Abstract
BACKGROUND
A significant number of patients receiving therapy with anti-tumor necrosis factor (TNF) agents for Crohn’s disease (CD) experience primary or secondary non-response. The aim of this study was to assess if patients with non-response to anti-TNF agents have increased expression of alternative cytokine pathways.
METHODS
We designed a prospective, cross-sectional study that included patients with CD receiving anti-TNF undergoing colonoscopy with adequate serum trough drug levels (≥8 μg/mL) and without anti-drug antibodies. Inflammatory cytokines and cell adhesions markers measured included intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), interleukin-(IL) 8, IL-1β and IL-6. The primary outcome was the presence of active endoscopic inflammation defined as the presence of at least one ulceration ≥5 mm.
RESULTS
47 patients were included. Patients with active inflammation had significantly higher levels of ICAM-1 and IL-1β when compared to those without intestinal inflammation (45.9 vs. 35.8 ng/mL, p<0.0001 and 3.2 vs. 1.5 pg/mL, p=0.002 respectively). There were no significant differences in the other study variables. Using receiving operating curves, ICAM and IL-1β had a good correlation (ROC≥0.8) with inflammation in this cohort of patients with “anti-TNF resistance.” The results were similar in the group of patients with previous anti-TNF exposure.
CONCLUSION
Our study suggests that patients who have active inflammation with seemingly adequate serum anti-TNF levels have increased levels of specific inflammatory pathways that may serve as biomarkers of non-response as well as potential targets of therapy in anti-TNF non-responders.
Keywords: Crohn’s disease, anti-tumor necrosis factor, cytokines
INTRODUCTION
Multiple cytokines have been implicated in the pathogenesis of Crohn’s disease and several recombinant antibodies against pro-inflammatory cytokines have been studied for its therapy. The first targeted biologic approved was infliximab, an anti-tumor necrosis factor antibody and subsequently two others have been found efficacious and subsequently approved (adalimumab and certolizumab pegol)1,2. Unfortunately, a significant proportion of patients demonstrate primary or secondary non-response to these drugs3. Among non-responders to therapy, some may have low serum drug levels which could be explained by under-dosing or high drug clearance4. On a high number of these patients, increase the drug dose can be of benefit5. Development of immunogenicity against the drug is also associated with loss of response6. However, some patients receiving anti-TNFs experience primary or secondary non-response despite having adequate serum drug levels and in the absence of neutralizing antibodies. Furthermore, we know that only a minority of anti-TNF non-responders will respond to a second anti-TNF7,8. One potential explanation for the persistence of active disease in this “anti-TNF resistant” group is the upregulation of alternative inflammatory pathways that are not dependent on TNF.
As the number of non-responders to anti-TNFs rise, we face the challenge of identifying potential therapeutic alternatives in these patients. Identifying alternative pathways that are driving the disease in these cases can ultimately lead to the development of targeted therapies. The aim of this study was to assess if patients with non-response to anti-TNFs despite adequate drug levels and the absence of anti-drug antibodies have a higher expression of alternative inflammatory cytokines or soluble cell adhesion molecules (CAMs) when compared to anti-TNF responders. We also sought to compare inflammatory pathway profiles between those patients with exposure to one versus more than one anti-TNF agent.
METHODS
Patient and Settings
We performed a prospective cross-sectional study including patients with a confirmed diagnosis of Crohn’s disease who were receiving therapy with either infliximab or adalimumab for at least 14 weeks and with adequate serum anti-TNF levels with no detectable anti-drug antibodies. Patients were selected based on drug levels among the population of patients that are followed in the outpatient clinic at the University of Miami. Samples are systematically drawn and banked for research purposes. We defined adequate anti-TNF level as a serum trough level ≥8 μg/mL based on previous published studies using the same assay (homogeneous mobility shift assay) and given high rates of remission at this cutoff9,10. Additionally, in order to be included in the study and have an objective assessment of disease activity, patients had to have a colonoscopy within 4 weeks of the blood draw and at least 14 weeks after starting anti-TNF therapy. Colonoscopies were performed as standard of care. We then sub-divided the cohort of patients into those in endoscopic remission and those with active mucosal inflammation. We defined endoscopic remission as a lack of ulcerations ≥5mm in the intestinal lumen at ileo-colonoscopy or capsule endoscopy. All patients were followed at the Crohn’s and Colitis Center of the University of Miami (Miami, Florida). The study was approved by the University of Miami Miller School of Medicine Institutional Review Board. At the time a patient was enrolled, a complete history and assessment of disease was completed (see below). All serum samples were frozen at −80° Celsius.
