Abstract
Background
Altered epithelial physical and functional barrier properties along with TH1/TH2 immune dysregulation are features of allergic asthma. Regulation of junction proteins to improve barrier function of airway epithelial cells has the potential for alleviation of allergic airway inflammation. Objective: We sought to determine the immunomodulatory effect of knob protein of the adenoviral capsid on allergic asthma and to investigate its mechanism of action on airway epithelial junction proteins and barrier function.
Methods
Airway inflammation, including junction protein expression, was evaluated in allergen-challenged mice with and without treatment with knob. Human bronchial epithelial cells were exposed to knob, and its effects on expression of junction proteins and barrier integrity were determined.
Results
Administration of knob to allergen-challenged mice suppressed airway inflammation (eosinophilia, airway hyperresponsiveness, and IL-5 levels) and prevented allergen-induced loss of airway epithelial occludin and E-cadherin expression. Additionally, knob decreased expression of TH2-promoting inflammatory mediators, specifically IL-33, by murine lung epithelial cells. At a cellular level, treatment of human bronchial epithelial cells with knob activated c-Jun N-terminal kinase, increased expression of occludin and E-cadherin, and enhanced epithelial barrier integrity.
Conclusion
Increased expression of junction proteins mediated by knob leading to enhanced epithelial barrier function might mitigate the allergen-induced airway inflammatory response, including asthma.
Keywords: Knob protein, adenoviral capsid, allergic airway inflammation, asthma, E-cadherin, occludin, airway epithelium, barrier integrity
Epithelial cells lining the airways serve as the first line of defense against allergens. The pivotal involvement and contribution of the airway epithelium during the development of allergic inflammation, remodeling, and bronchial hyperreactivity has received much attention in the recent past.1–4 This has led to a paradigm shift suggesting that asthma is an epithelial disorder caused by altered epithelial physical and functional barrier properties that are associated with a TH2-type inflammatory response.1,5,6 Increasingly, studies with biopsy specimens, epithelial cells, or both from asthmatic patients (reviewed by Georas and Rezaee3), as well as patients with allergic rhinitis,7 cultured in vitro have indicated that epithelial junction protein expression and barrier function are defective in these subjects, which could lead to entry of allergens into the airway tissue, thus activating the immune system. These studies have shown that biopsy specimens and epithelial cells from patients display disrupted or reduced junction protein expression and decreased transepithelial electrical resistance (TER) correlating inversely with permeability (number of eosinophils within the epithelium or paracellular flux) relative to healthy subjects.7,8 Studies with human bronchial epithelial cells (Hu-BECs; cell lines and cells isolated from asthmatic patients) have demonstrated that downregulation of E-cadherin, an epithelial junction protein, by means of RNA silencing or exposure to aeroallergens, such as house dust mite, results in loss of cell-cell contact and is associated with increased production of proinflammatory cytokines, promoting TH2-driven responses.4 Studies in rats9 and mouse models of allergic airway inflammation10,11 have further supported these observations of decreased/altered epithelial junction protein expression in response to allergen exposure.
Considering that airway epithelial cells form a barrier to the outside world through junction proteins (eg, tight junctions [TJs], adherens junctions, and desmosomes) that promote cell-cell adhesion and maintain barrier integrity,12 these molecules could serve as important targets to enhance the barrier function of epithelial cells and minimize the burden of allergic asthma in patients. Along these lines, recent studies have shown that CpG-DNA can enhance barrier integrity of Hu-BECs by inducing expression of TJ molecules.13
Although respiratory viruses, especially rhinoviruses, are known to promote asthma development and contribute to asthma exacerbations in human subjects14 and mouse models,15,16 studies indicate that adenoviruses (eg, replication-deficient human type 5 adenovirus [RD-Ad5]) have an opposite effect and inhibit the development of allergic airway inflammation in mice.17,18 Despite this observed positive outcome on airway inflammation, it is not feasible to use adenoviral vectors as a therapeutic tool because of innate and adaptive responses to the virus/vectors, as observed in gene therapy studies.19,20 In the current study we have investigated this observation and demonstrate that the knob protein of the adenoviral capsid attenuates allergic airway inflammation by inducing expression of airway epithelial junction proteins in mice, as well as in Hu-BECs, and enhancing epithelial barrier integrity.
METHODS
Preparation of adenoviral capsid and knob protein
E1/E3-deleted replication-deficient Ad5 vector containing the cytomegalovirus promoter and green fluorescent protein reporter gene (RD-Ad5) was propagated in HEK293 cells and isolated by using cesium chloride equilibrium-density gradient centrifugation.21 Capsid was obtained as a byproduct (top band) during isolation of RD-Ad5. Recombinant soluble knob protein was produced in Escherichia coli as an N-terminal histidine (His)–tagged fusion protein and purified by using metal chelate affinity chromatography, as described previously.22 Details are provided in the Methods section in this article’s Online Repository at www.jacionline.org. The amino acid sequence encoding this protein encompasses 15 residues of the last repeat of the fiber shaft, followed by the globular C-terminal domain, which comprises the receptor-binding region of the Ad5 fiber. The capsid and knob preparations were assessed by using 10% SDS-PAGE under denaturing conditions, followed by Coomassie Blue staining. The absence of viral nucleic acid in the capsid preparations was ascertained by using agarose gel electrophoresis (0.7%), followed by ethidium bromide staining.
Murine model of acute allergic airway inflammation
BALB/c mice (8–10 weeks) maintained under standard pathogen-free conditions were used. All studies involving mice were performed according to standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Mice were sensitized and challenged with ovalbumin (OVA; Grade V; Sigma-Aldrich, St Louis, Mo), as previously described.23 Some OVA-exposed mice received RD-Ad5 (1e10 virus particles per dose), capsid (100 μg per dose), or knob protein (1 μg per dose) at the same time as sensitization, as outlined in Fig 1, A. Mice administered aluminum hydroxide alone for sensitization followed by saline instead of OVA for challenge with or without RD-Ad5, capsid, or knob served as control groups.
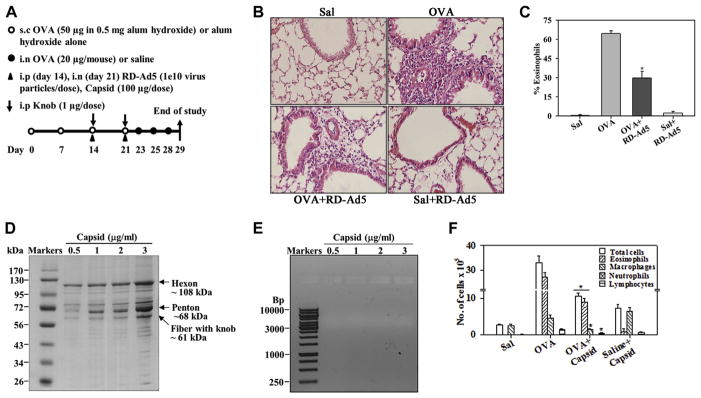
FIG. 1.
