Abstract
Background
In uncommon tremor disorders, clinical efficacy and optimal anatomical targets for Deep Brain Stimulation (DBS) remain inadequately studied and insufficiently quantified.
Methods
We performed a systematic review of PubMed.gov and ClinicalTrials.gov. Relevant articles were identified using the following keywords: “tremor,” “Holmes tremor,” “orthostatic tremor,” “multiple sclerosis,” “multiple sclerosis tremor,” “neuropathy,” “neuropathic tremor,” “fragile X–associated tremor/ataxia syndrome,” and “fragile X.”
Results
We identified a total of 263 cases treated with DBS for uncommon tremor disorders. Of these, 44 had Holmes tremor (HT), 18 orthostatic tremor (OT), 177 multiple sclerosis (MS)-associated tremor, 14 neuropathy-associated tremor, and 10 fragile X–associated tremor/ataxia syndrome (FXTAS). DBS resulted in favorable, albeit partial, clinical improvements in HT cases receiving Vim-DBS alone or in combination with additional targets. A sustained improvement was reported in OT cases treated with bilateral Vim-DBS, while the two cases treated with unilateral Vim-DBS demonstrated only a transient effect. MS-associated tremor responded to dual-target Vim-/VO-DBS, but the inability to account for the progression of MS-associated disability impeded the assessment of its long-term clinical efficacy. Neuropathy-associated tremor substantially improved with Vim-DBS. In FXTAS patients, while Vim-DBS was effective in improving tremor, equivocal results were observed in those with ataxia.
Conclusions
DBS of select targets may represent an effective therapeutic strategy for uncommon tremor disorders, although the level of evidence is currently in its incipient form and based on single cases or limited case series. An international registry is therefore warranted to clarify selection criteria, long-term results, and optimal surgical targets.
Keywords: DBS, tremor, FXTAS, OT, Holmes, multiple sclerosis, neuropathy
INTRODUCTION
Tremor, defined as an involuntary, rhythmic, oscillatory movement of a body part [1] is a key semeiological feature of Parkinson disease (PD) and essential tremor (ET) [2, 3], as well as the cardinal symptom of less common but equally disabling movement disorders, namely Holmes tremor (HT), orthostatic tremor (OT), and fragile X–associated tremor/ataxia syndrome (FXTAS) [1, 4]. In addition, tremor represents a secondary yet disabling feature of immune-mediated neurological disorders such as multiple sclerosis (MS) and demyelinating peripheral neuropathies [1].
Tremulous disorders are classified basing on two axes, consisting of (a) the clinical and semeiological characteristics of tremor and (b) the underlying etiology [1]. HT is a rest, postural, and intention low frequency (<5 Hz) tremor [1] usually associated with an ischemic or demyelinating lesion of the cerebello-thalamo-cortical or dentate-rubro-olivary pathways, with superimposed nigrostriatal dysfunction [1, 5, 6]. OT is a 13-18 Hz tremor affecting the weight-bearing limbs and resulting in a sensation of unsteadiness and imbalance when standing [1, 4, 7]. Although the exact location of its generator remains unclear, available evidence suggests the involvement of the cerebellum and pons [7, 8]. MS-associated tremor is a heterogeneous syndromic entity that includes at least 3 different subtypes of tremor [1, 4]: a 4-5 Hz cerebellar tremor predominantly involving the upper extremities; a 3-5 Hz ataxic tremor of the head (“head titubation”) and the trunk; and a 7-12 Hz mild-amplitude postural hand tremor [9]. Neuropathy-associated tremor is a posture and intention 3-6 Hz tremor predominantly involving the upper limbs [1, 10], and possibly associated with an altered peripheral sensory input, which might prevent the cerebellum ability to correct limb position and velocity during voluntary movements [11, 12]. Finally, FXTAS-associated tremor is a posture and intention tremor associated with ataxia, parkinsonism, cognitive decline, peripheral neuropathy, autonomic dysfunction, and psychiatric disorders [13, 14], and caused by a trinucleotide repeat expansion in the premutation range (CGG block lengths 55–200) in the FMR1 gene.
While pharmacological therapies, mostly beta-blockers, benzodiazepines, and anticonvulsants represent the first line of treatment for common tremor disorders [15] uncommon tremors may be refractory to oral medications and, in selected cases, require advanced surgical treatments such as deep brain stimulation (DBS) [16, 17]. The risk/benefit profile of DBS in uncommon tremor disorders, however, remain to be clarified.
In this review, we sought to analyze the literature related to the safety and efficacy of DBS in uncommon tremor disorders, discussing clinical indications, anatomical targets, and programming strategies.
MATERIALS AND METHODS
We reviewed the literature for studies reporting the outcome of DBS in patients with HT, OT, MS-associated tremor, neuropathy-associated tremor, or FXTAS. Relevant articles were identified through electronic search of PubMed.gov and ClinicalTrials.gov using the following keywords: “tremor,” “Holmes tremor,” “orthostatic tremor,” “multiple sclerosis,” “multiple sclerosis tremor,” “neuropathy,” “neuropathic tremor,” “fragile X– associated tremor/ataxia syndrome,” and “fragile X.” No language restrictions were applied.
We selected studies involving humans. There were no restrictions applied to gender, age, disease duration, or disease severity. The data extracted included the following: a) DBS targets including, but not limited to, ventral intermediate (Vim) nucleus, ventro-lateral (VL), ventralis oralis anterior (VOA) and ventralis oralis posterior (VOP) nuclei, posterior subthalamic area (PSA), subthalamic nucleus (STN), globus pallidus pars interna (GPi), and zona incerta (ZI); b) magnitude of response, according to the available presentation of clinical data; c) follow-up duration; d) adverse events (AEs); and e) stimulation settings.
RESULTS
A total of 263 cases of uncommon tremor disorders treated with DBS were identified through a systematic review of PubMed.gov and ClinicalTrials.gov. Of these, 44 had Holmes tremor (HT); 18 had orthostatic tremor (OT); 177 had multiple sclerosis (MS)-associated tremor; 14 had neuropathy-associated tremor; and 10 had fragile X–associated tremor/ataxia syndrome (FXTAS).
Holmes tremor
We identified 24 studies (17 single case reports and 7 case series), which totaled 44 patients treated with DBS for HT (Table 1).
Table 1.
Summary of literature data on Holmes Tremor cases treated with Deep Brain Stimulation.
| Author | No. of patients | Target | Follow up | Voltage | Pulse width (μs) | Frequency (Hz) | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Kudo et al. | 1 | Bilateral Vim | N/A | Rt. 2.2 & Left 1.7 | 100 | 120–150 | Almost complete disappearance of tremor | Transient dysarthria |
| Pahwa et al. | 1 | Unilateral Vim | 10 m | 3.7 | 90 | 170 | Improvement in postural & resting tremor | Patient developed infection over the IPG site. |
| Romanelli et al. | 1 | Unilateral Vim + STN | 2 yrs. | Vim 4.3 & STN 2.0 | Vim 90 & STN 90 | Vim 185 & STN 145 | Combined Vim-STN stimulation resolved all components of tremor | No peri- & post-operative complications were observed. |
| Samadani et al | 1 | Unilateral Vim | N/A | 2.5 | 90 | 185 | 57% increase in dexterity, 4 points decrease in functional disability in TRS | Not Reported |
| Nikkhah et al. | 2 | Unilateral Vim | 7 & 6 m respectively | 2.4/3.4 | 60/90 | 130/130 | Tremor resolved by 80% along with improvement in dystonic symptoms | Transient facial paresthesia |
| Piette et al. | 1 | Unilateral Vim | 18 m | N/A | N/A | N/A | Tremor improvement | Not Reported |
| Goto et al. | 1 | Unilateral Vim + GPi pallidotomy | 24 m (Vim- DBS) and 12 m (pallidotomy) | 3.4 | 90 | 160 | Tremor abolished | No peri- & post-operative complications were observed. |
| Foote et al. | 3 | Unilateral Vim/VOP border + Unilateral VOA/VOP border | 12, 6 & 8 m respectively | Vim 4.1/3.0/3.6 VOA 4.0/3.1/2.9 | Vim 90/60/120 VOA 90/60/90 | Vim 185/160/185 VOA 185/145/135 | Variable improvement in total TRS (38.4% – 66.67%) | Not Reported |
| Lim et al. | 1 | Unilateral Vim/VOA + GPi | 8 m | Vim 3.6, Vo 3.5 & GPi 6.0 | Vim 150, Vo 90 & GPi 210 | Vim 185, Vo 185 & GPi 160 | Tremor suppression | Not Reported |
| Plaha et al. | 1 | Bilateral ZI | 12 m | 2.48 (mean with other pts) | 120 (mean with other pts) | 147 (mean with other pts) | 70.2% of tremor improvement | dysphagia for a period of about 3 months post-surgery |
| Diederich et al. | 2 | Unilateral Vim | 7 & 5 yrs. respectively | N/A | N/A | N/A | Substantially ameliorated postural > rest > intention component | No peri- & post-operative complications were observed. |
