC assay [5]. Consistent with this, we also did not see a significant difference in the levels of granzyme A and granzyme B released by NK cells exposed to trastuzumab-bound SKOV-3 cells in the absence or presence of MEDI3622. A limitation of some in vitro ADCC assays, however, is that they assess the cumulative lytic hit by NK cells at typically high effector:target ratios. Approaches using custom-designed cell imaging systems have revealed that at low effector:target ratios, NK cells can kill target cells in a sequential or serial manner over a prolonged period [44–46]. It will be interesting to examine the effects of blocking ADAM17 on ADCC under conditions favoring serial killing, as the retention of cell surface CD16A on the activated NK cells would be predicted to affect the kinetics and efficiency of repeated engagement of antibody-bound tumor cells. The mouse cross-reactivity by MEDI3622 also provides an opportunity for in vivo pre-clinical studies. Unfortunately, mouse CD16 does not undergo ectodomain shedding [4], and thus future studies will focus on a human hematopoietic cell and tumor xenograft mouse model.
Negative immunologic regulators help keep immune responses in check. Inhibitory receptors on NK cells play a constitutive role in suppressing activation signals [47]. ADAM17 fits the role of a negative checkpoint regulator of CD16A. CD16A shedding, however, occurs in a rapid manner and is perhaps a means of tuning the signaling of this potent activating receptor. Of interest is that tumor-associated NK cells have been shown to have decreased levels of CD16A in patients with ovarian cancer [14, 17]. Thus, prolonged ADAM17 induction may occur in the tumor microenvironment and contribute to suppressing NK cell effector functions. By blocking ADAM17 activity it may be possible to sustain NK cell production of anti-tumor cytokines in the presence of therapeutic antibodies for ovarian cancer as well as various other malignancies. It has been reported that ADAM17 can also directly degrade secreted IFNγ [48], revealing another advantage for blocking ADAM17 as a cancer therapy. We cannot rule out that this may have contributed to the higher levels of IFNγ by NK cells when treated with MEDI3622. However, we do show that NK92 cells expressing ncCD16A released higher levels of IFNγ in the presence of antibody-bound tumor cells than NK92-CD16A cells, and that this was not further increased when NK92-ncCD16A were treated with MEDI3622.
In closing, therapeutic mAbs have become one of the fastest growing classes of drugs, and tumor-targeting mAbs are the most widely used and characterized immunotherapy for hematologic and solid tumors [49]. Several clinically successful antibodies utilize NK cell effector functions as a mechanism of action [2, 10]. MEDI3622 in combination with these therapeutic antibodies may provide a promising combination therapy to improve NK cell activity in the tumor microenvironment, in particular by enhancing their production of IFNγ to overcome tumor immunosuppression.
Acknowledgements
We thank Robert Hullsiek, Daniel Mendez, and Dr. Jianming Wu for technical assistance.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ADAM17
A disintegrin and metalloproteinase-17
- APC
Allophycocyanin
Author contributions
Conception and design: HKM and BW. Development of methodology: HKM and BW. Analysis and interpretation of data: HKM, NP, and BW. Writing, review, and/or revision of the manuscript: HKM, NP, and BW. Technical and material support: HKM, NP, EFM. Study supervision: BW.
Funding
This study was supported by the Grant R01CA203348 (Walcheck) from the National Institutes of Health.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was approved by the Institutional Review Board and the Animal Care and Use Committee at the University of Minnesota, 9708M00134 and 1612-34435A, respectively, PI: Bruce Walcheck.
Informed consent
Informed consent was received from each individual who donated peripheral blood for this study.
Animal source
Adam17 flox/flox (Adam17tm1.2Bbl/J) mice and Vav1-Cre mice (B6.Cg-Tg(Vav1-cre)A2Kio/J) were purchased from Jackson Laboratories.
Cell line authentication
The tumor-cell lines in this study are used to determine NK cell reactivity and so the critical feature is their tumor-associated antigen expression (i.e., CD20 and HER2). Cells were routinely checked to ensure consistent levels of antigen expression.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison D, Phillips JH, Lanier LL. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II) J Immunol. 1991;147:3459–3465. [PubMed] [Google Scholar]
- 4.Wang Y, Wu J, Newton R, Bahaie NS, Long C, Walcheck B. ADAM17 cleaves CD16b (FcgammaRIIIb) in human neutrophils. Biochim Biophys Acta. 2013;1833:680–685. doi: 10.1016/j.bbamcr.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romee R, Foley B, Lenvik T, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peruzzi G, Femnou L, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. Membrane-type 6 matrix metalloproteinase regulates the activation-induced downmodulation of CD16 in human primary NK cells. J Immunol. 2013;191:1883–1894. doi: 10.4049/jimmunol.1300313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajoie L, Congy-Jolivet N, Bolzec A, et al. ADAM17-mediated shedding of FcgammaRIIIA on human NK cells: identification of the cleavage site and relationship with activation. J Immunol. 2014;192:741–751. doi: 10.4049/jimmunol.1301024. [DOI] [PubMed] [Google Scholar]
- 8.Jing Y, Ni Z, Wu J, Higgins L, Markowski TW, Kaufman DS, Walcheck B. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One. 2015;10:e0121788. doi: 10.1371/journal.pone.0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone Roberti Maggiore U, Bellati F, Ruscito I, Gasparri ML, Alessandri F, Venturini PL, Ferrero S. Monoclonal antibodies therapies for ovarian cancer. Expert Opin Biol Ther. 2013;13:739–764. doi: 10.1517/14712598.2013.767328. [DOI] [PubMed] [Google Scholar]
