Abstract
Background
Desmoplastic small round cell tumor (DSRCT) is an aggressive soft tissue sarcoma affecting children and young adults with 5-year overall survival (OS) of approximately 20%. Despite generally poor prognosis, long-term survival does occur. However, no evidence-based system exists to risk-stratify patients at diagnosis.
Methods
We retrospectively reviewed all DSRCT cases diagnosed at our institution between January 2000 and September 2016. Demographics, diagnostic imaging, and clinical data were reviewed. Univariate and multivariate Cox proportional hazard modeling was used to evaluate associations between imaging characteristics and OS.
Results
There were 130 patients (85% male; median age at presentation: 21.2 y) with confirmed DSRCT and sufficient imaging and clinical information for analysis. Median 5-year OS was 28% (95%CI:19–37%). In univariate analysis, shorter OS was associated with presence of liver lesions (hazard ratio [HR] 2.1, 95%CI:1.28–3.45), chest lesions (HR 1.86, 95%CI:1.11–3.1), and ascites (HR 1.69, 95%CI:1.06–2.7). In multivariate analysis, liver involvement and ascites were predictive and were used to stratify risk (intermediate=no liver involvement or ascites; high=either liver involvement or ascites; very high=both liver involvement and ascites). Intermediate-risk patients had a 5-year survival of 61% (95%CI:40–76%) versus 16% (95%CI:6–29%) among high-risk patients and 8% (95%CI:1–29%) among very high risk patients.
Conclusion
Patients with DSRCT can be risk-stratified at diagnosis based on specific imaging characteristics.
Keywords: Desmoplastic Small Round Cell Tumor, DSRCT, Sarcoma, Pediatric surgery, Surgical oncology, Cancer staging
INTRODUCTION
Desmoplastic small round cell tumor (DSRCT) is a rare cancer arising from the peritoneal lining of the abdominal cavity. It predominantly affects male children and young adults, and has a highly aggressive clinical phenotype [1]. Tumors exhibit multilineage differentiation, with positive immunohistochemical staining for mesenchymal, epithelial, and neural markers [2, 3]. Additionally, a translocation (11:22) (p13:q12) which results in a functional fusion protein between Ewing sarcoma (EWS) and Wilms tumor (WT1) genes is present in greater than 95% of patients [1, 4, 5]. Patients characteristically present with widespread abdominal involvement, with frequent extra-peritoneal metastases [6]. Among the few large reported series of this rare disease, 5-year overall survival has been estimated at approximately 15–30% despite aggressive therapy [1, 7, 8].
When possible, multimodal treatment including complete macroscopic debulking surgery, chemotherapy, and adjuvant radiation has been shown to yield the best patient outcomes [6, 9]. Novel treatments for DSRCT have been proposed, including alternative chemotherapy regimens [10], hyperthermic intraperitoneal chemotherapy (HIPEC) [11], and radioimmunotherapy [12]. The benefits of these novel treatment options have not been established though [8], and overall survival in the most recent DSRCT series is similar to those described 20 years ago [1, 8].
Despite advances in diagnosis of DSRCT, no evidence-based clinical staging system has been established to guide patient and clinician decision making at diagnosis. A staging system has been proposed by Hayes-Jordan and colleagues, but this system has not been validated [11]. In this study, we propose an imaging based risk stratification system that predicts survival at the time of diagnosis.
METHODS
With institutional review board approval (#16-1506), patient records at our institution were queried for all cases of DSRCT diagnosed between January 2000 and September 2016. Cases were confirmed by pathology review including molecular genetic and immunohistochemical analyses. Cases were excluded if sufficient clinical data and/or diagnostic imaging was unavailable. All cases were included regardless of patient age. We obtained data on patient demographics, clinical presentation, diagnostic and treatment information, and survival. The completeness of cytoreductive surgery was determined based on careful review of operative reports. Length of follow up was defined as the time from diagnosis to the last known contact with the patient or patient death.