| Peker et al. | 1 | Unilateral Vim | 2.5 yrs. | 4.8 | 120 | 180 | Tremor diminished by 90% | Not Reported |
| Bandt et al. | 1 | Unilateral LF + ZI | 16 m | 3 | 120 | 170 | Almost complete resolution of tremor | No peri- & post-operative complications were observed |
| Acar et al | 1 | Bilateral Vim | 3 m | 4.0 | 90 | 180 | Improvement in tremor | Not Reported |
| Aydin et al. 2013 | 1 | Unilateral GPi + Vim | 6 m | GPi3.0 & Vim 3.0 | GPi 210 & Vim 90 | GPi 130 & Vim 100 | Resting component of tremor improved | No peri- & post-operative complications were observed. |
| Castrop et al. | 2 | Unilateral Vim | 8 & 7 yrs. respectively | N/A | N/A | N/A | Tremor suppression sustained even when during OFF stimulation | Not Reported |
| Issar et al. | 1 | Bilateral Vim | N/A | Rt. 3.0 & Left 3.0 | Rt. 90 & Left 90 | Rt.130 & Left 130 | Moderate improvement and dystonic movements persisted | Dystonic movements, paresthesia, ataxia |
| Follett et al. | 1 | Bilateral Vim | 12 m | Rt. 2.5 & Left 2.0 | Rt. 60 & Left 60 | Rt. 185 & Left 185 | Improvement in TETRAS score from 3 to 1 on right and 3.5 to 0 on left side | No peri- & post-operative complications were observed. |
| Grabska et al. | 1 | Unilateral VOA + ZI | First at 6 m then at 4 yrs. | 1.8 | 60 | 185 | Postural tremor improved at 6 m and remained stable throughout the years | Four years after DBS, pt. developed an infection around the site of electrode implantation. |
| Kobayashi et al. | 4 | Unilateral Vo/Vim + STN | 25 m | N/A | Vo/Vim & STN 210 | Vo/Vim & STN 135 | Tremor improved, TRS score lower with simultaneous Vim & SA stimulation | No peri- & post-operative complications were observed. |
| Kilbane et al | 4 | Unilateral GPi (2), Unilateral Vim + VOA + GPi (1), Unilateral Vim + GPi (1) | 36, 18, 52, 29 m | GPi 2.5/2.8/4.5/2.0 | GPi 90/90/90/120 | GPi 185/185/145/145 | 78.8% mean improvement in tremor severity using FTM tremor rating scale | No peri- & post-operative complications were observed. |
| Espinoza- Martinez et al. | 10 | Unilateral GPi (5), Bilateral GPi (2), Unilateral Vim (2), Bilateral Vim (1) | >2 yrs. | Variable | Variable | Variable | 64% mean improvement in tremor | No peri- & post-operative complications were observed. |
| Aydin et al. 2017 | 1 | Unilateral GPi + Vim | 6 m | Vim 3.0 & GPi 3.0 | Vim 90 & GPi 210 | Vim 100 & GPi 130 | Tremor especially resting component improved | No peri- & post-operative complications were observed. |
| Toda et al. | 1 | Vo +STN | 6 yrs. | N/A | N/A | N/A | Tremor especially resting component improved | Not Reported |
GPi: Global Pallidus internus, LF: Lenticular fasciculus, STN: Subthalamic nucleus, Vim: Ventralis intermedius, Vo: Ventralis oralis, VOA: Ventralis oralis anterior, VOP: Ventralis oralis posterior, ZI: Zona incerta, FTM: Fahn-Tolosa-Marin tremor rating scale, IPG: Implantable pulse generator, TETRAS: The essential tremor rating assessment scale, TRS: Tremor rating scale, N/A: not available in the respective study, m: months, yrs.: years.
There were 31 patients treated with Vim-DBS: 13 received unilateral Vim-DBS, 5 bilateral Vim-DBS, and 13 Vim-DBS plus an additional target (GPi n= 5; STN n= 5; VOA/VOP border n= 3). Tremor improved in all cases, with a reduction ≥ 80% in 10/31 patients (32.3%). The extent of improvement was not specified in 12/31 patients (38.7%). The DBS electrode was removed in 1 patient treated with unilateral Vim-DBS due to lack of efficacy. Time to follow-up varied from 3 to 52 months. AEs were reported in 4/31 patients (12.9%), consisting in 1 infection of the implantable pulse generator (IPG), 1 case of facial paresthesia, 1 case of transient dysarthria, and 1 case of ataxia, paresthesia and dystonic movements. Stimulation frequency ranged from 100 to 180 Hz, intensity from 1.7 to 4.8 V, and pulse width from 60 to 210 sec (Table 1) [18–37].
GPi-DBS was used in 14 patients with HT: 7 received unilateral GPi-DBS, 2 bilateral GPi-DBS, and 5 GPi-DBS plus Vim-DBS. Tremor improved in all cases. A reduction ≥ 80% occurred in at least one component of tremor in 11/14 patients (78.6%). Follow-up spanned from 6 to 52 months. All but one study reported no occurrence of AEs, and 1 study did not report AEs. Stimulation frequency ranged from 130 to 185 Hz, intensity from 2.0 to 6.0 V, and pulse width from 90 to 210 sec (Table 1) [26, 30, 35–37].
STN-DBS was used in 6 patients with HT: 5 received unilateral STN-DBS plus Vim-DBS, and 1 STN-DBS plus Vo-DBS. Tremor improved in all cases, with a reduction ≥ 80% in 1/6 patients (16.7%). The follow-up duration ranged from 24 to 72 months. All but one study reported no occurrence of AEs, and 1 study did not report AEs. Stimulation frequency ranged from 135 to 145 Hz, intensity was 2.0 V, and pulse width varied from 90 to 210 sec (Table 1) [21, 34, 38] .
Three cases were treated with ZI-DBS (1 bilateral ZI, 1 unilateral VOA/ZI, 1 unilateral Lenticular fasciculus/ZI). Tremor improved in all cases, with a reduction ≥ 80% in 1/3 patients (33.4%). The follow-up duration ranged from 12 to 48 months. There was a lead infection 4 years after surgery, requiring removal of the system, and one case of transient dysphagia. Stimulation frequency ranged from 145 to 185 Hz, intensity from 1.8 to 3.0 V, and pulse width from 60 to 120 sec (Table 1) [39–41].
Orthostatic tremor
We identified 9 studies (6 single case reports and 3 case series), reporting a total of 18 patients treated with DBS for OT (Table 2).
Table 2.
Summary of literature data on Orthostatic Tremor cases treated with Deep Brain Stimulation.
| Author | No. of patients | Target | Follow up (m/yr.) | Voltage | Pulse width (μs) | Frequency (Hz) | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Espay et al. | 2 | Bilateral Vim (1) & Unilateral Vim (1) | 18 m | 1st patient Right 4.0 & Left 4.0 2nd patient 1.5 | 1st patient Right 90 & Left 60 2nd patient 90 | 1st patient Right 185 & Left 185 2nd patient 160 | Improvement in patient with B/L stimulation, tremor recurrence in U/L patient after 3 months | No peri- & post-operative complications were observed |
| Guridi et al. | 1 | Bilateral Vim | 4 yrs. | Right 2.0 & Left 2.0 | Right 60 & Left 60 | Right 130 & Left 160 | Marked cessation of tremor | Not Reported |
| Magarinos- Ascone et al. | 1 | Bilateral Vim | 12 m | N/A | Right 90 & Left 90 | Right 185 & Left 185 | Reduction in tremor amplitude and patient could stand without any help or leg tremor | Not Reported |
| Yaltho et al. | 1 | Bilateral Vim | 6 m | Right 2.6 & Left 2.1 | Right 90 & Left 90 | Right 170 & Left 135 | Improvement in OT and hand tremor, manifested as improved ability to stand | Not Reported |
| Lyons et al. | 1 | Bilateral Vim | 30 m | Right 2.2 & Left 2.7 | Right 90 & Left 90 | Right 185 & Left 185 | 80% improvement in OT in left leg and 50% improvement in right leg, able to stand 7 minutes | Infection and skin erosion around the IPG which required its removal & a new one was placed 2 months later. |
| Contarino et al. | 1 | Bilateral Vim | 5 yrs. | N/A | N/A | N/A | Initially symptomatic improvement, although benefit lessened gradually to no “optimal clinical” | Persistent pain along the extension wires which required surgical replacement of non-flexible wires for flexible ones. |
| Coleman et al. | 2 | Bilateral Vim | 16 & 7 m respectively | 1st patient (Right 2.6 & Left 3.0) 2nd patient (Right 2.1 & Left 1.9) | 1st patient (Right 60 & Left 60) 2nd patient (Right 60 & Left 60) | 1st patient (Right 140 & Left 140) 2nd patient (Right 170 & Left 170) | Standing time improved to 15 minutes in 1st patient and to > 4 minutes in 2nd patient | All patients complained of stimulation-induced side effects such as paresthesia, dysarthria, imbalance, & light-headedness. Additionally, pt. #1 complained that his hands were clumsier & pt. #2 was hospitalized for brief generalized tonic-clonic seizures. |
| Lehn et al. | 1 | Bilateral Vim | 12 m | Right 2.6 & Left 2.05 | Right 75 & Left 75 | Right 130 & Left 130 | Improved standing time from 3 min to 5 minutes | Not Reported |
| Merola et al. | 8* | Bilateral Vim | Mean 38 m | N/A | N/A | N/A | Amelioration of OT symptoms and 21.6% improvement in the composite ADL/iADL | One patient developed infection leading to the removal of the entire DBS system, two patients had lead misplacement requiring surgical revision, and another patient complained of focal limb dystonia. Stimulation-induced side effects such as speech difficulties and ataxia were also reported. |
Vim: Ventralis intermedius, ADL: Activities of daily living, iADL: Instrumental activities of daily living, IPG: Implantable pulse generator, N/A: not available in the respective study, m: months, yrs.: years.
