Abstract
Background
Impaired cerebellar development is an important determinant of adverse motor and cognitive outcomes in very preterm (VPT) infants. However, longitudinal MRI studies investigating cerebellar m aturational trajectories from birth through childhood and associated neurodevelopmental outcomes are lacking.
Objective
To compare cerebellar volume and growth from term-equivalent age (TEA) to 7 years between VPT (<30 weeks’ gestation or <1250g) and full-term children, and to assess the association between these measures, perinatal factors and 7-year outcomes in VPT children, and whether these relationships varied by sex.
Methods
Prospective cohort study of 224 VPT and 46 full-term infants. Cerebellar volumes were measured on MRI at TEA and 7 years. Useable MRI data at either time-point were collected for 207 VPT and 43 full-term children. Cerebellar growth from TEA to 7 years was compared between VPT and full-term children. Associations with perinatal risk factors and 7-year outcomes were investigated in VPT children.
Results
VPT children had smaller TEA and 7-year cerebellar volumes, and reduced growth. Perinatal factors were associated with smaller cerebellar volume and growth between TEA and 7 years, namely, postnatal corticosteroids for TEA volume, and female sex, earlier birth gestation, white and deep nuclear grey matter injury for 7-year volume and growth. Smaller TEA and 7-year volumes, and reduced growth were associated with poorer 7-year IQ, language and motor function, with differential relationships observed for male and female children.
Conclusion
Cerebellar growth from TEA to 7 years is impaired in VPT children, and relates to early perinatal factors and 7-year neurodevelopmental outcomes.
Keywords: Brain, Cerebellum, Longitudinal Studies, Outcome Assessment, Premature Birth, Magnetic Resonance Imaging
Introduction
Cerebellar abnormalities, including hemorrhage and impaired development, are increasingly recognized as complications of very preterm (VPT) birth (1, 2), with mounting evidence for subsequent adverse effects on motor and cognitive outcomes (3–7). Studies using volumetric MRI analyses have shown that cerebellar volume is smaller in VPT compared with term-born infants (8–16). These volume changes have been attributed to prematurity itself (8, 14), as well as to the presence of both cerebral (8–13, 16) and cerebellar injury (9, 15, 16). Most studies have been cross-sectional, and while they have provided valuable insight into cerebellar development, little is known about the relationships between neonatal cerebellar abnormality, the cerebellum’s subsequent maturational trajectory and the long-term neurodevelopmental impairments commonly observed in VPT infants.
The vulnerability of the immature cerebellum is well established, and appears to be related to the cerebellum’s dynamic growth during the last trimester of gestation (2). However, few studies have examined the cerebellum’s growth trajectory after term equivalency in VPT infants. Parker and colleagues investigated cerebellar maturation between adolescence and adulthood in ex-preterm individuals (6), and more recently Lee and colleagues reported on the trajectory of cerebellar growth from infancy to 4 years of age following preterm birth (17). Both studies highlighted the cerebellum’s maturational vulnerability and its association with functional impairment at different developmental stages. We have previously reported smaller cerebellar volumes in VPT infants compared with full-term controls at term-equivalent age (TEA), with a weak correlation between cerebellar volume and cognitive and motor development at 2 years, mediated by cerebral white matter injury (WMI) (10). In a separate, larger cohort of moderate and late preterm infants, we recently demonstrated strong positive associations between cerebellar volume at TEA and language and motor scores at 2 years, even after adjustment for known perinatal risk factors (18). The extent to which these early findings correspond to longer-term neurodevelopmental outcomes, however, remains controversial (19, 20).
The first aim of this study was to compare cerebellar volume and growth between VPT and full-term children, from TEA to age 7 years. We hypothesized that VPT children would display smaller cerebellar volumes compared with full term children, in the neonatal period and at age 7 years, and reduced growth over this period. Secondly, we aimed to explore perinatal factors associated with cerebellar development from TEA to 7 years in VPT children, including cerebral injury, neonatal therapies and environmental factors. We hypothesized that these perinatal risk factors would be associated with impaired cerebellar development in VPT children from TEA to 7 years. Thirdly, we aimed to determine whether reduced growth and smaller cerebellar volumes at either age would be associated with neurodevelopmental outcomes at 7 years. We hypothesized that smaller cerebellar volumes in the neonatal period, at age 7 years, and reduced cerebellar growth would be associated with motor and cognitive impairments at age 7 years in VPT children. Due to the established sexual dimorphism of the cerebellum (21), we further sought to examine sex differences in cerebellar size and development, hypothesizing reduced cerebellar development in VPT female counterparts. Additionally, given evidence for sex differences in neurodevelopmental outcomes in VPT children (22), we sought to investigate these sex differences in relationships between cerebellar development and 7-year neurodevelopmental outcomes, hypothesizing that male and female VPT children would demonstrate differential functional vulnerabilities associated with reduced cerebellar development.
Materials and Methods
Participants
Participants were VPT and full-term infants born between April 11, 2001, and April 26, 2004 and recruited as part of a prospective, longitudinal, observational cohort study conducted at The Royal Women’s Hospital in Melbourne, Australia, and consisted of 224 VPT infants (<30 weeks’ gestational age (GA) or <1250 g birth weight) and a concurrent control group of 46 infants born full-term (37–42 weeks’ GA) and of normal birth weight (≥2500 g) (Fig. 1). Eligible VPT infants included those born without congenital abnormalities or syndromes known to affect development and who survived the neonatal period. MRI scans were acquired at TEA with infants who had an MRI scan between 37 and 43 weeks’ postmenstrual age (PMA) included in the current study. Children returned for follow-up MRI and neurodevelopmental assessment at 7 years of age at the Royal Children’s Hospital, Melbourne, Australia (VPT: n=198, full-term: n=43). Cerebellar TEA volumes suitable for analysis were generated for 201 VPT infants and 41 full-term infants (Fig. 1). At the 7-year follow-up, usable MRI data were collected for 114 VPT (51%) children and 29 full-term (63%) children; 108 VPT children (48%) and 27 full-term children (59%) had usable MRI data at both ages (Fig. 1). Usable MRI data at either infancy or 7 years were collected for 207 VPT children (98%) and 43 full-term children (93%).
Figure 1. Flowchart of participants.
MRI numbers reflect usable images suitable for this analysis. PMA – postmenstrual age; TEA – term-equivalent age; VPT – very preterm.
MRI
Neonatal MRI brain scans were acquired during natural sleep at TEA using a 1.5T General Electric MRI scanner (Signa LX Echospeed System; General Electric, Fairfield, Connecticut) at the Royal Children’s Hospital, Melbourne, Australia. Coronal T2-weighted, dual-echo, fast spin-echo images with interleaved acquisition were acquired with slice thickness 1.7 to 3 mm; repetition time 4000 ms; echo times 60 ms (first echo) and 160 ms (second echo); field of view 22 × 16 cm2; matrix 256 × 192 interpolated to 512 × 512.
Follow-up MRI scans were acquired at 7 years using a 3T Siemens MAGNETOM TrioTim system (Siemens, Erlangen, Germany) at the Royal Children’s Hospital, Melbourne, Australia. Sagittal, 3-dimensional, rapid gradient-echo T1-weighted images were acquired with slice thickness 0.8 mm; repetition time 1900 ms; echo time 2.27 ms; field of view 210 × 210 mm; matrix 256 × 256.
Volumetric Segmentation
Neonatal cerebellar volumes were generated using manual segmentation of the T2-weighted structural scans as previously described (10). A semi-automated brain segmentation approach utilizing a 40-week infant template (23) was used to classify brain tissue into its various components, the sum of which generated values for intracranial volume (ICV) (10).
Seven-year cerebellar volumes were generated using automated segmentation of the T1-weighted structural scans (FreeSurfer, version 4.4.0; http://surfer.nmr.mgh.harvard.edu) with manual editing. ICV, including white matter, cortical and subcortical gray matter, and cerebrospinal fluid, was also estimated for each subject using FreeSurfer. Cerebellar growth Z-scores were calculated for participants with data at both time-points, using the term control group’s mean cerebellar volume change from TEA to 7 years and standard deviation. ICV growth Z-scores were calculated similarly.
Perinatal and Sociodemographic Data
Perinatal data were obtained from chart review and socio-demographic information was obtained from a questionnaire completed by the child’s primary caregiver at the time of recruitment. Clinically relevant perinatal data included sex, birth GA, birth weight standard deviations score (BWSDS), intermittent positive pressure ventilation (IPPV) duration, infection (defined as either ≥1 episode of proven sepsis or necrotizing enterocolitis), postnatal corticosteroid exposure, morphine dose (mg/kg), and intraventricular hemorrhage (IVH) grade. IPPV duration was log-transformed to allow for better visualization. Total WMI, deep nuclear gray matter (DNGM) and cerebellar abnormalities, which have previously been related to 7-year neurodevelopmental outcomes (24), were assessed on T2-weighted scans by an experienced neonatal neurologist independent of knowledge of long-term outcomes, based on an established scoring system (25).