Cross sectional imaging acquired at the time of diagnosis was reviewed by an attending pediatric radiologist (AP). Thirty-one specific anatomic sites of potential tumor involvement were selected and examined for each patient with available imaging. These factors were selected based on clinical judgement of the investigators and a previous report of imaging characteristics of DSRCT [13]. “Any liver lesion” was defined as serosal or parenchymal liver lesions, and “any chest lesion” was defined as any solid lesion in the thoracic cavity.
Statistical Analyses
To assess survival in our cohort in the context of previously published studies, overall survival from diagnosis (OSfD) was calculated from the date of diagnosis until death. Patients with complete imaging data and who underwent surgery were included in formal statistical analyses (n=113). Overall survival (OS) was also calculated from the time of surgery until death. Patients who were alive at last follow up were censored. Annual and median survival estimates were provided along with 95% CI and Kaplan Meier (KM) plots. To allow for stable estimates, only those imaging factors present in at least 10 patients were included in analysis. Univariable and multivariable Cox proportional hazards regression were used to assess the relationship between imaging factors and OS. Factors with p-values less than or equal to 0.05 were considered for multivariable analyses. The proportional hazards assumption was checked with Schöenfeld residuals [14]. Informative missingness was checked with the log-rank test and with a missing indicator in the Cox analyses (data not shown) [15, 16].
To build the multivariable model, backwards selection was used with all variables significant on univariate analyses entered and removal criteria of 0.05. Selection methods were employed to prevent overfitting given the number of factors versus events and due to the collinearity of the imaging features. Discrimination was assessed with a bootstrap adjusted concordance probability estimate (CPE) with 500 iterations. Based on the selected model, a risk score system was developed. KM plots and estimates were used to display the findings of the scoring system. P-values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
RESULTS
Patient and Treatment Characteristics
A total of 157 patients with DSRCT diagnosed during the study period were identified, and 130 patients had diagnostic cross sectional imaging available for review. Of these, 113 patients had complete imaging data and were included in survival analysis. Among the cohort of 130 patients initially identified, the median age was 21 years (range: 6–62). The majority of patients were male (110/130, 85%) and white (88/130, 68%). Median follow up time for the patients alive at last follow up was 30.3 months (range: 1.0–198.5 months). The most common presenting symptoms were pain (53%) and mass (20%). The majority of patients underwent surgery (123/130, 95%), with 95 achieving gross total resection (77%), 20 with subtotal debulking (16%), and 8 with unknown extent of surgery (7%) (Table 1). Thirty-three factors including two combinatorial variables were assessed on imaging (Table 2). Lesions in the thoracic cavity were present in 81 (62.3%), liver lesions in 71 (54.6%), and ascites in 54 (41.5%).
Table 1.
Imaging characteristics examined and frequency at diagnosis
| Imaging Feature | Number with feature (%) |
|---|---|
| Chest | |
| Lung | 6 (4.6) |
| Pleura | 6 (4.6) |
| Internal Mammary LN | 26 (20) |
| Supradiaphragmatic LN | 70 (53.8) |
| Supraclavicular LN | 14 (10.8) |
| Mediastinal LN | 15 (11.5) |
| Any Chest Lesion | 81 (62.3) |
| Pleural Effusion | 17 (13.1) |
| Abdomen | |
| Infradiaphragmatic LN | 2 (1.5) |
| Mesenteric Mass | 121 (93.1) |
| Omental Caking | 61 (46.9) |
| Umbilical Mass | 14 (10.8) |
| Peritoneal Implants | 52 (40) |
| Retroperitoneal LN | 81 (62.3) |
| Liver Parenchyma | 35 (26.9) |
| Liver Serosa | 64 (49.2) |
| Any Liver Lesion | 71 (54.6) |
| Porta hepatis | 46 (35.4) |
| Falciform | 27 (20.8) |
| Ascites | 54 (41.5) |
| Spleen Parenchyma | 11 (8.5) |
| Spleen Serosa | 37 (28.5) |
| Spleen Hilum | 39 (30) |
| Max. Abdominal Mass Size, cm (Median (range)) | 9.7 (1.8–24.9) |
| Pelvis | |
| Pelvic lesion | 105 (80.8) |
| Pelvic LN | 75 (57.7) |
| Retrovesicular mass | 99 (76.2) |
| Retrovesicular Mass Size, cm (Median (range)) | 8.7 (2.2–18.3) |
| Sigmoid Encasement | 7 (5.4) |
| Other Features | |
| Bone | 6 (4.6) |
| Calcifications | 20 (15.4) |
Table 2.
