Abstract
Purpose
CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin that delivers a synergistic 5:1 drug ratio into leukemia cells to a greater extent than normal bone marrow cells. Prior clinical studies demonstrated a sustained drug ratio and exposure in vivo and prolonged survival versus standard-of-care cytarabine plus daunorubicin chemotherapy (7+3 regimen) in older patients with newly diagnosed secondary acute myeloid leukemia (sAML).
Patients and Methods
In this open-label, randomized, phase III trial, 309 patients age 60 to 75 years with newly diagnosed high-risk/sAML received one to two induction cycles of CPX-351 or 7+3 followed by consolidation therapy with a similar regimen. The primary end point was overall survival.
Results
CPX-351 significantly improved median overall survival versus 7+3 (9.56 v 5.95 months; hazard ratio, 0.69; 95% CI, 0.52 to 0.90; one-sided P = .003). Overall remission rate was also significantly higher with CPX-351 versus 7+3 (47.7% v 33.3%; two-sided P = .016). Improved outcomes were observed across age-groups and AML subtypes. The incidences of nonhematologic adverse events were comparable between arms, despite a longer treatment phase and prolonged time to neutrophil and platelet count recovery with CPX-351. Early mortality rates with CPX-351 and 7+3 were 5.9% and 10.6% (two-sided P = .149) through day 30 and 13.7% and 21.2% (two-sided P = .097) through day 60.
Conclusion
CPX-351 treatment is associated with significantly longer survival compared with conventional 7+3 in older adults with newly diagnosed sAML. The safety profile of CPX-351 was similar to that of conventional 7+3 therapy.
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by the expansion of malignant myeloid precursors in the blood and bone marrow. Although most cases arise de novo, AML can evolve from an antecedent hematologic disorder or as a late complication of chemotherapy or ionizing radiation.1 Secondary AML (sAML) accounts for approximately one quarter of all AMLs and occurs more frequently with advancing age.2 sAML also is associated with biologic features that contribute to poor outcomes independent of older age, including adverse/complex cytogenetics and multidrug resistance phenotype.2-6
For > 40 years, the 7+3 regimens (ie, cytarabine infused continuously for 7 days with three once-daily injections of an anthracycline) have been a standard for AML induction therapy.7,8 In older adults and patients with sAML, 7+3 induction is associated with lower remission rates, increased early mortality, and higher relapse rates than in younger adults and older patients with de novo AML.9,10 Attempts to improve 7+3 with the addition of other drugs or intensification of postremission therapy largely have failed to improve outcomes,11-13 although two agents14,15 were recently approved by the US Food and Drug Administration as additions to standard induction in specific settings.
CPX-351 (VYXEOS; Jazz Pharmaceuticals, Palo Alto, CA) is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a fixed 5:1 synergistic molar ratio.16-19 In animal models, CPX-351 demonstrated superior antileukemia activity versus free drugs when administered at the same drug ratios.16,19 In a randomized phase II study of patients age 60 to 75 years with newly diagnosed AML, higher remission rates were observed with CPX-351 than with 7+3 in the overall study population, with improved overall survival (OS) and event-free survival (EFS) in the subgroup of patients with sAML.20 On the basis of these results, we conducted a randomized phase III study to compare the efficacy and safety of CPX-351 to conventional 7+3, which recently led to the US Food and Drug Administration approval of daunorubicin:cytarabine 44:100 mg/m2 liposome (CPX-351) for the treatment of adults with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes.
PATIENTS AND METHODS
Study Design
This phase III, open-label study randomly assigned patients to receive CPX-351 or conventional cytarabine and daunorubicin (7+3 cohort). Patients were enrolled between December 2012 and November 2014 across 39 centers in the United States and Canada.
Patients were randomly assigned using a dynamic balancing randomization algorithm21 in a 1:1 ratio to receive CPX-351 or 7+3 as induction and consolidation chemotherapy. They were stratified by age (60 to 69 v 70 to 75 years) and AML type (five subtypes: therapy-related AML, AML with a history of myelodysplastic syndrome [MDS] with and without prior hypomethylating agents, AML with a history of chronic myelomonocytic leukemia [CMML], de novo AML with MDS-related cytogenetic abnormalities).
Patients could receive up to two cycles of induction chemotherapy to achieve complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi)22 followed by up to two cycles of consolidation therapy. The initial CPX-351 induction course consisted of 100 units/m2 (100 mg/m2 cytarabine and 44 mg/m2 daunorubicin) administered as a 90-minute infusion on days 1, 3, and 5. A second induction course (same dose) was administered on days 1 and 3 for patients who did not achieve hypoplastic marrow on a day 14 bone marrow assessment. For patients in CR/CRi after induction, postremission therapy consisted of up to two cycles of 65 units/m2 CPX-351 (65 mg/m2 cytarabine and 29 mg/m2 daunorubicin) on days 1 and 3. The 7+3 control cohort consisted of an initial induction course of cytarabine 100 mg/m2/d administered by 7-day continuous infusion with daunorubicin 60 mg/m2 on days 1 to 3. The second induction course and postremission consolidation courses consisted of cytarabine 100 mg/m2/d by 5-day continuous infusion with daunorubicin 60 mg/m2 on days 1 and 2. Allogeneic hematopoietic cell transplantation (HCT) was performed at the discretion of the treating physician.