Inhibition of allergen-induced eosinophilia by RD-Ad5 and adenoviral capsid. A, Outline of mouse allergen (OVA) challenge model with details of RD-Ad5, capsid, and knob administration. s.c., Subcutaneous; i.n., intranasal; i.p., intraperitoneal. B, Representative data of cellular infiltration of lung tissue from saline- and OVA-challenged mice with and without RD-Ad5. Scale bar = 50 μm. C, Eosinophils in BALF of mice described in Fig 1, B. D, Representative Coomassie-stained SDS-PAGE of capsid. E, Representative ethidium bromide–stained agarose gel of the capsid. F, BALF cell counts in saline- and OVA-challenged mice with and without capsid protein (n = 4 mice per group). Sal, Saline. *P < .001 in Fig 1, C, and P < .05 in Fig 1, F, compared with the OVA group.
Sample collection and analysis
Mice were killed 24 hours after the last allergen challenge. Lungs were lavaged with 1 mL of saline. Total cell numbers in the bronchoalveolar lavage fluid (BALF) were counted in a hemocytometer, and differential cell counts were determined from cytocentrifuged samples based on morphologic criteria after staining with the Hema 3 System (Fisher Diagnostics, Middletown, Va). BALF was centrifuged at 1000g to remove cellular debris and stored at −70°C for further evaluation. Lungs were perfused with 4% paraformaldehyde to preserve pulmonary structure, fixed in 4% paraformaldehyde for 48 hours at 4°C, and then paraffin embedded.
Measurement of airway hyperresponsiveness
Methacholine (MCh)–induced airway hyperresponsiveness (AHR) was assessed by using an invasive technique in control and allergen-challenged mice with or without knob treatment by using the FinePointe RC System (Buxco, Wilmington, NC), as previously described.23 Changes in pulmonary resistance in response to saline followed by increasing concentrations of inhaled MCh (3–25 mg/mL) were monitored continuously. Pulmonary resistance values after each dose of MCh are shown.
Measurement of BALF cytokines
TH1 (IL-2 and IFN-γ)/TH2 (IL-4, IL-5, and IL-13) cytokine and TNF-α levels in BALF were determined by using Flex Set kits (BD Biosciences, San Jose, Calif), according to the manufacturer’s methods, with a FACScan flow cytometer equipped with CellQuest Pro Software (BD Biosciences) for data acquisition and FlowJo Software (Tree Star, Ashland, Ore) for analysis. For IL-13, a FACSCanto II flow cytometer was used with FACSDiva software (BD Biosciences) for data acquisition and analysis. Levels of each cytokine were expressed as picograms of cytokine per milliliter of BALF. Levels of TGF-β1 in BALF, as well as IL-33 and eotaxin-2 in lung tissue and cell lysates prepared as described previously,24,25 were measured with ELISA kits (R&D Systems, Minneapolis, Minn).
Immunohistology
Hematoxylin and eosin staining (Thermo Fisher Scientific, Waltham, Mass) of paraffin-embedded lung tissue sections (4-μm thick) was performed to determine cellular infiltration. Detection and quantitation of lung tissue eosinophils was performed with rat mAb against murine eosinophil-specific major basic protein (MBP), with rat IgG as a control, as described previously.26 Occludin and E-cadherin expression in the airway epithelium were detected by using rabbit polyclonal antibodies against occludin (2.5 μg/mL; Abcam, Cambridge, United Kingdom) and E-cadherin (2 μg/mL; Santa Cruz Biotechnology, Dallas, Tex), respectively. Rabbit IgG was used as a control. Stained slides were examined with a Nikon Microphot EPI-FL microscope (Nikon, Melville, NY), and images were captured with an Olympus DP71 camera (Olympus, Center Valley, Pa). For quantitation of eosinophils, MBP+cells in randomly selected nonoverlapping microscopic fields were counted at ×400 magnification (12 ± 2 fields per slide for OVA groups and 5 fields per slide for control groups). Positively stained areas in the epithelium were quantitated with ImageJ image analysis software (National Institutes of Health, Bethesda, Md)27 and expressed as occludin- or E-cadherin–positive area (per square micrometer) per 100 μm of basement membrane length. Additional details are provided in the Methods section in this article’s Online Repository.
Cell culture
The BEAS-2B line (ATCC, Manassas, Va), a Hu-BEC cell line, was cultured in RPMI 1640–GlutaMax Medium containing 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Life Technologies, Grand Island, NY). 16HBE14o− cells (obtained by MTA from Dr Dieter Gruenert, Department of Otolaryngology, UCSF School of Medicine) were cultured in Corning Cellgro MEM Eagle/EBSS (Thermo Fisher Scientific, Pittsburgh, Pa) supplemented with FBS, penicillin, and streptomycin. MLE-12, a transformed murine lung epithelial cell line (ATCC), was cultured, as previously described.28 Primary Hu-BECs were isolated from bronchial tissue obtained from deidentified discarded lung specimens incidental to thoracic surgery at the Mayo Clinic (Rochester, Minn) for focal noninfectious causes (ie, lobectomies for cancer). Patients were nonasthmatic (male or female) and between 55 and 70 years of age.
Hu-BECs were isolated from sections of airways from normal-appearing lung areas identified by a pathologist (approved by the Mayo Clinic Institutional Review Board) and cultured in BEGM BulletKit Medium (Lonza, Walkersville, Md), as described previously.29 In all cases, cells were maintained in a 5% CO2 atmosphere at 37°C.
Treatment of cells with knob protein
Cells were seeded directly into 24-well plates (for gene and protein expression) or cultured on sterile poly-L-lysine–coated (10 μg/mL in PBS; Sigma-Aldrich) glass coverslips placed in 24-well plates (for immunofluorescence [IF] studies) at 2 × 105 per well and cultured overnight in medium containing low serum (2% FBS). Cells were then treated overnight with knob protein at a final concentration of 0.25 μg/mL in the same medium. For gene expression studies, TRIzol (Life Technologies) was added to cells seeded directly in the 24-well plates after removal of culture medium for extraction of total RNA and quantitative RT-PCR (qPCR). For Western blot analysis, cells in wells were washed with PBS (2 times), detached with 0.05% Trypsin-EDTA, and solubilized either in RIPA buffer (for p44/42 mitogen-activated protein kinase [MAPK], E-cadherin, and occludin) or 1× SDS gel-loading buffer (for c-Jun N-terminal kinase [JNK]) by means of sonication and then electrophoresed. For IF studies, coverslips were taken out of the 24-well plates and further processed, as described in detail later in this section.
