Abstract
Introduction
More than 90% of amyotrophic lateral sclerosis (ALS) patients have muscle cramps, and evidence-based treatments have not been available.
Methods
A multicenter double-blind placebo-controlled crossover trial of mexiletine 150 mg twice daily was conducted of ALS patients requesting treatment of symptomatic muscle cramps.
Results
Muscle cramp frequency was reduced in 18 of 20 patients; 13 reductions were attributed to treatment (P<.05). The average reduction was 1.8 cramps per day (a reduction from 5.3 with placebo to 3.5 with mexiletine) based on t tests. The estimated reduction of cramp severity was 15 units on a 100-unit scale (P=.01), from a baseline average of 46. No effect on fasciculations was noted. One patient discontinued the study because of dizziness, and another patient discontinued the study to start open-label mexiletine therapy. No serious adverse event occurred.
Discussion
Mexiletine is well-tolerated and effective medication for controlling the symptom of muscle cramps in ALS.
Keywords: amyotrophic lateral sclerosis, fasciculations, neuromuscular
Introduction
Muscle cramps affect more than 90% of persons with amyotrophic lateral sclerosis (ALS) (1,2). Muscle cramps are sudden, involuntary muscle contractions due to high-frequency motor unit discharges (3). Physiologically, the main mechanism behind muscle cramps in ALS is poorly characterized but may be due to an increase in persistent sodium ion (Na+) currents of lower motor neurons and changes in potassium ion conductance (4). The Na+ increase may be caused by axonal regeneration or collateral sprouting or by changes in Na+ channel gating. An alternative explanation may be passive membrane changes (5).
In 2003, quinine sulfate was the most frequently prescribed medication by ALS clinicians (6), but in 2006, the US Food and Drug Administration prohibited the marketing of quinine sulfate for muscle cramp treatment, citing a lack of documented effect and rare fatalities (7). Cochrane systematic review found low-quality evidence supporting a reduction in muscle cramps by quinine in contrast to the Food and Drug Administration's view (8). In 2008, while preparing for the present study, we surveyed 50 ALS clinicians in the United States (members of the Northeast ALS Consortium [NEALS]) regarding muscle cramp treatment. Of responders, 40 physicians prescribed baclofen and 38 prescribed gabapentin; several other agents were used, but only 3 physicians prescribed mexiletine. As of 2012, no consensus existed on how to treat muscle cramps in ALS (9,10).
Mexiletine is a Na+ channel blocker that reduces persistent sodium currents (11). Threshold tracking peripheral nerve excitability studies that evaluate the membrane properties of the axons at the stimulation site (12) have demonstrated that mexiletine reduces muscle cramps (13). Mexiletine also markedly reduced muscle cramps as a primary outcome measure in an open-label study of spinocerebellar ataxia type 3 (11). More recently, muscle cramps were measured as a secondary outcome in a study of mexiletine in ALS (14): Mexiletine was shown to markedly reduce muscle cramp severity and frequency while not having a measurable effect on ALS disease progression. This latter study did not only select patients who wanted treatment of muscle cramps. At any time, only a subset of ALS patients want medication treatment for muscle cramps, a fact that possibly reduces the applicability of the result (12). Factors determining whether an ALS patient wants treatment of muscle cramps are largely unknown, but more severe pain has been associated with the use of prescription medications (2).
Fasciculations are neuromuscular hyperexcitability phenomena and a hallmark symptom of ALS. No treatment today is proven to be effective against fasciculations. A membrane-stabilizing Na+ channel blocker such as mexiletine may reduce them, and this outcome has also been explored. At the time of this study, mexiletine had not been evaluated; now, a recent study provides evidence that it is ineffective (15).
Mexiletine is a medication approved by the US Food and Drug Administration (FDA) for the treatment of cardiac arrhythmias, with typical dosing for this indication between 200 and 400 mg 3 times per day. It has a half-life of 10 to 12 hours. Common dose-dependent adverse events have included gastrointestinal distress, lightheadedness, tremor, and coordination difficulties.
The present trial aimed to determine whether mexiletine reduces the frequency of muscle cramps and the severity of muscle cramps and fasciculations for patients with ALS.