Patient and treatment characteristics (n=130)
| N (%) | ||
|---|---|---|
| Patient Details | ||
| Age at Diagnosis, years | Median (range) (N=130) | 21.2 (5.9–62.3) |
| Gender | Male | 110 (84.6) |
| Female | 20 (15.4) | |
| Race | White | 88 (67.7) |
| Black | 16 (12.3) | |
| Hispanic | 10 (7.7) | |
| Asian | 5 (3.8) | |
| Unknown | 11 (8.5) | |
| Presenting Symptom* | Abdominal/Flank/Back pain | 74 (53.2) |
| Mass | 28 (20.1) | |
| Urinary | 6 (4.3) | |
| Constipation | 10 (7.2) | |
| Other | 11 (7.9) | |
| None/Unknown | 10 (7.2) | |
| Molecular Confirmation | Confirmed translocation | 108 (83.1) |
| Test not Performed | 21 (16.2) | |
| Negative | 1 (0.8) | |
| Treatment Details | ||
| Surgical Status | Gross total resection | 95 (73.1) |
| Subtotal debulking | 20 (15.4) | |
| Debulking with unknown completeness | 8 (6.2) | |
| No surgery | 7 (5.4) | |
| Radiation Treatment | Consolidation radiation | 59 (45.4) |
| None | 63 (48.5) | |
| Unknown radiation treatment | 8 (6.2) | |
| HIPEC | 3 (2.3) | |
| Intraperitoneal radioimmunotherapy | 31 (23.8) | |
Numbers represent frequency with percent of total number of patients (N=130) in parentheses unless otherwise specified.
Symptoms include total number of presenting symptoms, which was greater than total number of patients.
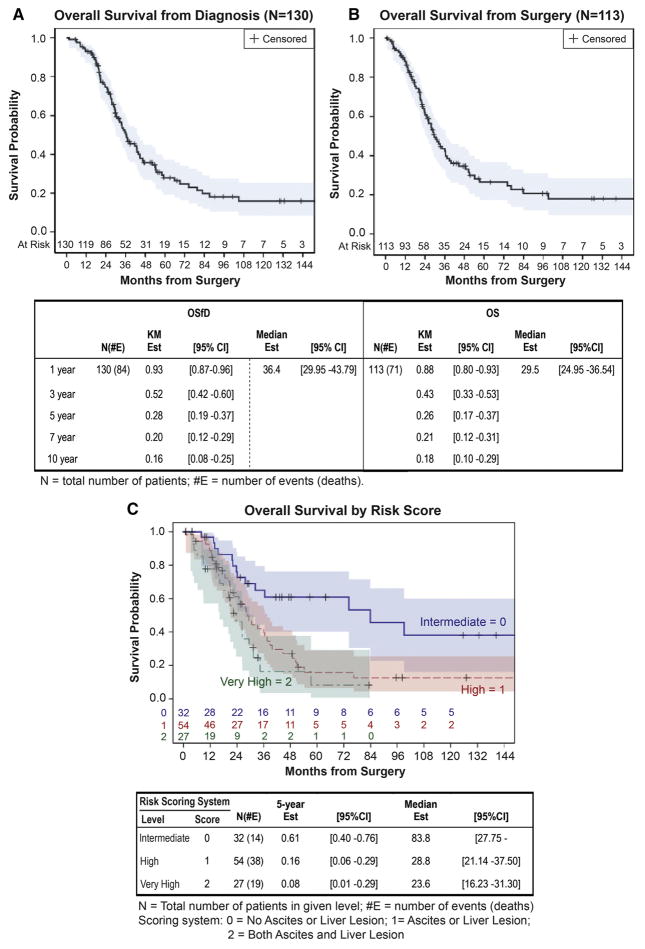
Overall Survival Estimates
For all 130 patients, 84 patients had died by the end of follow up and the median survival estimate from diagnosis was 36 months (95%CI: 30–44 months). Five-year and 10-year survival estimates were 28% (95%CI: 19–37%) and 16% (95%CI: 8–25%), respectively (Figure 1A). In the surgical cohort with complete imaging data (N=113), 71 patients died by the end of follow up with a median survival estimate from surgery of 30 months (95%CI: 25–37 months). Five- and 10-year estimates were 26% (95%CI: 17–37%) and 18% (95%CI: 10–29%), respectively (Figure 1B). No events occurred in the 7 patients at risk after 10 years.
