Abstract
Background
Computerized cognitive rehabilitation training (CCRT) may be beneficial for alleviating persisting neurocognitive deficits in Ugandan severe malaria survivors. We completed a randomized controlled trial of CCRT for both severe malaria and non-malaria cohorts of children.
Methods
150 school-age severe malaria and 150 non-malaria children were randomized to three treatment arms: 24 sessions of Captain’s Log CCRT for attention, working memory and nonverbal reasoning, in which training on each of 9 tasks difficulty increased with proficiency; a limited CCRT arm that did not titrate to proficiency but randomly cycled across the simplest to moderate level of training; and a passive control arm. Before and after 2 months of CCRT intervention and one year following, children were tested with the Kaufman Assessment Battery for Children, 2nd edition (KABC-II), computerized CogState cognitive tests, the Behavior Rating Inventory for Executive Function (BRIEF), and the Achenbach Child Behavior Checklist (CBCL).
Results
Malaria children assigned to the limited-CCRT intervention arm were significantly better than passive controls on KABC-II Mental Processing Index (P=0.04), Sequential Processing (working memory) (P=0.02) and the Conceptual Thinking subtest (planning/reasoning) (P=0.02). At one year post-training, the limited CCRT malaria children had more rapid CogState card detection (attention) (P=0.02), and improved BRIEF Global Executive Index (P=0.01) as compared to passive controls. Non-malaria children receiving CCRT significantly benefited only on KABC-II Conceptual Thinking (both full- and limited-CCRT; P<0.01), CogState Groton maze chase and learning (P<0.01), and CogState card identification (P=0.05, full CCRT only). Improvements in KABC-II Conceptual Thinking planning subtest for the non-malaria children persisted to one-year follow-up only for the full-CCRT intervention arm.
Conclusion
. For severe malaria survivors, limited CCRT improved attention and memory outcomes more than full CCRT, perhaps because of the greater repetition and practice on relevant training tasks in the absence of the performance titration for full CCRT. There were fewer significant cognitive and behavior benefits for the non-malaria children, with the exception of the planning/reasoning subtest of Conceptual Thinking, with stronger full- compared to limited-CCRT improvements persisting to one-year follow-up.
Clinical Trials Registration
ClinicalTrials.gov Identifier NCT01743417, Submitted September 5, 2012
Keywords: Computer Training, Cognitive Rehabilitation, Severe Malaria Anemia, Cerebral Malaria, Neuropsychology, Child Development
BACKGROUND
Cognitive impairment following severe malaria is a well-documented problem of great public health significance in sub-Saharan Africa (Kihara et al., 2009; Kihara, Carter, & Newton, 2006). Our group completed the first prospective studies evaluating the neurocognitive effects of cerebral malaria (CM), documenting that one in four surviving children had neurocognitive deficits at 6-month (Boivin et al., 2007) and 2-yr post-illness follow-up (Bergemann et al., 2012; John et al., 2008). More recent studies with Ugandan survivors of severe malaria have also evidenced persisting mental health problems in children (Idro et al., 2016; Ssenkusu et al., 2016).
We then piloted a computerized cognitive rehabilitation training (CCRT) program produced by BrainTrain Corporation called Captain’s Log (Sandford, 2007) to enhance cognitive performance in these Ugandan CM children (Boivin et al., 2007; John et al., 2008). Ugandan CM survivors receiving CCRT had significantly greater gains on a visual-motor target chasing task (Groton Maze Chase; CogState computerized battery) and greater efficiency on a maze learning task (Groton Maze Learning) compared to controls (Bangirana, Giordani, et al., 2009). CCRT intervention also resulted in significant improvements on the Achenbach Child Behavior Checklist (CBCL) in the area of Internalizing Symptoms (e.g., anxiety, depression, somatic complaints). In a separate study, we administered CCRT to severe malaria survivors in Kampala, Uganda three months after illness (Bangirana et al., 2011). Twenty-eight school-age children were randomized to sixteen sessions of CCRT (twice a week for 8 weeks) and 33 to a passive control group. Significant training benefits were observed in the intervention group only for the Kaufman Assessment Battery for Children (KABC) learning domain, but not attention, working memory, or academic performance (reading, writing, arithmetic) abilities.
As these computerized cognitive rehabilitation studies were taking place with Ugandan children surviving severe malaria, our group was also applying Captain’s Log CCRT with Ugandan children perinatally infected with HIV and who had survived into their school-age years (Boivin et al., 2010; Boivin, Nakasujja, Sikorskii, Opoka, & Giordani, 2016). Sixty impoverished rural children with HIV with neurocognitive disabilities, as established by the KABC test, were randomized to either 10 training sessions of Captain’s Log CCRT or not. All children were evaluated with the computerized CogState cognitive performance evaluation (Darby, Maruff, Collie, & McStephen, 2002; Maruff et al., 2009) before and after the training period. Compared to the control group, the CCRT intervention children showed significant improvement on the CogState card detection task of simple attention and speed of correct moves on a Groton Maze Learning Task, similar to our previous findings with Ugandan children surviving cerebral malaria (Bangirana, Giordani, et al., 2009).
These positive preliminary findings were the impetus for a subsequent clinical trial with children with HIV in this same rural area of Uganda, with the goal of evaluating the cognitive and behavioral benefits of a more extensive regimen of CCRT intervention. The CCRT arm received 24 one-hour sessions over 2 months, with CCRT programmed for games targeting working memory, attention, and visual–spatial analysis. These games progressed in difficulty as the child’s performance improved. The second arm was a ‘‘limited CCRT’’ with the same games rotated randomly from simple to moderate levels of training. The third arm was a passive control group receiving no training. All children were assessed at enrollment, 2 months (immediately following CCRT), and 3 months after CCRT completion. The CCRT group had significantly greater gains through three months of follow-up compared to passive controls on overall Kaufman Assessment Battery for Children–second edition (KABC-II) mental processing index, planning and reasoning, and knowledge (crystalized cognitive skills). The limited CCRT group performed better than controls on KABC-II learning, similar to previous findings with Ugandan severe malaria survivors (Bangirana, Boivin, & Giordani, 2013).
In the pediatric HIV CCRT clinical trial, both CCRT arms had significant improvements on CogState Groton maze learning, though not on CogState attention/memory tasks, a computerized test of variables of attention (TOVA) measure of impulsivity, or behavior rating inventory for executive function (BRIEF) or Achenbach Child Behavior Checklist (CBCL) ratings by the child’s caregiver. A subsequent pediatric HIV CCRT study with rural Ugandan children in this region involved the initial field trial of a Michigan State University Games for Entertainment and Learning laboratory’s Brain Powered Games (BPG) package (Novak, Giordani, Boivin, & Winn, 2017). A version of these games with an African village and music motif was used with 33 school-age children with HIV For 24 training sessions over a two-month period, similar to the previous Captain’s Log CCRT clinical trial with children with HIV in that region (Giordani et al., 2015). The difference was that the BPG game package could be administered by tablet in the children home setting by field trainers, and results uploaded by internet through a mobile network platform. With BPG CCRT training, significant improvements were seen with TOVA of attention and CogState measures of processing speed, maze chase and learning. Results from these multiple clinical studies of evaluating the neuropsychological benefits of CCRT in both Ugandan pediatric HIV and severe malaria school-age survivors provided the foundation for the present study (Bangirana, Boivin, et al., 2013). The present study is the most rigorous randomized controlled trial (RCT) to date in evaluating the effects of computerized cognitive rehabilitation training in African children with acquired brain injury from infectious disease. This is because the present study extends the follow-up evaluation to one year following completion of CCRT training, and includes the full- and limited-CCRT intervention arms along with an active control arm for both severe malaria and non-malaria cohorts from the same home environments.
METHODS
Source of Children Enrolled in the Present Study
The present study dovetailed with an observational study (R01NS055349) of the pathogenesis of severe malaria (cerebral malaria (CM) and severe malaria anemia (SMA) in surviving children, along with non-malaria (community control) children from their households (Bangirana et al., 2015; Bangirana et al., 2014). The severe malaria and non-malaria cohort children were eligible for enrollment in the present study once they completed their two-year follow-up assessments in the source study within which this RCT was nested. Only retinopathy positive children with a diagnosis of cerebral malaria noted in Table 1 were classified as having cerebral malaria in both the source and the present study, since this criterion increases the sensitivity and specificity of the diagnosis to over 95% (Lewallen et al., 2008).
Table 1. Severe malaria survivors.