7-year Neurodevelopmental Assessment
At 7 years’ corrected age, children returned for an extensive follow-up assessment covering various neurodevelopmental domains including general intelligence, academic achievement and motor functioning. Selected outcome measures relevant to this study are outlined below.
General intellectual functioning was assessed using the Full Scale IQ (FSIQ) score of the four-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI) (26). Verbal and performance IQ subdomains were also calculated.
Language ability was assessed using the Core Language Index (CLI) from the Clinical Evaluation of Language Fundamentals Fourth Edition (CELF-IV), Australian Standardised Edition (27). Additionally, the following language subdomains were calculated and reported: auditory comprehension using the Receptive Language Index (RLI) and language production using the Expressive Language Index (ELI).
Attention was assessed using the Score! subtest from the Test of Everyday Attention for Children (TEA-Ch) (28), with performance determined by the number of correctly identified targets (maximum 10).
Working memory was assessed using the Backward Digit Recall subtest from the Working Memory Test Battery for Children (WMTB-C) (29), with performance based on the child’s ability to recall sequences of digits in the reverse order to that presented.
Motor function was assessed using the Movement Assessment Battery for Children (MABC2) (30) and included measurement of both gross and fine-motor skills, with three subscores (balance, manual dexterity, and aiming and catching) summed to give a total motor score.
Age standardized scores are reported for the WASI, CELF-IV, WMTB-C (all Mean=100, SD=15), TEA-Ch and MABC2 (both Mean=10, SD=3). Children’s ages were corrected for prematurity to avoid bias in test scores (31).
Statistical Analysis
Data were analyzed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and STATA 13.1 (StataCorp, Texas, USA). To address the first aim of this study, cerebellar volumes and growth from TEA to 7 years was examined using a two-level linear mixed-effects model, which included a random intercept to capture correlations within repeated measures from participants. We also considered a three-level model accounting for correlations between multiple births but there was little evidence of clustering by family. The model included main effects of group and time-point, and an interaction term between group and time-point to assess whether cerebellar growth trajectories differed between VPT and full-term infants. All participants with usable MRI data at either TEA or 7 years were included in this analysis. Primary analyses were adjusted for sex and postmenstrual age at scan, and secondary analyses further adjusted for ICV to account for potential inter-subject variability in head size. Analyses were initially conducted for all participants, and then performed separately for males and females to examine differential effects of sex on cerebellar growth.
For the second aim, associations between perinatal risk factors and cerebellar volumes at TEA and 7 years were examined within the VPT group using linear regression models. Associations with cerebellar growth Z-scores were also evaluated for VPT infants who had data at both time-points. Analyses were initially performed separately for each predictor/outcome combination adjusted for postmenstrual age at scan, and subsequently adjusted for ICV.
For the third aim, associations between cerebellar volumes at TEA and 7 years, cerebellar growth and neurodevelopmental outcomes in VPT infants were examined using linear regression. The analysis of cerebellar growth Z-score associations was restricted to infants with data at both time-points. Analyses were performed separately for each cerebellar measure, adjusted for postmenstrual age at scan, and subsequently adjusted for ICV and maternal education; the latter due to its established association with cognitive achievement (32). Models exploring associations between cerebellar growth and outcomes were further carried out separately in males and females.
The assumptions of normality and homoscedasticity of residuals in the regression models were assessed using quantile-quantile (Q-Q) plots and plots of residuals versus fitted values. There was no evidence for violations of either of these assumptions. All results are presented as regression coefficients with 95% confidence intervals and p-values from 2-tailed tests. Given the multiple associations tested for Aims 2 and 3, and the potential for false positives (Type I errors), results from these aims were corrected for multiple comparisons using the False Discovery Rate (FDR) method (33). FDR correction was applied for each set of inferences, that is, within each time-point, and separately to primary and secondary analyses according to modern statistical practice (34). Given the exploratory nature of this study, both uncorrected and FDR-corrected results are presented.
Experimental Design
Volumetric analyses and neurodevelopmental assessments were performed by neuroscientists and experienced assessors blinded to clinical history including prematurity. The sample size for the study was based on the size of the original cohort and the number of children with useable MRI data available hence there was no a priori sample size calculation.
Results
There were no differences in clinical characteristics between participants who had useable MRI data at TEA and those who did not. Clinical characteristics were generally similar between participants with usable MRI data at 7 years and those without, except that participants with MRI data had lower total neonatal WMI scores (p < 0.001) and less postnatal corticosteroid exposure (p = 0.013). Table 1 provides a summary of participant characteristics for subjects with useable MRI at either time point. As expected, compared with the full-term group, the VPT group had a greater incidence of neonatal complications and a higher rate of multiple births. The full-term group was slightly older at the TEA scan than those born VPT, with a similar proportion of males. Compared with full-term infants, VPT infants had higher total WMI (p < 0.001) and DNGM injury (p < 0.001) scores; IVH was only observed in VPT infants. Cerebellar abnormality was present in 10 VPT and 3 full-term infants (p = 0.575) and comprised mostly of signal abnormality and small hemorrhages, all of which were punctate unilateral except for 1 VPT infant with punctate bilateral lesions. At 7-years, the VPT group performed more poorly than full-term controls in measures of IQ (p < 0.001), language (p < 0.001), working memory (p < 0.001) and motor ability (p = 0.012).
Table 1.
Participant Characteristics
| Characteristic | VPT (n=207) | Full term (n=43) |
|---|---|---|
| Perinatal data | ||
| Birth GA (weeks), mean (SD) | 27.5 (1.9) | 38.9 (1.2) |
| Birth weight (g), mean (SD) | 969 (226) | 3309 (490) |
| Postmenstrual age at MRI (weeks), mean (SD) | 40.5 (1.1) | 41.0 (1.2) |
| Weight at MRI (g), mean (SD) | 3015 (535) | 3483 (468) |
| Small for gestational age, n (%) | 18 (9) | 1 (2) |
| Male sex, n (%) | 102 (49) | 24 (56) |
| Multiple births, n (%) | 93 (45) | 2 (5) |
| IPPV, n (%) | 155 (75) | 1 (2) |
| Infection†, n (%) | 75 (36) | 1 (2) |
| Total WMI score, mean (SD) | 3.1 (2.1) | 1.2 (1.2) |
| Total DNGM injury score, mean (SD) | 0.9 (1.0) | 0.3 (0.6) |
| Cerebellar abnormality†, n (%) | 10 (5) | 3 (7) |
| IVH any grade, n (%) | 27 (13) | 0 (0) |
| Grade 1–2, n (%) | 19 (9) | 0 (0) |
| Grade 3–4, n (%) | 8 (4) | 0 (0) |
| Postnatal corticosteroids, n (%) | 18 (9) | 0 (0) |
| Morphine dose (mg/kg), mean (SD) | 0.19 (0.65) | 0 (0) |
| 7-Year data | ||
| Age at MRI (years), mean (SD) | 7.5 (0.3) | 7.6 (0.2) |
| Full-scale IQ, mean (SD) | 98 (13) | 109 (13) |
| Verbal IQ, mean (SD) | 99 (13) | 108 (14) |
| Performance IQ, mean (SD) | 98 (14) | 110 (16) |
| Language ability, mean (SD) | 94 (16) | 109 (11) |
| Receptive language, mean (SD) | 91 (15) | 103 (11) |
| Expressive language, mean (SD) | 97 (16) | 111 (12) |
| Attention, mean (SD) | 8 (4) | 8 (3) |
| Working memory, mean (SD) | 88 (15) | 101 (17) |
| Motor function, mean (SD) | 9 (3) | 11 (3) |
| Balance, mean (SD) | 10 (4) | 12 (3) |
| Manual dexterity, mean (SD) | 8 (3) | 9 (3) |
| Aiming and catching, mean (SD) | 11 (3) | 12 (3) |
DNGM – deep nuclear gray matter; GA – gestational age; IPPV – intermittent positive pressure ventilation; IQ – intelligence quotient; IVH – intraventricular hemorrhage; PMA – postmenstrual age; SD – standard deviation; VPT – very preterm; WMI – white matter injury.