RNA isolation and qPCR analysis
Total RNA was extracted from cells with TRIzol and reverse transcribed into cDNA by using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, Calif), according to the manufacturer’s protocol. Gene expression was examined by using qPCR with primers specific for various human or mouse genes synthesized by Integrated DNATechnologies (Coralville, Iowa). A list of the primer pairs used and conditions of amplification are detailed in the Methods section and Table E1 in this article’s Online Repository at www.jacionline.org. Results were expressed as fold change in expression relative to expression in untreated controls cells by using the ΔΔCT method (2−ΔΔCT).
Western blots
Cell lysates were electrophoresed (8% [E-cadherin] or 10% [occludin, extracellular signal-regulated kinase [ERK] 1/2, and stress-activated protein kinase [SAPK]/JNK] SDS-PAGE), transferred to polyvinylidene difluoride membranes, blocked, and incubated overnight at 4°C with the primary antibody. The antibodies used were phospho-SAPK/JNK (Thr183/Tyr185) (81E11) rabbit mAb (0.3 μg/mL), SAPK/JNK rabbit polyclonal antibodies (0.32 μg/mL), phopho-p44/42 MAPK (phospho-ERK1/2, T202/Y204) rabbit polyclonal antibodies (0.048 μg/mL), and p44/42 MAPK rabbit mAb (ERK1/2, 0.027 μg/mL) from Cell Signaling Technology (Danvers, Mass), as well as rabbit polyclonal antibodies against E-cadherin and occludin (0.2 μg/mL; both from Santa Cruz Biotechnology). Bound antibodies were detected by using peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H+L) or goat anti-rabbit IgG (H+L; 0.26 μg/mL; Jackson ImmunoResearch Laboratories, West Grove, Pa). Expression levels of β-actin were monitored by using peroxidase-conjugated mAb against actin (0.06 μg/mL; Santa Cruz Biotechnology). Protein bands were detected with Western Bright ECL (Advansta, Menlo Park, Calif) and visualized on x-ray films. The intensity of the detected bands was quantified by using ImageJ image analysis software. Expression levels of phospho-p44/42 MAPK and phospho-SAPK/JNK were normalized against total p44/42 MAPK and SAPK/JNK, respectively. Expression levels of E-cadherin and occludin were normalized against those of β-actin.
IF staining
Cells on poly-L-lysine–coated coverslips were fixed with chilled 100% methanol at −20°C for 10 minutes. After blocking (5% goat serum, 1% BSA, and 0.1% Tween-20 in PBS) for 1 hour at room temperature, cells were washed 2 times with PBS and incubated overnight with rabbit polyclonal antibodies against occludin (2 μg/mL; Abcam) or E-cadherin (0.8 μg/mL; Santa Cruz Biotechnology) of human origin at 4°C. Normal rabbit IgG was used as the isotype control (Thermo Fisher Scientific). Rhodamine Red-X affinity-purified goat anti-rabbit IgG (3 μg/mL for 1 hour at room temperature; Jackson ImmunoResearch Laboratories) was used for detection of bound antibodies, and cells were evaluated by using confocal microscopy.
Assessment of epithelial barrier integrity
16HBE14o− cells were seeded in noncoated Snapwell Inserts (0.4 μm; Corning Life Sciences, Tewksbury, Mass) at 1 × 105 cell/well. Knob protein was added to some wells at a final concentration of 0.25 μg/mL, and TER was monitored every other day until day 9 by using an epithelial voltohmmeter (EVOM; World Precision Instruments, Sarasota, Fla). Cells were replenished with fresh medium with or without knob protein on days 2, 4, 6, and 8. Inserts without cells were used to monitor background TER.
In some experiments knob-treated and control cells were exposed to a protein extract of Alternaria alternata (Greer Laboratories, Lenoir, NC) on day 5 after plating when cells were confluent. The extract was added apically in regular medium (ie, without knob protein) at 100 μg/well (40 μg/mL), as described previously,30 and TER was monitored up to 12 hours. To measure paracellular permeability, fluorescein isothiocyanate (FITC)–Dextran (4 kDa) was added to the apical medium (3 mg/mL) of knob-treated and control cells on day 5 after cells were replenished with fresh medium (without knob protein). Passage of FITC-Dextran was monitored by measuring fluorescence (FLUOstar OPTIMA; BMG Labtech, Cary, NC) in basal medium every 3 hours.
In some experiments 16HBE14o− cells grown under submerged conditions with or without knob treatment until confluence (days 5–6) were transferred to conditions of air-liquid interface (ALI). After measuring TER, apical medium was removed, and basal medium was replaced with 2.5 mL of cell culture medium with or without knob protein. Medium was changed every other day, and TER was measured up to day 22 after ALI. Subsequently, inserts were processed for IF staining for E-cadherin and occludin, as described earlier.
Statistical analysis
Combined data (means ± SEMs) of experiments performed at least 3 times in duplicate or triplicate are shown for in vitro studies. Statistical significance between 2 groups was determined by using a 2-tailed unpaired Student t test. Comparisons between control and multiple treatment conditions were carried out by means of ANOVA, followed by the Dunnett posttest. For animal studies, combined data of 2 to 3 experiments (n = 9–12 mice for saline and OVA groups [with and without treatment] and 6–7 mice for saline plus treatment group, unless otherwise indicated) are shown. A 2-tailed test was used to first establish statistical significance between OVA and OVA plus RD-Ad5/OVA plus capsid/OVA plus knob groups in airway cellular recruitment (Fig 1, C and F, and Fig 2, B and D), and a 1-tailed test was used for analyses of all other studies (Fig 2, F; Fig 3, A and B; and Fig 4, B and D). A P value of less than .05 was considered significant.
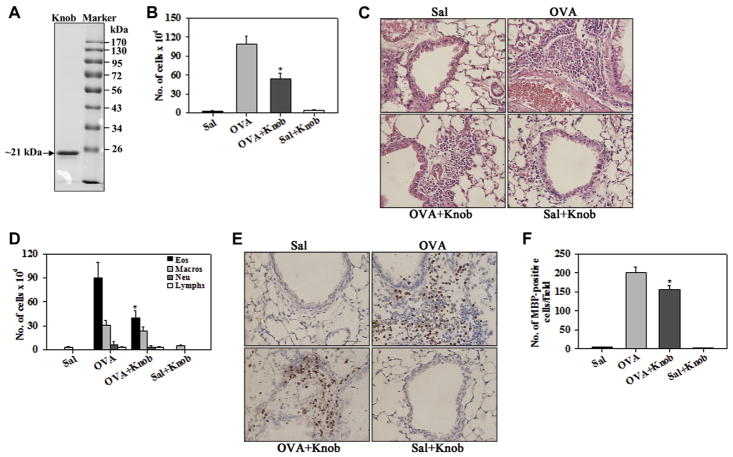
FIG. 2.