Eligibility Criteria
Patients were age 60 to 75 years with newly diagnosed therapy-related AML, AML with antecedent MDS or CMML, or de novo AML with MDS-related cytogenetic abnormalities (per 2008 WHO criteria).1 Those who previously received hypomethylating agents, such as azacitidine or decitabine for MDS or CMML, were eligible. Patients with de novo AML were required to have MDS-related cytogenetic abnormalities; cytogenetic screening was available through a central laboratory (University of Rochester, Rochester, NY), with results available within 48 hours of sample receipt. Those with acute promyelocytic leukemia, core-binding factor leukemia known at screening, active CNS leukemia, active second malignancies, or prior cumulative anthracycline exposure of > 368 mg/m2 daunorubicin or equivalent were excluded from the study.
End Points and Assessments
The primary end point was OS. Secondary end points were remission rate (CR, CR + CRi; assessed according to the Revised International Working Group Criteria for AML22), remission duration, and EFS (time since random assignment to date of induction failure, relapse from CR + CRi, or death as a result of any cause). Cumulative incidence of relapse at 6 months and 1 year were calculated among patients who achieved remission; events included relapse from CR + CRi and death, and patients were censored at the time of HCT (Data Supplement). Safety outcomes were adverse events, laboratory assessments, and early (30- and 60-day) mortality.
Statistical Analyses
With accrual within 2 years, minimum follow-up of 1.2 years, and an estimated median OS of 0.526 years in the 7+3 treatment group,20 a sample size of 270 patients was expected to result in 236 deaths, giving this trial 93.7% power and a one-sided α of .025 to detect a hazard ratio (HR) of 0.635 between treatment arms. An additional 30 patients were accrued to account for ineligible patients and patients who withdrew consent.
Efficacy analyses were performed in the intention-to-treat population; safety was evaluated in all treated patients. Time-to-event end points were evaluated using a stratified log-rank test to compare treatment groups. The Kaplan-Meier method was used to estimate the distribution of these end points over time. HRs and 95% CIs were estimated using a Cox proportional hazards regression model stratified by age and AML subtype. A Mantel-Haenszel test was used to compare remission rates and other binary end points.
Study Oversight
The trial protocol and modifications were approved by the independent ethics committee or institutional review board at each study site, and the study was conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent.