Defined as either ≥1 episode of proven sepsis or necrotizing enterocolitis;
Mostly signal abnormality and small hemorrhages, all punctate unilateral except for 1 VPT infant with punctate bilateral lesions
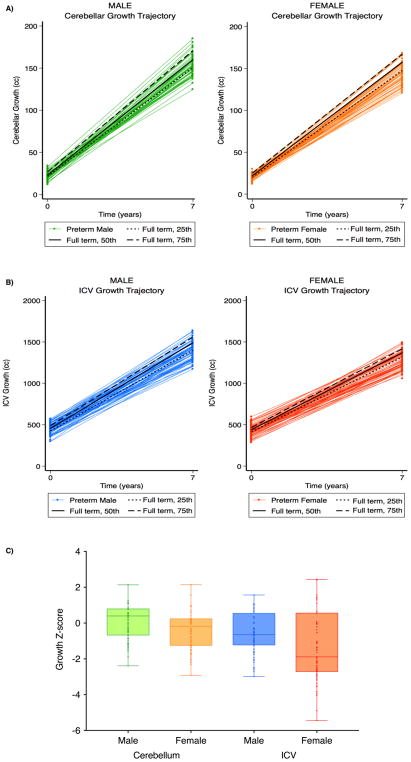
Linear mixed-effects models demonstrated differences in cerebellar volume between VPT and full-term infants at TEA and at 7 years, with the VPT group demonstrating smaller cerebellar volume at both time-points. These differences remained in models adjusting for ICV (Table 2). The effect of time (i.e., growth) also appeared to vary by group, with the VPT group showing slightly reduced growth than the full-term group, although this difference was attenuated in models adjusting for ICV. Fitting separate models for males and females demonstrated that while VPT males and females both had smaller cerebellums than full-term controls at TEA, this association was only observed for females at 7 years, with similar results in models adjusting for ICV. The effect of time also appeared to vary by group for females, but not males (Table 2, Fig. 2). Excluding children with cerebellar anomalies had little effect on any of these findings (Supplementary Table 1).
Table 2.
Comparison of Cerebellar Volume and Growth between VPT and full-term Infants
| Group | TEA Volume (cc) (VPT=201; Full term=41) | 7-Year Volume (cc) (VPT=114; Full term=29) | Growth (cc) (VPT=108; Full term=27) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VPT, mean (SD) | 21.22 (3.70) | 148.60 (13.61) | 127.00 (12.47) | ||||||||
| Full-term, mean (SD) | 23.35 (3.80) | 158.95 (13.64) | 134.82 (12.38) | ||||||||
|
| |||||||||||
| Linear Mixed Effects | |||||||||||
|
| |||||||||||
| N | Effect of Group at TEA | Effect of Group at 7 Years | Group x Time Interaction | ||||||||
|
| |||||||||||
| Model 1† | VPT | FT* | Coefficient (95% CI) | t | df | P-value | Coefficient (95% CI) | t | df | P-value | P-value |
| All | 207 | 43 | −1.98 (−3.18, −0.78) | −3.25 | 251 | 0.001 | −8.83 (−13.96, −3.70) | −3.39 | 251 | 0.001 | 0.008 |
| Males only | 102 | 24 | −1.93 (−3.70, −0.15) | −2.15 | 126 | 0.034 | −4.43 (−11.11, 2.25) | −1.31 | 126 | 0.191 | 0.446 |
| Females only | 105 | 19 | −2.04 (−3.66, −0.43) | −2.51 | 124 | 0.013 | −12.58 (−19.14, −6.03) | −3.8 | 124 | < 0.001 | 0.001 |
| Model 2‡ | VPT | FT* | Coefficient (95% CI) | t | df | P-value | Coefficient (95% CI) | t | df | P-value | P-value |
| All | 200 | 40 | −1.72 (−2.79, −0.66) | −3.19 | 237 | 0.002 | −5.11 (−9.46, −0.78) | −2.32 | 237 | 0.021 | 0.118 |
| Males only | 99 | 21 | −1.73 (−3.17, −0.29) | −2.37 | 118 | 0.019 | −0.65 (−6.14, 4.84) | −0.23 | 118 | 0.815 | 0.691 |
| Females only | 101 | 19 | −1.78 (−3.33, −0.23) | −2.27 | 118 | 0.025 | −9.41 (−15.55, −3.26) | −3.03 | 118 | 0.003 | 0.014 |
SD – standard deviation; TEA – term equivalent age; VPT – very preterm
Reference group;
Analysis adjusted for sex and postmenstrual age at MRI, sex analyses adjusted for postmenstrual age at MRI only;
Analysis adjusted for sex, postmenstrual age at MRI and intracranial volume, sex analyses adjusted for postmenstrual age at MRI and intracranial volume only
Figure 2. Sex differences in cerebellar and whole brain growth in very preterm infants compared with full-term controls.
(A, left panel) Male very preterm versus full-term cerebellar growth trajectories. Green spaghetti plots indicate individual male cerebellar growth, and black lines indicate 25th, 50th and 75th percentile growth trajectories for full-term males. (A, right panel) Female very preterm versus full-term cerebellar growth trajectories. Orange spaghetti plots indicate individual female cerebellar growth, and black lines indicate 25th, 50th and 75th percentile growth trajectories for full-term females. (B, left panel) Male very preterm versus full-term whole brain growth trajectories. Blue spaghetti plots indicate individual male whole brain growth, and black lines indicate 25th, 50th and 75th percentile growth trajectories for full-term males. (B, right panel) Female very preterm versus full-term whole brain growth trajectories. Red spaghetti plots indicate individual female whole brain growth, and black lines indicate 25th, 50th and 75th percentile growth trajectories for full-term females. (C) Sex differences in cerebellar and ICV growth Z-score shifts for very preterm infants relative to full-term peers. cc – cubic centimeters; ICV – intracranial volume.
Perinatal associations
Within the VPT group, cerebellar TEA volume was positively associated with birth weight SDS, and negatively associated with IPPV duration, infection, total DNGM score, postnatal corticosteroid exposure and morphine dose (Table 3). With the exception of birth GA and morphine dose, these associations remained statistically significant following correction for multiple comparisons. In models adjusting for ICV, only the association with postnatal corticosteroid exposure remained, although this weakened after correction for multiple comparisons [p = 0.009; FDR-corrected p (pFDR) = 0.09] (Fig. 3A, Supplementary Table 1).
Table 3.
Associations between cerebellar volumes, perinatal factors and neurodevelopmental outcomes in VPT children
| TEA Volume (cc) | 7-Year Volume (cc) | Growth Z-score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Perinatal Factor* | Coefficient (95% CI) | F | df | P-value | Coefficient (95% CI) | F | df | P-value | Coefficient (95% CI) | F | df | P-value |
| Sex | 0.59 (−0.30, 1.48) | 35.92 | 2,198 | 0.190 | 12.31 (7.77, 16.86) | 14.46 | 2,111 | < 0.001 | 0.99 (0.65, 1.33) | 16.98 | 2,105 | < 0.001 |
| Birth GA | 0.25 (0.01, 0.49) | 37.72 | 2,198 | 0.037 | 1.86 (0.54, 3.19) | 3.95 | 2,111 | 0.006 | 0.12 (0.02, 0.22) | 2.88 | 2,105 | 0.023 |
| Birth weight SDS | 0.59 (0.13, 1.05) | 39.13 | 2,198 | 0.012 | 2.62 (−0.43, 5.66) | 1.51 | 2,111 | 0.091 | 0.19 (−0.03, 0.41) | 1.62 | 2,105 | 0.096 |
| IPPV† | −0.53 (−0.85, −0.22) | 37.38 | 2,149 | 0.001 | −1.45 (−3.27, 0.37) | 1.33 | 2,84 | 0.118 | −0.08 (0.06, −0.22) | 0.67 | 2,81 | 0.278 |
| Infection | −1.26 (−2.17, −0.35) | 39.74 | 2,198 | 0.007 | −0.22 (−5.71, 5.28) | 0.06 | 2,111 | 0.937 | −0.02 (−0.43, 0.40) | 0.21 | 2,105 | 0.942 |
| Total WMI score | −0.17 (−0.38, 0.03) | 38.94 | 2,196 | 0.097 | −2.83 (−4.30, −1.36) | 7.31 | 2,111 | 0.006 | −0.19 (−0.30, −0.07) | 5.60 | 2,105 | 0.001 |
| Total DNGM score | −1.08 (−1.52, −0.65) | 53.78 | 2,196 | < 0.001 | −5.87 (−8.47, −3.28) | 10.11 | 2,111 | < 0.001 | −0.40 (−0.60, −0.20) | 7.80 | 2,105 | < 0.001 |
| IVH | −0.24 (−0.77, 0.30) | 35.23 | 2,194 | 0.386 | −1.42 (−4.68, 1.84) | 0.43 | 2,108 | 0.341 | −0.11 (−0.35, 0.13) | 0.72 | 2,102 | 0.376 |
| Postnatal corticosteroids | −3.22 (−4.73, −1.72) | 46.47 | 2,197 | < 0.001 | 0.61 (−11.76, 12.97) | 0.14 | 2,110 | 0.923 | 0.47 (−0.46, 1.41) | 0.67 | 2,104 | 0.316 |
| Morphine dose | −0.69 (−1.36, −0.02) | 37.56 | 2,198 | 0.043 | 1.60 (−4.35, 7.55) | 0.20 | 2,111 | 0.596 | 0.18 (−0.26, 0.61) | 0.52 | 2,105 | 0.429 |
cc – cubic centimeters; CI – confidence interval; df – degrees of freedom; DNGM – deep nuclear gray matter; GA – gestational age; IPPV – intermittent positive pressure ventilation; IVH – intraventricular hemorrhage; SDS – standard deviation score; TEA – term equivalent age; VPT – very preterm; WMI – white matter injury
Results of linear regression analyses for associations between perinatal factors and cerebellar volume/growth in VPT subjects, adjusted for postmenstrual age at MRI
Log-transformed
Bold p-values denote associations that are significant following FDR correction (pFDR < 0.05)
Figure 3. Perinatal associations of cerebellar volume and growth from TEA to 7 years in very preterm subjects.