Knob domain of the fiber protein of capsid suppresses allergen-induced airway cellular inflammation. A, Representative SDS-PAGE of recombinant knob (2 μg per lane). B and C, Total numbers of inflammatory cells in BALF and lung tissue of saline- and OVA-challenged mice with and without knob. D–F, BALF differential cell (Fig 2, D) and lung tissue eosinophil (Fig 2, E and F) counts in mice described in Fig 2, B. Scale bar = 50 μm. Sal, Saline. *P < .05 compared with the OVA group.
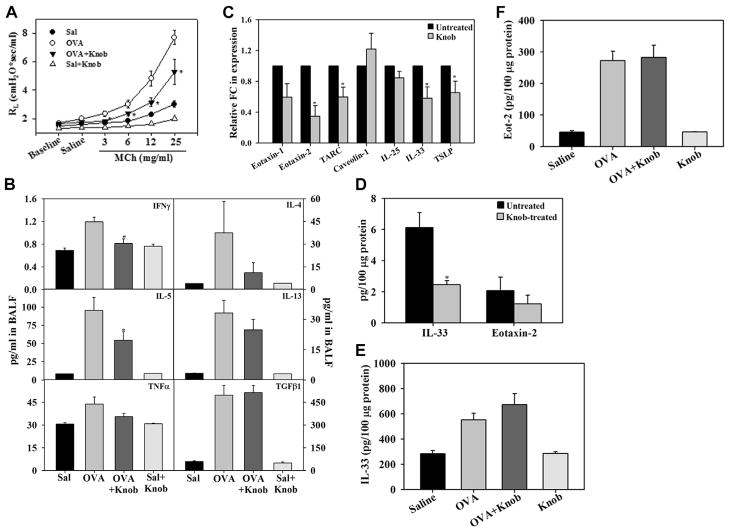
FIG. 3.
Knob reduces allergen-induced airway reactivity and IL-5 levels. A, Pulmonary resistance in saline-and OVA-challenged mice with and without knob treatment. B, Levels of indicated cytokines in BALF of mice identified in Fig 3, A. C, Gene expression of the indicated epithelially derived inflammatory mediators in knob-treated MLE-12 cells. D, IL-33 and eotaxin-2 protein expression in knob-treated MLE-12 cells. E and F, IL-33 and eotaxin-2 levels in lung tissue of mice identified in Fig 3, A. *P < .05 compared with the OVA group in Fig 3, A and B, and with untreated cells in Fig 3, C and D.
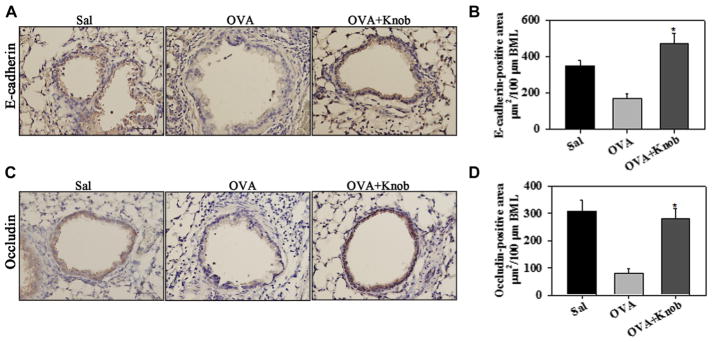
FIG. 4.
Induction of E-cadherin and occludin in the airway epithelium of knob-treated allergen-challenged mice. E-cadherin (A and B) and occludin (C and D) expression and quantitation, respectively, are shown in saline- and OVA-challenged mice with and without knob treatment (n = 5 to 6 mice per group). Scale bar = 50 μm. *P < .001 compared with the OVA group in Fig 4, B and D.
RESULTS
Inhibition of allergen-induced eosinophilia by RD-Ad5 and the adenoviral capsid
Earlier studies have demonstrated that RD-Ad5 has an anti-inflammatory effect on airway immune responses in the context of allergic inflammation.17,18 Given the innate inflammatory responses and toxicity associated with the use of adenoviral vectors,19 we first examined whether intact/whole RD-Ad5 (capsid and viral nucleic acid) was required for the inhibitory effect on allergen-induced airway inflammation. Consistent with previous findings, administration of RD-Ad5 to mice during the sensitization phase of allergen (OVA) exposure, as outlined in Fig 1, A, resulted in substantially inhibiting cellular inflammation in the lung tissue and airway eosinophilia (Fig 1, B and C). Next, the capsid was isolated from RD-Ad5 and first assessed for the presence of the major Ad5 capsid proteins and absence of viral nucleic acid. SDS-PAGE of the capsid indicated the presence of the major Ad5 capsid proteins (ie, hexon [approximately 108 kDa], penton base [approximately 68 kDa], and fiber with knob [approximately 61 kDa]), with no detectable viral nucleic acid based on ethidium bromide staining after agarose gel electrophoresis (Fig 1, D and E). Treatment of OVA-challenged mice with the adenoviral capsid instead of intact RD-Ad5 demonstrated a similar reduction in numbers of total cells, as well as eosinophils, in the BALF relative to OVA-challenged mice that did not receive capsid (Fig 1, F). In addition, a significant reduction in macrophage and lymphocyte numbers was also noted. Administration of capsid alone (control) induced minimal cellular recruitment (mostly macrophages) to the airways. These findings suggest that the capsid alone (devoid of viral nucleic acid) is sufficient to induce a suppressive effect on allergen-induced airway eosinophilia.