Predictive Variables
Phenotype of disease was classified according to the Montreal classification11. Briefly, Crohn’s disease was categorized as ileal, colonic, or ileo-colonic, with or without upper gastrointestinal tract involvement and stricturing or fistulizing disease. We also recorded concomitant drugs used at the time the of the blood draw. 5-aminosalicylates (5ASA) and corticosteroid (CS) use were noted if prescribed for 30 or more consecutive days prior to the index date (the day the blood sample was drawn). Rectally administered topical steroids (e.g., enemas or suppositories) were not considered in the analysis. Immunomodulators (azathioprine, 6-mercaptopurine, and methotrexate) were considered if taken for ≥60 days at any dose prior to the index date. Exposure to biologics was recorded and stratified by drug (infliximab or adalimumab). No patients on anti-integrins, ustekinumab, or an experimental therapy were included
Outcome: Inflammatory Cytokines and Cell Adhesion Molecule Levels
Our primary outcome were cytokine and soluble cellular adhesion molecule levels that have been associated with disease activity in IBD, including TNF, vascular cell adhesion protein-1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and IL-1β, 6 and 812,13. VCAM-1 and ICAM-1 are two cytokine-inducible glycoproteins that have a pivotal role in the migration of leukocytes through endothelium14. Both, ICAM-1 and VCAM-1 are expressed by endothelial cells after activation by several cytokines including TNF and IL-1β, and serum concentrations of both glycoproteins have been found to be higher in patients with active Crohn’s disease12. While other CAMs have been identified as having a role in Crohn’s disease, these two are soluble and can be measured in serum and hence can be potentially useful in routine clinical care. IL-1β has been shown to to be highly expressed in anti-TNF non-responders15. We also measured IL-6 levels in these patients as previous studies have found that subjects with Crohn’s disease present with a high expression of this cytokine and blocking IL-6 signaling was effective in suppressing intestinal inflammation16. Moreover, Phase I clinical trials showed that the administration of a monoclonal antibody against IL-6 had a beneficial effect in the treatment of active Crohn’s disease17. IL-8 was also measured, as this has been associated with active mucosal inflammation in patients with inflammatory bowel diseases18,19
Protein expression levels in human serum samples were determined using the vascular injury panel multiplex kit from Meso Scale Discovery (MSD) (Maryland, USA). Briefly, 25l of 1:1000 diluted serum samples and appropriate standards and controls were added to pre-blocked multiplex plates that had pre-bound capture antibodies. After a 2-hour sample incubation on a shaker, multiplex plates were washed with phosphate buffered saline with 0.05% Tween followed by 1-hour incubation with the detection antibody. The plates were washed again and read on the MSD instrument after the addition of 150l read buffer. The protein expression levels of IL1, IL6 and IL8 were measured using the human pro-inflammatory panel by MSD (Maryland, USA) as per the manufacturer’s instructions. In summary, 50l of 1:2 diluted serum samples and appropriate standards and controls were added to pre-blocked multiplex plates that had pre-bound capture antibodies. After a 2-hour sample incubation on a shaker, multiplex plates were washed 3 times with at least 150l/well of wash buffer followed by a 2-hour incubation with the detection antibody. The plates were washed again and read on the MSD instrument after the addition of 150l/well of read buffer followed by data analysis.