The paper reports data of 17 patients, whose 9 are already reported in other studies included in the Table.
Vim-DBS was used in 18 patients: 17 received bilateral Vim-DBS, and 1 unilateral Vim-DBS. Tremor amplitude reduction or increased latency to symptom onset after standing, was reported in 15/18 patients (83.3%). One patient treated with bilateral Vim-DBS reported no improvement, and the patient receiving unilateral Vim-DBS reported only a temporary improvement, which lasted less than 6 months. Most of patients experienced a mild/moderate waning of tremor improvement over time. The follow-up duration ranged from 6 to 102 months. A total of 14 AEs was reported in 13/18 patients (72.2%), consisting in 1 skin infection over IPG, 2 surgical revisions due to cervical pain and lead dislocation, 1 generalized tonic-clonic seizure 3 days after surgery, 2 transient paresthesia, 2 gait ataxia, 1 unilateral foot dystonia, 1 case of dizziness and 4 cases of speech difficulties. Stimulation frequency varied from 130 to 185 Hz, intensity from 1.5 to 4.0 V, and pulse width from 60 to 90 sec (Table 2) [42–50].
Multiple sclerosis-associated Tremor
We identified 26 studies (3 single case reports, 22 case series, and 1 randomized clinical trial), for a total of 177 patients treated with DBS for MS-associated tremor (Table 3).
Table 3.
Summary of literature data on multiple sclerosis-associated tremor cases treated with Deep Brain Stimulation.
| Author | No. of patients | Target | Follow up (m/yr.) | Voltage | Pulse width (μs) | Frequency (Hz) | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Nguyen et al. | 1 | Unilateral Vim | 17 m | 1.5 – 4.5 | 60 – 210 | 130 | Both proximal & distal component of tremor improved | No peri- & post-operative complications were observed |
| Siegfried et al. | 9 | Unilateral Vim (8) & Bilateral Vim (1) | N/A | N/A | N/A | N/A | Tremor activity suppressed. | Not Reported |
| Benabid et al. | 4 | Bilateral Vim | ≥ 6 m | 0.5 – 8 | 60 | 130 – 185 | Two patients had a good or fair benefit but improvement lasted only a few months | Transient neurological deficit due to micro hematoma. |
| Geny et al. | 13 | Unilateral Vim | 8 – 26 m | N/A | 60 – 210 | 130 | Tremor amplitude decreased in nine (69%) & functional disability improved in 12 cases (92%) | Asthenia and transient lower limb paresis. |
| Whittle et al. | 4 | Bilateral VL Thalamus | N/A | N/A | N/A | N/A | Improvement in tremor and functional status | Not Reported |
| Montgomery et al. | 14 | Unilateral Vim | < 3 m - >12 m | Range 2.5 – 3.8 | Range 90 – 120 | Range 145 – 185 | Improvement in tremor. Pt. developed mild tolerance to Vim- DBS | One exacerbation of multiple sclerosis that resulted in reduced mobility. One intracerebral hematoma which spontaneously resolved. |
| Schulder et al. | 5 | Unilateral Vim | > 6 m | N/A | N/A | N/A | All patients demonstrated reduction in tremor | No peri- & post-operative complications were observed. Two patients experienced MS exacerbations that responded to iv steroids. |
| Taha et al. | 2 | Bilateral Vim (1) & Unilateral Vim + Contralateral thalamotomy (1) | 10 m | N/A | 60 | 185 | All patients demonstrated improved grade of tremor | Imbalance. |
| Krauss et al. | 2 | Vim** | 3 – 24 m | N/A | 210 | 130 | Symptomatic improvement in tremor | Intraoperative complications consisted of cortical venous infarction, intraventricular hemorrhage and cardiovascular problems. Stimulation-induced adverse effects consisted of dysarthria and gait disturbances, more frequent in patients with bilateral stimulation. Two cases of wire breakage.* |
| Hooper et al. | 10 | Unilateral Vim | 12 m | Mean 3.2 | Mean 110 | Mean 160 | Significant improvement of postural tremor; less marked amelioration of kinetic tremor | Transient paresthesia. Two patients had thalamic-capsular hemorrhages at the site of electrode implant and two patients had seizures. |
| Berk et al. | 12 | Unilateral Vim | First at 2 m then 1 yr. | N/A | N/A | N/A | Reduction in tremor at 2 m that sustained for 1 yr. Trends in improved dressing, writing & personal hygiene (ADLs) were seen initially | Two wound infection treated with antibiotics. Stimulation-induced transient paresthesia was also reported. Two patients complained of transient postoperative urinary retention. |
| Nandi et al. | 10 | Bilateral VOP + ZI | 3 – 24 m | N/A | N/A | N/A | Tremor was suppressed by a combination of VOP & ZI stimulation (64% and 36% improvement for postural and intention tremor, respectively) | Transient hemiparesis, mild dysarthria, 2 wound infection and one episode of seizure. |
| Foote et al. | 1 | Unilateral Vim/VOP border + Unilateral VOA/VOP border | 21 m | Vim/VOP 3.6 + VOA/VOP 2.9 | Vim/VOP 120 + VOA/VOP 90 | Vim/VOP 185 + VOA/VOP 135 | Activation of both electrodes was associated with the greatest symptom reduction. | Not Reported |
| Hamel et al. | 2 | Bilateral VL thalamus +STN | 1 to several yrs. | 2.0 – 3.6 | 60 | 130 – 145 | Suppression of intention tremor more effective with STN stimulation than VL thalamic stimulation | Adverse effects include stimulation-induced paresthesia, dysarthria and gait ataxia which were more pronounced with bilateral stimulation* |
| Herzog et al. | 11 | Unilateral (5) & Bilateral Vim + STN (6) | 6 – 25 m | Variable | Variable | Variable | Reduced postural tremor in the terminal phase of goal-directed movement, more pronounced with STN stimulation | Not Reported |
| Plaha et al. | 4 | Bilateral ZI | Mean 12 m | 1.9 | 210 | 40 | Significant improvement in all components of tremor | One pt. complained of prolonged lethargy and reduced mobility postoperatively that resolved within 3 months. |
| Schuurman et al. | 10 | Bilateral Vim (5) & thalamotomy (5) | 5 yrs. | N/A | N/A | N/A | Tremor reduction was achieved, but after long follow up thalamic DBS was less efficacious than thalamotomy | Patients who underwent thalamotomy reported severe gait/balance disturbances. Dysarthria and ataxia were associated with thalamic stimulation. |
| Moore et al. | 1 | Unilateral Vim | 1 yr. | 4.5 | 120 | 185 | Tremor improved and the patient was able to perform daily activities. But he died after 1 yr. | No peri- & post-operative complications were observed. |
| Mandat et al. | 5 | Unilateral Vim | 3 m | 3.6 | 180 | 185 | Tremor reduction was 40% and mean ADL score improved by 18% | No peri- & post-operative complications were observed |
| Torres et al. | 10 | Unilateral Vim (9) & Bilateral Vim (1) | 3 yrs. | Range 2 – 3.6 | Range 60 – 150 | Range 145 – 185 | Half of the patients had reduction in tremor scores at 1 yr., 60% of them continued benefiting after 36 months | Three patients had a intraoperative seizure. One pt. developed infection that required DBS hardware removal. |
| Thevathasan et al. | 11 | Unilateral VOP + ZI (6) & Bilateral VOP + ZI (5) | Mean 5.2 yr. | Mean 3.8 | Mean 216 | Mean 130 or 180 | Sustained improvement in tremor in 50% of limbs affected by tremor | One pt. developed scalp erosion over extension cables that necessitated the removal of DBS system. Another pt. experienced self-limited seizure postoperatively. |
| Hassan et al. | 9 | Unilateral thalamotomy (6) & Unilateral Vim (3) | 22–127 m (for Vim pts) | N/A | N/A | N/A | Improvement lasted for at least 5 years in 2 patients, for one years in the other patient | Progression of multiple sclerosis and developed complications related to it. |
| Hosseini et al. | 9 | Unilateral Vim | 6 m | 2 – 3.6 (7 pts)/3.5 (1 pts)/2.7 (1 pts) | 80 (7 pts)/120 (1 pts)/80 (1 pts) | 130 (7 pts)/150 (1 pts)/130 (1 pts) | Kinetic and postural tremor score improved at 1 month after surgery that sustained by the end of 6 m | Aggravation of dysarthria and worsening of tremor were reported post operatively. |
| Zakaria et al. | 16 | Unilateral Vim (2) & Bilateral Vim (14) | Mean 11.6 m | N/A | N/A | N/A | Tremor was significantly reduced. Sub analysis of ADLs showed improvement in feeding, dressing etc. | Two patients developed infection that eventually leads to the removal and insertion of new electrodes. Another patient required replacement of the connecting leads to the battery due to malfunction with high impedance. |
| Mehanna et al. | 2 | Unilateral Vim + VOA (1) & Unilateral Vim + Raprl (1) | 6 yrs. | 1.8 + 2.8 (Vim + VOA)/4.1 + 2.5 (Vim + Raprl) | 60 + 90 (Vim + VOA)/90 + 90 (Vim + Raprl) | 130 | Only in Vim + VOA patient was observed an improvement with the double ON compared to stimulating the Vim alone | Not Reported |
| Oliveria et al. | 11 | Unilateral Vim + Vo (first randomly assigned to single lead stimulation and then dual stimulation 3 m later) | 6 m | 1.5 – 4.2 | 60 – 150 | 135 or 185 | Mean improvement in TRS score by 29.6% with dual lead stimulation | Two patients developed postsurgical infections that resolved with antibiotics. One case required DBS system removal. Extension wire was fractured and replaced in one patient. Selflimited intraoperative seizure, transient altered mental status & MS exacerbation were also reported. |
Raprl: pre-lemniscal radiations, STN: Subthalamic nucleus, Vim: Ventralis intermedius, VL: Ventro-lateral, VOA: Ventralis oralis anterior, VOP: Ventralis oralis posterior, ZI: Zona incerta, ADLs: Activities of daily life, iADL: instrumental Activities of daily life, TETRAS: The essential tremor rating assessment scale, TRS: Tremor rating scale, m: months, yrs.: years.