Knob domain of the fiber protein of capsid suppresses allergic airway inflammation
Because of the innate and adaptive responses associated with the use of adenoviral vectors/capsid as therapeutic options,19,20 we investigated whether a protein component of capsid rather than the intact capsid can suppress allergen-induced airway inflammation. The knob domain of the fiber protein of RD-Ad5 interacts with host cells through Coxsackie B virus and adenovirus receptor during virus attachment.31 Additionally, studies have shown that the fiber knob of another adenovirus (Ad serotype 35) can function as a potent regulator (downmodulation) of human T-cell activation.32,33
We examined the effect of RD-Ad5 knob in our model of allergic airway inflammation. Recombinant soluble knob protein was produced as described in the Methods section and formed a monomer of approximately 21 kDa on PAGE under denaturing conditions (Fig 2, A). Administration of knob during the sensitization phase to OVA-challenged mice resulted in a marked reduction in the number of inflammatory cells recruited in the BALF, as well as lung tissue, relative to that in OVA-challenged mice that did not receive knob (Fig 2, B and C). In addition, knob-treated OVA-challenged mice had significantly reduced eosinophilia based on differential cell counts in the BALF (Fig 2, D) and immunohistochemistry for MBP+ cells in the lung tissue (Fig 2, E and F). Administration of knob alone resulted in minimal cellular recruitment similar to that observed in saline-exposed control mice. These findings with knob parallel the suppressive effect of RD-Ad5 (Fig 1, C) and capsid (Fig 1, F) on allergen-induced airway eosinophilia.
Administration of knob to OVA-challenged mice also resulted in a significant reduction in AHR to inhaled MCh compared with OVA-challenged mice that did not receive knob, although levels remained higher than in control mice (Fig 3, A). Along with reduced cellular inflammation, OVA-challenged mice administered knob demonstrated significantly lower IL-5 and IFN-γ levels in the BALF relative to OVA-challenged mice that did not receive knob, whereas levels of IL-4, IL-13, TNF-α, and TGF-β1 were not statistically different relative to those in untreated mice (Fig 3, B). Additionally, the effect of knob on expression of select epithelially derived TH2-promoting cytokines (thymic stromal lymphopoietin [TSLP], IL-33, and IL-25), chemokines (thymus and activation-regulated chemokine [TARC], eotaxin-1, and eotaxin-2) and caveolin-1 was examined in MLE-12 murine lung epithelial cells by using qPCR. Knob-treated cells exhibited significantly lower expression of eotaxin-2, IL-33, TARC, and TSLP (Fig 3, C), whereas no effect was noted on expression of eotaxin-1, IL-25, and caveolin-1. Based on this, we examined expression of a select TH2-promoting cytokine (IL-33) and chemokine (eotaxin-2) at the protein level in these cells. IL-33 levels were significantly lower after treatment with knob, whereas the level of eotaxin-2 was not significantly different (Fig 3, D). However, levels of these 2 mediators in the lungs of knob-treated OVA-challenged mice remained unaltered relative to those of untreated mice (Fig 3, E and F).
Administration of knob reverses allergen-induced loss of airway epithelial junction proteins
Given the dysfunction of airway epithelial barrier properties in asthmatic patients2,3,34 and the anti-inflammatory effect of knob noted in the airways of mice, we examined whether knob treatment affects expression of epithelial junction proteins that maintain barrier integrity. Consistent with previously published findings in allergen-exposed mice,35 OVA-challenged mice exhibited loss of airway epithelial E-cadherin (Fig 4, A and B) and occludin (Fig 4, C and D) compared with saline-exposed control mice when examined by immunohistochemistry. In OVA-challenged mice that received knob, this effect was reversed. Expression of E-cadherin and occludin was significantly greater in these mice than in OVA-challenged mice that did not receive knob, restoring levels to those observed in saline-exposed mice.
Knob induces expression of occludin and E-cadherin in Hu-BECs
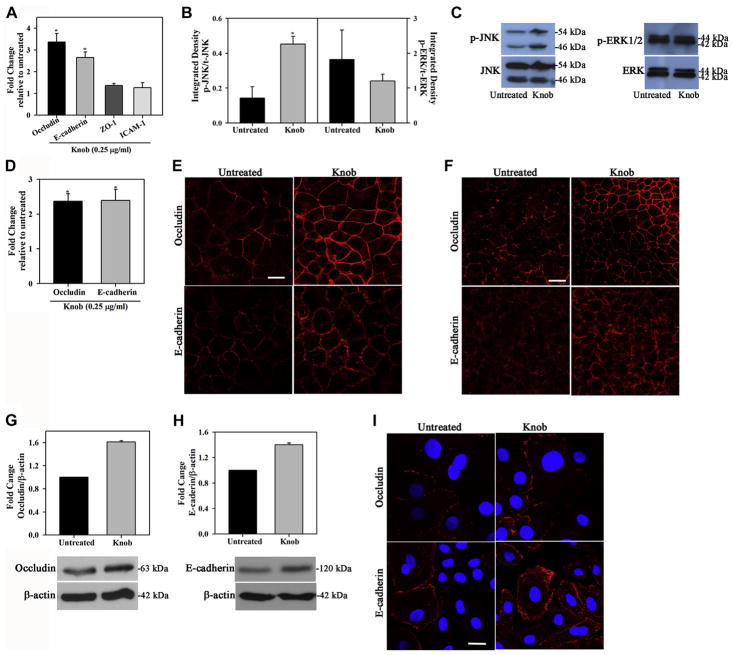
Hu-BECs (cell lines and primary cells) were treated with knob in vitro to further examine the effect of knob on junction protein expression at a cellular level. Treatment of BEAS-2B Hu-BECs with knob significantly induced expression of occludin and E-cadherin by means of qPCR (Fig 5, A) without affecting cell viability (see Table E2 in this article’s Online Repository at www.jacionline.org), whereas only a marginal increase in expression of zonula occludens 1 and intercellular adhesion molecule 1 was noted. Along with induction of occludin and E-cadherin expression, knob treatment of BEAS-2B cells resulted in activation of JNK with significantly increased levels of phosphorylated JNK but had no effect on ERK1/2 at this time point (Fig 5, B and C). Because BEAS-2B cells have been shown to have a limited capacity to differentiate and develop TJs, even under ALI conditions,36 we confirmed the ability of knob to induce expression of occludin and E-cadherin in 16HBE14o− cells, another extensively used Hu-BEC. A similar induction of occludin and E-cadherin was noted in knob-treated 16HBE14o− cells (Fig 5, D). Consistent with the increased expression at the mRNA level, IF staining with specific antibodies revealed increased expression of occludin and E-cadherin in confluent cultures of 16HBE14o− cells under regular and ALI culture (Fig 5, E and F, respectively) after treatment with knob. This is further supported by the increased protein expression of occludin and E-cadherin observed in Western blots of knob-treated 16HBE14o− cells (Fig 5, G and H). Finally, cultures (regular) of primary Hu-BECs also exhibited increased expression of occludin and E-cadherin after treatment with knob (Fig 5, I), although the level of basal and knob-induced expression of these molecules varied between the cell types.
FIG. 5.