Methods
A multicenter double-blind, within-patient crossover trial was conducted with 20 patients. The major inclusion criteria were ALS diagnosis by modified El Escorial criteria, Awaji version (clinically definitive, probable, or possible) (16); wish to treat muscle cramps with medication; and no contraindication to mexiletine, including heart and liver disease. Other medications taken for the expressed purpose of preventing muscle cramps were disallowed. Patients who had 2 or more cramps and were able to complete the diary for 1 week (screening epoch) were randomly assigned in a 1:1 ratio to mexiletine or placebo for their first epoch. The initial power calculation was based on an expected total of 30 patients with ALS entering this 2-treatment (placebo and mexiletine) crossover study and an expected <10% drop-out rate resulting in 27 patients completing the study. The primary statistical outcome of the trial was the within-subject difference in number of cramps on each treatment. Based on the gabapentin trial conducted by the WALS group (17), we had modeled that if the true difference between treatments was on average 1.2 cramps per day (23% reduction from 5.6 to 4.3 in treated vs no reduction in placebo), the trial would have an 80% power to detect a treatment difference using a 2-sided 5% significance level. This conclusion was based on the assumption that the within-patient standard deviation of the response variable was 1.55, the same within-patient standard deviation observed in the gabapentin trial. The study was halted at 23 patients randomly assigned because of slow enrollment, time constraints in the form of contract expiration and the larger cramp frequency effect size (−69%) seen by Weiss et al (14).
Randomization was stratified by riluzole use. The Excel random number generator (randbetween) was used to generate the sequence of randomized treatment assignments. The study statistician (D.M.) generated the allocation sequence, the blinded investigators and study personnel enrolled participants, and the blinded study pharmacist dispensed the interventions. After the 1-week screening period, patients were randomly assigned. For day 1 to day 3, patients took study-blinded capsules once at bedtime and for day 4 through day 14 took study capsules twice daily. Identical-appearing study capsules contained placebo or mexiletine 150 mg (prepared by University of Iowa Pharmaceuticals). Each treatment epoch was 2 weeks with an intervening 1-week washout, for a minimum trial duration of 6 weeks, including the screening week.
Electrocardiography and liver enzymes were tested for safety 3 times (screening epoch, end of epoch 1, and end of epoch 2). In larger populations with longer and higher exposure to mexiletine, disturbances in liver functions have been reported. Among patients with known cardiac conduction defects, electrocardiographic changes have been known to develop.
Patients were enrolled at the University of California sites Davis, Irvine, San Diego, and Los Angeles, and 1 patient participated by telemedicine. Telemedicine participation was offered to all ALS patients in California, via visits at a local physician's office using a videoconferencing system (Polycom Inc) to communicate with the University of California, Davis study team. A telemedicine patient's physical examination and safety monitoring could be performed at the patient's local physician's office. The telemedicine study was advertised through local support groups, newsletters from the ALS Association and the Muscular Dystrophy Association, by PatientsLikeMe, and through the research notification system from the Agency for Toxic Substances and Disease Registry throughout the state. In addition, the study was listed on ClinicalTrials.gov and online at trial recruitment sites, including NEALS and the ALS Therapy Development Institute.
The trial was approved by the University of California, Davis, Institutional Review Board in February 2013; the other University of California sites relied on University of California, Davis, as a central institutional review board. Trial enrollment was completed in December 2015. All patients signed written informed consent and received no stipend for study participation. All procedures were in accordance with the ethics standards of the responsible committee on human research and with the Helsinki Declaration of 1975, as revised in 1983. Data on race/ethnicity were collected to comply with National Institutes of Health guidelines; race/ethnicity was self-identified by the patients.
The primary outcome measure was daily muscle cramp count, completed by the patient at bedtime. All muscle cramps in the preceding 24 hours were recorded. Diary counts of muscle cramps have excellent face validity and have been shown to be responsive to clinical change (14). We also used a daily, self-administered 100-unit visual analog scale (VAS) to rate the severity of muscle cramps. This global severity rating scale was comprised of all aspects of the muscle cramps, including frequency, duration, and intensity. In addition, a global severity rating of fasciculations was collected and contained all aspects of fasciculations, including frequency and intensity. For global severity of muscle cramps and fasciculations, higher VAS numbers represented more severe symptoms. Muscle cramp severity scales have good face validity and have been shown to detect clinical change in several studies (14,18). Fasciculation ratings have been used previously (19). Patients were educated by study personnel and received an informational handout to help differentiate cramps, fasciculations, and spasticity.