Statistics Analysis
Descriptive statistics were used to examine the baseline characteristics of both study groups (in remission or active disease). Continuous variables were compared using Student’s t-test or the Mann–Whitney U-test (for nonparametric variables). The χ2 test was used to evaluate distributions of categorical variables. Correlation of inflammatory cytokine levels was performed using Spearman’s rank correlation test. Receiver operating characteristic (ROC) curves were generated in order to evaluate the performance of each cytokine in predicting active disease.
RESULTS
Forty-seven patients met inclusion criteria. Mean age of the study group was 39 (SD: 16) and 24 (51.1%) were men. Twenty-two (46.8%) had active endoscopic disease. More than half of the patients were on adalimumab (59.6%) and the rest on infliximab. Nineteen patients were on the second anti-TNF and one patient was on a third anti-TNFs. The median IFX level was 12.1 μg/mL (IQR: 8.6–21.1) while the median adalimumab level was 13.3 μg/mL (IQR: 11.7–22.5). The baseline characteristics of the study population are shown in Table 1. There was no significant difference in time on the anti-TNF between those patients with or without inflammation: 13 months (IQR:12–23.5) and 14 months (IQR: 9–41.8) respectively (p=0.44). Among patients with active disease, all had experienced some benefit.
Table 1.
Baseline characteristics of the study population
| Age [mean in years (SD)] | 39 (16) |
| Male gender [n (%)] | 24 (51.1) |
| Active smoking [n (%)] | 3 (1.3) |
| On adalimumab (vs. infliximab) [n (%)] | 28 (59.6) |
| Previous exposure to more than one anti-TNF [n (%)] | 20 (42.6) |
| Patients receiving steroids [n (%)] | 6 (12.8) |
| Patients receiving combination therapy with immunomodulators [n (%)] | 20 (42.6) |
| C-Reactive Protein [median in mg/L (IQR)] | 1.7 (0.7–5.3) |
| Patients with active endoscopic disease [n (%)] | 22 (46.8) |
| Crohn’s Disease Phenotype | |
| Ileal disease [n (%)] | 15 (31.9) |
| Ileo-colonic disease [n (%)] | 13 (27.7) |
| Colonic disease [n (%)] | 10 (21.3) |
| Fibro-stenotic disease [n (%)] | 21 (44.7) |
| Internal penetrating/perforating disease [n (%)] | 12 (25.5) |
| Peri-anal disease [n (%)] | 20 (42.6) |
When comparing cellular adhesion molecule levels, patients with active inflammation had significantly higher levels of ICAM-1 compared to those without intestinal inflammation (45.9 vs. 35.8 ng/mL, p<0.0001), but there was no significant difference in VCAM-1 levels (Table 2–Figure 1).
Table 2.
Differences between those patients with and without endoscopic inflammation
| Cytokine | Active inflammation (n=22) |
No inflammation (n=25) |
P value |
|---|---|---|---|
| ICAM [median in ng/mL (IQR)] | 306.9 (294–328) | 214.8 (207.6–248.8) | <0.0001 |
| VCAM [median in ng/mL (IQR)] | 500.1 (214–639) | 521.1 (444–581.2) | 0.48 |
| IL-8 [median in ng/mL (IQR)] | 10.3(7.8–27.3) | 14.9 (10.3–26.3) | 0.32 |
| IL-1B [median in ng/mL (IQR)] | 2.9 (2.4–3.9) | 0.82 (0.6–2.4) | <0.0001 |
| IL-6 [median in ng/mL (IQR)] | 3.6 (2–5.7) | 2.9 (2.5–4.7) | 0.33 |
| TNF [median in ng/mL (IQR)] | 12 (7–16.6) | 8.2 (6.5–12.8) | 0.089 |
| C-reactive protein [median in mg/dL (IQR)] | 2.5 (0.7–5.3) | 1.2 (0.5–5.7) | 0.26 |
| Anti-TNF level [median in μg/mL (IQR)] | 12.1 (9.6–19.8) | 13.3 (11.7–19.8) | 0.09 |
| Hemoglobin [mean in gr/dL (SD)] | 13.6 (13.3–15.5) | 13.9 (12.3–14.6) | 0.66 |
| Age [mean in years (SD)] | 40 (17) | 38 (15) | 0.65 |
| Time on anti-TNF [median in months (IQR)] | 13 (12–24) | 14 (9–41.8) | 0.44 |
| On combination therapy with an immunomodulator [n (%)] | 8 (36.4) | 12 (48) | 0.42 |
| Previous non-response to another anti-TNF [n (%)] | 7 (31.8) | 15 (68.2) | 0.16 |
(*) statistically significant
Figure 1.