Knob induces expression of occludin and E-cadherin in Hu-BECs. A, Occludin and E-cadherin gene expression in knob-treated BEAS-2B cells. B and C, JNK and ERK1/2 expression in knob-treated BEAS-2B cells. D, Occludin and E-cadherin gene expression in knob-treated 16HBE14o− cells. E and F, Knob-induced occludin and E-cadherin protein expression in 16HBE14o− cells under regular and ALI conditions, respectively. G and H, Induction of occludin and E-cadherin in 16HBE14o− cells by knob by using Western blotting. I, Knob-induced expression of occludin and E-cadherin in primary Hu-BECs. *P < .05 compared with untreated cells.
Knob enhances epithelial barrier integrity
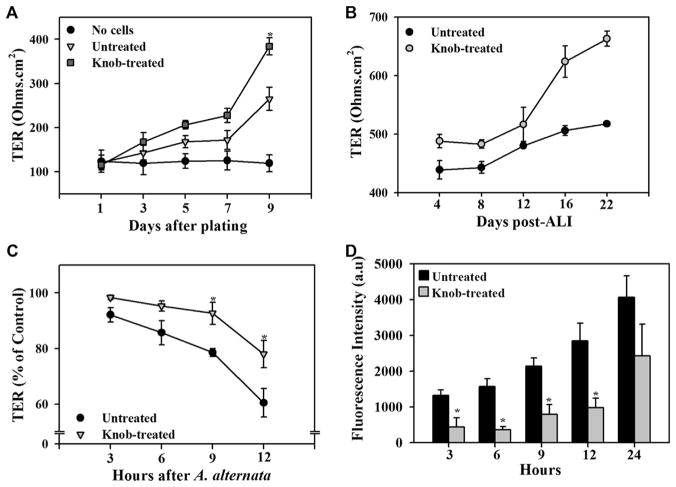
Next, we examined the effect of knob on TER, an indicator of barrier integrity, in monolayers of 16HBE14o− cells. Cultures under regular conditions containing knob on the apical and basal side or ALI cultures with knob on the basal side had higher TER than cultures in medium alone (Fig 6, A and B).
FIG. 6.
Knob enhances barrier integrity. A and B, Effect of knob on TER under regular and ALI culture conditions, respectively. Representative data (mean ± STD) of 3 independent experiments are shown in Fig 6, B. C, TER in knob-treated cultures exposed to Alternaria alternata extract. D, Paracellular permeability across knob-treated cultures. *P < .05 compared with untreated cells.
We examined whether knob can protect epithelial barrier integrity. In these studies the effect of Alternaria alternata extract, which is known to disrupt epithelial barrier integrity,30 was examined in knob-treated versus untreated 16HBE14o− cells cultured under regular conditions. Although barrier integrity of knob-treated and untreated 16HBE14o− cell cultures was affected by exposure to Alternaria alternata extract, the change in TER of knob-treated 16HBE14o− monolayers was significantly less than that observed in untreated monolayers after 9 hours (Fig 6, C).
Finally, paracellular permeability of FITC-Dextran was significantly lower across monolayers of 16HBE14o− cells treated with knob (Fig 6, D). Taken together, these findings suggest that knob can enhance barrier integrity and exert a protective effect on the epithelial barrier by inducing expression of TJ and adherens junction proteins, such as occludin and E-cadherin.
DISCUSSION
Studies in human subjects (reviewed by Georas and Rezaee3) and animal models10,11 provide evidence that there is a dysfunction of barrier properties of the airway epithelium in asthmatic patients that leads to orchestration of a TH2-type inflammatory response. Therefore strategies to resist/prevent breakdown of epithelial barrier function can serve as therapeutic options for mitigating the allergen-induced inflammatory response and reducing asthma symptoms. Unlike other respiratory tract viruses, such as respiratory syncytial virus and rhinovirus, which augment airway eosinophilia in allergen-exposed mice,15,16,37 our studies, along with previous investigations,17,18 indicate that RD-Ad5 inhibits airway eosinophilia in OVA-exposed mice. Importantly, we found that reduced eosinophilia is evident even when the adenoviral capsid protein is administered by itself (ie, without viral nucleic acid), thus establishing that the anti-inflammatory effect of RD-Ad5 is mediated by the protein coat of RD-Ad5.
Despite the observed positive outcome of RD-Ad5 or its capsid on allergic airway inflammation, adenoviral vectors/capsids are associated with innate and adaptive immune responses19,20 and therefore are not viable therapeutic options. To circumvent this, we examined whether the knob domain of the fiber protein of the RD-Ad5 capsid, which interacts with host cells during viral attachment,31 could attenuate allergen-induced cellular inflammation, as noted with the intact capsid. Previous studies with knob from another adenovirus (Ad serotype 35) have shown that this protein can function as a regulator (downmodulation) of human T-cell activation.32,33 OVA-challenged mice treated with knob in our study not only exhibited marked attenuation of airway eosinophilia but also significantly lower allergen-induced AHR, as well as IL-5 and IFN-γ levels, in the BALF relative to untreated OVA-challenged mice. We examined whether knob alters expression specifically of epithelial-derived inflammatory mediators that can contribute to the development of a TH2 phenotype (ie, IL-25, TSLP, IL-33, TARC, eotaxin-1, and eotaxin-2). Studies in MLE-12 mouse lung epithelial cells demonstrated that expression of TSLP, IL-33, TARC, and eotaxin-2 was lower at the mRNA level in knob-treated cells. Expression of epithelial-derived IL-33 known to be released during epithelial barrier dysfunction/activation caused by allergen exposure2,3,34 was significantly lower at the protein level in knob-treated cells. However, no difference in IL-33 expression was noted in total lung tissue lysates of OVA-challenged mice after knob treatment. This could be due to the fact that, in addition to epithelial cells, other cells in the lungs (ie, dendritic cells, fibroblasts, mast cells, and macrophages) are known to release IL-33, and the effect of knob on these cells is not known. Nonetheless, decreased expression of IL-33 by airway epithelial cells in response to knob, as observed in vitro, could in part contribute to amelioration of inflammation in vivo through suppression of autocrine effects (eg, IL-33–induced secretion of IL-8 by airway epithelial cells),38 as well as paracrine effects on type 2 innate lymphoid cells,39 to reduce type 2 inflammation.
Recent studies have shown that type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting TJs through IL-13 in an IL-33–induced model of airway inflammation.40 Most notably, our studies demonstrate that in addition to suppressing a TH2-type immune response, treatment with knob results in prevention of allergen-induced decrease of epithelial occludin and E-cadherin, with expression levels of both junction proteins being restored to those observed in control mice.