Analysis
Data from each epoch were evaluated using 3 methods. Mean responses of drug versus placebo were compared using paired t test for each participant. Next, a linear mixed-effects model was fitted to estimate the treatment effects on the measures. The model included terms for fixed effects: a constant, a placebo, and a drug difference effect. Random effects were added to allow for different constants and different placebo and drug effects of each participant. The fixed drug difference effect was tested against zero to determine whether treatment with mexiletine reduced numbers of muscle cramps and severities of muscle cramps and fasciculations. Finally, data of each patient were condensed to mean and median for each treatment. We used the pkcross function (Stata Corp LP), specifically designed for crossover data, to test for treatment, period, and order of treatment effects. Analyses were performed in Stata software version 12.
Results
Baseline patient characteristics are summarized in Table 1. Of 28 patients who signed consent, 5 did not meet inclusion criteria because of past myocardial infarction (n=1), long QT syndrome (n=1), AV block (n=1), no muscle cramps (n=1), and hospitalization during the screening period that resulted in inability to complete a screening diary and follow-up (n=1) (Figure 1). Twenty-three patients were randomly assigned to treatment; 21 patients completed the study, but1 patient did not return the outcome diary, leaving 20 completers for analysis. Of the 2 patients who did not complete the study, 1 patient discontinued mexiletine because of dizziness. This symptom was mild, and the patient was willing to restart the study drug, but had not taken it for a period longer than allowed per protocol. Consequently, that patient was withdrawn. One patient had such marked improvement from baseline after the start of the blinded study drug that he was unwilling to withdraw from the drug during washout. This latter patient was prescribed open-label mexiletine and had continued suppression of muscle cramps. At the end of the study, it was confirmed that the patient had received active drug during his first epoch. A total of 5 patients reported mild adverse effects while taking mexiletine and 3 while taking placebo (Table 2). The recruitment period for the trial was July 2013 to December 2015. The protocol did not require that the ALS diagnosis was established in person at a University of California ALS clinic. However, all patients who enrolled in the trial had undergone such an evaluation.
Table 1. Baseline Characteristics of Enrolled Participants.
| Characteristic | Value | Range |
|---|---|---|
| Total Patients (N=23) | ||
| Age at enrollment, mean (SD), y | 62.4 (12.5) | 35-78 |
| Male sex, No. (%) | 15 (65) | |
| Hispanic ethnicity, No. (%) | 3 (13) | |
| Riluzole use, No. (%) | 15 (65) | |
| No. of cramps per 24 h | 7 (7) | 1-25 |
| Cramp severity, unita | 43 (23) | 8-95 |
| Fasciculation severity, unita | 32 (26) | 0-100 |
| Patients From Both Phases (n=20) | ||
| Age at enrollment, mean (SD), y | 62.6 (13.2) | 35-78 |
| Disease duration, mean (SD), mo | 33.6 (27.5) | 4-118 |
| ALSFRS-R score, mean (SD) | 33.2 (9.3) | 12-45 |
| Male sex, No. (%) | 14 (70) | |
| Hispanic ethnicity, No. (%) | 5 (25) | |
| No. of cramps per 24 h | 6 (6) | 1-24 |
| Cramp severity, unita | 46 (23) | 16-95 |
| Fasciculation severity, unita | 34 (26) | 0-100 |
| ALS diagnosis | ||
| Definitive | 11 (55) | |
| Probable | 6 (30) | |
| Possible | 3 (15) | |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, ALS Functional Rating Scale–Revised.
Severity rated on the 100-unit visual analog scale.
Figure 1.
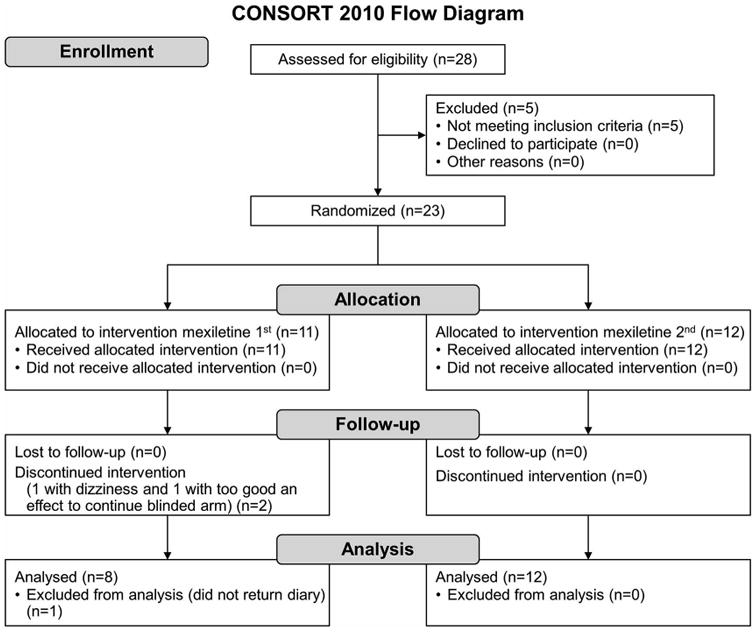
Flow Diagram of Patient Study Participation.