An independent data and safety monitoring board oversaw the trial by assessing safety and efficacy. The study sponsor, Celator Pharmaceuticals, a subsidiary of Jazz Pharmaceuticals, designed the study. Statistical analyses were performed by Celator/Jazz. All authors had confidential access to the data, assume responsibility for the accuracy and completeness of the data, and vouch for the fidelity of the trial to the protocol. The first two and last two authors wrote the first manuscript draft with assistance from professional medical writers funded by Jazz. All authors reviewed the manuscript through several revisions and made the decision to submit it for publication.
RESULTS
Patient Population
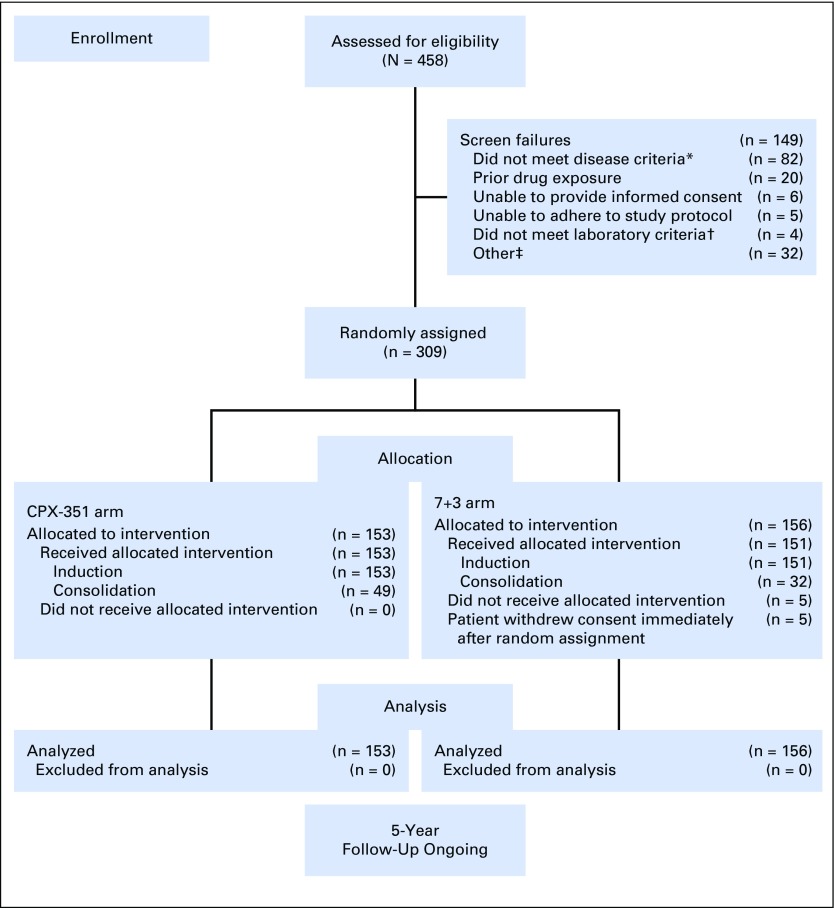
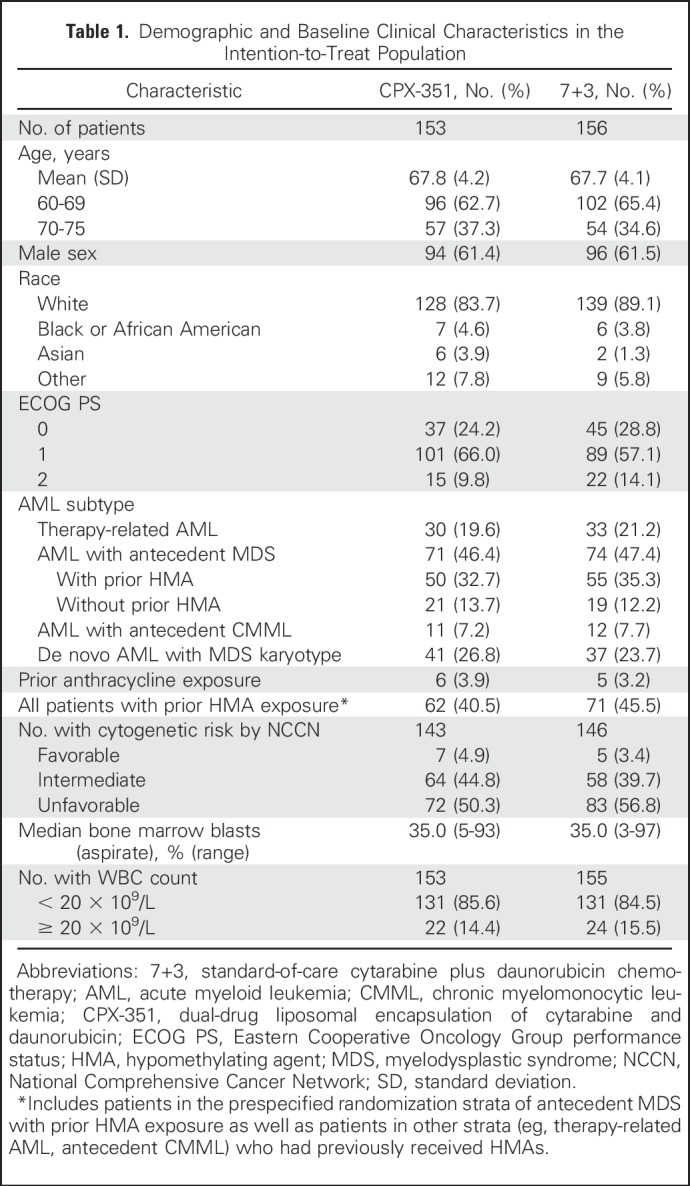
A total of 309 patients were randomly assigned to CPX-351 (n = 153) or 7+3 (n = 156) and included in the intention-to-treat population for efficacy analyses (Fig 1). The safety population included all patients in the CPX-351 cohort and 151 patients from the 7+3 cohort (five patients withdrew consent before the receipt of treatment). Patient baseline characteristics were balanced between treatment cohorts (Table 1).
Fig 1.
CONSORT diagram. (*) Patients without confirmation of therapy-related acute myeloid leukemia (AML), antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML), or de novo AML with MDS-related cytogenetic abnormalities (n = 43; many of these screen failures were in patients with de novo AML without MDS-related cytogenetic abnormalities because results of the required bone marrow biopsy specimens were not available at the patients’ initial diagnosis); without pathologic diagnosis of AML according to WHO criteria with ≥ 20% blasts in the peripheral blood or bone marrow (n = 30); with a history of myeloproliferative neoplasms, except CMML (n = 6); or with acute promyelocytic leukemia (t[15;17]) or favorable cytogenetics (t[8;21] or inv16 if known at the time of random assignment; n = 3). (†) Patients without serum creatinine < 2.0 mg/dL, serum total bilirubin < 2.0 mg/dL, and serum ALT or AST less than three times the upper limit of normal. (‡) Other includes patients with myocardial impairment of any cause that resulted in heart failure by New York Heart Association criteria (n = 3); active (uncontrolled, metastatic) second malignancies (n = 1); active fungal infection, hepatitis B or C, or HIV (n = 1); cardiac ejection fraction < 50% (n = 1); incorrect age (n = 1); secondary malignancy in remission (n = 1); and unspecified (n = 24). 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin.
Table 1.