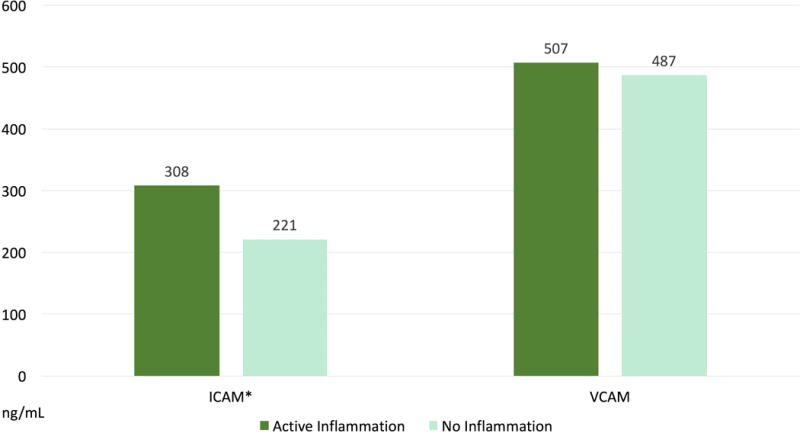
Differences in serum soluble adhesion molecule levels between those patients with and without active endoscopic inflammation despite adequate drug levels. (*) are statistically significant
Patients with active inflammation had significantly higher levels of IL-1β when compared to those without intestinal inflammation (3.2 vs. 1.5 pg/mL, p=0.002 respectively). There was no significant difference in the other cytokine levels nor CRP when comparing those patients with and without active disease (Table 2–Figure 2). Those patients with active disease despite adequate anti-TNF levels were not more likely to be smokers (OR:0.5 [95%CI:0.04–5.9], p=0.6) or to be receiving monotherapy rather than combination therapy with an immunomodulator (OR: 0.76 [95%CI: 0.2–2.4], p=0.6).
Figure 2.
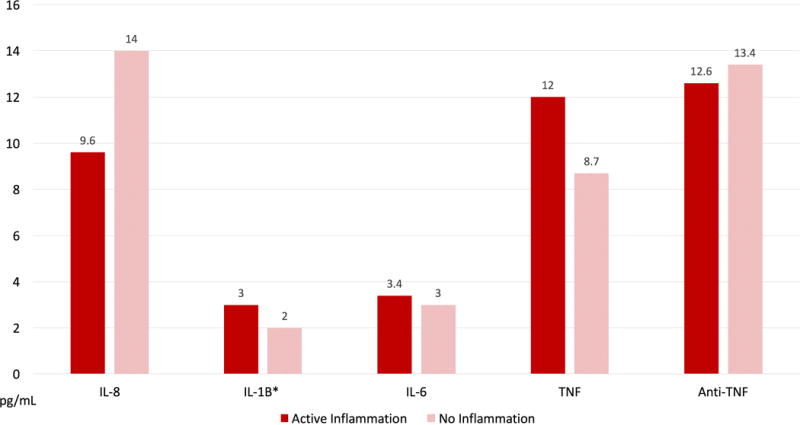
Differences in serum cytokine levels between those patients with and without active endoscopic inflammation despite adequate drug levels. (*) are statistically significant
When calculating receiving operating characteristic curves, ICAM-1 and IL-1β demonstrated a good correlation (ROC of 0.89 and 0.88 respectively – Figure 3A and 3B) with inflammation in this cohort of patients with anti-TNF resistance (active endoscopic inflammation with adequate serum anti-TNF levels). Receiving operating characteristic curves for all cytokines and CAMs are shown in Table 3.