Table 2. Adverse Effects Reported in Each 14-day Treatment Epoch.
| Adverse Effect | Mexiletine, No. | Placebo, No. |
|---|---|---|
| Patient reporting any adverse effect | 5 | 3 |
| Fall | 1 | 1 |
| Dizziness/achy | 1 | 0 |
| Headache | 1 | 1 |
| Congestion | 0 | 1 |
| Chest tightness | 1 | 0 |
| Dry mouth | 1 | 0 |
| Increased urination | 1 | 0 |
| Itchiness | 1 | 0 |
| Nausea | 1 | 0 |
| Diarrhea | 1 | 0 |
| Total events | 9 | 3 |
Number of Muscle Cramps
Of the 20 available patients, 18 had reductions in the prespecified primary outcome measure of mean number of muscle cramps when taking mexiletine (Figure 2). For 13 patients, paired t tests showed statistically significant reductions (P<0.05; 9 had P<0.01). The chance probability of 18 of 20 reductions was <10−10. Two of the 20 patients had statistically significant increases of muscle cramp counts while receiving mexiletine. Individual patient estimates for treatment effect ranged from a reduction of 7 to an increase of 4 muscle cramps per day. The average reduction over the whole epoch was 1.8 muscle cramps per day (a reduction from 5.3 with placebo to 3.5 with mexiletine) based on paired t tests. These results were confirmed by the linear mixed-effects model. The standard deviation of the random effect for treatment was 2.3, indicating a high variation in treatment effect. A pkcross analysis of the mean number of muscle cramps for each patient showed a treatment effect (P=0.008) but no effect from period (P=0.39) or sequence (P=0.70) of treatments (Table 3). No sex effects were tallied; no age effects on cramps were found from mexiletine therapy, but older patients had more cramps (0.6 cramps/day per decade of age; P<0.001). Patients taking riluzole (15/20) had fewer cramps—0.19 per day—but the effect of mexiletine was unchanged. Four patients reported no muscle cramps at all during the last 3 days of their mexiletine epoch and 2 during their placebo epoch.
Figure 2.
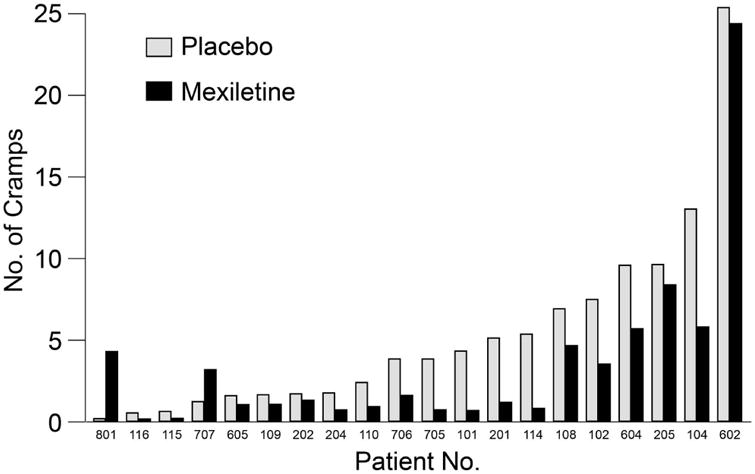
Number of Cramps by Patient and Treatment. Mean observed numbers of cramps over 24 hours during mexiletine and placebo treatments by patient.