This novel observation was examined further at a cellular level. Knob induced expression of occludin and E-cadherin in Hu-BECs (cell lines and primary cells) at the mRNA and protein levels. Induction of these junction proteins was associated with increased levels of phosphorylated JNK, suggesting increased JNK1 kinase activity. In previous studies with A549 alveolar type II–derived epithelial cells, the knob domain of RD-Ad5 has been shown to rapidly (within 20 minutes) induce ERK1/2 and JNK1 kinase activity in a Coxsackie B virus and adenovirus receptor–dependent manner.41 However, it is important to note that the 2 studies differ not only in the cells used but also the concentration of knob (10-fold lower in the current study) and duration of treatment.
At a functional level, knob treatment enhanced barrier integrity of 16HBE14o− cultures, as indicated by the increased TER (under regular and ALI conditions), reduced paracellular permeability, and resistance to barrier disruption when exposed to Alternaria alternata extract. Alternatively, knob could be exerting a protective effect by inhibiting protease activity of Alternaria alternata30 or suppressing Alternaria alternata–induced matrix metalloprotease secretion,42 both of which act on epithelial monolayers to decrease TER.30,43 In contrast to our observations with knob treatment, infection with rhinovirus has been shown to decrease expression of E-cadherin, occludin, and zonula occludens 1 along with a reduction in TER in nasal epithelial cells,44 as well as in biopsy specimens (transtissue resistance) from patients with chronic rhinosinusitis.45 Along these lines, infection of 16HBE14o− cells with rhinovirus was shown to result in damage to the respiratory epithelial barrier and facilitate allergen penetration, thus contributing to increased allergic inflammation.46
In summary, our studies have led to the novel finding that knob protein can induce expression of E-cadherin and occludin and enhance epithelial barrier integrity. In vivo administration of knob to allergen-challenged mice suppresses TH2-type immune responses (eosinophilia, AHR, and IL-5 levels) and prevents the allergen-induced decrease in epithelial E-cadherin and occludin expression. These findings together indicate a novel role for knob in protecting epithelial barrier integrity and suggest that knob can serve as a potential therapeutic agent for allergic asthma by enhancing airway barrier function.
METHODS
Preparation of knob protein
Plasmid constructs (pRSET A; Life Technologies) that produce knob protein were transformed into BL21(DE3)pLysS competent bacteria (Life Technologies) and grown in LB supplemented with 100 μg of ampicillin (Sigma-Aldrich) and 25 μg/mL chloramphenicol (Sigma-Aldrich). Transformants were induced with 0.4 mmol/L isopropyl-β-d-thiogalactopyranoside (Promega, Madison, Wis) for 12 hours at 37°C to induce recombinant knob protein. Recombinant knob protein was purified from cell lysates by using cOmplete His-Tag Purification Resin (Roche Life Sciences, Indianapolis, Ind) and passed through Detoxi-Gel Endotoxin Removing Gel (Thermo Fisher Scientific), according to the manufacturer’s instructions. Residual endotoxin content was determined by using Limulus Amebocyte Lysate PYROGENT Plus (sensitivity = 0.06 EU/mL; Lonza, Walkersville, Md) and found to be less than detectable levels.
Conditions for qPCR
qPCR was performed with iTaq Universal SYBR Green Supermix 200 (Bio-Rad Laboratories). The reaction was carried out in an iQ 5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories). The relative amount of target mRNA was calculated based on its threshold cycle, as suggested by the software (iQ 5 Optical System software), in comparison with the threshold cycle of the housekeeping gene β-actin. After denaturation, conditions for gene expression were as follows: 50 cycles of 30 seconds at 95°C followed by 30 seconds at 60°C each cycle for most genes. For murine Tslp, 60 cycles of amplification with 30 seconds at 95°C followed by 30 seconds at 54°C each cycle were performed; for murine Il25, 60 cycles with 30 seconds at 92°C followed by 30 seconds at 52°C each cycle were performed; and for murine Il33, 55 cycles with 30 seconds at 92°C followed by 30 seconds at 56°C each cycle were performed.
Immunohistology
For all immunohistologic analysis, tissue sections were subjected to antigen retrieval, followed by quenching of endogenous peroxidase activity before staining with specific antibodies. Sections were briefly counterstained (for 5 seconds) with hematoxylin. Appropriate VECTASTAIN ABC Kits using biotinylated secondary antibodies (Vector Laboratories, Burlingame, Calif) and the Peroxidase AEC (3-amino-9-ethylcarbazole) substrate kit (Vector Laboratories) were used for detection. Stained slides were examined with a Nikon Microphot EPI-FL microscope (Nikon, Tokyo, Japan), and images were captured with an Olympus DP71 camera (Olympus, Center Valley, Pa). For quantitation of eosinophils, MBP+ cells in randomly selected nonoverlapping microscopic fields were counted at ×400 magnification (12 ± 2 fields per slide for OVA groups and 5 fields per slide for control groups). For quantitation of E-cadherin and occludin, positively stained areas in the epithelium of all similarly sized airways with total basement membrane length in the range of 550 to 650 μm were quantitated for each mouse.
Supplementary Material
Key messages.
Airway epithelial barrier function is defective in asthmatic patients.
Knob protein of the adenoviral capsid induces expression of E-cadherin and occludin, improves airway epithelial barrier integrity in vitro, and attenuates airway inflammation in allergen-challenged mice.
Knob can potentially mitigate allergic airway inflammation, including asthma, by protecting epithelial barrier function.
Acknowledgments
Supported by the University of Minnesota College of Veterinary Medicine.