Figure 1.
Kaplan Meier survival plots for (A) overall survival from diagnosis (OSfD), (B) from time of debulking surgery (OS), and (C) overall survival stratified by risk scoring system.
Univariate Analysis
The cohort included 113 patients with complete imaging that were included in survival analysis. Twenty-four imaging characteristics were assessed for their association with overall survival (Table 3). Patients with any chest lesion (HR: 1.86, 95%CI: 1.11–3.1, p=0.018), internal mammary lymphadenopathy (HR: 1.89, 95%CI: 1.1–3.23, p=0.020), supradiaphragmatic lymphadenopathy (HR: 1.65, 95%CI: 1.02–2.67, p=0.042), any liver lesion (HR: 2.1, 95%CI: 1.28–3.45, p=0.003), liver serosal lesions (HR: 2.03, 95%CI: 1.25–3.29, p=0.004), liver parenchymal lesions (HR: 2.14, 95%CI: 1.28–3.57, p=0.004), porta hepatis lesions (HR: 1.92, 95%CI: 1.19–3.1, p=0.007), ascites (HR: 1.69, 95%CI: 1.06–2.7, p=0.029), retrovesicular mass (HR: 1.98, 95%CI: 1.09–3.57, p=0.024), or pelvic lymphadenopathy (HR: 1.63, 95%CI: 1–2.67, p=0.050) were at a higher risk of death compared to patients without these features. No other factors were significantly associated with survival (p=0.09–>0.95).
Table 3.
Univariable and Multivariable Cox Proportional Hazards Analyses (N=113)
| Univariable | Final Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Chest | ||||
| Supraclavicular LN | 1.33 (0.68–2.62) | 0.41 | ||
| Internal Mammary LN | 1.89 (1.1–3.23) | 0.020 | ||
| Mediastinal LN | 1.72 (0.89–3.31) | 0.11 | ||
| Supradiaphragmatic LN | 1.65 (1.02–2.67) | 0.042 | ||
| Any Chest Lesion | 1.86 (1.11–3.1) | 0.018 | ||
| Pleural Effusion | 1.68 (0.88–3.22) | 0.12 | ||
| Abdomen | ||||
| Omental Caking | 1 (0.62–1.6) | >0.95 | ||
| Umbilical Mass | 1.82 (0.9–3.68) | 0.09 | ||
| Peritoneal Implants | 1.42 (0.88–2.29) | 0.15 | ||
| Liver Parenchyma | 2.14 (1.28–3.57) | 0.004 | ||
| Liver Serosa | 2.03 (1.25–3.29) | 0.004 | ||
| Any Liver Lesion | 2.1 (1.28–3.45) | 0.003 | 2.06 (1.25–3.38) | 0.004 |
| Porta hepatis | 1.92 (1.19–3.1) | 0.007 | ||
| Falciform | 1.55 (0.91–2.63) | 0.11 | ||
| Ascites | 1.69 (1.06–2.7) | 0.029 | 1.64 (1.02–2.62) | 0.040 |
| Spleen Parenchyma | 1.92 (0.91–4.03) | 0.09 | ||
| Spleen Serosa | 1.42 (0.85–2.39) | 0.18 | ||
| Spleen Hilum | 1.37 (0.83–2.25) | 0.22 | ||
| Retroperitoneal LN | 1.47 (0.9–2.39) | 0.12 | ||
| Max. Mass Size, cm | 0.97 (0.92–1.02) | 0.29 | ||
| Pelvis | ||||
| Pelvic | 1.71 (0.89–3.26) | 0.11 | ||
| Pelvic LN | 1.63 (1–2.67) | 0.050 | ||
| Retrovesicular Mass | 1.98 (1.09–3.57) | 0.024 | ||
| Other | ||||
| Calcifications | 1.1 (0.59–2.04) | 0.77 | ||
Hazard ratio represents comparison of Present to Absent with Absent as the reference group for all factors other than size.