Table 3. Responses of the 20 Participants by Treatment and Treatment Order.
| Treatment Sequence | No. of Patients | Treatment | ||
|---|---|---|---|---|
| Placebo | Mexiletine | Reduction | ||
| Mean (SD) number cramps/d | ||||
| Placebo first | 12 | 4.7 (4.2) | 2.5 (2.8) | 2.2 (2.2) |
| Mexiletine first | 8 | 6.1 (8.1) | 4.9 (8.0) | 1.2 (2.9) |
| Mean (SD) cramp severity/d, unita | ||||
| Placebo first | 12 | 44.7 (26.7) | 32.8 (27.7) | 12.0 (18.1) |
| Mexiletine first | 8 | 43.6 (31.0) | 23.1 (16.8) | 20.5 (40.6) |
| Mean (SD) fasciculation severity/d, unita | ||||
| Placebo first | 12 | 31.7 (26.7) | 31.7 (29.0) | −0.1 (8.5) |
| Mexiletine first | 8 | 25.8 (32.7) | 32.4 (34.4) | −6.6 (23.2) |
Severity rated on the 100-unit visual analog scale.
Muscle Cramp Severity
A reduction in muscle cramp severity, the secondary outcome, was shown in the linear mixed-effects model. The estimated reduction was 15 units on the VAS (P=0.01) compared with whole epochs. Analysis with the pkcross function confirmed this finding, based on mean severity of muscle cramps. The treatment effect was statistically significant (P=0.02), whereas neither period (P=0.53) nor sequence of treatments (P=0.46) was significant. The data are shown in Figure 3.
Figure 3.
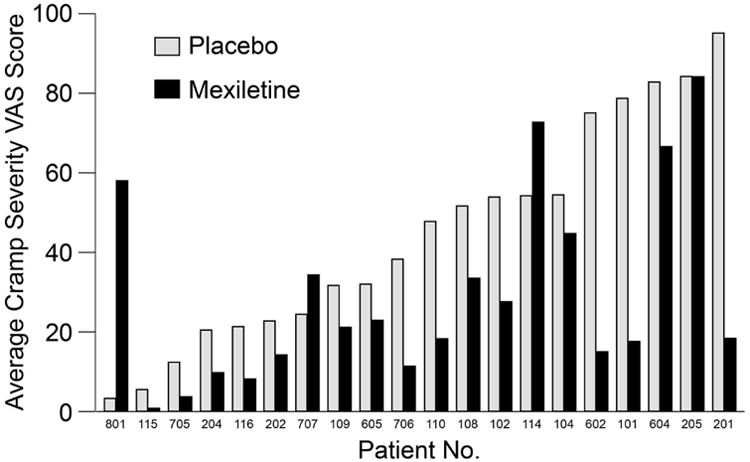
Average Muscle Cramp Severity by Patient and Treatment. VAS indicates visual analog scale (range, 1-100).
Fasciculation Severity
Neither the linear mixed-effects model nor pkcross found a treatment effect of mexiletine on fasciculation severity. The t test found 8 patients with reduced versus 12 with increased severity.
Safety
No statistically significant change occurred in QTc time. The mean (SD) QTc changed by 3.94 (24.3) ms (n=17; range, −27 to 55) with mexiletine versus 4.16 (26.1) ms (n=19; range, −36 to 83 ms) with placebo treatment. The longest QTc change occurred with placebo and was 83 ms. No clinically significant laboratory abnormalities were noted.
Discussion
We found a reduction of muscle cramps in patients with ALS when taking mexiletine at a dose of 150 mg, twice daily compared with placebo. Four patients became completely cramp-free and 18 of the 20 patients completing the trial recorded fewer muscle cramps when taking the dose studied in this trial. The studied dose was well tolerated, and dosing is considered low in the context of the drug's recommended maximal daily dose of 1,200 mg. The effect of mexiletine on cramps in ALS is consistent with results in a recently published paper from a longer, concurrent parallel arm mexiletine study by Weiss et al (14). While an effect of mexiletine on cramps was reported, this study also failed to show that mexiletine has a measurable effect in altering the disease course over a period as short as the present study duration of 2 weeks, making this an unlikely confounder. The notable differences between the study by Weiss et al (14) and the present study were that muscle cramp frequency was the primary outcome in this present study, a shorter treatment duration of 2 weeks was studied by our group versus a longer treatment duration of 12 weeks, and more restrictive inclusion criteria were used in the study by Weiss et al, including disease duration <3 years, and vital capacity >50%. In an attempt to make the results generalizable to the ALS clinic population with muscle cramps, we enrolled ALS patients who wanted medication treatment of muscle cramps and applied few exclusion criteria.