The point estimates represent the change in cerebellar volume (cc) and cerebellar growth Z-score for every unit change in the independent variable. Results are presented based on individual linear regression models for each perinatal variable adjusted for postmenstrual age at MRI (black circles) and further for ICV (blue triangles). The error bars represent the 95% confidence intervals. Sex=male. cc – cubic centimeters; DNGM – deep nuclear gray matter; GA – gestational age; ICV – intracranial volume; IPPV – intermittent positive pressure ventilation; IVH – intraventricular hemorrhage; TEA – term-equivalent age; WMI – white matter injury.
At 7 years of age, smaller cerebellar volume was observed in females than males, in children born at earlier GA, and in children with higher WMI and DNGM scores (Table 3). These associations remained significant following correction for multiple comparisons. In models adjusting for ICV, only the associations with birth GA and total WMI score remained, even after correction for multiple comparisons (Fig. 3B, Supplementary Table 1).
Consistent with findings at 7 years, there was reduced cerebellar growth in females, in children born at earlier GA, and in children with higher total WMI and DNGM score (Table 3). With the exception of birth GA all associations remained significant following correction for multiple comparisons. In models adjusting for ICV, associations with sex, birth GA, total WMI score and DNGM score all remained, even after correction for multiple comparisons (Fig. 3C, Supplementary Table 1).
Neurodevelopmental outcomes
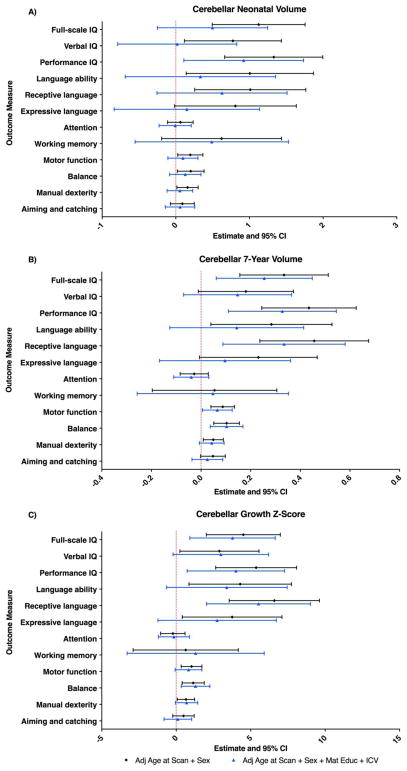
Analyses of cerebellar volumes at TEA demonstrated positive associations with several neurodevelopmental outcomes, including IQ, language, and motor functioning (Table 4). There were no observed associations with attention or working memory. Upon investigation of outcome subdomains, cerebellar volumes at TEA were positively associated with performance and verbal IQ, receptive language, balance and manual dexterity (Table 4). All associations remained significant following correction for multiple comparisons. In models adjusting for maternal education and ICV only the association with performance IQ remained, although this weakened after correction for multiple comparisons (p = 0.026; pFDR = 0.312) (Fig. 4A, Supplementary Table 2).
Table 4.
Associations between cerebellar volumes, perinatal factors and neurodevelopmental outcomes in VPT children
| TEA Volume (cc) | 7-Year Volume (cc) | Growth Z-score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Neurodevelopmental Outcome† | Coefficient (95% CI) | F | df | P-value | Coefficient (95% CI) | F | df | P-value | Coefficient (95% CI) | F | df | P-value |
| Full-scale IQ | 1.12 (0.49, 1.75) | 6.45 | 3,165 | 0.001 | 0.33 (0.16, 0.51) | 7.28 | 3,110 | < 0.001 | 4.51 (2.03, 6.99) | 8.38 | 3,104 | <0.001 |
| Performance IQ | 1.33 (0.66, 2.00) | 6.61 | 3,166 | < 0.001 | 0.43 (0.24, 0.63) | 7.10 | 4,109 | 0.005 | 5.37 (2.65, 8.08) | 7.75 | 3,104 | <0.001 |
| Verbal IQ | 0.77 (0.12, 1.43) | 4.34 | 3,167 | 0.021 | 0.18 (−0.01, 0.37) | 3.33 | 3,110 | 0.064 | 2.91 (0.25, 5.56) | 4.90 | 3,104 | 0.033 |
| Language ability | 1.01 (0.14, 1.87) | 3.05 | 3,159 | 0.023 | 0.28 (0.04, 0.53) | 3.59 | 3,107 | 0.023 | 4.30 (0.85, 7.74) | 3.31 | 3,101 | 0.015 |
| Receptive language | 1.01 (0.26, 1.77) | 3.59 | 3,158 | 0.009 | 0.46 (0.24, 0.68) | 6.52 | 3,107 | < 0.001 | 6.59 (3.56, 9.62) | 7.00 | 3,101 | <0.001 |
| Expressive language | 0.81 (−0.02, 1.64) | 2.69 | 3,159 | 0.055 | 0.23 (−0.01, 0.47) | 3.38 | 3,106 | 0.056 | 3.76 (0.41, 7.10) | 2.90 | 3,100 | 0.028 |
| Attention | 0.06 (−0.11, 0.24) | 0.86 | 3,158 | 0.483 | −0.03 (−0.08, 0.03) | 0.80 | 3,106 | 0.337 | −0.23 (−1.04, 0.59) | 0.61 | 3,100 | 0.587 |
| Working memory | 0.62 (−0.19, 1.43) | 2.32 | 3,153 | 0.134 | 0.05 (−0.20, 0.31) | 0.47 | 3,105 | 0.669 | 0.64 (−2.89, 4.17) | 1.43 | 3,99 | 0.720 |
| Motor function | 0.20 (0.03, 0.37) | 2.84 | 3,146 | 0.024 | 0.09 (0.04, 014) | 5.11 | 3,101 | < 0.001 | 1.03 (0.33, 1.73) | 3.96 | 3,95 | 0.004 |
| Balance | 0.20 (0.02, 0.38) | 3.09 | 3,147 | 0.029 | 0.10 (0.05, 0.15) | 6.75 | 3,102 | < 0.001 | 1.14 (0.40, 1.88) | 5.35 | 3,96 | 0.003 |
| Manual dexterity | 0.16 (0.01, 0.30) | 3.02 | 3,151 | 0.031 | 0.05 (0.01, 0.09) | 3.94 | 3,104 | 0.016 | 0.65 (0.07, 1.24) | 3.31 | 3,98 | 0.028 |
| Aiming and catching | 0.09 (−0.07, 0.25) | 1.61 | 3,149 | 0.276 | 0.05 (−0.002, 0.10) | 2.13 | 3,102 | 0.059 | 0.49 (−0.23, 1.21) | 2.56 | 3,96 | 0.179 |
cc – cubic centimeters; CI – confidence interval; df – degrees of freedom; IQ – intelligence quotient; TEA – term equivalent age; VPT – very preterm
Results of linear regression analyses for associations between cerebellar volume/growth and 7-year neurodevelopmental outcomes in VPT subjects, adjusted for sex and postmenstrual age at MRI
Bold p-values denote associations that are significant following FDR correction (pFDR < 0.05)
Figure 4. Associations of cerebellar volume and growth from TEA to 7 years with 7-year neurodevelopmental outcomes in very preterm subjects.