Figure 3.
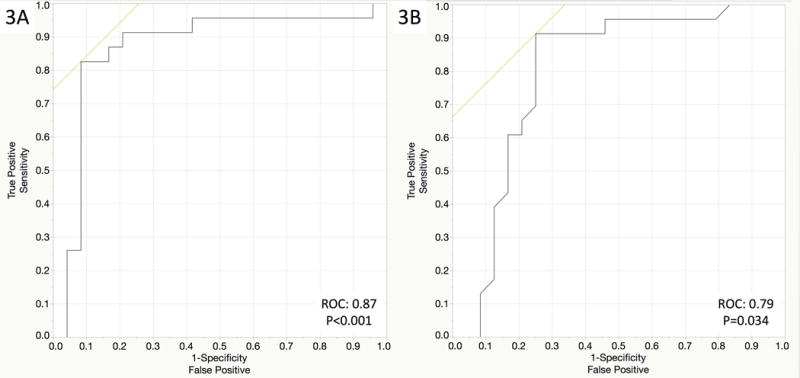
Area under the curve showing the correlation between active endoscopic disease and ICAM-1 (Figure 3A) and IL-1β (Figure 3B)
Table 3.
Association of multiple inflammatory cytokines with endoscopic inflammation in Anti-TNF resistance patients
| Biomarker | ROC for inflammation | P value |
|---|---|---|
| C-reactive protein | 0.6 | 0.31 |
| Intercellular Adhesion Molecule-1 | 0.89 | <0.001 |
| Vascular cell adhesion molecule-1 | 0.57 | 0.48 |
| Interleukin-8 | 0.4 | 0.63 |
| Interleukin-1β | 0.88 | 0.003 |
| Interleukin-6 | 0.59 | 0.21 |
| Tumor necrosis factor-α | 0.65 | 0.07 |
We also compared those patients with exposure to one versus more than one anti-TNF agent (20 patients, 43.5% of the total study group). Those patients on their index anti-TNF agent were not more likely to be in endoscopic remission when compared to those patients with multiple prior anti-TNF therapies (OR: 0.72 [95%CI: 0.2–2.9], p=0.64). Interestingly, those patients with exposure to ≥1 anti-TNF therapies had statistically significantly higher levels of VCAM-1 and IL-6 than patients with on their first anti-TNF agent but there were no differences in other cytokine levels (Table 4). When analyzing only the group of patients with exposure to ≥1 anti-TNF therapies, those with active inflammation had a significantly higher median level of ICAM-1 and IL-1β when compared to those without intestinal inflammation (313.6 vs. 224.8 ng/mL, p=0.002 and 4 vs. 1.1 pg/mL, p=0.0013 respectively) with no differences in other cytokine levels.
Table 4.