With regard to dosing, the number of participants exposed to the 150-mg, twice daily dose of mexiletine was 20 in both randomized placebo-controlled studies. Weiss et al (14) also evaluated a higher mexiletine dose of 900 mg per day, which was intolerable to 32% of patients but had an even greater effect in controlling muscle cramps. It is our opinion that a symptomatic cramp treatment should be well tolerated and restricted to patients with cramps that the patients believe warrant treatment, as in the present study. In contrast, a disease-modifying agent might be considered for use by patients even with tolerability problems. We suggest that in clinical practice, the 150-mg, twice daily dose is a reasonable starting dose on the basis of the experience of these 2 studies. This also conforms to our personal experience and practice pattern. The dose can be increased if necessary and anecdotally, and as shown in the study by Weiss et al, a higher dose may further reduce muscle cramps if the 150-mg, twice daily dose does not provide satisfactory results. Gradually increasing the dose by 150 mg every few days until effect, adverse effects, or the maximal approved dose (1,200 mg/day) is reached is a strategy that we have used clinically. Periodic review of the indication for continued therapy is advised, but our personal experience has been that many patients benefit from treatment until the end of their disease.
Prior randomized trials have evaluated muscle cramps as secondary outcomes in ALS trials. They include trials of riluzole (20,21), xaliproden (22), and gabapentin (17), but the results have been negative. The trial of tetrahydrocannabinol by Weber et al (19) is the only other prospective randomized trial conducted to evaluate cramps as the primary outcome measure. Their trial used a crossover study method similar to ours, but no effect of tetrahydrocannabinol was seen. The only positive previous trial with muscle cramps as a primary outcome measure in ALS, to our knowledge, is an open-label study of levetiracetam (18). The result of that study has not been replicated in a blinded study, and levetiracetam has not won widespread use (2). Riluzole, which also blocks persistent Na+ currents, has not been shown to have an effect on cramps in larger studies (20,21). Of particular interest, the combination of mexiletine and riluzole did not appear to have an effect different from what was seen with mexiletine alone in the present study. Differences between the effect of riluzole and of mexiletine on Na+ currents have been explored (15), but no definitive conclusions have been reached. This study suggests that modulation of persistent Na+ currents affected by mexiletine reduces cramp generation, as suggested by electrophysiologic studies (13). The recently FDA-approved drug edaravone was not studied; no known interactions between the 2 drugs exist. Edaravone would not be expected to have an effect on muscle cramps on the basis of its mechanism as a free radical scavenger, but no data have been published.
The lack of an effect of mexiletine on fasciculations is consistent with clinical observations, but unfortunately, this symptom currently has no effective treatment option. A recent study using the same dose of mexiletine also supports this finding of no effect of mexiletine on fasciculations (15).
The primary limitation of the present study is the small sample size. However, the crossover design enabled us to have sufficient power to detect the effect of mexiletine on cramp number per unit time. Crossover designs can have problems with confounding period and sequence effects, but in our situation, neither was significant. In the study design, the randomization and crossover among participants should reduce the risk of a bias from a differential progression of cramp symptoms, but cramp frequency is known to vary over time (1,2). This variability also may explain why 2 participants had increased cramps during the mexiletine phase of the trial. No period effect was observed statistically, arguing that this did not occur.
This study also measured the direct patient experience of muscle cramps and did not use an electrophysiologic biomarker such as threshold tracking, which was done in a prior study of mexiletine (13). Other types of involuntary muscle contractions or other symptoms may have been misidentified as muscle cramps by the participants in this study, and another objective outcome measure would have been useful to help validate the patient-reported primary outcome measure.
In another limitation of the study, detailed descriptive information was not collected regarding other symptomatic treatments of cramps or exacerbating factors. For example, we did not try to account for other factors that could affect reported muscle cramps over the short 2-week treatment period, such as specific activities or amount of hydration.
No quality-of-life (QoL) measures were obtained in this study. Although symptomatic treatment is aimed at improving the patient's QoL in a global sense, muscle cramps are not measured by generic QoL measures, including the 36-Item Short Form Survey or ALS-specific ones, such as Amyotrophic Lateral Sclerosis Assessment Questionnaire 5 or the ALS-Specific Quality of Life–Revised. Hence, we did not expect to be able to detect a drug effect using a QoL measure.
Our study provided 1 important methodological insight that may be of value beyond the ALS clinical trial community. Contrary to our expectation, only 1 patient participated through telemedicine, despite multiple recruitment strategies to promote such participation.