Demographic characteristics and outcomes at baseline by trial arm (N=150 total).
| Characteristic | CCRT N=51 | Limited CCRT N=54 | Control N=45 |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Sex | |||
| Male | 32 (62.75) | 32 (59.26 | 27 (60.00) |
| Female | 19 (37.25) | 22 (40.74) | 18 (40.00) |
| Malaria exposure | |||
| Cerebral | 31 (60.78) | 34 (62.96) | 28 (62.22) |
| Severe | 20 (39.22) | 20 (37.04) | 17 (37.78) |
| Mean (St Dev) | Mean (St Dev) | Mean (St Dev) | |
| Age in years | 7.39 (1.74) | 6.74 (1.33) | 6.85 (1.68) |
| Height-for-age z-score | −1.03 (0.98) | −0.95 (0.96) | −1.12 (0.92) |
| Weight-for-age z-score | −0.91 (0.80) | −0.96 (0.96) | −0.92 (0.77) |
| HOME score | 37.38 (14.38) | 36.09 (9.98) | 38.32 (12.28) |
| SES score | 10.58 (3.19) | 9.63 (2.93) | 8.96 (2.61) |
| KABC learning domain | 79.47 (13.12) | 74.80 (11.65) | 76.44 (14.95)) |
| KABC pattern reasoning scale | 3.72 (2.39) | 4.21 (2.63) | 4.96 (2.60) |
| KABC story completion scale | 5.20 (2.68) | 5.94 (2.63) | 6.42 (2.88) |
| KABC conceptual thinking scale | 10.37 (4.66) | 7.15 (5.01) | 8.69 (6.14) |
| KABC sequential processing domain | 75.24 (9.61) | 75.20 (10.64) | 77.53 (10.87) |
| KABC simultaneous processing domain | 67.02 (13.22) | 64.31 (13.54) | 66.51 (10.90) |
| KABC delayed recall domain | 78.51 (12.74) | 75.56 (11.32) | 78.20 (12.71) |
| KABC mental processing index | 66.51 (9.73) | 64.88 (9.25) | 66.84 (8.89) |
| CogState, correct moves per second, maze chase | 0.09 (0.13) | 0.07 (0.09) | 0.10 (0.16) |
| CogState, correct moves per second, maze learning | 0.08 (0.15) | 0.05 (0.12) | 0.05 (0.11) |
| CogState, detection time | 2.86 (0.12) | 2.80 (0.41) | 2.59 (0.86) |
| CogState, identification time | 2.97 (0.13) | 2.68 (0.90) | 2.74 (0.79) |
| CogState, one-card learning accuracy | 0.41 (0.17) | 0.38 (0.18) | 0.44 (0.23) |
| CogState, one-back card memory accuracy | 0.41 (0.25) | 0.34 (0.26) | 0.38 (0.27) |
| BRIEF Global Executive Composite | 53.76 (13.57) | 56.44 (13.75) | 56.80 (12.08) |
| CBCL Internalizing Symptoms | 61.16 (8.26) | 60.97 (8.46) | 61.61 (9.36) |
| CBCL Externalizing Symptoms | 61.53 (11.47) | 60.55 (8.82) | 61.65 (8.15) |
| CBCL Total Symptoms | 59.44 (10.85) | 59.24 (9.05) | 60.46 (9.52) |
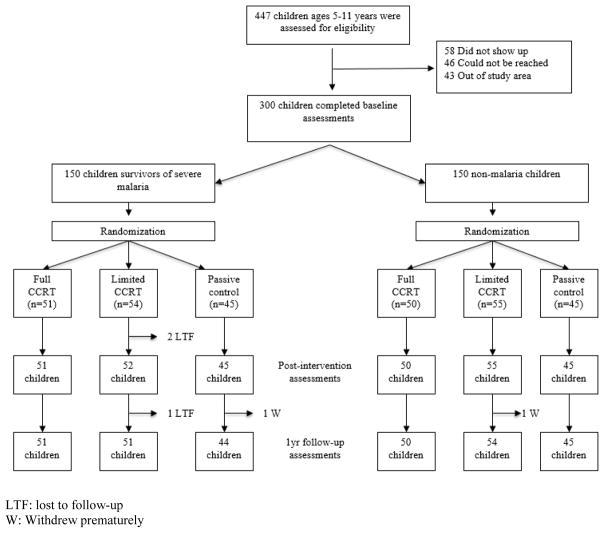
Eligible children from the pathogenesis study were stratified as severe malaria survivors (N=150) and non-malaria comparison children (N=150) (Figure 1). One hundred and forty seven eligible children from the source study were not enrolled in the present study: 58 did not come to their scheduled enrollment appointment by the end of the enrollment period, 46 could not be reached, and 43 had moved out of the study area b y the time of enrollment eligibility (Figure 1). Enrollment continued from eli gible children who had completed the pathogenesis R01 source study until the target enrollment of 150 severe malaria and 150 non-malaria children was reached for the present study. The 147 children from the pathogenesis R01 study who were not enrolled in the present study did not differ significantly from the 300 enrolled children in the present study in terms of sex distribution, malaria group, anthropometric measures at pathogenesis study enrollment, Caldwell Home Observation for the Measurement of the Environment (HOME) total score, or socio-economic status (SES) score.
Figure 1.
Flow diagram of the progress through the phases of the randomized controlled trial for Computerized Cognitive Rehabilitation Treatment in school-age children surviving malaria in Kampala, Uganda.
LTF: lost to follow-up
W: Withdrew prematurely
Study Recruitment, Enrollment, and Retention
Institutional Review Board approval for this study was obtained from Makerere University School of Medicine, University of Michigan, and Michigan State University. Research permission was obtained from the Uganda National Council for Science and Technology. Enrollment, assessment, and training took place from August 2012 to August 2016. Children surviving severe malaria were recruited in the source study from the Acute Care ward of Mulago Hospital (national referral and teaching hospital of Makerere University in Kampala, Uganda) after obtaining written informed consent in the local language from their parent(s) and signed assent from children 7 years of age and older.
Inclusion Criteria
1) Aged 5 to 12 years of age from the households of the severe malaria study children, and did not meet any of the exclusion criteria listed below; 2) Signed consent from the parent/guardian, assent from children aged 7 years and older; 3). Completion of their 24 months of follow-up testing in the severe malaria pathogenesis source study.
Exclusion Criteria
Same as the exclusion criteria used in the pathogenesis of severe malaria source study protocol (Bangirana, Menk, John, Boivin, & Hodges, 2013; Bangirana et al., 2015; Bangirana et al., 2014). Exclusion criteria for all children included (1) known chronic illness requiring medical care; (2) known developmental delay; or (3) history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. Additional exclusion criteria for children with SMA included (1) impaired consciousness at physical examination, (2) other clinical evidence of central nervous system disease, or (3) >1 seizure before admission. Additional exclusion criteria for community controls included (1) illness requiring medical care within the previous 4 weeks or (2) major medical or neurologic abnormalities at screening physical examination. In addition, the present study required that eligible children not have severe neurologic complications from the malaria episode that hindered the child’s ability to comprehend instructions and perform manual tasks on the computer. This was evidenced by a child’s ability to complete the computerized tests of variables of attention (TOVA), which was always the first test administered in the neuropsychological assessment battery. In the present study without exception, children able to complete the computerized TOVA test were always able to continue on to complete the KABC-II and CogState tests as well. They were also able to perform the required tasks on the CCRT training program.
Randomization and Masking
Following enrollment, severe malaria and non-malaria children were randomized to one of three treatment arms (full CCRT, limited CCRT, passive control) using a computer generated list of random numbers. Children assigned to either full or limited CCRT completed three training sessions per week for eight weeks (24 training session – lasting about an hour). Training usually took place in a private setting after school near the child’s home, under the supervision of research assistant.
Procedure for Computerized Cognitive Rehabilitation Training (CCRT)
Captain's Log® by BrainTrain is a comprehensive set of computerized cognitive training program with five modules - Developmental, Visual Motor Skills, Conceptual Skills, Numeric Concepts with Memory Skills, and Attention Skills (Sandford, 2007). These training modules were selected by a team of three Ugandan psychologists at Makerere University in Kampala. After reviewing all 35 possible training tasks, they selected the 9 training tasks which they considered to be most culturally fair while having 3 tasks emphasizing visual-spatial working memory, 3 tasks emphasizing vigilance attention, and 3 tasks emphasizing nonverbal reasoning. These same 9 training module tasks were used in both of our preliminary studies with severe malaria survivors (Bangirana et al., 2011; Bangirana, Giordani, et al., 2009), and in children with HIV in Uganda (Boivin et al., 2008; Boivin et al., 2010; Boivin et al., 2016).
Research assistants supervised the computerized CCRT training program, translating any screen instructions in English by speaking them to the children in the local language (Luganda), with sessions programmed to run for 45 to 60 minutes. Children typically no longer needed spoken instructions for the various training tasks after the first couple of sessions. Training was done in a quiet setting near the child’s home after school so as to avoid bringing unnecessary attention to the child and possible stigmatization by classmates, or disruption of training by distractions and interruptions in the home environment. The CCRT field trainers were generally aware of children’s trial arm assignment and malaria cohort status, although the research assistants who did the neuropsychological assessments were not.