Demographic and Baseline Clinical Characteristics in the Intention-to-Treat Population
Efficacy
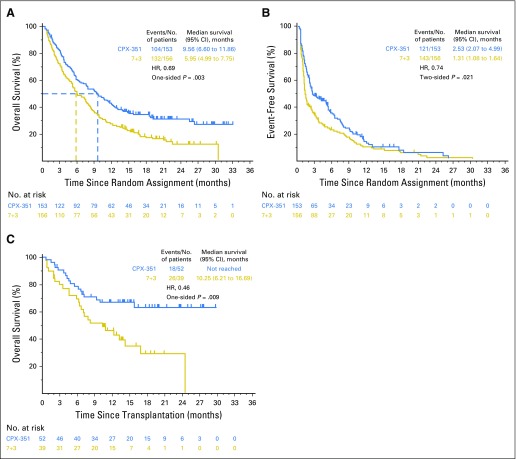
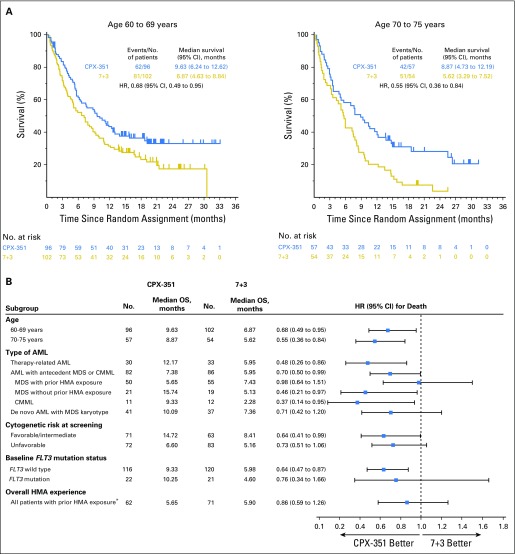
Survival was analyzed after 236 deaths occurred (104 in CPX-351 cohort, 132 in 7+3 cohort). With a median follow-up of 20.7 months, CPX-351 significantly improved OS compared with 7+3 (median, 9.56 v 5.95 months; HR, 0.69; 95% CI, 0.52 to 0.90; one-sided P = .003; Fig 2A); Kaplan-Meier estimates of 1-year OS were 41.5% and 27.6% and of 2-year OS were 31.1% and 12.3%. Prespecified subgroup analyses by age and AML subtype and post hoc subgroup analyses indicated significantly improved OS with CPX-351 versus 7+3, irrespective of age (Kaplan-Meier OS shown in Fig 3A) and in patients with wild-type FMS-like tyrosine kinase 3 (FLT3), therapy-related AML, AML with antecedent MDS or CMML, and favorable/intermediate cytogenetic risk classification (Fig 3B). For additional post hoc analyses see the Data Supplement. Univariable and multivariable analyses of baseline factors associated with OS are listed in the Data Supplement. For the multivariable analysis, significant factors were Eastern Cooperative Oncology Group performance status, karyotype risk classification, platelet count, WBC count, and treatment group.
Fig 2.
Median overall survival (OS) and event-free survival (EFS). Kaplan-Meier estimates of (A) OS and (B) EFS are shown for the overall intention-to-treat population, and Kaplan-Meier estimates of (C) OS landmarked from the date of transplantation are shown for patients in the intention-to-treat population who received a hematopoietic cell transplant. EFS was defined as the time from random assignment to the date of induction treatment failure, relapse from complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi), or death as a result of any cause, whichever came first. Patients alive and not known to have any of these events were censored on the latter of their last dates of disease assessment or hematology assessment. For patients who achieved CR or CRi, duration of remission was measured from the date of remission (CR or CRi) until the date of relapse or death as a result of any cause. Patients not known to have relapsed or died at the last follow-up were censored in a similar fashion as described for EFS. 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin; HR, hazard ratio.
Fig 3.
Kaplan-Meier estimates of overall survival by (A) age subgroup and (B) baseline patient characteristics. (*) Includes patients in the prespecified randomization strata of antecedent myelodysplastic syndrome (MDS) with prior hypomethylating agent (HMA) exposure as well as patients in other strata (eg, therapy-related acute myeloid leukemia [AML], antecedent chronic myelomonocytic leukemia [CMML]) who had previously received HMAs. Some patients received HMAs for therapy-related MDS, which then progressed to AML, and these patients may have been classified as having either therapy-related AML or antecedent MDS with prior HMA exposure. 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin; FLT3, FMS-like tyrosine kinase 3; HR, hazard ratio; OS, overall survival.