HR=Hazard ratio; CI = confidence interval.
We also assessed if any demographic factors were associated with OS. No associations were found for gender (HR: 1.23, 95%CI: 0.63–2.4, p=0.54), age (HR: 1.07, 95%CI: 0.67–1.71, p=0.78), or race [(African American HR: 0.82, 95%CI: 0.41–1.68, p=0.59), (Hispanic HR: 1.54, 95%CI: 0.48–4.95, p=0.47), (Asian HR: 2.35, 95%CI: 0.93–5.95, p=0.07), white as reference].
Multivariate analysis
Due to the known combinatorial nature of “any liver lesion” and “any chest lesion” these variables were considered for multivariable analysis without the component variables used to define them. The six significant univariate factors were entered into a backward selection model. After selection, only “any liver lesion” (HR: 2.06, 95%CI: 1.25–3.38, p=0.004) and presence of ascites at diagnosis (HR: 1.64, 95%CI: 1.02–2.62, p=0.040) were associated with overall survival. The bias adjusted CPE for this model was 0.61 (95%CI: 0.54–0.66) (Table 3).
Both ascites and any liver lesion were assigned 1 point to the scoring system, creating a possible score of 2 points. Using the existing dataset, 32 patients had 0 points (intermediate risk), 54 patients had 1 point (high risk), and 27 patients had 2 points (very high risk). The 5-year survival probability was 61% (95%CI: 40–76) for 0 points, 16% (95%CI: 6–29%) for 1 point, and 8% (95%CI: 1–29%) for 2 points (Figure 1C).
DISCUSSION
This study represents the largest series to date of patients with desmoplastic small round cell tumor. It is also the first to establish a statistically robust risk stratification system for the disease. Cancer staging systems are of paramount importance to both patients and clinicians, especially at the time of diagnosis. Robust staging systems quantify prognosis, determine appropriate treatment and establish comparable patient groups that may be studied in a standardized fashion [17].
The ability to quantify prognosis affects patient and family decisions, especially with respect to pursuing aggressive therapy [18]. Until now, all patients with a diagnosis of DSRCT have received a uniformly poor prognosis of approximately with 20% overall survival at 5 years. Our review demonstrates that distinct groups of patients with better and worse survival outcomes can be identified based on cross-sectional imaging findings at diagnosis. When employed in a clinical setting, this risk stratification system can substantially contribute to patient and clinician expectations and inform decision making. Additionally, this risk stratification system has the potential to guide research, especially clinical trials that to this point have included low numbers of patients with diverse clinical characteristics.
The demographic characteristics of patients included in our study are similar to previously published studies, with a preponderance of white male patients, and a median age of diagnosis of approximately 20 years with a wide range of patient ages [1, 6, 7, 9, 19–21]. Our study also substantiates previous reports that show that the vast majority of DSRCT patients have dominant disease in the abdominal cavity with common sites of extraperitoneal metastases in retroperitoneal lymph nodes, liver, spleen, and chest.