Based on our preliminary studies (Bangirana, Boivin, et al., 2013; Boivin et al., 2010), all children assigned to either the full or limited CCRT treatment arms started at the simplest program level for each task. Typically, Captain’s Log adjusts the difficulty level of each task to the performance level of the child (titration). As the child achieves mastery at a given level of difficulty, that task is automatically adjusted at the next level of difficulty (e.g. full CCRT). There are 32 levels of difficulty for each task. The ability of Captain’s Log to step a child through successively more difficult levels of training as the child achieves mastery on a given task is an important defining feature of the cognitive rehabilitation that CCRT is meant to achieve. For the limited CCRT group, we programmed Captain’s Log® so that it rotated randomly from simple to moderate levels of training for the entire session (non-titration CCRT treatment arm). In every other respect this training arm was like the full CCRT training arm, in that the children in either the full or limited CCRT received the same nine training program. The difference was that the full CCRT training program was titrated to become progressively more difficult as the child gained proficiency on a given training program. This was not the case for the limited CCRT intervention arm. For the limited-CCRT intervention arm, we presented the same training program tasks in the same order, only at random levels of difficulty. This allowed the limited-CCRT children to receive the same training program exposure as the children receiving the full CCRT. However, the key difference between full and limited CCRT was the progressive nature of the training difficulty that titrated to the child’s level of proficiency for full CCRT. This is the most important feature of a true CCRT intervention, as opposed to simply playing games on a computer (Rabiner, Murray, Skinner, & Malone, 2010).
Neuropsychological assessment took place at the Mulago Hospital paediatric severe malaria clinic before the intervention (baseline), post-intervention (after 24 CCRT sessions, at about 8 weeks), and 1 year after completion of CCRT training. Training and testing was done in the local language (Luganda) by Bachelor’s Degree graduates trained in Psychology from Makerere University trained by M.J.B. and B.G.. Testers were masked to intervention arm for the study children, and their malaria cohort status. Testing always started with the TOVA test, a computerized test which quickly allowed testers to determine if the children could understand instructions and had the visual and motor proficiency capability to complete the rest of the battery (KABC-II, computerized CogState tests). Since all the children in the present study had successfully completed the same tests as part of the two-year assessment program for the neuropathogenesis of severe malaria source study in which our study was embedded, none of our study children were disqualified at this stage.
Neuropsychological and Behavioral Outcomes for CCRT Intervention
The principal outcomes for the present trial were improved attention (CogState card detection and identification and Groton maze chasing tasks); working memory and learning (KABC-II sequential processing and learning global domains, and CogState one-back card memory and card learning and Groton maze learning tasks); and nonverbal reasoning (KABC-II simultaneous processing global domain and planning subtests, along with the Nonverbal Index (NVI) of global cognitive performance). The caregiver-rated BRIEF Global Executive Composite index and the CBCL Internalizing, Externalizing, and Total symptoms were also principal outcomes in terms of behavioural and psychosocial problems for the study children.
Kaufman Assessment Battery for Children – Second Edition (KABC-II)
(Kaufman & Kaufman, 2004). Within the Luria model of neuropsychological evaluation, the KABC-II evaluates sequential (working memory) and simultaneous processing (visual-spatial analysis and problem solving). This edition also evaluates learning as well as the executive function domain of planning/reasoning. We previously established the factor structure of the KABC-II with Ugandan children surviving cerebral malaria (Bangirana, Musisi, et al., 2009), and it’s construct validity in terms of the sensitivity of this test to quality of the home and educational environment of urban Ugandan children surviving severe malaria (Bangirana, Menk, et al., 2013). In terms of the present study goals, the KABC-II has been previously used in preliminary clinical studies of CCRT benefit among severe malaria survivors (Bangirana et al., 2011; Bangirana, Boivin, et al., 2013; Bangirana, Giordani, et al., 2009). A recent systematic review has supported the validity of the KABC in the neuropsychological evaluation of pediatric HIV in a number of African countries (van Wyhe, van de Water, Boivin, Cotton, & Thomas, 2017).
Computerized cognitive skills assessment battery (CogState)
(CogState, 2007). This assessment provides attention, working memory, learning, and nonverbal reasoning measures in a computerized game-like format with playing cards. Ugandan school-age children are usually familiar with such playing cards. This test was used to evaluate these outcomes in our preliminary studies with Captain’s LogR CCRT intervention (Bangirana, Giordani, et al., 2009; Boivin et al., 2010). It also includes the Groton maze learning task as a measure of planning and visual tracking. For all CogState assessment tasks, equivalent stimuli are randomly chosen for each response trial. Therefore, repeated assessments can take place with minimum confounding from practice effects, even with multiple assessments in a single day (Mollica, Maruff, Collie, & Vance, 2005). The correspondence validity between the CogState and the KABC-II tests for Ugandan children has been previously documented by our group (Bangirana, Sikorskii, Giordani, Nakasujja, & Boivin, 2015).
Behavioral Inventory for Executive Function (BRIEF)
(Gioia, Isquith, Guy, & Kenworthy, 2003). The BRIEF is a parent-reported checklist for behavioral problems in children associated with executive function (e.g., problem solving planning, reasoning, behavior and emotional control). Its construct validity has been established with school-age Ugandan children with HIV and with children surviving severe malaria (Familiar et al., 2015).
Achenbach Child Behavior Checklist (CBCL) – School Age Version
(Achenbach, 1991, 2010). This is a psychiatric screening questionnaire for emotional (Internalizing Symptoms), behavior (Externalizing Symptoms), and total psychiatric symptoms and problems for the study child. This questionnaire is read to the principal caregiver out load in Luganda, so that she does not have to be literate to respond to the items. Also, the mother or caregiver could ask for clarification on any item that is not clear to her. It has been validated with severe malaria survivors in Uganda by our group (Bangirana, Nakasujja, et al., 2009; Familiar et al., 2015). The scale measures for this instrument were standardized by age and sex using the available global cross-cultural norms (Achenbach & Rescorla, 2007).
Caldwell Home Observation for the Measurement of the Environment (HOME)
(Bradley, Caldwell, Brisby, Magee, & et al., 1992). The middle childhood version of the Caldwell Home Observation for the Measurement of the Environment (HOME) was used to assess the stimulation and learning opportunities offered by the child’s home environment. The HOME score has been shown to be associated with the KABC performance in severe malaria survivors (Bangirana, John, et al., 2009) (Boivin et al., 1996).
The Socioeconomic Status (SES)
evaluation score was based the checklist of material possessions as opposed to income, characteristics of the home structure and living space, and parental education and occupational levels. Our SES assessment was developed for use in Democratic Republic of Congo (Boivin & Giordani, 1993; Boivin et al., 1993), and later adapted for Ugandan research with cerebral malaria survivors (Bangirana, Menk, et al., 2013).
Statistical Analyses
Power calculations for the present RCT sample sizes were based on preliminary CCRT intervention studies with Ugandan CM survivors in whom the KABC-II, CogState, and CBCL measures were used to assess intervention benefit (Bangirana et al., 2011; Bangirana, Boivin, et al., 2013; Bangirana, Giordani, et al., 2009). Using a two-sample t test (CCRT training versus passive control), sample size of 50 children per treatment arm was sufficient to detect pairwise trial arm differences of 0.57 of the standard deviation or greater for the principal outcomes with power of 0.80 or greater in two-tailed tests with 0.05 level of significance.
The KABC-II manual with American norms was used to obtain standardized and scaled scores to adjust for age. These norms were also required in order to compute the KABC-II Mental Processing Index and the Nonverbal Index, which were our two principal outcomes measuring overall cognitive ability in the present study. The BRIEF and CBCL age- and gender-based norms were used for those standardized comparisons. However, raw score performance outcomes were used for the TOVA and the CogState outcome analyses.
All analyses were performed separately for each of the two cohorts: severe malaria survivors (cerebral malaria and severe malaria anemia subgroups combined) and their non-malaria household reference children (community controls). Baseline characteristics of the sample were summarized and characteristics of drop-outs were compared by trial arm. The least square (LS) means of the outcome variables and their 95% confidence intervals (CIs) were obtained from linear mixed effects (LME) models with two repeated measures: (1) immediately after CCRT training (two months after intake into the trial), and (2) twelve months following completion of CCRT training (one-year follow-up). Outcome analyses were adjusted for age, sex, physical growth (weight and height standardized scores using the WHO 2016 norms), socioeconomic status (SES), and quality of caregiving and home environment (Caldwell HOME scale). These demographic factors were selected a priori based on their reported associations with the neuropsychological and behavioral outcomes used in this study (Boivin & Giordani, 2009, 2013). In addition to these control variables, baseline measures of the outcome variables were used as covariates for added control for any pre-intervention influences. The differences among LS means by trial arm were tested for each outcome variable at each time point.
Role of the Funding Source
The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
Figure 1 summarizes the flow of the participants through the trial; baseline characteristics are in Table 1 for the severe malaria survivors and Table 2 for the non-malaria children. There were no differences by trial arm in baseline characteristics among those who dropped out.
Table 2. Non-malaria children.