- 12.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 13.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC. Drug Des. Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 15.Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox MC, Battella S, La Scaleia R, et al. Tumor-associated and immunochemotherapy-dependent long-term alterations of the peripheral blood NK cell compartment in DLBCL patients. Oncoimmunology. 2015;4:e990773. doi: 10.4161/2162402X.2014.990773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felices M, Chu S, Kodal B, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145:453–461. doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granzin M, Soltenborn S, Muller S, Kollet J, Berg M, Cerwenka A, Childs RW, Huppert V. Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy. 2015;17:621–632. doi: 10.1016/j.jcyt.2015.03.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy MJ, Mullooly M, O’Donovan N, Sukor S, Crown J, Pierce A, McGowan PM. The ADAMs family of proteases: new biomarkers and therapeutic targets for cancer? Clin. Proteomics. 2011;8:9. doi: 10.1186/1559-0275-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280:19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- 21.Shen H, Li L, Zhou S, et al. The role of ADAM17 in tumorigenesis and progression of breast cancer. Tumour Biol. 2016 doi: 10.1007/s13277-016-5418-y. [DOI] [PubMed] [Google Scholar]
- 22.Mustafi R, Dougherty U, Mustafi D, et al. ADAM17 is a tumor promoter and therapeutic target in western diet-associated colon cancer. Clin Cancer Res. 2017;23:549–561. doi: 10.1158/1078-0432.CCR-15-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchanan PC, Boylan KLM, Walcheck B, Heinze R, Geller MA, Argenta PA, Skubitz APN. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J Biol Chem. 2017;292:6339–6351. doi: 10.1074/jbc.M116.746859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tape CJ, Willems SH, Dombernowsky SL, Stanley PL, Fogarasi M, Ouwehand W, McCafferty J, Murphy G. Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci USA. 2011;108:5578–5583. doi: 10.1073/pnas.1017067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards FM, Tape CJ, Jodrell DI, Murphy G. Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS One. 2012;7:e40597. doi: 10.1371/journal.pone.0040597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok HF, Botkjaer KA, Tape CJ, Huang Y, McCafferty J, Murphy G. Development of a ‘mouse and human cross-reactive’ affinity-matured exosite inhibitory human antibody specific to TACE (ADAM17) for cancer immunotherapy. Protein Eng Des Sel. 2014;27:179–190. doi: 10.1093/protein/gzu010. [DOI] [PubMed] [Google Scholar]
- 27.Caiazza F, McGowan PM, Mullooly M, et al. Targeting ADAM-17 with an inhibitory monoclonal antibody has antitumour effects in triple-negative breast cancer cells. Br J Cancer. 2015;112:1895–1903. doi: 10.1038/bjc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios-Doria J, Sabol D, Chesebrough J, et al. A monoclonal antibody to ADAM17 inhibits tumor growth by inhibiting EGFR and non-EGFR-mediated pathways. Mol Cancer Ther. 2015;14:1637–1649. doi: 10.1158/1535-7163.MCT-14-1040. [DOI] [PubMed] [Google Scholar]
- 29.Dosch J, Ziemke E, Wan S, et al. Targeting ADAM17 inhibits human colorectal adenocarcinoma progression and tumor-initiating cell frequency. Oncotarget. 2017;8:65090–65099. doi: 10.18632/oncotarget.17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng L, Cook K, Xu L, Cheng L, Damschroder M, Gao C, Wu H, Dall’Acqua WF. Molecular basis for the mechanism of action of an anti-TACE antibody. mAbs. 2016;8:1598–1605. doi: 10.1080/19420862.2016.1226716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 32.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91:299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kammertoens T, Friese C, Arina A, et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature. 2017;545:98–102. doi: 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra HK, Long C, Bahaie NS, Walcheck B. Regulation of CXCR2 expression and function by a disintegrin and metalloprotease-17 (ADAM17) J Leukoc Biol. 2015;97:447–454. doi: 10.1189/jlb.3HI0714-340R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra HK, Johnson TJ, Seelig DM, Walcheck B. Targeting ADAM17 in leukocytes increases neutrophil recruitment and reduces bacterial spread during polymicrobial sepsis. J Leukoc Biol. 2016;100:999–1004. doi: 10.1189/jlb.3VMAB1115-496RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 39.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- 40.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci USA. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Crit Rev Oncog. 2014;19:91–105. doi: 10.1615/CritRevOncog.2014011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perussia B, Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984;132:1410–1415. [PubMed] [Google Scholar]
- 43.Mishra HK, Ma J, Walcheck B. Ectodomain shedding by ADAM17: its role in neutrophil recruitment and the impairment of this process during sepsis. Front Cell Infect Microbiol. 2017;7:138. doi: 10.3389/fcimb.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi PJ, Mitchison TJ. Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc Natl Acad Sci USA. 2013;110:6488–6493. doi: 10.1073/pnas.1221312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanherberghen B, Olofsson PE, Forslund E, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–1334. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 46.Guldevall K, Brandt L, Forslund E, et al. Microchip screening platform for single cell assessment of NK cell cytotoxicity. Front Immunol. 2016;7:119. doi: 10.3389/fimmu.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanzaki H, Shinohara F, Suzuki M, et al. A-disintegrin and metalloproteinase (ADAM) 17 enzymatically degrades interferon-gamma. Sci Rep. 2016;6:32259. doi: 10.1038/srep32259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battella S, Cox MC, Santoni A, Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J Leukoc Biol. 2016;99:87–96. doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]