Differences between those patients with and without previous exposure to an anti-tumor necrosis factor agent
| Cytokine | Previous Exposure to Anti-TNF agent (20) | No Previous Exposure to Anti-TNF agent (27) | P value |
|---|---|---|---|
| ICAM [median in ng/mL (IQR)] | 239.5 (207.6–322.8) | 291.7 (239.5–311.2) | 0.33 |
| VCAM [median in ng/mL (IQR)] | 552 (485.8–697.5) | 452.9 (391-7–557.6) | 0.04* |
| IL-8 [median in ng/mL (IQR)] | 11.5 (7.7–35.1) | 13.9 (9.2–26.8) | 0.52 |
| IL-1B [median in ng/mL (IQR)] | 1.8 (0.7–3.0) | 2.7 (1.1–3.9) | 0.11 |
| IL-6 [median in ng/mL (IQR)] | 3.5 (2.8–6.2) | 2.7 (1.9–4.1) | 0.042* |
| TNF [median in ng/mL (IQR)] | 10.6 (7.1–18.5) | 8.7 (6.5–14.6) | 0.47 |
| C-reactive protein [median in mg/dL (IQR)] | 2.1 (0.5–7.1) | 1.2 (0.7–4.9) | 0.14 |
| Anti-TNF level [median in μg/mL (IQR)] | 13 (10.6–19.4) | 15.5 (10.6–19.8) | 0.47 |
| Hemoglobin [mean in gr/dL (SD)] | 13.2 (12.4–14.1) | 14 (13.4–15.7) | 0.06 |
| Age [mean in years (SD)] | 43 (18) | 36 (14) | 0.17 |
| Time on anti-TNF [median in months (IQR)] | 12 (12–24) | 16 (8.5–34.5) | 0.24 |
statistically significant
CONCLUSION
In this study, we examined the expression of several inflammatory pathways that have been implicated in Crohn’s disease in patients with non-response to anti-TNF agents despite optimal pharmacokinetic profiles. As the use of anti-TNF agents has increased, so too has the burden of patients losing response to these drugs. Much of the loss of response has been attributed to low drug levels and/or antibodies to anti-TNFs. In the current study, we examined only patients with serum levels of anti-TNF that are generally considered adequate (≥ 8 μg/ml) who also had no evidence of neutralizing antibodies. We divided those patients into those that had achieved endoscopic remission or not—strictly defined as no ulcerations greater than 5mm. We found that the group of patients with active endoscopic disease had a higher level of ICAM-1 and IL-1β when compared to those in remission, independent of whether they had been exposed to one or more than one anti-TNF agent; other cytokines including TNF were not different when comparing those with and without active endoscopic disease. We also found that patients with previous exposure to multiple anti-TNF therapies, whether in remission or not, had higher levels of VCAM and IL-6 when compared to those receiving their first anti-TNF.
The results suggest a possible role for targeting non-TNF inflammatory cytokines that are dysregulated in patients on anti-TNF therapy in the development of new agents for patients with anti-TNF resistance. As newer agents that target other inflammatory pathways become available, measuring alternative inflammatory pathways in these patients may help guide therapeutic interventions and identify an ideal second line agent with an alternative mechanism.
Leal et al. did a transcriptional analysis from intestinal biopsy specimens of Crohn’s disease patients who did not respond to anti-TNF therapy. They found that those patients maintained increased expression of IL-1β, IL-17A and S100A8 but had a significant modulation of other genes commonly upregulated in active Crohn’s disease, including IL-6 and IL-23 p19. In our study, we also found a higher level of IL-1β in the serum of those non-responders while we did not find a difference in IL-6 levels between groups. One difference in our studies is that we measured serum protein by ELISA rather than RNA. IL-6 has been found to be elevated in patients with Crohn’s disease on no therapy and also associated with disease activity20. Persistent elevation of IL-6 levels has been associated with a higher risk of clinical relapse20, but based on our findings and in the study by Leal et al, this may not be a pivotal inflammatory mediator in anti-TNF resistant patients. Moreover, anti-IL-6 therapy may carry other risks.
We also found a higher level of ICAM-1 in those patients with active endoscopic disease. Previous studies have found that patients with inflammatory bowel diseases (IBD) have a higher expression of ICAM-1 when compared to controls, and blocking it in animal models of IBD improved inflammation. This led to a clinical trial with alicaforsen (a selective inhibitor of ICAM-1 expression). However, it showed no clinical benefit even in patients with previous exposure to anti-TNFs21,22. It is unclear if those patients with previous exposure to anti-TNF had been optimized, especially considering that at the time the study was performed (2001–2004) therapeutic drug monitoring was not readily available and certainly not routinely integrated into clinical practice. There were also other limitations to the delivery and mechanism of action of alicaforsen and therefore ICAM-targeted therapy may still have some utility. The study did find that the sub-group of patients with a high CRP showed response22. Furthermore, there is evidence that alicaforsen is efficacious in the treatment of pouchitis23.