The point estimates represent the change in outcome score for every unit increase in cerebellar volume (cc) and growth Z-score. Results are presented based on individual linear regression models for each outcome measure adjusted for postmenstrual age at MRI and sex (black circles), and further for maternal education and ICV (blue triangles). The error bars represent the 95% confidence intervals. cc – cubic centimeters; ICV – intracranial volume; TEA – term-equivalent age.
Cerebellar volumes at 7 years were positively associated with IQ, language and motor functioning (Table 4). In models adjusting for maternal education and ICV, these associations largely were largely comparable, except for overall language ability (Fig. 4B, Supplementary Table 2). In both models, investigation of outcome subdomains demonstrated the strongest associations for performance IQ, receptive language, and motor balance, all of which remained significant following correction for multiple comparisons (Fig. 4B, Supplementary Table 2).
Examination of cerebellar growth demonstrated reduced growth was also associated with poorer IQ, language and motor function, all of which remained significant following correction for multiple comparisons (Table 4). The strongest outcome subdomain associations were observed for receptive language, and motor balance, even after adjusting for ICV and maternal education, and after controlling for multiple comparisons (Fig. 4C, Supplementary Table 2).
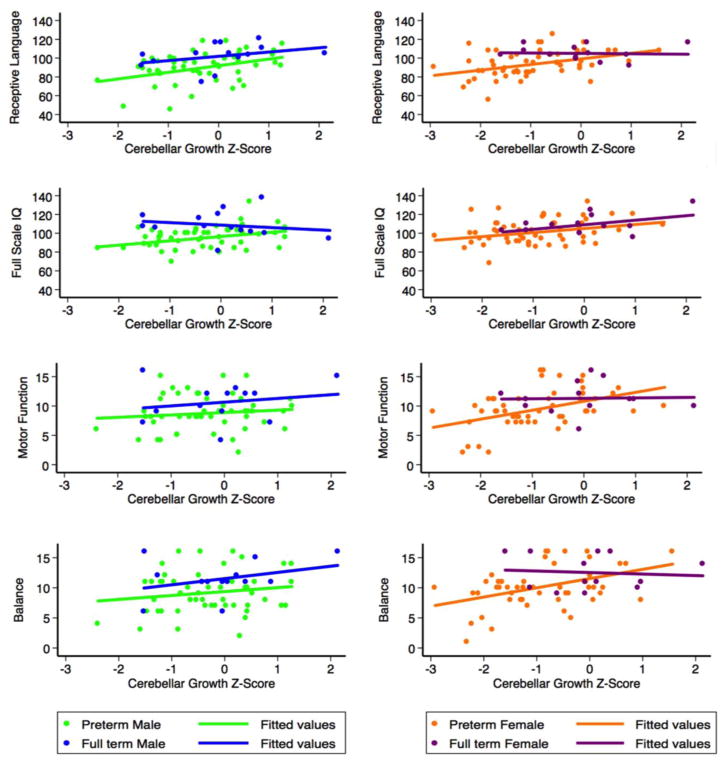
Exploring relationships between cerebellar growth and IQ, language and motor function outcomes separately for males and females demonstrated somewhat differential patterns of maturational vulnerability (Fig. 5). For female VPT children, greater growth was associated with better receptive language [coefficient (95% CI) 5.97 (2.12, 9.83), F(2,51) = 5.02, p = 0.003, pFDR = 0.01] and IQ [coefficient (95% CI) 4.34 (0.69, 7.99), F(2,52) = 4.05, p = 0.021, pFDR = 0.031], particularly verbal IQ [4.64 (0.88, 8.39), F(2,52) = 4.67, p = 0.016, pFDR = 0.031]. Greater cerebellar growth in female VPT children was also associated with better overall motor function [1.52 (0.61, 2.43), F(2,47) = 5.62, p = 0.002, pFDR = 0.01]. This association was observed within all three subdomains, including balance [1.54 (0.55, 2.52), F(2,47) = 4.93, p = 0.003, pFDR = 0.01], aiming and catching [1.28 (0.38, 2.19), F(2,47) = 4.10, p = 0.006, pFDR = 0.015], and manual dexterity [0.91 (0.14, 1.68), F(2,47) = 3.16, p = 0.022, pFDR = 0.031]. These associations largely remained in models adjusting for ICV and maternal education, although only the association with receptive language remained significant following correction for multiple comparisons [receptive language (F(4,38) = 6.75, p = 0.019, pFDR = 0.0143); verbal IQ (F(4,39) = 3.73, p = 0.043, pFDR = 0.108); overall motor function (F(4,37) = 3.43; p = 0.022, pFDR = 0.073); balance (F(4,37) = 3.20, p = 0.007, pFDR = 0.07)].
Figure 5. Sex differences in the relationship between cerebellar growth and outcome associations at age 7.
Very preterm males and females are represented by green and orange circles, respectively. Full-term male and female relationships are also shown for comparison, and are represented by blue and purple circles, respectively. Mean fit lines are represented by green and orange lines for very preterm male and female infants, respectively, and by blue and purple lines for full-term male and female infants, respectively.
For VPT males, greater cerebellar growth was associated with receptive language [6.99 (2.20, 11.79), F(2,48) = 4.80, p = 0.005, pFDR = 0.025] and IQ [4.56 (1.19, 7.94), F(2,50) = 4.58, p = 0.009, pFDR = 0.03], particularly performance IQ [7.22 (2.20, 11.13), F(2,50) = 8.98, p = 0.001, pFDR = 0.01], but there was little association with overall motor function (F(2,46) = 2.81, p = 0.458, pFDR = 0.509) or motor subdomains. Only the associations with IQ remained significant in models adjusting for ICV and maternal education, although this weakened after correction for multiple comparisons [IQ (F(4,41) = 5.38, p = 0.012, pFDR = 0.1); performance IQ (F(4,41) = 4.45, p = 0.020, pFDR = 0.1)].
Discussion
This study represents the first account of cerebellar growth and long-term follow-up from infancy to 7 years in VPT children. Importantly, we demonstrate the lasting impact of early cerebellar vulnerability in children born VPT, alongside perinatal associations and 7-year neurodevelopmental correlates of cerebellar development. We report several robust associations between cerebellar growth, perinatal factors and neurodevelopmental outcomes that remained significant following correction for multiple comparisons. In particular, female sex, earlier GA, WMI and DNGM injury were significantly associated with reduced cerebellar growth in VPT children from term equivalent to age 7 years, including in models adjusting for ICV. A similar pattern of perinatal factor associations was observed for 7-year cerebellar volume. We also identified some evidence suggesting an association between postnatal corticosteroid exposure and smaller TEA cerebellar volume, although this weakened following FDR-correction in models adjusting for ICV. Our findings relating to neurodevelopmental outcomes demonstrated robust associations with IQ, receptive language and balance. While these relationships were observed for TEA cerebellar volume, they were particularly robust for 7-year cerebellar volume and growth. Results from the sex analyses further demonstrated differential relationships between cerebellar growth and neurodevelopmental outcomes in male and female VPT children. Our study findings should be interpreted as exploratory, warranting further and confirmatory analysis.
Preterm Cerebellar Development from Infancy to 7 Years
Our findings of smaller cerebellar volume in VPT compared with full-term infants are consistent with previous reports in infancy (8–16) and later development (3–6). We demonstrate novel evidence for reduced cerebellar growth in VPT compared with full-term children from TEA to 7 years, corroborating earlier reports of cerebellar maturation (17). For VPT infants, critical phases of cerebellar growth take place in the neonatal intensive care unit, where very early exposure to life ex-utero can disrupt the highly-regulated processes that govern cerebellar development (35), likely leading to the volumetric and growth impairments observed in this study. Postulated mechanisms for these alterations in VPT children include decreased expansion of cerebellar granule cells, possibly as a consequence of reduced sonic hedgehog (SHH) expression (36), in addition to secondary effects from supratentorial brain injury with secondary Wallerian degeneration and mechanisms associated with perinatal risk factors unrelated to obvious destructive parenchymal disease (2). Our findings further indicate differential cerebellar maturational trajectories in VPT females and males relative to full-term peers, with reduced growth particularly evident in VPT females by 7 years; in part not surprising given larger cerebellums are typically observed in males (21).
Direct mechanisms for cerebellar underdevelopment are also implicated. These commonly involve a prominent hemorrhagic component, resulting in cerebellar atrophy and subsequent growth failure (37). A role for germinal matrix bleeding within the subpial external granular cell layer has been proposed (2); with cerebellar hemorrhage frequently occurring, and sharing similar etiologic factors, with IVH (38). Of note, overlapping incidences of cerebellar hemorrhage and high grade IVH were observed in our cohort. Importantly, cerebellar hemorrhage has been previously shown to be associated with abnormal neurological outcomes in preschool children born preterm (39). However, we were unable to assess these relationships due to the relatively low rate of cerebellar hemorrhage in our cohort, possibly due to missed incidences of small cerebellar hemorrhages below the spatial resolution of our T2 sequence, or hemorrhages that may have resolved without sequela by the time of the MRI scan. Nonetheless, to confirm that our observations of reduced cerebellar volume in VPT subjects were unrelated to direct cerebellar injury, we performed a sensitivity analysis excluding subjects with cerebellar abnormality, which did not change our main conclusions.