Demographic characteristics and outcomes at baseline by trial arm (N=150 total).
| Characteristic | CCRT N=50 | Limited CCRT N=55 | Control N=45 |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Sex | |||
| Male | 23 (46.00) | 24 (43.64) | 20 (44.44) |
| Female | 27 (54.00) | 31 (56.36) | 25 (55.56) |
| Mean (St Dev) | Mean (St Dev) | Mean (St Dev) | |
| Age in years | 6.99 (2.06) | 7.08 (1.85) | 7.00 (1.76) |
| Height-for-age z-score | −1.12 (1.03) | −0.77 (1.13) | −0.85 (1.11) |
| Weight-for-age z-score | −0.84 (1.02) | −0.80 (0.89) | −0.78 (0.89) |
| HOME score | 41.51 (12.97) | 41.25 (9.95) | 40.23 (12.64) |
| SES score | 10.36 (3.56) | 10.53 (3.09) | 9.09 (2.16) |
| KABC learning domain | 76.92 (15.17) | 77.29 (11.78) | 77.18 (12.17) |
| KABC pattern reasoning scale | 5.74 (3.09) | 4.56 (2.45) | 4.13 (3.08) |
| KABC story completion scale | 6.50 (3.13) | 5.96 (3.27) | 5.67 (3.23) |
| KABC conceptual thinking scale | 9.54 (5.94) | 8.67 (4.91) | 9.06 (5.29) |
| KABC sequential processing domain | 78.80 (10.22) | 78.42 (9.12) | 81.20 (12.09) |
| KABC simultaneous processing domain | 67.72 (13.92) | 66.67 (13.70) | 65.36 (14.27) |
| KABC delayed recall domain | 81.34 (12.77) | 79.67 (12.75 ) | 80.53 (14.31) |
| KABC mental processing index | 68.96 (11.44) | 67.16 (8.32) | 67.47 (10.58) |
| CogState, correct moves per second, maze chase | 0.06 (0.08) | 0.10 (0.14) | 0.09 (0.13) |
| CogState, correct moves per second, maze learning | 0.05 (0.10) | 0.05 (0.10) | 0.05 (0.08) |
| CogState, detection time | 2.81 (0.42) | 2.80 (0.41) | 2.66 (0.74) |
| CogState, identification time | 2.87 (0.60) | 2.86 (0.58) | 2.86 (0.64) |
| CogState, one-card learning accuracy | 0.41 (0.18) | 0.43 (0.20) | 0.42 (0.18) |
| CogState, one-back card memory accuracy | 0.37 (0.29) | 0.40 (0.25) | 0.40 (0.31) |
| BRIEF Global Executive Composite | 51.92 (13.77) | 50.29 (11.18) | 46.33 (10.14) |
| CBCL Internalizing Symptoms | 55.41 (10.69) | 59.04 (9.75) | 55.37 (10.06) |
| CBCL Externalizing Symptoms | 56.62 (10.24) | 58.55 (8.87) | 54.48 (9.04) |
| CBCL Total Symptoms | 54.47 (10.61) | 56.52 (9.21) | 52.50 (9.85) |
Severe malaria survivors (Table 3) receiving limited CCRT intervention were significantly better (P=0.05) than controls on KABC-II Mental Processing Index (limited CCRT adjusted mean 71.75, 95% CI (69.87, 73.84), controls adjusted mean 68.65, 95% CI (66.54, 70.76)). Limited CCRT was also superior to full CCRT on Sequential Processing (P=0.02) (limited CCRT adjusted mean 79.94, 95% CI (77.47, 82.42); full CCRT adjusted mean 75.24, 95% CI (72.74, 77.75)). The difference between limited and full CCRT diminished at one year follow-up, but limited CCRT was significantly better than controls (P=0.02). Limited CCRT performed significantly better than controls on the KABC-II scale of Conceptual Thinking (P<0·01) (limited CCRT adjusted mean 12.11, 95% CI (11.14, 13.08); controls adjusted mean 9.48, 95% CI (8.45, 10.50)), but not at one-year follow-up. Limited CCRT had more rapid CogState card detection (a measure of attention) at one-year follow-up (P=0·02) (adjusted mean=2.58 msec, 95% CI (2.45, 2.71) compared to controls adjusted mean 2.79, 95% CI (2.65, 2.93), and full CCRT adjusted mean 2.83, 95% CI (2.70, 2.96). Limited CCRT had fewer behavior problems as measured by the BRIEF Global Executive Composite (P=0·01) at one-year follow-up (limited CCRT adjusted mean 47.12, 95% CI (44.28, 49.97) as compared to controls adjusted mean 53.18, 95% CI (50.15, 56.20). Statistical results for other outcomes for the KABC-II, TOVA, CogState, BRIEF, and CBCL assessments are presented in detail in Table 3.
Table 3. Severe malaria survivors.
Post-training least square means and 95% confidence intervals (CIs) by trial arm adjusted for age, sex, weight, height, HOME score, material possessions socio-economic score, and malaria exposure (cerebral versus severe malarial anemia). Least square (LS) means of the outcome variables were obtained from linear mixed effects (LME) models with two repeated measures: (1) immediately after CCRT training (post-training) and (2) 12 months following completion of CCRT training (one year follow-up). Baseline measures of the outcome variables were used as covariates for added control for any pre-intervention influences.
| CCRT LS Mean (95% CI) | Limited CCRT LS Mean (95% CI) | Control LS Mean (95% CI) | P-value for arm comparison | |
|---|---|---|---|---|
| KABC learning domain | ||||
| Post-training | 82.11 (79.62, 84.60) | 84.22 (81.75, 86.68)* | 80.07 (77.45, 82.68) | 0.07 |
| One year follow up | 77.05 (74.56, 79.54) | 76.21 (73.73, 78.70) | 76.10 (73.46, 78.74) | 0.85 |
| KABC pattern reasoning scale | ||||
| Post-training | 3.97 (3.42, 4.51) | 4.04 (3.50, 4.58) | 4.22 (3.64, 4.79) | 0.82 |
| One year follow up | 3.28 (2.73, 3.83) | 2.79 (2.24, 3.34) | 2.85 (2.27, 3.44) | 0.40 |
| KABC story completion scale | ||||
| Post-training | 6.10 (5.51, 6.69) | 5.93 (5.35, 6.52) | 6.55 (5.93, 7.17) | 0.34 |
| One year follow up | 4.86 (4.27, 5.45) | 4.65 (4.07, 5.24) | 4.91 (4.29, 5.54) | 0.81 |
| KABC conceptual thinking domain | ||||
| Post-training | 10.86 (9.87, 11.85) | 12.11 (11.14, 13.08)* | 9.48 (8.45, 10.50) | <0.01 |
| One year follow up | 11.70 (10.72, 12.68) | 12.15 (11.17, 13.14) | 12.02 (10.98, 13.06) | 0.80 |
| KABC sequential processing domain | ||||
| Post-training | 75.24 (72.74, 77.75) | 79.94 (77.47, 82.42)# | 78.08 (75.43, 80.73) | 0.02 |
| One year follow up | 74.15 (71.64, 76.65) | 77.31 (74.81, 75.82)* | 73.14 (70.46, 75.82) | 0.06 |
| KABC simultaneous processing domain | ||||
| Post-training | 68.62 (65.79, 71.45) | 71.50 (68.70, 74.29) | 69.66 (66.68, 72.64) | 0.34 |
| One year follow up | 65.35 (62.52, 68.18) | 68.46 (65.64, 71.30) | 65.43 (62.42, 68.44) | 0.21 |
| KABC delayed recall domain | ||||
| Post-training | 80.86 (78.11, 83.60) | 83.24 (80.53, 85.96) | 80.78 (77.90, 83.67) | 0.36 |
| One year follow up | 77.05 (74.31, 79.79) | 74.69 (71.95, 77.43) | 76.22 (73.27,79.17) | 0.47 |
| KABC mental processing index | ||||
| Post-training | 68.80 (66.79, 70.80) | 71.75 (69.87, 73.84)* | 68.65 (66.54, 70.76) | 0.05 |
| One year follow up | 65.23 (63.22, 67.23) | 65.75 (63.75, 67.76) | 64.11 (61.98, 66.25) | 0.53 |
| CogState, correct moves per second, maze chase | ||||
| Post-training | 0.23 (0.18, 0.28) | 0.18 (0.13, 0.23) | 0.17 (0.12, 0.22) | 0.16 |
| One year follow up | 0.17 (0.12, 0.22) | 0.21 (0.16, 0.26) | 0.17 (0.12, 0.22) | 0.40 |
| CogState, correct moves per second, maze learning | ||||
| Post-training | 0.10 (0.07, 0.14) | 0.13 (0.09, 0.16) | 0.09 (0.05, 0.13) | 0.42 |
| One year follow up | 0.10 (0.06, 0.14) | 0.09 (0.05, 0.13) | 0.12 (0.08, 0.16) | 0.69 |
| CogState, detection time | ||||
| Post-training | 2.79 (2.66, 2.91) | 2.80 (2.67, 2.93) | 2.91 (2.77, 3.05) | 0.40 |
| One year follow up | 2.83 (2.70, 2.96) | 2.58 (2.45, 2.71)*# | 2.79 (2.65, 2.93) | 0.02 |
| CogState, identification time | ||||
| Post-training | 2.93 (2.81, 3.04) | 2.97 (2.85, 3.10) | 2.96 (2.83, 3.09) | 0.84 |
| One year follow up | 2.88 (2.76, 3.00) | 2.94 (2.82, 3.06) | 3.02 (2.89, 3.15) | 0.30 |
| CogState, one-card learning accuracy | ||||
| Post-training | 0.41 (0.36, 0.46) | 0.46 (0.41, 0.51) | 0.48 (0.42, 0.53) | 0.18 |
| CogState, one-back card memory accuracy | ||||
| Post-training | 0.40 (0.34, 0.46)* | 0.43 (0.37, 0.49)* | 0.52 (0.46, 0.59) | 0.02 |
| One year follow up | 0.47 (0.41, 0.53) | 0.49 (0.43, 0.55) | 0.54 (0.47, 0.60) | 0.32 |
| BRIEF Global Executive Composite | ||||
| Post-training | 52.63 (49.78, 55.47) | 55.03 (52.22, 57.84) | 56.24 (53.24, 59.23) | 0.21 |
| One year follow up | 49.18 (46.33, 52.02) | 47.12 (44.28, 49.97)* | 53.18 (50.15, 56.20) | 0.01 |
| CBCL Internalizing Symptoms | ||||
| Post-training | 60.69 (58.19, 63.18) | 62.97 (60.37, 65.56) | 59.18 (56.13, 62.23) | 0.16 |
| One year follow up | 56.13 (53.64, 58.63) | 58.54 (55.90, 61.18) | 57.61 (54.57, 60.64) | 0.41 |
| CBCL Externalizing Symptoms | ||||
| Post-training | 59.08 (56.62, 61.53) | 60.40 (57.84, 62.95) | 61.20 (58.20, 64.20) | 0.53 |
| One year follow up | 56.42 (53.96, 58.88) | 59.05 (56.46, 61.64) | 60.00 (57.01, 62.99) | 0.14 |
| CBCL Total Symptoms | ||||
| Post-training | 57.46 (55.14, 59.79) | 59.62 (57.20, 62.04) | 58.96 (56.12, 61.80) | 0.43 |
| One year follow up | 54.70 (52.38, 57.03) | 55.51 (53.05, 57.97) | 57.09 (54.25, 59.92) | 0.43 |
indicates significant difference from control;
indicates difference between limited CCRT and CCRT.