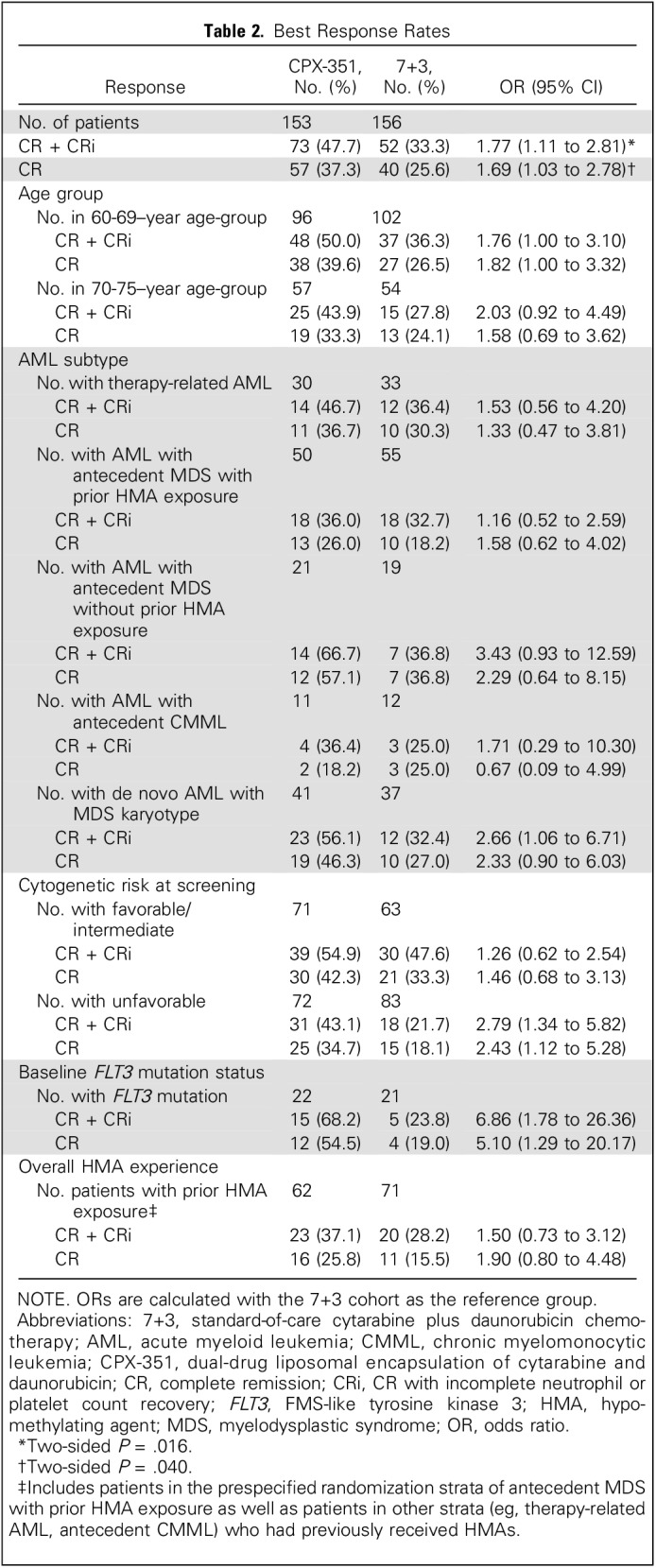
CPX-351 was associated with a significantly higher overall remission rate (CR + CRi) versus 7+3 (47.7% v 33.3%; two-sided P = .016) and CR rate (37.3% v 25.6%; two-sided P = .040; Table 2). CR + CRi rates among patients with one induction cycle were 55.2% (58 of 105 patients) for CPX-351 versus 34.0% (34 of 100 patients) for 7+3, and among patients with two induction cycles, the rates were 31.3% (15 of 48 patients) for CPX-351 versus 35.3% (18 of 51 patients) for 7+3. Higher CR + CRi rates in the CPX-351 cohort were observed across age groups and AML subtypes, including patients with MDS-related cytogenetics (Table 2). Median EFS was significantly prolonged with CPX-351 versus 7+3 (2.53 v 1.31 months; HR, 0.74; 95% CI, 0.58 to 0.96; two-sided P = .021; Fig 2B). Median remission duration was similar between CPX-351 and 7+3 (6.93 v 6.11 months; two-sided P = .291).
Table 2.
Best Response Rates
Ninety-one (29.4%) of 309 patients underwent allogeneic HCT (52 [34.0%] of 153 in the CPX-351 cohort; 39 [25.0%] of 156 in the 7+3 cohort; two-sided P = .098); characteristics of patients who underwent HCT are listed in the Data Supplement. The majority of patients who underwent allogeneic HCT were in CR or CRi in both the CPX-351 cohort (30 [57.7%] of 52 and 10 [19.2%] of 52, respectively) and the 7+3 cohort (19 [48.7%] of 39 and five [12.8%] of 39, respectively). An exploratory landmark survival analysis from the time of HCT favored CPX-351 (HR, 0.46; 95% CI, 0.24 to 0.89; one-sided P = .009; Fig 2C).
Treatment Exposure
All patients in the safety analysis received the first induction; similar proportions in each cohort received a second induction (CPX-351, 31.4%; 7+3, 33.8%). A higher proportion of CPX-351 patients achieved CR + CRi and proceeded to receive the first (32.0%) and second (15.0%) postremission consolidation cycles compared with the 7+3 cohort (21.2% and 7.9%, respectively). The higher proportion of patients who proceeded to consolidation resulted in a longer median length of the overall treatment phase with CPX-351 (62 days) versus 7+3 (41 days).