Perinatal Associations
Although there appeared to be some overlap in perinatal factor associations with cerebellar volume at TEA and 7 years, we identified factors that were associated with cerebellar volume and growth between TEA and 7 years, primarily: postnatal corticosteroids for TEA volume; and female sex, earlier GA at birth, WMI and DNGM injury for 7-year volume and cerebellar growth.
Our observed association between postnatal corticosteroid exposure and TEA cerebellar volume are in line with reports documenting the immature cerebellum’s vulnerability to postnatal glucocorticoids (40). While not surprising given the high concentration of glucocorticoid receptors in the cerebellum (41), this association weakened following correction for multiple comparisons in models adjusted for ICV, and further did not persist into early childhood, suggesting that postnatal corticosteroid exposure may not explain the observed alterations in cerebellar developmental trajectories. Increasing morphine dose, prolonged IPPV, infection and birth weight SDS were associated with smaller neonatal cerebellar volume. However, with the exception of morphine dose, these relationships weakened following adjustment for ICV, and none were evident at age 7 years. Morphine has been previously shown to be associated with reduced neonatal cerebellar volume and adverse neurodevelopmental outcomes (42), although median cumulative doses were much higher in that study than in our cohort, indicating a possible dose-response effect.
The observed relationship between GA at birth and cerebellar volume at age 7 years and cerebellar growth suggests greater deviations from normal maturational trajectories in infants born at earlier gestational ages. Interestingly, this appeared to be the case for female VPT children in particular. Sex has been postulated to influence the mechanisms by which the developing brain is affected, with males appearing to be particularly vulnerable to the deleterious effects of VPT birth (43). It may be that our findings reflect cerebellar catch-up growth as a potential over-compensatory, albeit aberrant, response to VPT birth. This growth ‘overdrive’ may in turn contribute to the constellation of adverse outcomes more commonly observed in male VPT survivors.
Neonatal WMI was associated with 7-year cerebellar volume, consistent with previous work highlighting the potentially deleterious impact of early supratentorial injury on later cerebellar development (9–11, 44). WMI was further related to reduced cerebellar growth, albeit not independently. These associations have been postulated to be due to disrupted trophic interactions via reciprocal cerebro-cerebellar and cerebello-cerebral white matter pathways (9, 45). Based on this hypothesis, injury to the cerebrum during a developmentally vulnerable period may lead to inadequate wiring and poor trophic interaction with the cerebellum, and ultimately cerebellar underdevelopment. Similarly, primary cerebellar abnormality would lead to altered cerebral development. Although WMI was related to cerebellar development, we did not find an independent effect of IVH on cerebellar volumes or growth. This differs from other reports (13) and may relate in part to the relative infrequency of high grade IVH in our cohort, and/or the large association of immaturity with IVH.
The observed association with DNGM injury is also not surprising given the cerebellum is connected via extensive mono- and polysynaptic pathways to key subcortical centres including the hippocampus, amygdala, hypothalamus, basal ganglia, thalamus and brainstem (46, 47). In particular there is a growing body of evidence implicating the existence of functionally integrated networks linking the cerebellum, basal ganglia and thalamic nuclei (48, 49). These pathways likely contribute to cerebellar growth and development and may provide some of the neurobiological underpinnings for the cognitive deficits observed following cerebellar dysfunction.
Neurodevelopmental outcomes
We found positive associations between cerebellar development and both motor and cognitive outcomes, consistent with the growing body of evidence highlighting the cerebellum’s role beyond motor control (50). Notably, 7-year volumes and growth were more commonly associated with neurodevelopmental outcomes than TEA volumes. In particular, cerebellar volume at 7-years and cerebellar growth were both highly associated with IQ, language and motor balance, even after controlling for ICV and maternal education; with the most robust findings relating to IQ, receptive language and balance. In contrast, covariate adjustment weakened most relationships between TEA volumes and outcomes. We have previously shown that cerebral tissue volumes at TEA were more strongly associated with language and motor outcome than 7-year volumes or growth during this period (51). These contrasting findings highlight the cerebellum’s protracted developmental vulnerability and allude to the emergence of clinical consequences that may be unique to cerebellar maturational disruption beyond the neonatal period. We must also consider the implications of controlling for ICV and the associated difficulties in interpreting our findings. While this approach is commonly used in region-of-interest volumetric analyses to minimize confounding (52), it is important to consider whether ICV or cerebellar volume is the more likely predictor of neurodevelopmental outcomes, and further how they may relate to each other along a common causal pathway given the evidence for crossed trophic interactions between the cerebellum and cerebrum (9, 16). Results from our secondary analyses adjusted for ICV support the concept of selective cerebellar vulnerability being associated with functional outcomes in VPT children. However, it is plausible that cerebellar vulnerability contributes to ICV changes in VPT children via impaired cerebello-cerebral pathways. Furthermore, it has been argued that removing all variance associated with head size may remove important volumetric differences related to protective or compensatory mechanisms (53). In the current study, we therefore report findings from both an ICV-adjusted and unadjusted analysis, with the aim of providing answers to different, complementary questions (54).
Recent work by Lee and colleagues failed to find similar cerebellar volume associations with 4-year outcomes (17), suggesting the consequences of early cerebellar deficits may not be evident until later in childhood. While some aspects of development and performance at age 4 years should be reasonably aligned with performance at age 7, such as for example motor performance and related developmental coordination disorders (55), differences within IQ, language complexity and attention are more likely to be detected later in childhood, coinciding with the expanded and increasing academic and cognitive demands associated with school age. Importantly, assessment of these aspects of development is limited in early childhood. Indeed, Lee et al. report cerebellar development associations with a limited set of neurodevelopmental outcomes at age 4, restricted to measures of full-scale IQ assessed using the Wechsler Abbreviated Scale of Intelligence, and visual motor integration, visual perception, and motor coordination assessed using the Beery-Buktenica Test of Visual Motor Integration. While it is possible that the differences between our findings, particularly for IQ and motor coordination, are due to Lee et al.’s developmental assessments in early childhood lacking the sensitivity to detect impairments (56), they may also be due to the relatively small sample size of their study (only 41 VPT children with MRI data at the 4-year follow-up compared with 114 in our study). Other existing studies have largely reported cross-sectional findings of cerebellar volumetric deficits in infancy and childhood, primarily in association with WMI, IVH and/or cerebellar injury (10, 39, 57, 58). Our findings thus provide the first account of longitudinal cerebellar growth associations with neurodevelopmental impairment that appear to be independent of cerebral growth. We postulate that early cerebellar disruption in the VPT infant sets in motion an altered growth trajectory that is associated with adverse neurodevelopmental consequences emerging later in childhood. We have previously demonstrated using diffusion tensor tractography a potential role for alterations at the microstructural level, in mediating this relationship (45).
This study highlights an important role for cerebellar maturation in shaping the course of early motor and cognitive development in VPT infants, particularly for balance coordination, general intelligence and receptive language. Our findings for the former are in line with the cerebellum’s well-established role in balance and postural control. Indeed, patients with cerebellar injury commonly present with balance and gait disturbances (59). Interestingly, however, in a previous study of adolescents (14–15 years) born VPT (3), no association was demonstrated between cerebellar volume and any motor neurological signs, which was postulated by the authors to reflect developmental plasticity of the cerebellum and its cortico-cerebellar circuits and functional compensation over time (3, 60). It is possible that our observed relationships with motor function reflect an earlier developmental window before such functional compensation has taken place. Our findings for IQ are consistent with previous work suggesting a relationship between smaller cerebellar size and lower general intelligence in adolescents born VPT (3, 6). In line with evolutionary theory, human intelligence and cognitive ability have been postulated to be attributed, at least in part, to the phylogenetic increase in cerebellar size, particularly the striking lateral expansion of the cerebellar hemispheres in hominoids (61, 62).