Our study also sought to investigate differences in cytokine profiles between those patients exposed to one vs more than one anti-TNF agent. We found that independently of the presence of active disease, the group of patients with exposure to multiple anti-TNF agents had significantly higher levels of VCAM-1 and IL-6 when compared to those on their first anti-TNF. This observation suggests that exposure to different anti-TNF therapies may change the inflammatory milieu. Even when patients respond to second anti-TNF, they may be have a cytokine environment that sets them up for refractoriness to the class. Thus far, anti-IL-6 therapy for Crohn’s disease has shown some efficacy in a pilot study but is associated with toxicity17. Nevertheless, IL-6 may be a good target for resetting the “immunostat” in patients that have been previously exposed to TNF suppression.
We recognize that our study has strengths and limitations. A strength of our study lies in the careful phenotyping of the patients and the matched endoscopy data alongside levels of serum drug and anti-drug antibodies that allow us to identify patients with therapeutic drug levels with active or inactive disease. One could argue, however, that levels of drug > 8μg/ml are not sufficient for all patients. While that may be true, it could also mean that some patients require higher levels because they have already become partially refractory or serum levels do not represent cytokine activity at the level of the tissue in question. In addition, it is often impractical, impossible, or too costly to achieve higher levels of anti-TNF in patients who have lost response. With respect to the inflammatory markers we examined, we chose pathways that have been implicated in IBD and for which adequate assays exist in serum. Tissue levels may have yielded different results than serum, as our group described that anti-TNF levels in tissue can vary between areas of inflammation and no inflammation; however, analysis of serum is far more practical24. Is also important to point out that the group of patients with active disease did have a numerically higher level of TNF and would be interesting to know if a larger study could be able to differentiate subtler differences. Another limitation relates to how patients were determined to have active disease. The study used “ulcers ≥ 5 mm” and not a more established scoring system such as the simple endoscopic score for Crohn’s disease. Finally, we examined known pathways associated with intestinal inflammation. We did not perform an unbiased analysis of tissue from anti-TNF non-responders to understand whether other pathways may be involved. Nevertheless, the pathways we looked at were ones that are feasible to target.
In conclusion, in this pilot study we found that patients with Crohn’s disease and “anti-TNF resistance” have higher serum levels of ICAM-1 and IL-1β when compared to those who responded to anti-TNF therapy. While larger studies are needed, novel therapies targeting these cytokine pathways could prove to be efficacious in patients with non-response or loss of response to anti-TNF agents.
Footnotes
Conflicts of Interest
Andres J. Yarur, MD: consultant and part of the speaker bureau for Prometheus Laboratories.
Anjali Jain, PhD: employee of Prometheus Laboratories.
Maria A Quintero, MD: no conflicts of interest
Frank Czul, MD: no conflicts of interest
Amar R. Deshpande, MD: no conflicts of interest
David H. Kerman, MD: no conflicts of interest
Maria T. Abreu, MD: conception and design of the study, analysis and interpretation of data, and final approval of the submitted version submitted.
Contributor Information
Andres J. Yarur, Division of Gastroenterology and Hepatology, Medical College of Wisconsin, Milwaukee, WI.
Anjali Jain, Prometheus Laboratories, San Diego CA.
Maria A Quintero, Division of Gastroenterology, University of Miami Miller School of Medicine, Miami, FL.
Frank Czul, Division of Gastroenterology, University of Miami Miller School of Medicine, Miami, FL.
Amar R. Deshpande, Division of Gastroenterology, University of Miami Miller School of Medicine, Miami, FL.
David H. Kerman, Division of Gastroenterology, University of Miami Miller School of Medicine, Miami, FL.