Safety
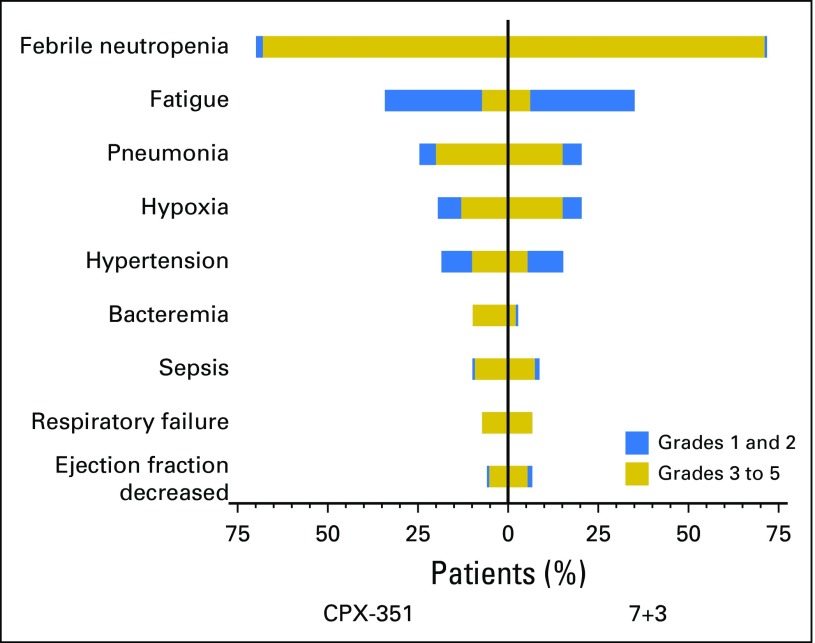
The types of adverse events, proportions of patients who experienced them, and severities of events were comparable between treatment cohorts (Fig 4). The most frequently reported grade 3 to 5 adverse events in the CPX-351 and 7+3 cohorts were febrile neutropenia (68.0% v 70.9%), pneumonia (19.6% v 14.6%), and hypoxia (13.1% v 15.2%). Five patients experienced adverse events that led to treatment discontinuation (CPX-351: cardiac failure, cardiomyopathy, and acute renal failure [one patient each]; 7+3: decreased ejection fraction [two patients]). Although the proportions of patients with adverse events were comparable between cohorts, the CPX-351 cohort had a longer median treatment phase and, thus, a longer period of adverse event reporting; the rate of adverse events (reported during or within 30 days of the end of treatment) per patient-year was evaluated to normalize this effect. The median rate of adverse events per patient-year was 75.68 with CPX-351 versus 87.22 with 7+3.
Fig 4.
Most frequently reported adverse events. The percentage of patients with grade 1 and 2 and grade 3 to 5 events are shown for all adverse events that occurred in > 5% of patients in either treatment group as grade 3 to 5 events. 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin.
The median time to neutrophil (≥ 500/μL) and platelet (≥ 50,000/μL) recovery in patients who achieved CR + CRi after initial induction chemotherapy was longer with CPX-351 (35.0 and 36.5 days, respectively) versus 7+3 (29 and 29 days; Data Supplement). Rates of infection-related events of any grade (92.8% v 92.7%) and grades 3 to 5 (83.7% v 86.1%) were similar with CPX-351 and 7+3, with low rates of grade 5 infection-related events in both groups (7.2% v 2.6%). Bleeding events of any grade were more common with CPX-351 than with 7+3 (74.5% v 59.6%), as were grade 3 to 5 events (11.8% v 8.6%); however, rates of grade 5 bleeding-related events were equal (2.6% in both cohorts).
The overall number of deaths was 106 in the CPX-351 arm (69.3%) and 128 in the 7+3 arm (84.8%); causes of death were similar between treatment arms (Data Supplement). Early mortality rates with CPX-351 and 7+3 were 5.9% and 10.6% (two-sided P = .149) through day 30 and 13.7% and 21.2% (two-sided P = .097) through day 60.
DISCUSSION
The survival of patients with sAML has remained disappointingly short.23 CPX-351 is an example of the CombiPlex platform (Celator Pharmaceuticals), which, in contrast to traditional combination chemotherapy regimens, identifies synergistic drug ratios in vitro and uses an appropriate nanoscale carrier to enhance drug delivery. CPX-351 maintains a 5:1 molar ratio of cytarabine:daunorubicin coencapsulated within a bilamellar liposome, enabling intracellular delivery of the synergistic drug ratio and enhancing uptake in leukemia cells to a greater extent than normal cells.16-19 In the current study, CPX-351 significantly improved OS, remission rates, and EFS versus conventional 7+3 in older adults with newly diagnosed high-risk/sAML. CPX-351 efficacy was maintained across AML subtypes and age strata, with improved OS and high rates of HCT in patients both 60 to 69 and 70 to 75 years of age.
Although the efficacy of CPX-351 ultimately relies on the activity of cytarabine and daunorubicin, CPX-351 possesses unique pharmacokinetic and pharmacodynamic properties. CPX-351 liposomes provide persistent exposure of cytarabine and daunorubicin in bone marrow, with greater uptake in leukemic cells than in normal cells at a near-constant 5:1 drug ratio16,19 that cannot be sustained through administration of free cytarabine and daunorubicin as a result of their independent and dissimilar pharmacokinetics.18,24,25 Persistence of CPX-351 liposomes in the plasma also prolongs drug exposure and preserves the synergistic 5:1 drug ratio, which may increase cytotoxic activity and leukemic cell killing relative to free drug.24,25 Consistent with this increase in drug exposure to the bone marrow was the longer time to recovery of neutrophils and platelets in patients treated with CPX-351. Although studies have shown increased multidrug resistance in older patients, particularly those with sAML,5,26 observations have suggested that CPX-351 may bypass drug efflux pumps by entering leukemia cells as intact liposomes,16 which possibly overcomes drug resistance mechanisms, such as multidrug resistance phenotype (eg, plasma membrane–localized P-glycoprotein) and rapid cytarabine deaminase−dependent cytarabine inactivation.