Abbreviations used
- AHR
Airway hyperresponsiveness
- ALI
Air-liquid interface
- BALF
Bronchoalveolar lavage fluid
- ERK
Extracellular signal-regulated kinase
- FITC
Fluorescein isothiocyanate
- Hu-BEC
Human bronchial epithelial cell
- IF
Immunofluorescence
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MBP
Major basic protein
- MCh
Methacholine
- OVA
Ovalbumin
- qPCR
Quantitative RT-PCR
- RD-Ad5
Replication-deficient human type 5 adenovirus
- SAPK
Stress-activated protein kinase
- TARC
Thymus and activation-regulated chemokine
- TER
Transepithelial electrical resistance
- TJ
Tight junction
- TSLP
Thymic stromal lymphopoietin
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–19. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–20. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijink IH, Nawijn MC, Hackett TL. Airway epithelial barrier function regulates the pathogenesis of allergic asthma. Clin Exp Allergy. 2014;44:620–30. doi: 10.1111/cea.12296. [DOI] [PubMed] [Google Scholar]
- 5.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Steelant B, Farre R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137:1043–53. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Hackett T-L, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45:1090–100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 9.Tillie-Leblond I, Gosset P, Le Berre R, Janin A, Prangere T, Tonnel AB, et al. Keratinocyte growth factor improves alterations of lung permeability and bronchial epithelium in allergic rats. Eur Respir J. 2007;30:31–9. doi: 10.1183/09031936.00011606. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-C, Chuang J-G, Su Y-Y, Chiang B-L, Lin Y-S, Chow L-P. The protease allergen pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J Biol Chem. 2011;286:26667–79. doi: 10.1074/jbc.M110.193987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post S, Nawijn MC, Hackett TL, Baranowska M, Gras R, van Oosterhout AJM, et al. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax. 2012;67:488–95. doi: 10.1136/thoraxjnl-2011-200606. [DOI] [PubMed] [Google Scholar]
- 12.Rezaee F, Georas SN. Breaking barriers. New Insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol. 2014;50:857–69. doi: 10.1165/rcmb.2013-0541RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo T, Wawrzyniak P, Morita H, Sugita K, Wanke K, Kast JI, et al. CpG-DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol. 2015;136:1413–6. doi: 10.1016/j.jaci.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE. Virus/allergen interaction in asthma exacerbation. Ann Am Thorac Soc. 2015;12(suppl):S137–43. doi: 10.1513/AnnalsATS.201503-153AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, et al. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol. 2010;185:2525–35. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke DL, Davis NH, Majithiya JB, Piper SC, Lewis A, Sleeman MA, et al. Development of a mouse model mimicking key aspects of a viral asthma exacerbation. Clin Sci. 2014;126:567–80. doi: 10.1042/CS20130149. [DOI] [PubMed] [Google Scholar]
- 17.Stampfli MR, Ritz SA, Neigh GS, Sime PJ, Lei XF, Xing Z, et al. Adenoviral infection inhibits allergic airways inflammation in mice. Clin Exp Allergy. 1998;28:1581–90. doi: 10.1046/j.1365-2222.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Suzuki S, Yamamoto N, Komatsu S, Inoue S, Hashiba T, et al. Immune responses against replication-deficient adenovirus inhibit ovalbumin-specific allergic reactions in mice. Hum Gene Ther. 2000;11:827–38. doi: 10.1089/10430340050015446. [DOI] [PubMed] [Google Scholar]
- 19.Gregory SM, Nazir SA, Metcalf JP. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011;6:357–74. doi: 10.2217/fvl.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25:265–84. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K, Wold WS. Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med. 2007;130:223–35. doi: 10.1385/1-59745-166-5:223. [DOI] [PubMed] [Google Scholar]
- 22.Medina-Kauwe LK, Leung V, Wu L, Kedes L. Assessing the binding and endocytosis activity of cellular receptors using GFP-ligand fusions. Biotechniques. 2000;29:602–9. doi: 10.2144/00293rr03. [DOI] [PubMed] [Google Scholar]
- 23.Bahaie NS, Hosseinkhani RM, Ge XN, Kang BN, Ha SG, Blumenthal MS, et al. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J Immunol. 2012;188:1479–90. doi: 10.4049/jimmunol.1102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge XN, Greenberg Y, Hosseinkhani MR, Long EK, Bahaie NS, Rao A, et al. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res. 2013;39:365–78. doi: 10.3109/01902148.2013.829537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marten E, Nielsen HC, Dammann CEL. Interdependent TTF1-ErbB4 interactions are critical for surfactant protein-B homeostasis in primary mouse lung alveolar type II cells. J Cell Commun Signal. 2015;9:207–15. doi: 10.1007/s12079-015-0299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge XN, Ha SG, Rao A, Greenberg YG, Rushdi MN, Esko JD, et al. Endothelial and leukocyte heparan sulfates regulate the development of allergen-induced airway remodeling in a mouse model. Glycobiology. 2014;24:715–27. doi: 10.1093/glycob/cwu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 28.Eneling K, Brion L, Pinto V, Pinho MJ, Sznajder JI, Mochizuki N, et al. Salt-inducible kinase 1 regulates E-cadherin expression and intercellular junction stability. FASEB J. 2012;26:3230–9. doi: 10.1096/fj.12-205609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339:815–24. doi: 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, Dennison PW, et al. Barrier disrupting effects of Alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 2013;8:e71278. doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby I, Davison E, Beavil AJ, Soh CPC, Wickham TJ, Roelvink PW, et al. Identification of contact residues and definition of the CAR-binding site of Adenovirus type 5 fiber protein. J Virol. 2000;74:2804–13. doi: 10.1128/jvi.74.6.2804-2813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams WC, Berenson RJ, Karlsson Hedestam GB, Lieber A, Koup RA, Lore K. Attenuation of CD4+ T-cell function by human adenovirus type 35 is mediated by the knob protein. J Gen Virol. 2012;93:1339–44. doi: 10.1099/vir.0.039222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay J, Carter D, Lieber A, Astier AL. Recombinant Ad35 adenoviral proteins as potent modulators of human T cell activation. Immunology. 2014;144:453–60. doi: 10.1111/imm.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loxham M, Davies DE, Blume C. Epithelial function and dysfunction in asthma. Clin Exp Allergy. 2014;44:1299–313. doi: 10.1111/cea.12309. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JR, Roos A, Berg T, Nord M, Fuxe J. Chronic respiratory aeroallergen exposure in mice induces epithelial-mesenchymal transition in the large airways. PLoS One. 2011;6:e16175. doi: 10.1371/journal.pone.0016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart CE, Torr EE, Mohd Jamili NH, Bosquillon C, Sayers I. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy. 2012;2012:11. doi: 10.1155/2012/943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaitov MR, Shilovskiy IP, Nikonova AA, Shershakova NN, Kamyshnikov OY, Babakhin AA, et al. Small interfering RNAs targeted to interleukin-4 and respiratory syncytial virus reduce airway inflammation in a mouse model of virus-induced asthma exacerbation. Hum Gene Ther. 2014;25:642–50. doi: 10.1089/hum.2013.142. [DOI] [PubMed] [Google Scholar]
- 38.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–50. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 39.Salmond RJ, Mirchandani AS, Besnard A-G, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130:1159–66. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141:300–10. e11. doi: 10.1016/j.jaci.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, et al. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–54. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–92. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, Karp P, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L751–62. doi: 10.1152/ajplung.90578.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo N-K, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010;120:346–52. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]
- 45.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130:1087–96. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Gangl K, Waltl EE, Vetr H, Cabauatan CR, Niespodziana K, Valenta R, et al. Infection with rhinovirus facilitates allergen penetration across a respiratory epithelial cell layer. Int Arch Allergy Immunol. 2015;166:291–6. doi: 10.1159/000430441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.