Similarly, the cerebellum’s evolutionary expansion has been proposed to be a preadaptation for language ability (61). Indeed, the cerebellum’s role in language has been extensively studied in healthy subjects and in lesion studies (see (63) for a review). Furthermore, various aspects of language impairment are included in the cerebellar cognitive affective syndrome (CCAS), a characteristic pattern of cognitive deficits observed following cerebellar injury (60, 64). However, less is known about these relationships in preterm cohorts. Two studies in preterm children with cerebellar hemorrhage have reported language deficits in association with vermis injury (57), and with hemispheric injury and impaired contralateral cerebral growth (58). Similar relationships were also observed with vermis injury in term-born infants (4). While these studies reported receptive and expressive language deficits, associations were only evident for receptive language in our cohort; potentially due to volumetric reductions in our cohort that are independent of cerebellar injury. Indeed, different mechanisms have been proposed to underlie indirect and direct cerebellar abnormality in the preterm infant (2).
Exploration of sex differences in outcome associations with cerebellar growth demonstrated different patterns of vulnerability for males and females. Male sex is a well-established risk factor for poor neurodevelopmental outcome in preterm infants (65) and in line with this, VPT females had consistently higher average scores than VPT males across all outcomes. However, full-term females also consistently scored better than full-term males. In VPT females, reduced cerebellar growth was associated with impairments in receptive language, IQ, particularly verbal IQ, and general motor ability; while for VPT males, unexpectedly, reduced cerebellar growth was only associated with impairments in receptive language and IQ, particularly performance IQ. Of note, the most robust association between cerebellar growth and outcome in our sex analysis was observed for receptive language in VPT female children. We postulate that these sex-specific vulnerabilities may be a consequence of sex differences in divergence from expected cerebellar developmental trajectories. Further studies may better establish the nature and implications of sexual dimorphism in cerebellar development.
Limitations
Despite our study strengths, our findings need to be considered with respect to some limitations, including those inherent to longitudinal studies. For example, manual versus automated cerebellar segmentation approaches were used for TEA and 7-year time-points, respectively, as these approaches are optimized to provide the most accurate volumetric analysis for each time-point. While we attempted to minimize potential sources of bias by manual inspection and editing of automated volumes, we accept that there may be systematic differences resulting from the two approaches. Due to inevitable MRI advances over the course of longitudinal studies, different acquisition software and hardware were also used at TEA and 7 years. Additionally, neonatal scans were acquired using varying slice thickness, with the larger thicknesses potentially resulting in partial volume errors, particularly at boundaries with similar signal intensity such as at the borders of cerebellum and cerebrum. Furthermore, given the highly foliated nature of the cerebellum, larger slice thicknesses may limit detection of subtle structural alterations within very thin gyri and sulci, although this is a limitation inherent to currently available cerebellar volumetric approaches and present even in adult studies (66). The contribution of such partial volume effects to our findings thus cannot be excluded, however their potential overall influence on the observed between-group differences is likely minimized by the relatively large size of the cerebellum. While clinical characteristics largely did not differ between participants with useable MRI data and those without, the possibility of selection bias or confounding due to participant dropout also cannot be excluded. Although we analyzed cerebellar growth and its associations with neurodevelopmental outcomes separately in males and females, we were underpowered to detect interactions. Future work focusing on delineating these complex interactions is nonetheless warranted, given increasing evidence of sex differences in cerebellar structure and function (21, 67, 68). Finally, we acknowledge the need for caution in interpreting our findings pertaining to associations with total brain injury scores, as such composite measures may not capture differences in the relative importance of individual injury scores.
Conclusion
Our finding that smaller cerebellar volume at TEA persists to 7 years highlights the cerebellum’s vulnerability to long-term maturational disruption following VPT birth, with deleterious functional consequences. Our findings further suggest that specific perinatal factors may confer time-dependent cerebellar vulnerability. The differing associations observed between cerebellar volume and postnatal corticosteroid exposure, sex, GA, WMI and DNGM injury from TEA to 7 years, highlight the importance of longitudinal investigation of preterm infants for delineating clinically important relationships that may be unapparent early in development. Similarly, these investigations have the potential to elucidate the long-term significance of associations only present early in life. Importantly, our findings that reduced cerebellar volumes and growth were associated with poorer 7-year IQ and language, in addition to motor performance, add to the growing body of literature shifting attention to the cerebellum as a new frontier for interrogating the neurobiological underpinnings of cognitive impairment in preterm infants.
Supplementary Material
Acknowledgments
The authors gratefully thank Merilyn Bear for recruitment, Michael Kean and the radiographers at Melbourne Children’s MRI Centre, the VIBeS and Developmental Imaging groups at the Murdoch Children’s Research Institute for their ideas and support, as well as the families and children who participated in this study. We also gratefully acknowledge Sara Cherkerzian for statistical input, and the contributions of Richard Beare, Divyen Shah, and Zohra Ahmadzai who generated the infant and 7-year cerebellar volumes.
Funding: This work was supported by Australia’s National Health and Medical Research Council (Centre for Clinical Research Excellence 546519 to LD, PA and TI; Centre for Research Excellence 1060733 to LD, PA and DT; Project grants 237117 to TI and LD, 491209 to PA, TI, LD, DT; Senior Research Fellowship 1081288 to PA; Career Development Fellowship 1085754 to DT; Early Career Fellowship 1012236 to DT; Career Development Fellowship 1127984 to KJL), National Institutes of Health (HD058056), United Cerebral Palsy Foundation (USA), Leila Y. Mathers Charitable Foundation (USA), the Brown Foundation (USA), and the Victorian Government’s Operational Infrastructure Support Program. This work was further conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Conflict of Interest Notification
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All phases of the study were approved by the Human Research Ethics Committees at The Royal Women’s Hospital and The Royal Children’s Hospital, Melbourne, Australia.
Informed Consent: Parental written informed consent was obtained for all individual participants included in the study.
References
- 1.Limperopoulos C. The vulnerable immature cerebellum. Seminars in Fetal and Neonatal Medicine. 2016;21(5):293–4. doi: 10.1016/j.siny.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Cerebellum of the Premature Infant: Rapidly Developing, Vulnerable, Clinically Important. Journal of Child Neurology. 2009;24(9):1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MHS, Stewart AL, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very prematurely. Brain. 2001;124(1):60–6. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 4.Limperopoulos C, Robertson RL, Sullivan NR, Bassan H, du Plessis AJ. Cerebellar injury in term infants: clinical characteristics, magnetic resonance imaging findings, and outcome. Pediatr Neurol. 2009;41(1):1–8. doi: 10.1016/j.pediatrneurol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Messerschmidt A, Fuiko R, Prayer D, Brugger P, Boltshauser E, Zoder G, et al. Disrupted cerebellar development in preterm infants is associated with impaired neurodevelopmental outcome. European Journal of Pediatrics. 2008;167(10):1141–7. doi: 10.1007/s00431-007-0647-0. [DOI] [PubMed] [Google Scholar]
- 6.Parker J, Mitchell A, Kalpakidou A, Walshe M, Jung HY, Nosarti C, et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain. 2008;131(Pt 5):1344–51. doi: 10.1093/brain/awn062. [DOI] [PubMed] [Google Scholar]