Multiple studies have demonstrated low remission rates and reduced survival in patients with high-risk AML, including sAML and AML with adverse-risk karyotypes.2,4,13,27,28 However, few randomized studies have independently reported the effect of novel therapies in these patients. High-dose cytarabine demonstrated higher remission and survival rates than standard-dose cytarabine in patients age ≤ 45 years with newly diagnosed high-risk AML but not in those age more than 45 years.29 A study of adults age ≤ 60 years demonstrated improved survival with the addition of cladribine but not fludarabine to 7+3 versus 7+3 alone in a small subgroup of patients with adverse karyotype.30 The addition of other agents, such as amonafide, a DNA intercalator that evades drug resistance mechanisms in high-risk AML, did not improve remission rates and survival when combined with standard-dose cytarabine.28 CPX-351 is the first agent to significantly improve survival and remission rates in older patients with AML with high-risk features.
In both study cohorts, the most frequent grade 3 to 5 adverse events of febrile neutropenia, pneumonia, and hypoxia occurred at similar rates. Patients randomly assigned to CPX-351 remained on study for approximately 50% longer than those in the 7+3 cohort. Despite differences in the length of treatment phase, the proportion of patients who experienced adverse events in the CPX-351 cohort was comparable with that of those in the 7+3 cohort, which culminated in a lower rate of adverse events per patient-year and suggests that CPX-351 may have a more favorable overall toxicity profile than 7+3. The rates of early (30- and 60-day) mortality seemed lower with CPX-351 despite an increase in the time to neutrophil and platelet recovery in CPX-351–treated patients. In addition, the improved post-HCT survival observed in patients randomly assigned to CPX-351 suggests that the response is deeper than that achieved with 7+3.31 Additional studies are warranted to confirm these findings.
Collectively, these clinical data support the adoption of CPX-351 for the initial treatment of adults with high-risk/sAML as well as the investigation of CPX-351 in other cohorts. For example, in preclinical studies, leukemia blasts from patients with FLT3-ITD were five-fold more sensitive to CPX-351 than wild-type blasts,32 and, in the current study, a trend toward improved survival was observed with CPX-351 versus 7+3 in the small subgroup of patients with baseline FLT3 mutations. These preliminary findings support additional study in patients with FLT3 mutations. In addition, many genetic mutations found in sAML are shared with MDS,33 and data have indicated that many cases of de novo AML share a similar mutation profile to clinically defined sAML, with similarly poor outcomes.34 Together, these observations suggest that CPX-351 may ultimately prove effective in high-risk patients with MDS and other high-risk de novo AML, particularly those in whom allogeneic HCT is contemplated. The combination of CPX-351 with small-molecule inhibitors (eg, enasidenib, midostaurin, venetoclax) and/or conjugated monoclonal antibodies also may improve efficacy in specific subsets of patients.
ACKNOWLEDGMENT
Medical writing and editorial assistance for the preparation of this article were provided by Daniel Famer and John Norwood of The Curry Rockefeller Group and Kimberly Brooks of SciFluent Communications under the direction of the authors; this assistance was financially supported by Jazz Pharmaceuticals.
Footnotes
Supported by Celator Pharmaceuticals, a subsidiary of Jazz Pharmaceuticals.