Adverse effects were reported including all patients without the possibility to distinguish the different types of tremors.
Laterality has not been specified among the patients.
Vim-DBS was used in 146 patients: 93 received unilateral Vim-DBS, 26 bilateral Vim-DBS, 25 Vim-DBS plus an additional target (STN n=11; VO n= 13; Pre-lemniscal radiations n=1), and 2 unspecified Vim laterality. Symptoms improved to some extent in 126/146 patients (83.6%). A transitory response was observed in 20/146 patients (13.7%). The follow-up duration ranged from 3 to 127 months. Several patients experienced AEs, encompassing MS exacerbation, seizures, intracerebral hematoma, gait/balance disturbance, asthenia and transient lower limb paresis, ataxia, dysarthria, paresthesias, and infections. Stimulation frequency ranged from 130 to 185 Hz, intensity from 0.5 to 8.0 V, and pulse width from 60 to 210 sec (Table 3)[25, 51–70].
ZI-DBS was used in 25 patients with MS-associated tremor: 4 received bilateral ZI-DBS, and 21 bilateral ZI-DBS plus VOP-DBS. Improvement of all the tremor components was reported for most of treated patients. Some cases gradually deteriorated across a follow-up of 12-62 months. AEs were reported in 8/25 patients (32%), consisting in 1 infection with scalp erosion leading to DBS removal, 2 other wound infections, 2 peri-operative seizure, 1 transient hemiparesis, 1 mild dysarthria, and 1 case of post-operative prolonged lethargy and reduced mobility. Stimulation frequency ranged from 40 to 105 Hz, intensity from 1.9 to 3.8 V, and pulse width from 90 to 330 sec (Table 3) [39, 71, 72].
VL thalamic-DBS was used in 6 patients with MS-associated tremor: 4 received bilateral VL-DBS and 2 VL-DBS plus STN-DBS. Tremor improved in all cases, but follow-up was limited to fewer than 12 months. One study did not report AEs, and 1 study reported stimulation-induced paresthesia, dysarthria and gait ataxia in a cohort of mixed patients, without specifying whether AEs occurred in MS patients. Stimulation frequency ranged from 130 to 145 Hz, intensity from 2.0 to 3.6 V, and pulse width was 60 sec in all cases (Table 3) [73, 74].
Neuropathy-associated tremor
We identified 9 studies (7 single case reports and 2 case series), reporting a total of 14 patients treated with DBS for tremor associated with neuropathy (Table 4).
Table 4.
Summary of literature data on neuropathy-associated tremors treated with Deep Brain Stimulation.
| Author | No. of patients | Target | Follow up (m/yr.) | Voltage | Pulse width (μs) | Frequency (Hz) | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Růzicka et al. | 1 | Unilateral Vim | 12 m | 1.5 | 90 | 145 | Both postural & kinetic components of tremor improved, no change in ataxia | No peri- & post-operative complications were observed |
| Blomstedt et al. | 1 | Unilateral PSA | 12 m | 3.0 | 60 | 185 | Improvement in hand and head tremor | Intraoperative stimulation induced side effects such as visual phenomena, speech alterations & paresthesias. |
| Breit et al. | 1 | Bilateral Vim | 12 m | Right 3.5 & Left 4.5 | 210 | 130 | Sustained tremor improvement despite worsening of neuropathy | Dysarthria and worsening of gait ataxia at high stimulation settings. |
| McMaster et al. | 1 | Unilateral Vim | 12 m | 1.0 | 60 | 130 | Tremor resolved completely and improvement in the performance of daily activities | Not Reported |
| Bayreuther et al. | 1 | Bilateral Vim | 6 m | Right 3.0 & Left 3.0 | Right 60 & Left 90 | Right 130 & Left 130 | Sustained dramatic improvement in tremor | Mild dysarthria was observed at high stimulation settings. |
| Shields et al. | 1 | Bilateral Vim | 19 m | Right 0.6 & Left 1.5 | Right 90 & Left 90 | Right 185 & Left 185 | Tremor and gait improved | No peri- & post-operative complications were observed |
| Weiss et al. 2011 | 1 | Bilateral Vim | 12 m | Right 3.1 & Left 3.5 | Right 180 & Left 180 | Right 180 & Left 180 | 59% improvement in FTM, 31% improved motor functionality in daily activities | Not Reported |
| Patel et al. | 5 | Bilateral Vim (4) & Unilateral Vim (1) | 0.5 - >9 yrs. | N/A | N/A | N/A | Tremors improved but patients developed habituation and required frequent reprogramming | Skin erosion over battery site. |
| Cabanes- Martinez et al. | 2 | Bilateral Vim (1) & Unilateral Vim (1) | 16 & 14 yrs. respectively | 2.2/2.5 | 90/120 | 135/135 | Marked reduction of tremor along with an improvement of quality of life | Mild transient paresthesia. |
PSA: Posterior subthalamic area, Vim: Ventralis intermedius, FTM: Fahn-Tolosa-Marin tremor rating scale, N/A: not available in the respective study, m: months, yrs.: years.
Vim-DBS was used in 13 patients: 9 received bilateral Vim-DBS, and 4 unilateral Vim-DBS. Tremor improved in all cases. There was a complete resolution of tremor in 1/14 patient (7.1%), and a “marked” or “dramatic” tremor improvement in 2/14 patients (14.3%). Follow-up ranged from 6 months to 9 years. AEs were reported in 4/13 patients (30.8%), with 1 case of mild transient paresthesia, 1 case of dysarthria and worsening of gait ataxia, 1 case of mild dysarthria, and 1 skin erosion over IPG. Two studies reported no occurrence of AEs, and 2 studies did not report AEs. Stimulation frequency varied from 130 to 185 Hz, intensity from 0.6 to 4.5 V, and pulse width from 60 to 210 sec (Table 4) [12, 75–81].
One patient received unilateral PSA-DBS with a significant improvement of symptoms over a follow-up of 12 months. Intraoperative occurrence of visual phenomena, speech alterations and paresthesia were reported. Stimulation frequency was 145 Hz, intensity 3.0 V, and pulse width 60 sec (Table 4) [82].
Fragile X–associated tremor/ataxia syndrome
We identified 8 studies (7 case reports and 1 case series), reporting a total of 10 patients treated with DBS for FXTAS (Table 5).
Table 5.
Summary of literature data on Fragile X-associated tremor/ataxia syndrome treated with Deep Brain Stimulation.
| Author | No. of patients | Target | Follow up (m/yr.) | Voltage | Pulse width (μs) | Frequency (Hz) | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Ferrara et al. | 1 | Bilateral Vim | 21 m | Right 4.0 & Left 4.0 | Right 90 & Left 90 | Right 185 & Left 160 | 56% improvement in FTM | Worsening of both gait and speech. |
| Senova et al. | 1 | Unilateral Vim | 6 m | 2.5 | 60 | 130 | 73.4% improvement in FTM and 30.2 % improvement in ataxia score | No peri- & post-operative complications were observed |
| Xie et al. | 1 | Unilateral Vim | 24 m | 4.4 | 150 | 185 | Improvement in tremor and daily living function | No peri- & post-operative complications were observed |
| Mehanna et al. | 1 | Staged Bilateral Vim | 6 m | N/A | N/A | N/A | Tremor improvement | After unilateral DBS implant, no peri- & post-operative complications were observed. Bilateral DBS implant resulted in worsening of ataxia, cognitive decline, dysarthria & apraxia even before turning on the stimulation. |
| Oyama et al. | 1 | Unilateral PSA | First at 6 m and then 8 m | 2.8 | 120 | 160 | 57.9% sustained improvement in TRS, no change in ataxia score | No peri- & post-operative complications were observed |
| Weiss et al.2015 | 3 | Bilateral Vim (2), Bilateral Vim/PSA (1) | 4 yrs. | Right 1.5/4.4/4.6 Left 1.7/5.4/4.9 | Right 150/90/120 Left 150/60/120 | Right 125/170/150 Left 125/170/150 | Mean improvement in FTM by 70% and improved ataxia (kinematic measures of gait) | Two patients developed slightly worsening of MMSE scores post- DBS. |
| Dos Santos Ghilardi et al. | 1 | Bilateral VOP/ZI | 30 m | Right 3.0 & Left 3.25 | Right 91 & Left 91 | Right 176 & Left 176 | Improvement of tremor and ataxia, functional gain in ADL | No peri- & post-operative complications were observed |
| Tamas et al. | 1 | First, bilateral Vim, then bilateral STN and at the end unilateral thalamotomy | 5 yrs. | N/A | N/A | N/A | Transient tremor cessation after Vim and STN while thalamotomy improved postural and kinetic tremor | Worsening of gait, scanning speech and nystagmus. |
PSA: Posterior subthalamic area, STN: Subthalamic nucleus, Vim: Ventralis intermedius, VOP: Ventralis oralis posterior, ZI: Zona incerta, ADL: Activities of daily life, FTM: Fahn-Tolosa-Marin tremor rating scale, N/A: Not available in the respective study, m: months, yrs.: years.