Compared to other published series, our study identifies a significantly higher percentage of patients with disease in sites other than the peritoneal lining of the abdominal cavity. This is especially true for thoracic disease and liver involvement which were 49% and 43% respectively. In a series of 38 patients described by Honoré and colleagues 37% of patients had liver metastases and 13.2% had lung metastases [6]. Similarly Schwarz et al. described 31% of patients with liver metastases and 16% of patients with chest disease in their series of 32 patients [22]. Our study likely exhibits higher rates of these characteristics due to the focused nature of our imaging evaluation protocol. Previous studies have defined types of metastases with broad categories like “extraperitoneal metastases” [6] or “extraabdominal metastases” [11] that mask the specific features of metastatic lesions. Other studies have used only a few specific terms such as lung or pleural metastases without identifying more subtle but more characteristic findings such as internal mammary lymphadenopathy. Additionally, previous studies that include descriptions of extent of disease rarely describe the methodology used to determine extent of disease. When limited to specific metastasis categories, such as liver parenchyma metastases (present in 27% of patients in our cohort) and lung metastases (present in 5% of our cohort), the frequency of metastatic lesions in this series is similar to those published in previous reports.
Our institution has previously described common imaging features characteristic to DSRCT [13]. The current study builds on that work by describing the frequency of each of these features in a large cohort of patients, and describing the ability of each to predict survival (Table 3). Our comprehensive radiologic review of this cohort allowed us to examine specific features of metastatic disease (e.g. serosal vs. parenchymal lesions) and their variable influence on survival.
A number of factors were predictive of survival in univariate analysis including multiple sites in the thoracic cavity as well as other factors associated with liver involvement (Table 3). Interestingly, all variables related to disease in the chest that were significant in univariate analysis did not retain significance in the multivariate model. Both serosal and parenchymal liver lesions were shown to be strongly predictive of survival. In order to develop a parsimonious multivariate model, these variables were combined into a single variable for any liver lesion, which was highly significant in univariate and multivariate analysis. This finding reflects the difficulty of effectively treating DSRCT in the liver. Presence of ascites was also a strong predictor of survival in univariate analysis and remained significant in the multivariate model independent of liver involvement, likely signaling the systemic severity of extensive disease. Comorbidities were not examined in our analysis as patients in the cohort were generally young and without other medical conditions at the time of diagnosis.
We used our multivariate model to create a scoring system for risk stratification at diagnosis (Figure 1C). Patients with neither ascites nor liver lesions were assigned a score of 0 (intermediate risk), patients with either of these features were assigned a score of 1 (high risk), and patients with both ascites and liver lesions were assigned a score of 2 (very high risk). While the 5-year survival estimates are quite disparate between intermediate (61%), high (16%), and very high risk patients (8%), the confidence intervals for the survival estimates of high and very high risk moderately overlap. Given that the scoring system was developed on this same data, the significance of these differences could not be evaluated here, but the similarity between high and very high risk patients may suggest that a meaningful difference is not present. However, the number of patients is small, and the current study may be underpowered to detect a difference between these groups. Testing and validation with an external data set is necessary to establish the significance of this scoring system.
A staging system for DSRCT was first proposed by Hayes-Jordan and colleagues [11]; however, this system is limited by the fact that it was based on a small number (n= 24) of heterogeneous patients. This staging system has not yet been validated and did not predict survival when utilized in an analysis by Honoré and colleagues [8]. The risk stratification system proposed in our study is derived from multivariate analysis in a relatively large cohort of similar patients. We believe our system builds on the work of Hayes-Jordan and colleagues and can contribute to the management and study of this patient group.
Our study had several limitations. The retrospective design made for inherent biases in selection and treatment. Further, our study took place at a single tertiary center, which may not be generalizable to all sites and patient groups.
In summary, this study represents the largest cohort of DSRCT patients reported to date, along with the most comprehensive radiologic review of extent of disease at diagnosis. We demonstrate that the presence of liver lesions and/or ascites at the time of diagnosis can stratify patients into intermediate, high, and very high risk groups. With this system, we have shown that there is a sizable group of patients diagnosed with DSRCT that have survival outcomes that are much better than the previously established 5-year survival of approximately 20%. It is important to note that patients surviving past 5 years cannot be considered cured as some patients in all risk groups suffered recurrence and death after this time point. As a result of our findings, we recommend that all patients diagnosed with DSRCT receive full-body cross sectional imaging with PET/CT scan to carefully evaluate the extent of disease at diagnosis both to direct treatment and to facilitate risk stratification.