- 7.Spittle AJ, Doyle LW, Anderson PJ, Inder TE, Lee KJ, Boyd RN, et al. Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum Dev. 2010;86(1):1–5. doi: 10.1016/j.earlhumdev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late Gestation Cerebellar Growth Is Rapid and Impeded by Premature Birth. Pediatrics. 2005;115(3):688–95. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 9.Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired Trophic Interactions Between the Cerebellum and the Cerebrum Among Preterm Infants. Pediatrics. 2005;116(4):844–50. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 10.Shah D, Anderson P, Carlin J, Pavlovic M, Howard K, Thompson D, et al. Reduction in Cerebellar Volumes in Preterm Infants: Relationship to White Matter Injury and Neurodevelopment at Two Years of Age. Pediatric Research. 2006;60(1):97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan L, Allsop J, Counsell SJ, Boardman JP, Edwards AD, Rutherford M. Smaller Cerebellar Volumes in Very Preterm Infants at Term-Equivalent Age are Associated with the Presence of Supratentorial Lesions. AJNR Am J Neuroradiol. 2006;27(3):573–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Tam E, Ferriero D, Xu D, Berman J, Vigneron D, Barkovich A, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009;66(1):102–6. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam E, Miller S, Studholme C, Chau V, Glidden D, Poskitt K, et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158(3):366–71. doi: 10.1016/j.jpeds.2010.09.005. Epub 2010 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Soelen ILC, Brouwer RM, Peper JS, van Beijsterveldt TCEM, van Leeuwen M, de Vries LS, et al. Effects of Gestational Age and Birth Weight on Brain Volumes in Healthy 9 Year-Old Children. The Journal of Pediatrics. 2010;156(6):896–901. doi: 10.1016/j.jpeds.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Bolduc M, Du Plessis AJ, Evans A, Guizard N, Zhang XUN, Robertson RL, et al. Cerebellar malformations alter regional cerebral development. Developmental Medicine & Child Neurology. 2011;53(12):1128–34. doi: 10.1111/j.1469-8749.2011.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. 2010;68(2):145–50. doi: 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- 17.Lee W, Al-Dossary H, Raybaud C, Young JM, Morgan BR, Whyte HE, et al. Longitudinal cerebellar growth following very preterm birth. Journal of magnetic resonance imaging: JMRI. 2015;43(6):1462–73. doi: 10.1002/jmri.25098. [DOI] [PubMed] [Google Scholar]
- 18.Cheong JLY, Thompson DK, Spittle AJ, Potter CR, Walsh JM, Burnett AC, et al. Brain volumes at term-equivalent age are associated with two-year neurodevelopment in moderate and late preterm children. The Journal of Pediatrics. 2016;174:91–7. doi: 10.1016/j.jpeds.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Spittle AJ, Spencer-Smith MM, Eeles AL, Lee KJ, Lorefice LE, Anderson PJ, et al. Does the Bayley-III Motor Scale at 2 years predict motor outcome at 4 years in very preterm children? Developmental Medicine & Child Neurology. 2013;55(5):448–52. doi: 10.1111/dmcn.12049. [DOI] [PubMed] [Google Scholar]
- 20.Luttikhuizen dos Santos ES, de Kieviet JF, Königs M, van Elburg RM, Oosterlaan J. Predictive value of the Bayley Scales of Infant Development on development of very preterm/very low birth weight children: A meta-analysis. Early Human Development. 2013;89(7):487–96. doi: 10.1016/j.earlhumdev.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22(6):1161–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145(2):242–9. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Anderson PJ, Treyvaud K, Neil JJ, Cheong JLY, Hunt RW, Thompson DK, et al. Associations of Newborn Brain Magnetic Resonance Imaging with Long-Term Neurodevelopmental Impairments in Very Preterm Children. J Pediatr. 2017;87:58–65 e1. doi: 10.1016/j.jpeds.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34(11):2208–14. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company; New York, NY: 1999. [Google Scholar]
- 27.Semel EM, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals (4th-Australian Standardised Edition) Marrickville, New South Wales, Australia: Harcourt Assessment; 2006. [Google Scholar]
- 28.Manly T, Robertson I, Anderson V, Nimmo-Smith I. The Test of Everyday Attention for Children. Suffolk, UK: Thames Valley Test Co; 1999. [Google Scholar]
- 29.Pickering S, Gathercole S. Working Memory Test Battery for Children - Manual. London: The Psychological Corporation; 2001. [Google Scholar]
- 30.Henderson SE, Sugden DA, Barnett AL. Movement Assessment Battery for Children-2 second edition (Movement ABC-2) London, UK: The Psychological Corporation; 2007. [Google Scholar]
- 31.Wilson-Ching M, Pascoe L, Doyle LW, Anderson PJ. Effects of correcting for prematurity on cognitive test scores in childhood. J Paediatr Child Health. 2014;50(3):182–8. doi: 10.1111/jpc.12475. [DOI] [PubMed] [Google Scholar]
- 32.Wong HS, Edwards P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Maternal and child health journal. 2013;17(9):1689–700. doi: 10.1007/s10995-012-1183-8. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 34.Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 35.Limperopoulos C. Cerebellar Injury in the Preterm Infant. In: Boltshauser E, Schmahmann J, editors. Cerebellar Disorders in Children Clinics in Developmental Medicine No 191–192. 1. London: Mac Keith Press; 2012. [Google Scholar]
- 36.Haldipur P, Bharti U, Alberti C, Sarkar C, Gulati G, Iyengar S, et al. Preterm Delivery Disrupts the Developmental Program of the Cerebellum. PLoS ONE. 2011;6(8):e23449. doi: 10.1371/journal.pone.0023449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boltshauser E, Schmahmann J, editors. Cerebellar Disorders in Children. 1. London: Mac Keith Press; 2012. [Google Scholar]
- 38.Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116(3):717–24. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 39.Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158(2):245–50. doi: 10.1016/j.jpeds.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam EWY, Chau V, Ferriero DM, Barkovich AJ, Poskitt KJ, Studholme C, et al. Preterm Cerebellar Growth Impairment After Postnatal Exposure to Glucocorticoids. Science Translational Medicine. 2011;3(105):105ra. doi: 10.1126/scitranslmed.3002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavlik A, Buresova M. The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain research. 1984;314(1):13–20. doi: 10.1016/0165-3806(84)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller Cerebellar Growth and Poorer Neurodevelopmental Outcomes in Very Preterm Infants Exposed to Neonatal Morphine. J Pediatr. 2016;172:81–7 e2. doi: 10.1016/j.jpeds.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. The Journal of Pediatrics. 2004;145(2):242–9. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Messerschmidt A, Brugger PC, Boltshauser E, Zoder G, Sterniste W, Birnbacher R, et al. Disruption of Cerebellar Development: Potential Complication of Extreme Prematurity. AJNR Am J Neuroradiol. 2005;26(7):1659–67. [PMC free article] [PubMed] [Google Scholar]
- 45.Shany E, Inder TE, Goshen S, Lee I, Neil JJ, Smyser CD, et al. Diffusion Tensor Tractography of the Cerebellar Peduncles in Prematurely Born 7-Year-Old Children. Cerebellum. 2016;16(2):314–25. doi: 10.1007/s12311-016-0796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106(5):2322–45. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Frontiers in Systems Neuroscience. 2013:7. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proceedings of the National Academy of Sciences. 2010;107(18):8452–6. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milardi D, Arrigo A, Anastasi G, Cacciola A, Marino S, Mormina E, et al. Extensive Direct Subcortical Cerebellum-Basal Ganglia Connections in Human Brain as Revealed by Constrained Spherical Deconvolution Tractography. Frontiers in neuroanatomy. 2016;10:29. doi: 10.3389/fnana.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13(1):151–77. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monson BB, Anderson PJ, Matthews LG, et al. Examination of the pattern of growth of cerebral tissue volumes from hospital discharge to early childhood in very preterm infants. JAMA Pediatrics. 2016;170(8):772–9. doi: 10.1001/jamapediatrics.2016.0781. [DOI] [PubMed] [Google Scholar]
- 52.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Voevodskaya O, Simmons A, Nordenskjöld R, Kullberg J, Ahlström H, Lind L, et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Frontiers in Aging Neuroscience. 2014;6:264. doi: 10.3389/fnagi.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ. Statistical adjustments for brain size in volumetric neuroimaging studies: Some practical implications in methods. Psychiatry research. 2011;193(2):113–22. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffiths A, Morgan P, Anderson PJ, Doyle LW, Lee KJ, Spittle AJ. Predictive value of the Movement Assessment Battery for Children - Second Edition at 4 years, for motor impairment at 8 years in children born preterm. Dev Med Child Neurol. 2017;59(5):490–6. doi: 10.1111/dmcn.13367. [DOI] [PubMed] [Google Scholar]
- 56.Aylward GP. The conundrum of prediction. Pediatrics. 2005;116(2):491–2. doi: 10.1542/peds.2005-1061. [DOI] [PubMed] [Google Scholar]
- 57.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, et al. Does Cerebellar Injury in Premature Infants Contribute to the High Prevalence of Long-term Cognitive, Learning, and Behavioral Disability in Survivors? Pediatrics. 2007;120(3):584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 58.Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the Premature Cerebellum: Outcome is Related to Remote Cortical Development. Cerebral Cortex. 2014;24(3):728–36. doi: 10.1093/cercor/bhs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2004;10(3):247–59. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- 60.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 61.Barton Robert A, Venditti C. Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Current Biology. 2014;24(20):2440–4. doi: 10.1016/j.cub.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 62.MacLeod CE, Zilles K, Schleicher A, Rilling JK, Gibson KR. Expansion of the neocerebellum in Hominoidea. Journal of Human Evolution. 2003;44(4):401–29. doi: 10.1016/s0047-2484(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 63.De Smet HJ, Paquier P, Verhoeven J, Mariën P. The cerebellum: Its role in language and related cognitive and affective functions. Brain and Language. 2013;127(3):334–42. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 65.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–40. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inglese M, Petracca M, Mormina E, Achiron A, Straus-Farber R, Miron S, et al. Cerebellar volume as imaging outcome in progressive multiple sclerosis. PLoS ONE. 2017;12(4):e0176519. doi: 10.1371/journal.pone.0176519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abel JM, Witt DM, Rissman EF. Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology. 2011;93(4):230–40. doi: 10.1159/000324402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM. Progress in brain research. Vol. 148. Elsevier; 2005. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations; pp. 341–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.