Vim-DBS was used in 8 patients: 4 received bilateral Vim-DBS, 2 unilateral Vim-DBS, 1 bilateral Vim-DBS plus PSA-DBS, and 1 bilateral Vim-DBS plus bilateral STN-DBS. Tremor improved in all cases, with a reduction ≥ 50% in 5/8 patients (62.5%). In 4 patients a concomitant improvement of ataxia was reported. The follow-up duration ranged from 6 to 60 months. AEs were assessed in all studies and reported in 5/8 patients (62.5%), consisting in 3 cases of ataxia and speech worsening (in 1 case with associated cognitive decline), and 2 cases of slight worsening of cognition after surgery. Two studies reported no occurrence of AEs. Stimulation frequency varied from 125 to 185 Hz, intensity from 1.5 to 5.4 V, and pulse width from 60 to 150 sec (Table 5) [83–88].
Vo/ZI-DBS was used in one patient with unquantified improvement of tremor and associated ataxia. Follow-up was 30 months, during which no AEs were reported. Stimulation settings were as follows: frequency= 176 Hz; intensity= 3.0 V (right) and 3.25 V (left); and pulse width= 90 sec (Table 5) [89].
PSA-DBS was used in one patient, resulting in a 58% improvement in tremor severity with no AEs occurrence. The follow-up duration was 8 months. Stimulation frequency was 160 Hz, intensity 2.8 V, and pulse width 120 sec (Table 5) [90].
DISCUSSION
We reviewed and summarized studies that reported the outcomes of DBS in uncommon tremor disorders, namely HT, OT, MS-associated tremor, neuropathy-associated tremor and FXTAS. Apart from one randomized clinical trial that tested the efficacy and safety of DBS in MS [70], the majority of studies consisted of single case reports or small case series. While this relevant limitation should be recognized, suggesting the possibility that a “file drawer effect” might have influenced our results, data reported in the literature seem to suggest that DBS may be clinically useful in uncommon tremor disorders.
The majority of cases received Vim-DBS, but other surgical targets were also tested, including GPi, ZI, PSA, VOA or VOP nuclei, STN, and VL thalamus (Figure 1). A few patients with HT and MS-associated tremor received dual target DBS, mostly Vim-DBS in association with GPi-DBS or STN-DBS. Available data does not permit one to draw final conclusions on the best target in each subtype of tremor. Nonetheless, promising results were observed with PSA-DBS and with dual target DBS. In fact, the most conventional Vim-DBS target was sometimes confounded by worsening pre-existent ataxia in FXTAS, MS-, and neuropathy-associated tremor. Similar complications have been reported in patients with OT, where the accuracy of Vim-DBS targeting is complicated by the somatotopic arrangement of thalamic fibers. In fact, the thalamic region corresponding to the lower limbs is located laterally and is in close proximity to the internal capsule. Such specific anatomical organization accounts for the programming’s narrow therapeutic window that is frequently observed in uncommon tremor disorders. Motor and sensory stimulation-induced side effects consist of speech impairment, sensory issues, motor pulling, and possibly gait ataxia. New directional DBS systems capable of greater anatomical resolution may potentially address these difficulties, leading to an improvement in the risk/benefit profile of DBS treatment in uncommon tremor disorders.
Figure 1. Main DBS targets for uncommon tremor disorders.
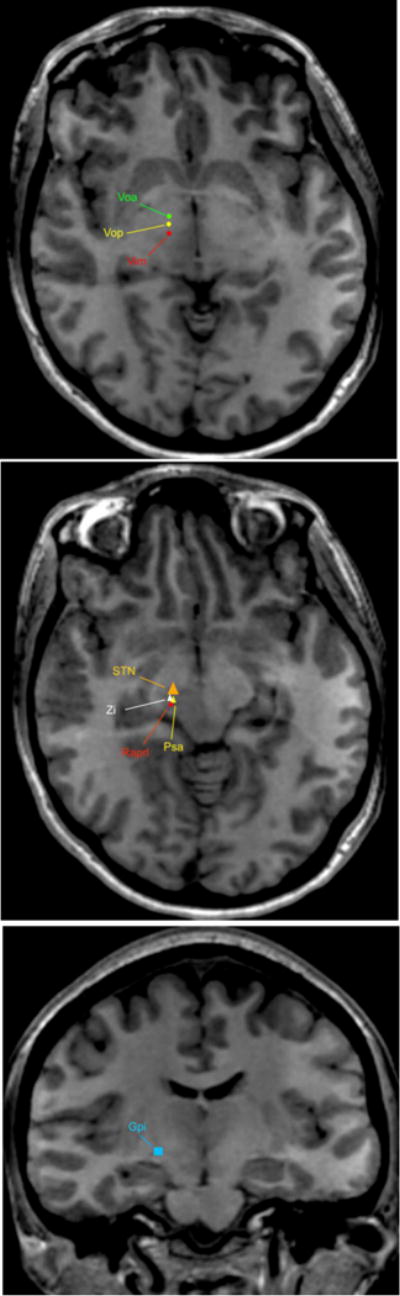
Holmes tremor = Vim; Voa; Vop; STN; Gpi
Orthostatic Tremor = Vim; Zi
MS-associated tremor = Vim; Voa; Vop; Zi; Raprl
FXTS = Vim; Vop; Zi; PSA
Neuropathy-associated tremor = Vim; PSA
DBS, Deep Brain Stimulation; FXTAS, Fragile X-associated tremor/ataxia syndrome; MS, multiple sclerosis; GPe, globus pallidus pars externa; GPi, globus pallidus pars interna; IC, internal capsule; MS, multiple sclerosis; PUT, putamen; PSA, posterior subthalamic area; Raprl, prelemniscal radiation; SN, substantia nigra; STN, subtalamic nucleus; TH, thalamus; Voa, ventralis oralis anterior; Vop, ventralis oralis; ZI, zona incerta.
Overall, our main findings can be summarized as follows:
- Holmes tremor: Vim-DBS, GPi-DBS, and STN-DBS appear to represent effective targets in HT, with a potential to modulate both cerebellar-thalamic and nigrostriatal outflows. However, given the multiple pathways involved in its pathogenesis, a combination of two targets might also be considered. In particular, positive results were observed in patients treated with Vim-DBS and GPi-DBS or with Vim-DBS and STN-DBS. The vast majority of studies reported a programming strategy based on single monopolar stimulation, with high frequency (160-180 Hz in most cases) and a pulse width of 60-90 sec (≥ 100 sec in few cases). Bipolar electrode configurations were occasionally used, mostly due to stimulation-induced side-effects. Vim-DBS can be considered as a viable therapeutic option for HT patients that have failed multiple oral treatments, including levodopa, dopamine agonists, anticholinergics, and anticonvulsants [15, 91].
- Orthostatic tremor: bilateral Vim-DBS demonstrated long-lasting efficacy in reducing tremor in most patients; a few cases treated with unilateral Vim-DBS reported transient and unsatisfactory results. The vast majority of studies reported a programming strategy based on single monopolar stimulation, with a frequency of 160-180 Hz (less frequently 130 Hz) and a pulse width of 60 or 90 sec. More complex programming strategies, such as bipolar configurations and interleaving, were occasionally used. Although partial efficacy in reducing OT has been demonstrated by benzodiazepines, followed by beta-blockers and anticonvulsants [7], the severity of OT symptoms usually progresses over time. This therefore lessens the efficacy of oral medications. Results from an international registry suggest that bilateral Vim-DBS might significantly improve the severity of symptoms in patients with incomplete response to oral medications.
- Multiple sclerosis-associated tremor: dual-lead thalamic DBS (one targeting the VIM border and one targeting the ventralis oralis anterior-ventralis oralis posterior border) has the highest level of clinical trial support [70], providing better control likely by stimulating the major pathways involved in MS-associated tremor, cerebello-thalamo-cortical and pallidal pathways. Almost all studies reported the use of single monopolar stimulation, with a frequency ranging from 130 to 180 Hz and variable pulse width, ranging from 60 to 210 sec, but typically higher than other tremors. Interestingly, a very short frequency stimulation (40 Hz) was reported as efficacious in controlling the tremor of 4 patients. The application of DBS in MS-associated tremor is debated. Approximately 46% of MS patients have some form of tremor, severe enough to impair daily living activities in 5.8% of cases [9]. Beta-blockers and botulinum toxin injection [92, 93] should represent the first line of treatment. DBS can be considered in cases with particularly disabling symptoms, refractory to oral medications.