Acknowledgments
Funding: Research at Memorial Sloan Kettering is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
Conflict of Interest Disclosure: The authors declare that they have no financial conflicts of interest.
Level of Evidence: Level IV
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerald WL, Ladanyi M, de Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16:3028–3036. doi: 10.1200/JCO.1998.16.9.3028. [DOI] [PubMed] [Google Scholar]
- 2.Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499–513. [PubMed] [Google Scholar]
- 3.Ordonez NG, el-Naggar AK, Ro JY, et al. Intra-abdominal desmoplastic small cell tumor: a light microscopic, immunocytochemical, ultrastructural, and flow cytometric study. Hum Pathol. 1993;24:850–865. doi: 10.1016/0046-8177(93)90135-4. [DOI] [PubMed] [Google Scholar]
- 4.Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–2840. [PubMed] [Google Scholar]
- 5.de Alava E, Ladanyi M, Rosai J, et al. Detection of chimeric transcripts in desmoplastic small round cell tumor and related developmental tumors by reverse transcriptase polymerase chain reaction. A specific diagnostic assay. Am J Pathol. 1995;147:1584–1591. [PMC free article] [PubMed] [Google Scholar]
- 6.Honore C, Amroun K, Vilcot L, et al. Abdominal desmoplastic small round cell tumor: multimodal treatment combining chemotherapy, surgery, and radiotherapy is the best option. Ann Surg Oncol. 2015;22:1073–1079. doi: 10.1245/s10434-014-4123-6. [DOI] [PubMed] [Google Scholar]
- 7.Bent MA, Padilla BE, Goldsby RE, et al. Clinical Characteristics and Outcomes of Pediatric Patients with Desmoplastic Small Round Cell Tumor. Rare Tumors. 2016;8:6145. doi: 10.4081/rt.2016.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honore C, Atallah V, Mir O, et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One. 2017;12:e0171639. doi: 10.1371/journal.pone.0171639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lal DR, Su WT, Wolden SL, et al. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005;40:251–255. doi: 10.1016/j.jpedsurg.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Aguilera D, Hayes-Jordan A, Anderson P, et al. Outpatient and home chemotherapy with novel local control strategies in desmoplastic small round cell tumor. Sarcoma. 2008;2008:261589. doi: 10.1155/2008/261589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes-Jordan A, Green H, Fitzgerald N, et al. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. J Pediatr Surg. 2010;45:1000–1006. doi: 10.1016/j.jpedsurg.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Hayes-Jordan A, LaQuaglia MP, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg. 2016;25:299–304. doi: 10.1053/j.sempedsurg.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora VC, Price AP, Fleming S, et al. Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol. 2013;43:93–102. doi: 10.1007/s00247-012-2485-0. [DOI] [PubMed] [Google Scholar]
- 14.Schoenfeld D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika. 1982;69:239–241. [Google Scholar]
- 15.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–165. doi: 10.2147/CLEP.S129785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton A, Altman DG. Missing covariate data within cancer prognostic studies: a review of current reporting and proposed guidelines. Brit J Cancer. 2004;91:4–8. doi: 10.1038/sj.bjc.6601907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 19.Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14:1526–1531. doi: 10.1200/JCO.1996.14.5.1526. [DOI] [PubMed] [Google Scholar]
- 20.Lae ME, Roche PC, Jin L, et al. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002;26:823–835. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lettieri CK, Garcia-Filion P, Hingorani P. Incidence and outcomes of desmoplastic small round cell tumor: results from the surveillance, epidemiology, and end results database. J Cancer Epidemiol. 2014;2014:680126. doi: 10.1155/2014/680126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz RE, Gerald WL, Kushner BH, et al. Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol. 1998;5:416–422. doi: 10.1007/BF02303860. [DOI] [PubMed] [Google Scholar]