Non-malaria children (Table 4) receiving full or limited CCRT benefited on KABC-II Conceptual Thinking scale compared to controls post-intervention (P=0·02) (full CCRT adjusted mean 12.26, 95% CI (11.23, 13.29); limited CCRT adjusted mean 11.74 (10.75, 12.72); controls adjusted mean 9.95 (8.84, 11.05)). The benefit remained significant at one year follow up for the full CCRT compared to controls but was attenuated for the limited CCRT.
Table 4. Non-malaria children.
Post-training least square means and 95% confidence intervals (CIs) by trial arm adjusted for age, sex, weight, height, HOME score, material possessions socio-economic score, and malaria exposure (cerebral versus severe malarial anemia). Least square (LS) means of the outcome variables were obtained from linear mixed effects (LME) models with two repeated measures: (1) immediately after CCRT training (post-training) and (2) 12 months following completion of CCRT training (one year follow-up). Baseline measures of the outcome variables used as covariates for added control for pre-intervention influences.
| CCRT LS Mean (95% CI) | Limited CCRT LS Mean (95% CI) | Control LS Mean (95% CI) | P-value for arm comparison | ||
|---|---|---|---|---|---|
| KABC learning domain | |||||
| Post-training | 84.73 (81.99, 87.46) | 84.49 (81.88, 87.11) | 83.90 (80.97, 86.83) | 0.92 | |
| One year follow up | 79.49 (76.75, 82.22) | 78.29 (75.66, 80.92) | 76.78 (73.77, 79.80) | 0.43 | |
| KABC pattern reasoning scale | |||||
| Post-training | 4.54 (3.95, 5.12) | 4.82 (4.27, 5.37) | 4.89 (4.27, 5.50) | 0.68 | |
| One year follow up | 3.92 (3.33, 4.50) | 3.90 (3.34, 4.45) | 3.90 (3.26, 4.54) | 0.99 | |
| KABC story completion scale | |||||
| Post-training | 6.98 (6.37,7.59)# | 6.12 (5.54, 6.70) | 6.16 (5.51, 6.81) | 0.08 | |
| One year follow up | 5.06 (4.45, 5.67) | 5.13 (5.54, 5.72) | 5.03 (4.35, 5.70) | 0.97 | |
| KABC conceptual thinking domain | |||||
| Post-training | 12.26 (11.23, 13.29)* | 11.74 (10.75, 12.72)* | 9.95 (8.84, 11.05) | <0.01 | |
| One year follow up | 13.42 (12.37, 14.47)* | 12.27 (11.27, 13.26) | 11.40 (10.26, 12.54) | 0.04 | |
| KABC sequential processing domain | |||||
| Post-training | 81.50 (79.09, 83.91) | 80.63 (78.32,82.93) | 79.92 (77.33, 82.51) | 0.68 | |
| One year follow up | 77.66 (75.25, 80.07) | 76.94 (74.62, 79.26) | 76.53 (73.85, 79.20) | 0.82 | |
| KABC simultaneous processing domain | |||||
| Post-training | 71.72 (68.79, 75.65) | 71.54 (68.74, 74.33) | 71.23 (68.09, 74.36) | 0.97 | |
| One year follow up | 69.02 (66.09, 71.95) | 66.13 (63.32, 68.95) | 66.64 (63.41, 69.87) | 0.34 | |
| KABC delayed recall domain | |||||
| Post-training | 83.17 (80.40, 85.94) | 83.58 (80.93, 86.23) | 84.07 (81.10, 87.04) | 0.91 | |
| One year follow up | 79.69 (76.92, 82.46) | 78.21 (75.54, 80.88) | 78.67 (75.60, 81.74) | 0.74 | |
| KABC mental processing index | |||||
| Post-training | 72.73 (70.44, 75.02) | 72.95 (70.77, 75.14) | 73.20 (70.75, 75.64) | 0.97 | |
| One year follow up | 68.33 (66.04, 70.62) | 67.02 (64.81, 69.22) | 66.64 (64.11, 69.17) | 0.58 | |
| CogState, correct moves per second, maze chase | |||||
| Post-training | 0.28 (0.23, 0.33)* | 0.24 (0.20, 0.29)* | 0.14 (0.09, 0.20) | <.01 | |
| One year follow up | 0.23 (0.18, 0.29)* | 0.25 (0.20, 0.30)* | 0.15 (0.09, 0.21) | 0.02 | |
| CogState, correct moves per second, maze learning | |||||
| Post-training | 0.13 (0.10, 0.16)* | 0.13 (0.10, 0.16)* | 0.07 (0.04, 0.11) | 0.02 | |
| One year follow up | 0.14 (0.11, 0.17) | 0.15 (0.12, 0.18)* | 0.11 (0.07, 0.14) | 0.21 | |
| CogState, detection time | |||||
| Post-training | 2.77 (2.63, 2.91) | 2.75 (2.62, 2.88) | 2.82 (2.67, 2.98) | 0.76 | |
| One year follow up | 2.74 (2.60, 2.88) | 2.72 (2.58, 2.85) | 2.72 (2.56, 2.88) | 0.96 | |
| CogState, identification time | |||||
| Post-training | 2.88 (2.81, 2.96)* | 2.93 (2.86, 3.00) | 3.02 (2.94, 3.10) | 0.05 | |
| One year follow up | 2.96 (2.88, 3.03) | 2.97 (2.90, 3.04) | 3.01 (2.93, 3.10) | 0.60 | |
| CogState, one-card learning accuracy | |||||
| Post-training | 0.46 (0.42, 0.51) | 0.45 (0.41, 0.50) | 0.44 (0.39, 0.49) | 0.75 | |
| One year follow up | 0.52 (0.47, 0.56)* | 0.47 (0.43, 0.51) | 0.44 (0.40,0.50) | 0.12 | |
| CogState, one-back card memory accuracy | |||||
| Post-training | 0.46 (0.39, 0.52) | 0.49 (0.42, 0.55) | 0.44 (0.37, 0.50) | 0.55 | |
| One year follow up | 0.54 (0.48, 0.61) | 0.47 (0.41, 0.54) | 0.45 (0.38, 0.52) | 0.15 | |
| BRIEF Global Executive Composite | |||||
| Post-training | 50.65 (48.03, 53.27) | 48.38 (45.88, 50.87) | 48.27 (45.44, 51.10) | 0.37 | |
| One year follow up | 45.11 (42.49, 47.73) | 47.24 (44.73, 49.76) | 47.14 (44.22, 50.05) | 0.45 | |
| CBCL Internalizing Symptoms | |||||
| Post-training | 56.27 (53.38, 59.17) | 56.93 (54.43, 59.45) | 56.09 (53.33, 58.85) | 0.89 | |
| One year follow up | 52.86 (49.92, 55.80) | 55.41 (52.87, 57.96) | 55.50 (52.56, 58.44) | 0.36 | |
| CBCL Externalizing Symptoms | |||||
| Post-training | 55.20 (52.51, 57.89) | 56.26 (53.92, 58.59) | 57.94 (55.36, 60.52) | 0.35 | |
| One year follow up | 52.13 (49.39, 54.86) | 55.56 (53.20, 57.93) | 55.18 (52.45, 57.92) | 0.15 | |
| CBCL Total Symptoms | |||||
| Post-training | 52.95 (50.23, 55.67) | 53.69 (51.34, 56.05) | 54.51 (51.90, 57.12) | 0.72 | |
| One year follow up | 48.72 (45.96, 51.48)# | 52.87 (50.49, 55.25) | 52.25 (49.49, 55.01) | 0.07 | |
indicates significant difference from control;
indicates difference between limited CCRT and CCRT.