Maria T. Abreu, Division of Gastroenterology, University of Miami Miller School of Medicine, Miami, FL.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002 May 4;359(9317):1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn“s disease. Crohn”s Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 3.Gisbert JP, Panés J. Loss of Response and Requirement of Infliximab Dose Intensification in Crohn’s Disease: A Review. Am J Gastroenterol. 2009 Jan 27;104(3):760–7. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 4.Yarur AJ, Rubin DT. Therapeutic Drug Monitoring of Anti-tumor Necrosis Factor Agents in Patients with Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2015 Jul;21(7):1709–18. doi: 10.1097/MIB.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 5.Afif W, Loftus EV, Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical Utility of Measuring Infliximab and Human Anti-Chimeric Antibody Concentrations in Patients With Inflammatory Bowel Disease. Am J Gastroenterol. 2010 Feb 9;105(5):1133–9. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baert F, Noman M, Vermeire S, Van Assche G, D Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003 Feb 13;348(7):601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human Anti–Tumor Necrosis Factor Monoclonal Antibody (Adalimumab) in Crohn’s Disease: the CLASSIC-I Trial. Gastroenterology. 2006 Feb;130(2):323–33. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel J-F, Panaccione R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007 Jun 19;146(12):829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 9.Yarur AJ, Jain A, Hauenstein SI, Quintero MA, Barkin JS, Deshpande AR, et al. Higher Adalimumab Levels Are Associated with Histologic and Endoscopic Remission in Patients with Crohnʼs Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016 Feb;22(2):409–15. doi: 10.1097/MIB.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 10.Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A, et al. Concentrations of 6-Thioguanine Nucleotide Correlate With Trough Levels of Infliximab in Patients With Inflammatory Bowel Disease on Combination Therapy. Clinical Gastroenterology and Hepatology. 2015 Jun;13(6):1118–1124.e3. doi: 10.1016/j.cgh.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005 Sep 1;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 12.Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, et al. Adhesion molecules in inflammatory bowel disease. Gut BMJ Group. 1995 May;36(5):724–30. doi: 10.1136/gut.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014 Apr 22;14(5):329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 14.Gahmberg CG, Tolvanen M, Kotovuori P. Leukocyte adhesion–structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur J Biochem. 1997 Apr 15;245(2):215–32. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 15.Leal RF, Planell N, Kajekar R, Lozano JJ, Ordas I, Dotti I, et al. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNF therapy. Gut. 2015 Jan 8;64(2):233–42. doi: 10.1136/gutjnl-2013-306518. [DOI] [PubMed] [Google Scholar]
- 16.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature Medicine. 2000 May;6(5):583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease☆. Gastroenterology. 2004 Apr;126(4):989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Katsuta T, Lim C, Shimoda K, Shibuta K, Mitra P, Banner BF, et al. Interleukin-8 and SDF1-alpha mRNA expression in colonic biopsies from patients with inflammatory bowel disease. Am J Gastroenterol. 2000 Nov;95(11):3157–64. doi: 10.1111/j.1572-0241.2000.03289.x. [DOI] [PubMed] [Google Scholar]
- 19.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut BMJ Group. 1996 Feb;38(2):216–22. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinisch W, Gasché C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, et al. Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999 Aug 1;94(8):2156–64. doi: 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- 21.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001 Dec;121(6):1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 22.Yacyshyn B, Chey WY, Wedel MK, Yu RZ, Paul D, Chuang E. A Randomized, Double-Masked, Placebo-Controlled Study of Alicaforsen, an Antisense Inhibitor of Intercellular Adhesion Molecule 1, for the Treatment of Subjects With Active Crohn’s Disease. Clinical Gastroenterology and Hepatology. 2007 Feb;5(2):215–20. doi: 10.1016/j.cgh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.van Deventer SJH. A randomised, controlled, double blind, escalating dose study of alicaforsen enema in active ulcerative colitis. Gut. 2004 Nov 1;53(11):1646–51. doi: 10.1136/gut.2003.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarur AJ, Jain A, Sussman DA, Barkin JS, Quintero MA, Princen F, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016 Jan 6;65(2):249–55. doi: 10.1136/gutjnl-2014-308099. [DOI] [PubMed] [Google Scholar]