- Neuropathy-associated tremor: Vim-DBS appears to provide substantial improvement, and improvement was reported in the only case treated with STN-DBS. The typical programming strategy was based on single monopolar stimulation, with a frequency of 130 or 180 Hz and a pulse width of 60 or 90 sec (in 3 cases > 100 sec). While the employment of DBS in neuropathy-associated tremor requires further confirmation by larger clinical trials, the significant impact played by tremor on functional activities in patients with immune-mediated neuropathies suggest that DBS might be a feasible therapeutic option for patients with neuropathy-associated tremor failing propranolol, primidone, and benzodiazepines [94].
- Fragile X–associated tremor/ataxia syndrome: Vim-DBS seems to be effective in improving tremor and sometimes also ataxia; however, a high rate of cases showed a worsening of ataxia, speech, and cognition after surgery, raising the question of the balance between the possible benefit and the potential harm deriving from DBS surgery in these patients. Most studies reported a stimulation frequency of 160-185 Hz and a pulse width of 90 sec or higher. Complex programming strategies such as bipolar or double monopolar stimulation were occasionally used, mostly due to stimulation-induced side-effects. Further controlled clinical trials would help clarify the extent of benefit achievable with Vim-DBS, as compared to oral medications, in patients with FXTAS.
CONCLUSIONS
Our review summarized the limited evidence available as well as some of the challenges associated with the use of DBS in uncommon tremor disorders. First, the inherent rarity of these conditions is indeed a limitation, which lends itself to certain publications being underpowered, and thus with an elevated risk of Type II errors. Second, the frequent association of uncommon tremor disorders with other movement disorders, such as ataxia, dystonia, and chorea, enhance the litany of confounders endemic to the analyses, which make disentanglement of cause-and-effect relationships challenging. Specifically, such clinical heterogeneity hampers the ability to determine the nature, and magnitude, of certain interventions and what outcomes they portend with great reliability. Third, the lack of validated scales for the objective measurements of disability and DBS-associated clinical benefits limit the generalizability of the results.
Given these limitations, particular efforts ought to be capture the entirety of the patient’s clinical picture, including identification of co-existing movement disorders. Other variables to consider may include, but are not limited to, relevant genotyping, especially in syndromes that have been not well characterized, selection of DBS target(s), and ascertainment of optimal stimulation settings. Recent and future advances in DBS technology, such as directional stimulation, leads with more contacts, and adaptive stimulation stand to improve outcomes when using DBS for uncommon tremor disorders. An international registry to gather data from DBS-treated patients with uncommon tremor disorders is warranted to clarify selection criteria, long-term results, and optimal surgical targets.
Acknowledgments
The authors are thankful to Dr. Alberto J. Espay for his intellectual contribution in the critical revision of the manuscript.
Footnotes
CONTRIBUTORSHIP STATEMENT
Dr. Artusi: study conception, data analysis, writing the first draft; Dr. Farooqi: data collection and analysis, manuscript revision; Dr. Romagnolo: data collection and analysis, manuscript revision; Dr. Marsili: data collection and analysis, manuscript revision; Dr. Balestrino: data collection and analysis, manuscript revision; Dr. Sokol: revision of the manuscript for important intellectual contents; Dr. Zibetti: revision of the manuscript for important intellectual contents; Dr. Duker: revision of the manuscript for important intellectual contents; Dr. Mandybur: revision of the manuscript for important intellectual contents; Dr. Lopiano: revision of the manuscript for important intellectual contents; Dr. Merola: study conception, data analysis, writing and revision of the first draft.
All the co-authors listed above gave their final approval of this manuscript version
CONFLICTS OF INTEREST
Dr. Artusi reports no conflict of interest; Dr. Farooqi reports no conflict of interest; Dr. Romagnolo has received grant support and speaker’s honoraria from AbbVie, speaker honoraria from Chiesi Farmaceutici and travel grants from Lusofarmaco and UCB Pharma; Dr. Marsili reports no conflict of interest; Dr. Balestrino reports no conflict of interest; Dr. Sokol reports no conflict of interest; Dr. Madybur is supported by the Mayfield Education research fund grant; he received honoraria from Medtronic and Boston Scientific; Dr. Wang has no disclosures; Dr. Zibetti has received speaker’s honoraria from Medtronic, Lundbeck, UCB Pharma, and AbbVie; Dr. Duker reports no conflict of interest; Dr. Lopiano has received honoraria for lecturing and travel grants from Medtronic, UCB Pharma, and AbbVie; Dr Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring, Cynapsus Therapeutics, and AbbVie. He has received grant support from Lundbeck and Abbott.
References
- 1.Bhatia KP, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 5.Remy P, de Recondo A, Defer G, et al. Peduncular “rubral” tremor and dopaminergic denervation: a PET study. Neurology. 1995;45:472–477. doi: 10.1212/wnl.45.3.472. [DOI] [PubMed] [Google Scholar]
- 6.Seidel S, Kasprian G, Leutmezer F, Prayer D, Auff E. Disruption of nigrostriatal and cerebellothalamic pathways in dopamine responsive Holmes’ tremor. J Neurol Neurosurg Psychiatry. 2009;80:921–923. doi: 10.1136/jnnp.2008.146324. [DOI] [PubMed] [Google Scholar]
- 7.Hassan A, Ahlskog JE, Matsumoto JY, et al. Orthostatic tremor: Clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458–464. doi: 10.1212/WNL.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 8.Gallea C, Popa T, García-Lorenzo D, et al. Orthostatic tremor: a cerebellar pathology? Brain. 2016;139:2182–2197. doi: 10.1093/brain/aww140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinker JR, Salter AR, Walker H, et al. Prevalence and characteristics of tremor in the NARCOMS multiple sclerosis registry: a cross-sectional survey. BMJ Open. 2015;5:e006714. doi: 10.1136/bmjopen-2014-006714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain PG. The management of tremor. J Neurol Neurosurg Psychiatry. 2002;72:I3–I9. doi: 10.1136/jnnp.72.suppl_1.i3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bain PG, Britton TC, Jenkins IH, et al. Tremor associated with benign IgM paraproteinaemic neuropathy. Brain. 1996;119:789–799. doi: 10.1093/brain/119.3.789. [DOI] [PubMed] [Google Scholar]
- 12.Weiss D, Govindan RB, Rilk A, et al. Central oscillators in a patient with neuropathic tremor: evidence from intraoperative local field potential recordings. Mov Disord. 2011;26:323–327. doi: 10.1002/mds.23374. [DOI] [PubMed] [Google Scholar]
- 13.Hagerman RJ, Hagerman P. Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol. 2016;12:403–412. doi: 10.1038/nrneurol.2016.82. [DOI] [PubMed] [Google Scholar]
- 14.Hagerman RJ, Hall DA, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puschmann A, Wszolek ZK. Diagnosis and treatment of common forms of tremor. Semin Neurol. 2011;31:65–77. doi: 10.1055/s-0031-1271312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyam JA, Pereira EAC, McCulloch P, et al. Implementing novel trial methods to evaluate surgery for essential tremor. Br J Neurosurg. 2015;29:334–339. doi: 10.3109/02688697.2014.997670. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Zamora A, Okun MS. Deep brain stimulation for the treatment of uncommon tremor syndromes. Expert Rev Neurother. 2016;16:983–997. doi: 10.1080/14737175.2016.1194756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Goto S, Nishikawa S, et al. Bilateral thalamic stimulation for Holmes’ tremor caused by unilateral brainstem lesion. Mov Disord. 2001;16:170–174. doi: 10.1002/1531-8257(200101)16:1<170::aid-mds1033>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Goto S, Yamada K. Combination of thalamic Vim stimulation and GPi pallidotomy synergistically abolishes Holmes’ tremor. J Neurol Neurosurg Psychiatry. 2004;75:1203–1204. doi: 10.1136/jnnp.2003.023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahwa R, Lyons KE, Kempf L, Wilkinson SB, Koller WC. Thalamic stimulation for midbrain tremor after partial hemangioma resection. Mov Disord. 2002;17:404–407. doi: 10.1002/mds.10084. [DOI] [PubMed] [Google Scholar]