Both full and limited CCRT did significantly better than controls on CogState Groton maze chase (P<0·01) (full CCRT adjusted mean correct moves per second 0.28, 95% CI (0.23, 0.33); limited CCRT adjusted mean 0.24, 95% CI (0.20, 0.29), controls adjusted mean 0.14, 95% CI (0.09, 0.20). This benefit persisted for both full and limited CCRT at one-year follow-up (P=0.02). Both full and limited CCRT also did significantly better than controls on CogState Groton maze learning (P=0.02) (full and limited CCRT had the same adjusted mean 0.13, 95% CI (0.10, 0.16) correct moves per second versus controls adjusted mean 0.07, 95% CI (0.04, 0.11), but not at one-year follow-up (P=0.21), at which time only limited CCRT remained superior to controls. Full CCRT non-malaria children also did significantly better than controls on the two-step attention task of CogState card identification speed (P<0.05) (full CCRT 2.88 msec, 95% CI (2.81, 2.96); controls adjusted mean 3.02, 95% CI (2.94, 3.10), but not at one-year follow-up. For the non-malaria children, aside from KABC-II conceptual thinking subtest and CogState correct moves per second Groton maze learning and maze chase measures, the CCRT treatment arms did not perform significantly better after training than the passive controls for the other KABC-II, CogState, BRIEF, or CBCL outcomes (Table 4). Statistical results for all outcomes for the non-malaria cohort for the KABC-II, TOVA, CogState, BRIEF, and CBCL assessments are presented in detail in Table 4.
DISCUSSION
Cerebral malaria and HIV infection are among the commonest CNS infections associated with long-term neurocognitive deficits in African children (Boivin & Giordani, 2009, 2013). Also, the high mortality associated with these conditions historically has made them a public health priority in many African countries. With better medical treatment and supportive care for these conditions now available, more children now survive into adulthood. More attention is now being paid to the quality of life of these children who live with persisting cognitive deficits. In other for these children to achieve their full potential, interventions have been called for like cognitive rehabilitation, speech and physical therapy and care-giver training (Boivin, Kakooza, Warf, Davidson, & Grigorenko, 2015).
Severe malaria survivors receiving limited CCRT intervention were significantly better than the passive control arm on KABC-II Mental Processing Index (MPI; composite of all cognitive domains), sequential processing (working memory), and the Conceptual Thinking subtest from the planning global domain, which is a measures of executive function and reasoning. These KABC-II findings were consistent with the KABC cognitive performance benefits for Captain’s LogR CCRT observed in our preliminary study (Bangirana et al., 2011) However, in the present study, these performance benefits persisted to one-year follow-up after training only for sequential processing.
For the non-malaria children, the full CCRT treatment arm was significantly better compared to passive controls following training only for the KABC-II subtest of conceptual thinking, and this persisted to one-year follow-up. Overall, CCRT training benefit on KABC-II outcomes was greater for the severe malaria than for the non-malaria children. Furthermore, for the severe malaria survivors, limited CCRT with its greater repetition and practice at simpler levels of training had greater benefit in terms of attention and working memory outcomes than full CCRT.
For the CogState measures, only detection speed, which is a simple measure of attention, favored limited CCRT compared to passive controls among malaria children at one-year follow-up. Positive effects of both full and limited CCRT on CogState one-back card memory accuracy were seen among malaria children post-training, but not at one year follow-up. Non-malaria children receiving full or limited CCRT were significantly better than passive controls on the CogState Groton maze chase (visual-motor tracking/attention) and Groton maze learning outcomes, consistent with our previous findings for this CCRT outcome benefit (Bangirana, Giordani, et al., 2009). These significant differences were also apparent at one-year follow-up only for maze chase, and not for maze learning.
According to Mahncke and colleagues (Mahncke, Bronstone, & Merzenich, 2006) CCRT is effective in remediating neurocognitive disability because it is designed to redress the four principal causes of negative brain plasticity. These are 1) reduced schedules of neural network activity; 2) noisy processing within the neural network; 3) weakened neuromodulatory control; and 4) negative learning transfer effect, interfering with new learning. Our present neuropsychological test battery assesses the effects of CCRT in remediating these four dimensions of negative neuroplasticity resulting from the effects of infections affecting brain function such as cerebral malaria (Boivin, 2002; Boivin et al., 2007; John et al., 2008). These include 1) outcomes pertaining to the processing speed in the CogState attention test outcomes (cared detection and identification) as enhanced by CCRT stimulation of neural network activity; 2) CCRT reduction of noisy processing as assessed by improved KABC-II Sequential and Simultaneous Processing performance; 3) CCRT strengthened neuromodulatory control as evidenced by improved KABC-II Planning performance; and 4) improved learning from CCRT as evidenced by KABC-II and CogState Groton maze learning and card learning performance outcomes. These are the principal causes of the persisting attention, working memory, and language development develops caused by severe malaria (Holding & Boivin, 2013).
A common concern with respect to proposed evidence for CCRT training benefit is whether the training simply improves certain skills related to the neuropsychological outcome tasks and does not actually improve brain/behavior function in terms of positive neuroplasticity. This concern was addressed in a recent RCT of CCRT emphasizing working memory (WM) training (see www.cogmed.edu) with normal school children in Cambridge, England (Astle, Barnes, Baker, Colclough, & Woolrich, 2015). Astle and colleagues compared a full CCRT treatment arm to children receiving a non-titrating version of Cogmed training. Using magnetoencephalography (MEG) to evaluate changes in resting state connectivity between brain regions underpinning WM performance, they concluded that CCRT in children enhances neurophysiological brain connectivity intra- and inter-hemispherically between brain regions known to be related to verbal and visual-spatial WM respectively (fronto-parietal networks and both lateral occipital complex and inferior temporal cortex) (Astle et al., 2015; Barnes, Woolrich, Baker, Colclough, & Astle, 2016).
Many CCRT interventions lack adequate validation studies that demonstrate lasting benefit and tie CCRT results to other medical illness indicators (Bangirana, Boivin, & Giordani, 2013). Although technical issues appear reasonably resolved for resource poor settings, considerations remain with regard to best approaches and influence of language issues or familiarity with presented stimuli. Cost issues related to such settings also are to be considered. Most available programs are expensive to purchase or lease due to extensive programming and development work, often making these inappropriate outside of research-supported settings. Increasing humanitarian interest generated by positive results in resource poor settings may help defray such costs or increased interest in open-source and non-copyright approaches may lead to wider use. Finally, CCRT can be made accessible to boys and girls in resource-constrained settings through smart phone or tablet devices on a mobile network platform or with downloaded apps, particularly in contexts where the school-based education of girls may not be as strongly prioritized as that of the boys in families struggling to pay school fees (M.J. Boivin, Dobias, & Giordani, 2013; Giordani et al., 2015; Novak et al., 2017).
Additional research will be necessary in terms of comparing the efficacy of self-titrating versus fixed presentation CCRT programs. Although developers of CCRT interventions argue that the self-titrating, increasingly challenging nature of CCRT programs in inherently important (Klingberg et al., 2005; Rabiner et al., 2010), sufficient comparisons to non-titrating cognitive training programs will need to be completed. The availability of feedback and the type/amount of feedback within the CCRT setting continue as other areas of interest and ones that may require careful consideration across cultural settings. Emphasis on feedback in terms of monetary representations or loud auditory feedback may not be appropriate in some cultures or settings. Ease and use of directions in local languages may be necessary, though the inherent game-like quality of most of these programs allowing for enhanced learning and understanding over time may reduce this concern.
With the recent proliferation of information communication technology in the rural areas of sub-Saharan Africa, use of the internet through mobile phone networks to provide these interventions to remote areas is possible scenario. For example, the entire country of Uganda presently has mobile network coverage, and this has become increasingly the case throughout sub-Sahara Africa. Mental health interventions like cognitive behavior therapy have been administered through the internet in high income countries with less costs but still highly effective in their treatment (Hedman et al., 2011; Ljotsson et al., 2011). Administration of CCRT through the internet has also proven effective enhancing usage of compensatory strategies resulting respondents being satisfaction with the intervention (Bergquist et al., 2009; Bergquist, Thompson, Gehl, & Munoz Pineda, 2010).