Mexiletine is an approved medication with more than 10,000 patients monitored in postmarketing studies. As a class Ib antiarrhythmic, it carries a black box warning common to all class I agents derived initially from the Cardiac Arrhythmia Suppression Trial (23). In that study of patients at 6 days to 2 years after myocardial infarction, increased rates of death and nonfatal cardiac arrest were seen among patients treated with the class Ic agents encainide and flecainide compared with rates seen in placebo-treated patients. This small, short-duration study does not contribute materially to the reported safety of the drug. Prior reports highlight that dose-related adverse events most commonly affect the central nervous system, producing dizziness, or gastrointestinal system, causing nausea or vomiting (24). Cardiac conduction is not generally affected for patients with normal conduction (as seen in this study), but in patients with cardiac conduction defects (excluded from this study), changes can occur (24).
In conclusion, a mexiletine dose of 150 mg orally twice daily was shown in a placebo-controlled randomized clinical trial to reduce the frequency of cramps over the first 2 weeks of treatment with infrequent adverse effects. Higher doses may provide additional benefit but more frequent adverse effects. Mexiletine is the only medication shown to reduce muscle cramp frequency and severity in ALS.
Supplementary Material
Acknowledgments
The trial was conducted by the following research coordinators: University of California, Davis, Michelle Cregan, CRA; University of California, Irvine, Brian Minton, CCRP; University of California, San Diego, Ivy Chippendale; and University of California, Los Angeles, Clara Sam, CRC.
Funding/Support and Role of the Sponsor: This trial was developed at the National Institute of Neurological Disorders and Stroke, a branch of the National Institutes of Health (NIH), Clinical Trials Methodology Course in 2008.
Support was provided by the University of California, Davis, Clinical and Translational Science Center; National Center for Research Resources UL1 RR024146 regulatory assistance; NIH UL1 TR 000002; and KL2 TR 000134. Additional study support was provided by the ALS Association 14CM190. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. None of the funding agencies were involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Data Access and Responsibility: Björn Oskarsson, MD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Björn Oskarsson, MD, takes responsibility for the integrity of the work as a whole, from inception to published article.
Resources: We acknowledge M. Weiss for helping procure the blinded study drug; University of Iowa Pharmaceuticals for providing study drug; University of California, Davis, Center for Health and Technology for providing facilities and services for the telemedicine patient; University of California, Davis, Institutional Review Board for providing assistance during the University of California Biomedical Research Acceleration, Integration, and Development, Institutional Review Board reliance development; the local ALS Association chapters in California (Greater Sacramento, Golden West, Irvine, and San Diego) for distributing recruitment material to patients; and the Western ALS Study Group for helping to organize the study. Recruitment for this study was in part made possible by the Agency for Toxic Substances and Disease Registry's National ALS Registry Research Notification Mechanism. (http://wwwn.cdc.gov/ALS/ALSClinicalResearch.aspx).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FDA
US Food and Drug Administration
- NEALS
Northeast ALS Consortium
- Na+
sodium ion
- QoL
quality of life
- VAS
visual analog scale
Footnotes
Ethical Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflict of Interest and Financial Disclosures: None of the authors have any conflict of interest to disclose.
The trial was registered on ClinicalTrials.gov, NCT01811355.