- 21.Romanelli P, Brontë-Stewart H, Courtney T, Heit G. Possible necessity for deep brain stimulation of both the ventralis intermedius and subthalamic nuclei to resolve Holmes tremor. Case report. J Neurosurg. 2003;99:566–571. doi: 10.3171/jns.2003.99.3.0566. [DOI] [PubMed] [Google Scholar]
- 22.Samadani U, Umemura A, Jaggi JL, et al. Thalamic deep brain stimulation for disabling tremor after excision of a midbrain cavernous angioma. Case report. J Neurosurg. 2003;98:888–890. doi: 10.3171/jns.2003.98.4.0888. [DOI] [PubMed] [Google Scholar]
- 23.Nikkhah G, Prokop T, Hellwig B, Lücking CH, Ostertag CB. Deep brain stimulation of the nucleus ventralis intermedius for Holmes (rubral) tremor and associated dystonia caused by upper brainstem lesions. Report of two cases. J Neurosurg. 2004;100:1079–1083. doi: 10.3171/jns.2004.100.6.1079. [DOI] [PubMed] [Google Scholar]
- 24.Piette T, Mescola P, Henriet M, et al. [A surgical approach to Holmes’ tremor associated with high-frequency synchronous bursts] Rev Neurol (Paris) 2004;160:707–711. doi: 10.1016/s0035-3787(04)71023-1. [DOI] [PubMed] [Google Scholar]
- 25.Foote KD, Seignourel P, Fernandez HH, et al. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 2006;58 doi: 10.1227/01.NEU.0000192692.95455.FD. ONS-280-285; discussion ONS-285-286. [DOI] [PubMed] [Google Scholar]
- 26.Lim DA, Khandhar SM, Heath S, et al. Multiple target deep brain stimulation for multiple sclerosis related and poststroke Holmes’ tremor. Stereotact Funct Neurosurg. 2007;85:144–149. doi: 10.1159/000099072. [DOI] [PubMed] [Google Scholar]
- 27.Diederich NJ, Verhagen Metman L, Bakay RA, Alesch F. Ventral intermediate thalamic stimulation in complex tremor syndromes. Stereotact Funct Neurosurg. 2008;86:167–172. doi: 10.1159/000120429. [DOI] [PubMed] [Google Scholar]
- 28.Peker S, Isik U, Akgun Y, Ozek M. Deep brain stimulation for Holmes’ tremor related to a thalamic abscess. Childs Nerv Syst. 2008;24:1057–1062. doi: 10.1007/s00381-008-0644-2. [DOI] [PubMed] [Google Scholar]
- 29.Acar G, Acar F, Bir LS, Kı ılay Z, Cırak B. Vim stimulation in Holmes’ tremor secondary to subarachnoid hemorrhage. Neurol Res. 2010;32:992–994. doi: 10.1179/016164110X12714125204272. [DOI] [PubMed] [Google Scholar]
- 30.Aydin S, Abuzayed B, Kiziltan G, et al. Unilateral thalamic Vim and GPi stimulation for the treatment of Holmes’ tremor caused by midbrain cavernoma: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2013;74:271–276. doi: 10.1055/s-0032-1322549. [DOI] [PubMed] [Google Scholar]
- 31.Castrop F, Jochim A, Berends LP, Haslinger B. Sustained suppression of holmes tremor after cessation of thalamic stimulation. Mov Disord. 2013;28:1456–1457. doi: 10.1002/mds.25398. [DOI] [PubMed] [Google Scholar]
- 32.Issar NM, Hedera P, Phibbs FT, Konrad PE, Neimat JS. Treating post-traumatic tremor with deep brain stimulation: report of five cases. Parkinsonism Relat Disord. 2013;19:1100–1105. doi: 10.1016/j.parkreldis.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Follett MA, Torres-Russotto D, Follett KA. Bilateral deep brain stimulation of the ventral intermediate nucleus of the thalamus for posttraumatic midbrain tremor. Neuromodulation. 2014;17:289–291. doi: 10.1111/ner.12096. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Katayama Y, Oshima H, et al. Multitarget, dual-electrode deep brain stimulation of the thalamus and subthalamic area for treatment of Holmes’ tremor. J Neurosurg. 2014;120:1025–1032. doi: 10.3171/2014.1.JNS12392. [DOI] [PubMed] [Google Scholar]
- 35.Espinoza Martinez JA, Arango GJ, Fonoff ET, et al. Deep brain stimulation of the globus pallidus internus or ventralis intermedius nucleus of thalamus for Holmes tremor. Neurosurg Rev. 2015;38:753–763. doi: 10.1007/s10143-015-0636-0. [DOI] [PubMed] [Google Scholar]
- 36.Kilbane C, Ramirez-Zamora A, Ryapolova-Webb E, et al. Pallidal stimulation for Holmes tremor: clinical outcomes and single-unit recordings in 4 cases. J Neurosurg. 2015;122:1306–1314. doi: 10.3171/2015.2.JNS141098. [DOI] [PubMed] [Google Scholar]
- 37.Aydın S, Canaz H, Erdogan ET, Durmaz N, Topcular B. Holmes’ Tremor with Shoulder Pain Treated by Deep Brain Stimulation of Unilateral Ventral Intermediate Thalamic Nucleus and Globus Pallidus Internus. J Mov Disord. 2017;10:92–95. doi: 10.14802/jmd.16051. https://doi.org/10.14802/jmd.16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toda H, Nishida N, Iwasaki K. Coaxial interleaved stimulation of the thalamus and subthalamus for treatment of Holmes tremor. Neurosurg Focus. 2017;42:V1. doi: 10.3171/2017.4.FocusVid.16510. [DOI] [PubMed] [Google Scholar]
- 39.Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79:504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- 40.Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. J Neurosurg. 2008;109:635–639. doi: 10.3171/JNS/2008/109/10/0635. [DOI] [PubMed] [Google Scholar]
- 41.Grabska N, Rud ińska M, Dec-Ćwiek M, et al. Deep brain stimulation in the treatment of Holmes tremor - a long-term case observation. Neurol Neurochir Pol. 2014;48:292–295. doi: 10.1016/j.pjnns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23:2357–2362. doi: 10.1002/mds.22271. [DOI] [PubMed] [Google Scholar]
- 43.Guridi J, Rodriguez-Oroz MC, Arbizu J, et al. Successful thalamic deep brain stimulation for orthostatic tremor. Mov Disord. 2008;23:1808–1811. doi: 10.1002/mds.22001. [DOI] [PubMed] [Google Scholar]
- 44.Magariños-Ascone C, Ruiz FM, Millán AS, et al. Electrophysiological evaluation of thalamic DBS for orthostatic tremor. Mov Disord. 2010;25:2476–2477. doi: 10.1002/mds.23333. [DOI] [PubMed] [Google Scholar]
- 45.Yaltho TC, Ondo WG. Thalamic Deep Brain Stimulation for Orthostatic Tremor. Tremor Other Hyperkinet Mov (N Y) 2011;1 doi: 10.7916/D8NZ86C1. pii: tre-01-26-56-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyons MK, Behbahani M, Boucher OK, Caviness JN, Evidente VG. Orthostatic tremor responds to bilateral thalamic deep brain stimulation. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D8TQ608K. pii: tre-02-30-85-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contarino MF, Bour LJ, Schuurman PR, et al. Thalamic deep brain stimulation for orthostatic tremor: Clinical and neurophysiological correlates. Parkinsonism Relat Disord. 2015;21:1005–1007. doi: 10.1016/j.parkreldis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Coleman RR, Starr PA, Katz M, et al. Bilateral Ventral Intermediate Nucleus Thalamic Deep Brain Stimulation in Orthostatic Tremor. Stereotact Funct Neurosurg. 2016;94:69–74. doi: 10.1159/000444127. [DOI] [PubMed] [Google Scholar]
- 49.Lehn AC, O’Gorman C, Olson S, Salari M. Thalamic Ventral Intermediate Nucleus Deep Brain Stimulation for Orthostatic Tremor. Tremor Other Hyperkinet Mov (N Y) 2017;7:479. doi: 10.7916/D8280JHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merola A, Fasano A, Hassan A, et al. Thalamic deep brain stimulation for orthostatic tremor: A multicenter international registry. Mov Disord. 2017;32:1240–1244. doi: 10.1002/mds.27082. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen JP, Degos JD. Thalamic stimulation and proximal tremor. A specific target in the nucleus ventrointermedius thalami. Arch Neurol. 1993;50:498–500. doi: 10.1001/archneur.1993.00540050050014. [DOI] [PubMed] [Google Scholar]
- 52.Siegfried J, Lippitz B. Chronic Electrical Stimulation of the VL-VPL Complex and of the Pallidum in the Treatment of Movement Disorders: Personal Experience since 1982. Stereotact Funct Neurosurg. 1994;62:71–75. doi: 10.1159/000098599. [DOI] [PubMed] [Google Scholar]
- 53.Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 54.Geny C, Nguyen J-P, Pollin B, et al. Improvement of severe postural cerebellar tremor in multiple sclerosis by chronic thalamic stimulation. Mov Disord. 1996;11:489–494. doi: 10.1002/mds.870110503. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery EB, Baker KB, Kinkel RP, Barnett G. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625–628. doi: 10.1212/wnl.53.3.625. [DOI] [PubMed] [Google Scholar]
- 56.Schulder M, Sernas T, Mahalick D, Adler R, Cook S. Thalamic stimulation in patients with multiple sclerosis. Stereotact Funct Neurosurg. 1999;72:196–201. doi: 10.1159/000029726. [DOI] [PubMed] [Google Scholar]