Study Limitations
The principal study limitation was the lack of a computerized intervention arm that did not involve cognitive training (active control). Unlike the passive control arm, this type of active control arm would have allowed us to better disentangle the effects of computer use experience from that of neurocognitive training itself on such computer-based outcome tests such as CogState. It would also have provided for adult interactions with the child in the active control arm, allowing us to isolate the psychosocial benefits of cognitive rehabilitation from the psychosocial enrichment embedded within the supervision of the CCRT sessions. This would have been helpful in interpreting the behavioral benefits occurring at one-year follow-up for the severe malaria survivors on the BRIEF measure of beh avior problems related to executive function. Another study limitation was the fact that the CCRT field trainers generally knew the malaria cohort and intervention arm status of the study children, although the neuropsychological testers did not.
CONCLUSIONS
The results from this study demonstrate that computerized cognitive training programs can be adapted for resource-poor and rural non-Western settings, and that children can be motivated to take part in these extended training regimens. Trainers consistently observed that the children seemed to like and be well-motivated to take part in these game-based training exercises, which is important since motivation has been associated with increased benefit from computerized training (Jaeggi et al., 2013). However, the neuropsychological and behavioral benefits were mostly limited to specific domains and did not usually extend to one-year follow-up post training. Continued “booster training” sessions may be needed in order for these neurocognitive benefits to persist, especially with survivors of severe malaria.
The titration feature specific to full CRRT is important for training benefit on some tasks (executive function planning). In contrast, the limited-CCRT arm resulted in better attention and working memory improvements with the severe malaria survivors. This was probably because of gains in processing speed from more repetition and practice on relevant tasks at the simpler levels of training that typified the limited-CCRT arm. Future studies of our group will evaluate ways of bringing CCRT interventions to scale at a school- or community-wide level taking advantage of increased use of smartphones as with the Brain Powered Games (BPG) (Giordani et al., 2015; Novak et al., 2017).
HIGHLIGHTS.
Computerized rehabilitation games improve cognition in severe malaria survivors
There were more cognitive benefits in the severe malaria than non-malaria cohorts
Most cognitive training benefits dissipated at one-year follow-up post training
Cognitive training that titrated to child’s performance level improved reasoning
More repetitive cognitive training at simpler levels improved attention & memory
Acknowledgments
Funding: National Institutes of Health (NIH) grant# R01 HD064416. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript, and in the decision to publish this manuscript.
Cognitive training, assessment, and on-site study management was conducted by members of the Global Health Uganda study team for this project at Mulago Hospital, Kampala, Uganda. Team members were Lyagoba Monica (study coordinator), Alex Mutebe (data management), Robert Tuke (medical officer), Susan Nalubwama (study nurse), Agatha Kuteesa, Michael Sengendo, Ethel Wandeka Nuwamanya, Maria Kateete, Richard Seviiri, Stella Akayo, Titus Sessanga, and Stella Butala. Without their efforts, this study would never have been accomplished. University of Michigan doctoral nursing students Katie Finn and medical student Jennifer Neva contributed as research interns for the study, as did MSU medical students Christopher Adam and Hailey Wouters, and Chicago School of Professional Psychology graduate student Jacquelyn Moore. The efforts of all of these students are very much appreciated.
Abbreviations
- RCT
Randomized Controlled Trial
- BRIEF
Behavior Rating Inventory of Executive Function
- CBCL
Achenbach Child Behavior Checklist
- CCRT
Computerized Cognitive Rehabilitation Training
- CI
confidence interval
- CM
cerebral malaria
- LME
Lineal Mixed Effects
- HOME
Caldwell Home Observation for Measurement of the Environment
- IRB
institutional review board
- KABC-II
Kaufman Assessment Battery for Children (2nd ed)
- MPI
Mental Processing Index
- SAS
Statistical Analysis System
- SES
socio-economic status
- SMA
severe malaria anemia
- TOVA
Tests of Variables of Attention
Footnotes
DECLARATIONS
Ethics approval and consent to participate: IRB approval for this study was obtained from Makerere University School of Medicine, University of Michigan, and Michigan State University. Research permission was obtained from the Uganda National Council for Science and Technology. Written informed consent was obtained from the principal caregiver of all study children, as well as written assent for children 7 yrs of age and older.
Competing interests: The authors declare that they have no competing interests.
Consent for publication: Not applicable
Availability of data and material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request, contingent upon completion of an approved data sharing agreement with Michigan State University.
Author Contributions: MJB served as study PI, had oversight over all phases of study conceptualization, design, grant writing, implementation, analyses, interpretation, programming and implementing the computer cognitive games packages for the interventions, wrote the complete first draft of the paper, and approved the final draft of the paper as submitted. NN also served as study PI, shared oversight over all phases of study implementation, submission of IRB approval in Uganda, and approved the final draft of the paper as submitted. AS was responsible for all analyses and tables, participated in the writing of the paper, and approved the final draft of the paper as submitted. HR-E shared scientific oversight of the study on site, participated in the writing of the paper, and approved the final draft of the paper as submitted. I.F-L shared scientific oversight of the study on site, participated in the writing of the paper, and approved the final draft of the paper as submitted. KM revised and helped finalize the protocol and standard operating procedures of the study, supported the IRB amendment, approval, and audit process, supported staff training in the implementation of study procedures, and approved the final draft of the paper as submitted. EMvdL directed the study team in the original implementation of the protocol and standard operating procedures of the study, supported the IRB application process, supported staff training in the implementation of study procedures, and approved the final draft of the paper as submitted. ROO supported administrative oversight of the study team, pediatric support and care for severe malaria study children, and approved the final draft of the paper as submitted. BG supported the PIs in their oversight over all phases of study conceptualization, design, grant writing, implementation, analyses, interpretation, and was instrumental in finalizing the configuration of the computerized cognitive rehabilitation programs, and approved the final draft of the paper as submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Integrative Guide ot the 1991 CBCL/4-18 YSR, and TRF Profiles. 1. Vol. 108. University of Vermont, Department of Psychology Pediatrics; 1991. p. e14. [Google Scholar]
- Achenbach TM. The Multicultural Supplement to the Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: ASEBA; 2010. [Google Scholar]
- Achenbach TM, Rescorla LA. Multicultural Supplement to the Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2007. [Google Scholar]
- Astle DE, Barnes JJ, Baker K, Colclough GL, Woolrich MW. Cognitive training enhances intrinsic brain connectivity in childhood. J Neurosci. 2015;35(16):6277–6283. doi: 10.1523/JNEUROSCI.4517-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Allebeck P, Boivin MJ, John CC, Page C, Ehnvall A, Musisi S. Cognition, behaviour and academic skills after cognitive rehabilitation in Ugandan children surviving severe malaria: a randomised trial. BMC Neurol. 2011;11:96. doi: 10.1186/1471-2377-11-96. 1471-2377-11-96 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Boivin MJ, Giordani B. Computerized Cognitive Rehabilitation Therapy (CCRT) for African Children: Evidence for Neuropsychological Benefit and Future Directions. In: Boivin MJ, Giordani B, editors. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience. Vol. 1. New York: Springer Science+Business Media; 2013. pp. 277–298. [Google Scholar]
- Bangirana P, Giordani B, John CC, Page C, Opoka RO, Boivin MJ. Immediate neuropsychological and behavioral benefits of computerized cognitive rehabilitation in Ugandan pediatric cerebral malaria survivors. J Dev Behav Pediatr. 2009;30(4):310–318. doi: 10.1097/DBP.0b013e3181b0f01b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, Boivin MJ. Socioeconomic predictors of cognition in Ugandan children: Implications for community based interventions. PLOS ONE. 2009;4(11):e7898. doi: 10.1371/journal.pone.0007898. doi:7810.1371/journal.pone.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Menk J, John CC, Boivin MJ, Hodges JS. The association between cognition and academic performance in Ugandan children surviving malaria with neurological involvement. PLOS ONE. 2013;8(2):e55653. doi: 10.1371/journal.pone.0055653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Musisi S, Allebeck P, Giordani B, John CC, Opoka RO, … Boivin MJ. A preliminary investigation of the construct validity of the KABC-II in Ugandan children with prior cerebral insult. African Health Sciences. 2009;9(3):186–192. [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Nakasujja N, Giordani B, Opoka RO, John CC, Boivin MJ. Reliability of the Luganda version of the Child Behaviour Checklist in measuring behavioural problems after cerebral malaria. Child Adolesc Psychiatry Ment Health. 2009;3:38. doi: 10.1186/1753-2000-3-38. 1753-2000-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, John CC. Neurocognitive domains affected by cerebral malaria and severe malarial anemia in children. Learning and Individual Differences. 2015 doi: 10.1016/j.lindif.2015.01.010. [DOI] [PMC free article] [PubMed]
- Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, … John CC. Severe malarial anemia is associated with longterm neurocognitive impairment. Clinical Infectious Diseases. 2014;59(3):336–344. doi: 10.1093/cid/ciu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangirana P, Sikorskii A, Giordani B, Nakasujja N, Boivin MJ. Validation of the CogState battery for rapid neurocognitive assessment in Ugandan school age children. Child Adolesc Psychiatry Ment Health. 2015;9:38. doi: 10.1186/s13034-015-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JJ, Woolrich MW, Baker K, Colclough GL, Astle DE. Electrophysiological measures of resting state functional connectivity and their relationship with working memory capacity in childhood. Dev Sci. 2016;19(1):19–31. doi: 10.1111/desc.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann TL, Bangirana P, Boivin MJ, Connett JE, Giordani BJ, John CC. Statistical approaches to assess the effects of disease on neurocognitive function over time. J Biomet Biostat. 2012;(S7:016):1–8. doi: 10.4172/2155-6180.S7-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist T, Gehl C, Mandrekar J, Lepore S, Hanna S, Osten A, Beaulieu W. The effect of internet-based cognitive rehabilitation in persons with memory impairments after severe traumatic brain injury. Brain Inj. 2009;23(10):790–799. doi: 10.1080/02699050903196688. 914076410. [DOI] [PubMed] [Google Scholar]
- Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed J E Health. 2010;16(4):417–423. doi: 10.1089/tmj.2009.0118. [DOI] [PubMed] [Google Scholar]
- Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23(5):353–364. doi: 10.1097/00004703-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, John CC. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119(2):e360–366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Tomac R, Parikh S, Opoka RO, Nakasujja N, … Giordani B. Neuropsychological benefits of computerized cognitive rehabilitation training in Ugandan children surviving cerebral malaria and children with HIV. BMC Proceedings. 2008;2(Suppl 1):P7. [Google Scholar]
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, Giordani B. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24(5):667–673. doi: 10.1037/a0019312. 2010-17509-013. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Dobias K, Giordani B. M. J. B. a. B. G. S. T. i. P. Neuropsychology, editor. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience, (Vol. Specialty Topics in Pediatric Neuropsychology) New York, NY: Springer Science+Business Media; 2013. Chapter 16 Postscript: Towards a universal brain/behavior omnibus in the neuropsychology of African children; pp. 329–333. [Google Scholar]
- Boivin MJ, Giordani B. Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. J Pediatr Psychol. 1993;18(2):249–264. doi: 10.1093/jpepsy/18.2.249. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Giordani B. Neuropsychological assessment of African children: evidence for a universal basis to cognitive ability. In: Chiao JY, editor. Cultural Neuroscience: Cultural Influences on Brain Function. Vol. 178. New York, NY: Elsevier Publications; 2009. pp. 113–135. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Giordani B, editors. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience. Vol. 1. New York, NY: Springer; 2013. [Google Scholar]
- Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N. Economic advantage and the cognitive ability of rural children in Zaire. J Psychol. 1996;130(1):95–107. doi: 10.1080/00223980.1996.9914992. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N, Muamba K. Effects of treatment for intestinal parasites and malaria on the cognitive abilities of schoolchildren in Zaire, Africa. Health Psychol. 1993;12(3):220–226. doi: 10.1037//0278-6133.12.3.220. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Kakooza AM, Warf BC, Davidson LL, Grigorenko EL. Reducing neurodevelopmental disorders and disability through research and interventions. Nature. 2015;527(7578):S155–160. doi: 10.1038/nature16029. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Nakasujja N, Sikorskii A, Opoka RO, Giordani B. A Randomized Controlled Trial to Evaluate if Computerized Cognitive Rehabilitation Improves Neurocognition in Ugandan Children with HIV. AIDS Res Hum Retroviruses. 2016;32(8):743–755. doi: 10.1089/AID.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Brisby J, Magee M, et al. The HOME Inventory: A new scale for families of pre- and early adolescent children with disabilities. Research in Developmental Disabilities. 1992;13(4):313–333. doi: 10.1016/0891-4222(92)90009-u. [DOI] [PubMed] [Google Scholar]
- CogState (Producer) [17-February-2018];CogState Computer programs. 2007 Retrieved from http://cogstate.com/go/home.
- Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59(7):1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- Familiar I, Ruisenor-Escudero H, Giordani B, Bangirana P, Nakasujja N, Opoka R, Boivin M. Use of the Behavior Rating Inventory of Executive Function and Child Behavior Checklist in Ugandan children with HIV or a history of severe malaria. J Dev Behav Pediatr. 2015;36(4):277–284. doi: 10.1097/DBP.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function® (BRIEF®) Lutz, FL: Psychological Assessment Resources (PAR); 2003. [Google Scholar]
- Giordani B, Novak B, Sikorskii A, Bangirana P, Nakasujja N, Winn BW, Boivin MJ. Designing and evaluating Brain Powered Games for cognitive training and rehabilitation in at-risk African children. Global Mental Health. 2015;2(e6):1–14. doi: 10.1017/gmh.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman E, Andersson E, Ljotsson B, Andersson G, Ruck C, Lindefors N. Cost-effectiveness of Internet-based cognitive behavior therapy vs. cognitive behavioral group therapy for social anxiety disorder: results from a randomized controlled trial. Behav Res Ther. 2011;49(11):729–736. doi: 10.1016/j.brat.2011.07.009. S0005-7967(11)00158-6. [DOI] [PubMed] [Google Scholar]
- Holding P, Boivin MJ. The assessment of neuropsychological outcomes in pediatric severe malaria. In: Boivin MJ, Giordani B, editors. Specialty Topics in Pediatric Neuropsychology (Vol. Neuropsychology of African Children: Risk and Resilience) New York, NY: Springer; 2013. pp. 235–275. [Google Scholar]
- Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO, … Nalugya J. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184. doi: 10.1186/s12936-016-1233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Tjalsma H, Kortman GA, … Zimmermann MB. Iron status and systemic inflammation, but not gut inflammation, strongly predict gender-specific concentrations of serum hepcidin in infants in rural Kenya. PLOS ONE. 2013;8(2):e57513. doi: 10.1371/journal.pone.0057513PONE-D-12-35305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, … Boivin MJ. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92–99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Assessment Battery for Children, Second Edition. Circle Pines, MN: American Guidance Service Publishing/Pearson Products Inc; 2004. [Google Scholar]
- Kihara M, Carter JA, Holding PA, Vargha-Khadem F, Scott RC, Idro R, … Newton CR. Impaired everyday memory associated with encephalopathy of severe malaria: the role of seizures and hippocampal damage. Malar J. 2009;8(1):273. doi: 10.1186/1475-2875-8-273. 1475-2875-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health. 2006;11(4):386–397. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, … Westerberg H. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008;102(11):1089–1094. doi: 10.1016/j.trstmh.2008.06.014. S0035-9203(08)00282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljotsson B, Hedman E, Andersson E, Hesser H, Lindfors P, Hursti T, … Andersson G. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol. 2011;106(8):1481–1491. doi: 10.1038/ajg.2011.139. ajg2011139. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. In: Moller AR, editor. Progress in Brain Research. Vol. 157. Amsterdam: Elsevier B.V; 2006. pp. 81–109. [DOI] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, Pietrzak RH. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- Mollica CM, Maruff P, Collie A, Vance A. Repeated assessment of cognition in children and the measurement of performance change. Child Neuropsychol. 2005;11(3):303–310. doi: 10.1080/092970490911306. [DOI] [PubMed] [Google Scholar]
- Novak B, Giordani B, Boivin M, Winn B. Potential uses of computer-based cognitive rehabilitation programs. In: Abubakar A, van de Vijver FJR, editors. Handbook of Applied Developmental Science in Sub-Saharan Africa. New York, NY: Springer Publishing; 2017. pp. 281–290. [Google Scholar]
- Rabiner DL, Murray DW, Skinner AT, Malone PS. A Randomized Trial of Two Promising Computer-Based Interventions for Students with Attention Difficulties. Journal of Abnormal Child Psychology. 2010;38:131–142. doi: 10.1007/s10802-009-9353-x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19697119. [DOI] [PubMed] [Google Scholar]
- Sandford JA. [23-December-2015];Captain's Log. 2007 Retrieved from http://www.braintrain.com/
- Ssenkusu JM, Hodges JS, Opoka RO, Idro R, Shapiro E, John CC, Bangirana P. Long-term Behavioral Problems in Children With Severe Malaria. Pediatrics. 2016;138(5):e20161965. doi: 10.1542/peds.2016-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyhe KS, van de Water T, Boivin MJ, Cotton MF, Thomas KG. Cross-cultural assessment of HIV-associated cognitive impairment using the Kaufman assessment battery for children: a systematic review. J Int AIDS Soc. 2017;20(1):1–11. doi: 10.7448/IAS.20.1.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]