References
- 1.Caress JB, Ciarlone SL, Sullivan EA, Griffin LP, Cartwright MS. Natural history of muscle cramps in amyotrophic lateral sclerosis. Muscle Nerve. 2016 Apr;53(4):513–7. doi: 10.1002/mus.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens HE, Joyce NC, Oskarsson B. National study of muscle cramps in ALS in the USA. Amyotroph Lateral Scler Frontotemporal Degener. 2017 Feb;18(1-2):32–36. doi: 10.1080/21678421.2016.1245755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller TM, Layzer RB. Muscle cramps. Muscle Nerve. 2005 Oct;32(4):431–42. doi: 10.1002/mus.20341. [DOI] [PubMed] [Google Scholar]
- 4.Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994 Jul;72(1):349–59. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- 5.Tamura N, Kuwabara S, Misawa S, Kanai K, Nakata M, Sawai S, et al. Increased nodal persistent Na+ currents in human neuropathy and motor neuron disease estimated by latent addition. Clin Neurophysiol. 2006 Nov;117(11):2451–8. doi: 10.1016/j.clinph.2006.07.309. Epub 2006 Sep 25. [DOI] [PubMed] [Google Scholar]
- 6.Forshew DA, Bromberg MB. A survey of clinicians' practice in the symptomatic treatment of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003 Dec;4(4):258–63. doi: 10.1080/14660820310017344. [DOI] [PubMed] [Google Scholar]
- 7.Silver Spring; MD: 2006. [cited 2016 Nov 17]. FDA Orders Unapproved Quinine Drugs from the Market and Cautions Consumers About “Off-Label” Use of Quinine to Treat Leg Cramps. [Internet] Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108799.htm. [Google Scholar]
- 8.El-Tawil S, Al Musa T, Valli H, Lunn MP, El-Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database Syst Rev. 2010 Dec 8;(12):CD005044. doi: 10.1002/14651858.CD005044.pub2. Update in: Cochrane Database Syst Rev. 2015;4:CD005044. [DOI] [PubMed] [Google Scholar]
- 9.Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2010 Feb 23;74(8):691–6. doi: 10.1212/WNL.0b013e3181d0ccca. [DOI] [PubMed] [Google Scholar]
- 10.Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2012 Apr 18;(4):CD004157. doi: 10.1002/14651858.CD004157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai K, Kuwabara S, Arai K, Sung JY, Ogawara K, Hattori T. Muscle cramp in Machado-Joseph disease: altered motor axonal excitability properties and mexiletine treatment. Brain. 2003 Apr;126(Pt 4):965–73. doi: 10.1093/brain/awg073. [DOI] [PubMed] [Google Scholar]
- 12.Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998 Feb;21(2):137–58. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara S, Misawa S, Tamura N, Kanai K, Hiraga A, Ogawara K, et al. The effects of mexiletine on excitability properties of human median motor axons. Clin Neurophysiol. 2005 Feb;116(2):284–9. doi: 10.1016/j.clinph.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Weiss MD, Macklin EA, Simmons Z, Knox AS, Greenblatt DJ, Atassi N, et al. Mexiletine ALS Study Group. A randomized trial of mexiletine in ALS: safety and effects on muscle cramps and progression. Neurology. 2016 Apr 19;86(16):1474–81. doi: 10.1212/WNL.0000000000002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya K, Misawa S, Kimura H, Noto Y, Sato Y, Sekiguchi Y, et al. A single blind randomized controlled clinical trial of mexiletine in amyotrophic lateral sclerosis: efficacy and safety of sodium channel blocker phase II trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5-6):353–8. doi: 10.3109/21678421.2015.1038277. [DOI] [PubMed] [Google Scholar]
- 16.de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008 Mar;119(3):497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- 17.Miller RG, Moore D, Young LA, Armon C, Barohn RJ, Bromberg MB, et al. WALS Study Group, Western Amyotrophic Lateral Sclerosis Study Group. Placebo-controlled trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 1996 Dec;47(6):1383–8. doi: 10.1212/wnl.47.6.1383. [DOI] [PubMed] [Google Scholar]
- 18.Bedlack RS, Pastula DM, Hawes J, Heydt D. Open-label pilot trial of levetiracetam for cramps and spasticity in patients with motor neuron disease. Amyotroph Lateral Scler. 2009 Aug;10(4):210–5. doi: 10.1080/17482960802430773. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010 Oct;81(10):1135–40. doi: 10.1136/jnnp.2009.200642. [DOI] [PubMed] [Google Scholar]
- 20.Bensimon G, Lacomblez L, Meininger V ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994 Mar 3;330(9):585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 21.Bensimon G, Lacomblez L, Delumeau JC, Bejuit R, Truffinet P, Meininger V Riluzole/ALS Study Group II. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol. 2002 May;249(5):609–15. doi: 10.1007/s004150200071. [DOI] [PubMed] [Google Scholar]
- 22.Meininger V, Bensimon G, Bradley WR, Brooks B, Douillet P, Eisen AA, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004 Jun;5(2):107–17. doi: 10.1080/14660820410019602. [DOI] [PubMed] [Google Scholar]
- 23.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991 Mar 21;324(12):781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 24.Fenster PE, Comess KA. Pharmacology and clinical use of mexiletine. Pharmacotherapy. 1986 Jan-Feb;6(1):1–9. doi: 10.1002/j.1875-9114.1986.tb03442.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