- 57.Taha JM, Janszen MA, Favre J. Thalamic deep brain stimulation for the treatment of head, voice, and bilateral limb tremor. J Neurosurg. 1999;91:68–72. doi: 10.3171/jns.1999.91.1.0068. [DOI] [PubMed] [Google Scholar]
- 58.Krauss JK, Simpson RK, Ondo WG, et al. Concepts and methods in chronic thalamic stimulation for treatment of tremor: technique and application. Neurosurgery. 2001;48:535–541. doi: 10.1097/00006123-200103000-00015. discussion 541-543. [DOI] [PubMed] [Google Scholar]
- 59.Hooper J, Taylor R, Pentland B, Whittle IR. A prospective study of thalamic deep brain stimulation for the treatment of movement disorders in multiple sclerosis. Br J Neurosurg. 2002;16:102–109. doi: 10.1080/02688690220131769. [DOI] [PubMed] [Google Scholar]
- 60.Berk C, Carr J, Sinden M, Martzke J, Honey CR. Thalamic deep brain stimulation for the treatment of tremor due to multiple sclerosis: a prospective study of tremor and quality of life. J Neurosurg. 2002;97:815–820. doi: 10.3171/jns.2002.97.4.0815. [DOI] [PubMed] [Google Scholar]
- 61.Herzog J, Hamel W, Wenzelburger R, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130:1608–1625. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- 62.Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23:1146–1153. doi: 10.1002/mds.22059. [DOI] [PubMed] [Google Scholar]
- 63.Moore GRW, Vitali AM, Leung E, et al. Thalamic stimulation in multiple sclerosis: evidence for a “demyelinative thalamotomy”. Mult Scler. 2009;15:1311–1321. doi: 10.1177/1352458509345914. [DOI] [PubMed] [Google Scholar]
- 64.Mandat T, Koziara H, Tutaj M, et al. Thalamic deep brain stimulation for tremor among multiple sclerosis patients. Neurol Neurochir Pol. 2010;44:542–545. doi: 10.1016/S0028-3843(14)60150-X. [DOI] [PubMed] [Google Scholar]
- 65.Torres CV, Moro E, Lopez-Rios A-L, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for tremor in patients with multiple sclerosis. Neurosurgery. 2010;67:646–651. doi: 10.1227/01.NEU.0000375506.18902.3E. [DOI] [PubMed] [Google Scholar]
- 66.Hassan A, Ahlskog JE, Rodriguez M, Matsumoto JY. Surgical therapy for multiple sclerosis tremor: a 12-year follow-up study. Eur J Neurol. 2012;19:764–768. doi: 10.1111/j.1468-1331.2011.03626.x. [DOI] [PubMed] [Google Scholar]
- 67.Hosseini H, Mandat T, Waubant E, et al. Unilateral thalamic deep brain stimulation for disabling kinetic tremor in multiple sclerosis. Neurosurgery. 2012;70:66–69. doi: 10.1227/NEU.0b013e31822da55c. [DOI] [PubMed] [Google Scholar]
- 68.Zakaria R, Vajramani G, Westmoreland L, et al. Tremor reduction and quality of life after deep brain stimulation for multiple sclerosis-associated tremor. Acta Neurochir (Wien) 2013;155:2359–2364. doi: 10.1007/s00701-013-1848-0. [DOI] [PubMed] [Google Scholar]
- 69.Mehanna R, Machado AG, Oravivattanakul S, Genc G, Cooper SE. Comparing two deep brain stimulation leads to one in refractory tremor. Cerebellum. 2014;13:425–432. doi: 10.1007/s12311-014-0552-9. [DOI] [PubMed] [Google Scholar]
- 70.Oliveria SF, Rodriguez RL, Bowers D, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16:691–700. doi: 10.1016/S1474-4422(17)30166-7. [DOI] [PubMed] [Google Scholar]
- 71.Nandi D, Aziz TZ. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21:31–39. doi: 10.1097/00004691-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Thevathasan W, Schweder P, Joint C, et al. Permanent tremor reduction during thalamic stimulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:419–422. doi: 10.1136/jnnp.2010.213900. [DOI] [PubMed] [Google Scholar]
- 73.Whittle IR, Yau YH, Hooper J. Mesodiencephalic targeting of stimulating electrodes in patients with tremor caused by multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:1210. [PMC free article] [PubMed] [Google Scholar]
- 74.Hamel W, Herzog J, Kopper F, et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien) 2007;149:749–758. doi: 10.1007/s00701-007-1230-1. [DOI] [PubMed] [Google Scholar]
- 75.Ricka E, Jech R, Zárubová K, Roth J, Urgosík D. VIM thalamic stimulation for tremor in a patient with IgM paraproteinaemic demyelinating neuropathy. Mov Disord. 2003;18:1192–1195. doi: 10.1002/mds.10510. [DOI] [PubMed] [Google Scholar]
- 76.Bayreuther C, Delmont E, Borg M, Fontaine D. Deep brain stimulation of the ventral intermediate thalamic nucleus for severe tremor in anti-MAG neuropathy. Mov Disord. 2009;24:2157–2158. doi: 10.1002/mds.22604. [DOI] [PubMed] [Google Scholar]
- 77.Breit S, Wächter T, Schöls L, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. J Neurol Neurosurg Psychiatry. 2009;80:235–236. doi: 10.1136/jnnp.2008.145656. [DOI] [PubMed] [Google Scholar]
- 78.McMaster J, Gibson G, Castro-Prado F, Vitali A, Honey CR. Neurosurgical treatment of tremor in anti-myelin-associated glycoprotein neuropathy. Neurology. 2009;73:1707–1708. doi: 10.1212/WNL.0b013e3181c1de66. [DOI] [PubMed] [Google Scholar]
- 79.Shields DC, Flaherty AW, Eskandar EN, Williams ZM. Ventral intermediate thalamic stimulation for monoclonal gammopathy-associated tremor: case report. Neurosurgery. 2011;68:E1464–1467. doi: 10.1227/NEU.0b013e3182124633. [DOI] [PubMed] [Google Scholar]
- 80.Patel N, Ondo W, Jimenez-Shahed J. Habituation and rebound to thalamic deep brain stimulation in long-term management of tremor associated with demyelinating neuropathy. Int J Neurosci. 2014;124:919–925. doi: 10.3109/00207454.2014.895345. [DOI] [PubMed] [Google Scholar]
- 81.Cabañes-Martínez L, Del Álamo de Pedro M, de Blas Beorlegui G, Bailly-Bailliere IR. Long-Term Effective Thalamic Deep Brain Stimulation for Neuropathic Tremor in Two Patients with Charcot-Marie-Tooth Disease. Stereotact Funct Neurosurg. 2017;95:102–106. doi: 10.1159/000457963. [DOI] [PubMed] [Google Scholar]
- 82.Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir (Wien) 2009;151:31–36. doi: 10.1007/s00701-008-0163-7. [DOI] [PubMed] [Google Scholar]
- 83.Ferrara JM, Adam OR, Ondo WG. Treatment of fragile-X-associated tremor/ataxia syndrome with deep brain stimulation. Mov Disord. 2009;24:149–151. doi: 10.1002/mds.22354. [DOI] [PubMed] [Google Scholar]
- 84.Senova S, Jarraya B, Iwamuro H, et al. Unilateral thalamic stimulation safely improved fragile X-associated tremor ataxia: a case report. Mov Disord. 2012;27:797–799. doi: 10.1002/mds.24923. [DOI] [PubMed] [Google Scholar]
- 85.Xie T, Goodman R, Browner N, et al. Treatment of fragile X-associated tremor/ataxia syndrome with unilateral deep brain stimulation. Mov Disord. 2012;27:799–800. doi: 10.1002/mds.24958. [DOI] [PubMed] [Google Scholar]
- 86.Mehanna R, Itin I. Which approach is better: bilateral versus unilateral thalamic deep brain stimulation in patients with fragile X-associated tremor ataxia syndrome. Cerebellum. 2014;13:222–225. doi: 10.1007/s12311-013-0530-7. [DOI] [PubMed] [Google Scholar]
- 87.Weiss D, Mielke C, Wächter T, et al. Long-term outcome of deep brain stimulation in fragile X-associated tremor/ataxia syndrome. Parkinsonism Relat Disord. 2015;21:310–313. doi: 10.1016/j.parkreldis.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Tamás G, Kovács N, Varga NÁ, et al. Deep brain stimulation or thalamotomy in fragile X-associated tremor/ataxia syndrome? Case report Neurol Neurochir Pol. 2016;50:303–308. doi: 10.1016/j.pjnns.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 89.dos Santos Ghilardi MG, Cury RG, dos Ângelos JS, et al. Long-term improvement of tremor and ataxia after bilateral DBS of VoP/zona incerta in FXTAS. Neurology. 2015;84:1904–1906. doi: 10.1212/WNL.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 90.Oyama G, Umemura A, Shimo Y, et al. Posterior subthalamic area deep brain stimulation for fragile X-associated tremor/ataxia syndrome. Neuromodulation. 2014;17:721–723. doi: 10.1111/ner.12150. [DOI] [PubMed] [Google Scholar]
- 91.di Biase L, Munhoz RP. Deep brain stimulation for the treatment of hyperkinetic movement disorders. Expert Rev Neurother. 2016;16:1067–1078. doi: 10.1080/14737175.2016.1196139. [DOI] [PubMed] [Google Scholar]
- 92.Koch M, Mostert J, Heersema D, De Keyser J. Tremor in multiple sclerosis. J Neurol. 2007;254:133–145. doi: 10.1007/s00415-006-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Der Walt A, Sung S, Spelman T, et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012;79:92–99. doi: 10.1212/WNL.0b013e31825dcdd9. [DOI] [PubMed] [Google Scholar]
- 94.Charles PD, Esper GJ, Davis TL, Maciunas RJ, Robertson D. Classification of tremor and update on treatment. Am Fam Physician. 1999;59:1565–1572. [PubMed] [Google Scholar]