Presented at the 2016 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT01696084.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Chiarella, Arthur C. Louie
Provision of study materials or patients: Jeffrey E. Lancet, Geoffrey L. Uy, Jorge E. Cortes, Laura F. Newell, Tara L. Lin, Ellen K. Ritchie, Robert K. Stuart, Stephen A. Strickland, Donna Hogge, Scott R. Solomon, Richard M. Stone, Dale L. Bixby, Jonathan E. Kolitz, Gary J. Schiller, Matthew J. Wieduwilt, Daniel H. Ryan, Bruno C. Medeiros
Collection and assembly of data: Jeffrey E. Lancet, Geoffrey L. Uy, Jorge E. Cortes, Laura F. Newell, Tara L. Lin, Ellen K. Ritchie. Robert K. Stuart, Stephen A. Strickland, Donna Hogge, Scott R. Solomon, Richard M. Stone, Dale L. Bixby, Jonathan E. Kolitz, Gary J. Schiller, Matthew J. Wieduwilt, Daniel H. Ryan, Bruno C. Medeiros
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jeffrey E. Lancet
Consulting or Advisory Role: Celgene, Janssen, Biosight, Jazz Pharmaceuticals, Astellas Pharma, Daiichi Sankyo
Research Funding: Pfizer (Inst)
Geoffrey L. Uy
Consulting or Advisory Role: GlycoMimetics, Novartis, Celator/Jazz Pharmaceuticals, Curis
Jorge E. Cortes
Consulting or Advisory Role: ARIAD Pharmaceuticals, Bristol-Myers Squibb, BioLineRX, Novartis, Pfizer, Amphivena Therapeutics, Janssen
Research Funding: ARIAD Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Pfizer (Inst), TEVA Pharmaceuticals Industries (Inst), Celgene (Inst), Arog Pharmaceuticals (Inst), Astellas Pharma (Inst), Ambit Biosciences (Inst), Celator/Jazz Pharmaceuticals (Inst), ImmunoGen (Inst), Incyte (Inst)
Laura F. Newell
No relationship to disclose
Tara L. Lin
Honoraria: Jazz Pharmaceuticals
Ellen K. Ritchie
Consulting or Advisory Role: Incyte, Celgene, Pfizer, Novartis
Speakers’ Bureau: Celgene, Incyte, Novartis, ARIAD Pharmaceuticals
Research Funding: Astellas Pharma (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), NS Pharma (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Celgene, Novartis
Robert K. Stuart
Consulting or Advisory Role: Sunesis Pharmaceuticals
Honoraria: Sunesis Pharmaceuticals
Research Funding: Agios, Astellas, Bayer AG, Celator/Jazz Pharmaceuticals, Incyte, Sunesis Pharmaceuticals
Travel, Accommodations, Expenses: Sunesis Pharmaceuticals
Stephen A. Strickland
Consulting or Advisory Role: Sunesis Pharmaceuticals, Tolero Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, Baxalta, CTI BioPharma, Daiichi Sankyo
Research Funding: Sunesis Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Donna Hogge
Consulting or Advisory Role: Novartis, Roche, Sanofi
Scott R. Solomon
No relationship to disclose
Richard M. Stone
Consulting or Advisory Role: Arog Pharmaceuticals, Amgen, argenx, Astellas Pharma, Celgene, Cornerstone BioPharma, Fujifilm, Jazz Pharmaceuticals, Ono Pharmaceutical, Otsuka, Pfizer, Roche/Genentech, Sumitomo Dainippon Pharma
Dale L. Bixby
No relationship to disclose
Jonathan E. Kolitz
Consulting or Advisory Role: Magellan Health, Novartis, Pharmacyclics, Gilead, Seattle Genetics
Stock or Other Ownership: Celator/Jazz Pharmaceuticals
Honoraria: Gilead, Magellan, Novartis
Research Funding: Boehringer Ingelheim, Cantex, Erytech, Millennium
Travel, Accommodations, Expenses: Novartis, Gilead, Seattle Genetics
Gary J. Schiller
Research Funding: Celator/Jazz Pharmaceuticals
Matthew J. Wieduwilt
Stock or Other Ownership: Reata Pharmaceuticals
Research Funding: Sigma Tau Pharmaceuticals (Inst)
Daniel H. Ryan
Stock or Other Ownership: AbbVie
Patents, Royalties, Other Intellectual Property: University of Rochester
Antje Hoering
No relationship to disclose
Kamalika Banerjee
Employment: Celator/Jazz Pharmaceuticals
Stock or Other Ownership: Celator/Jazz Pharmaceuticals
Arthur C. Louie
Employment: Celator/Jazz Pharmaceuticals
Stock or Other Ownership: Celator/Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Celator/Jazz Pharmaceuticals
Michael Chiarella
Employment: Celator/Jazz Pharmaceuticals
Stock or Other Ownership: Celator/Jazz Pharmaceuticals
Bruno C. Medeiros
Honoraria: Celator/Jazz Pharmaceuticals, Astellas Pharma, Novartis, Amgen
Consulting or Advisory Role: Novartis, Celgene, Celator/Jazz Pharmaceuticals, Astellas Pharma, Amgen, Pfizer, Roche/Genentech
Research Funding: Celator/Jazz Pharmaceuticals, Celgene, Novartis, MEI Pharma, Astellas Pharma, Merck/Schering Plough, Roche/Genentech
Travel, Accommodations, Expenses: Celgene, Celator/Jazz Pharmaceuticals, Novartis, Astellas Pharma
REFERENCES
- 1.Vardiman JW, Thiele J, Arber DA, et al. : The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 114:937-951, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. : Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: A national population-based cohort study. J Clin Oncol 33:3641-3649, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Miesner M, Haferlach C, Bacher U, et al. : Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: A comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood 116:2742-2751, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Hulegårdh E, Nilsson C, Lazarevic V, et al. : Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish Acute Leukemia Registry. Am J Hematol 90:208-214, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Leith CP, Kopecky KJ, Godwin J, et al. : Acute myeloid leukemia in the elderly: Assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood 89:3323-3329, 1997 [PubMed] [Google Scholar]
- 6.Grimwade D, Hills RK, Moorman AV, et al. : Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354-365, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Rai KR, Holland JF, Glidewell OJ, et al. : Treatment of acute myelocytic leukemia: A study by cancer and leukemia group B. Blood 58:1203-1212, 1981 [PubMed] [Google Scholar]
- 8.Yates JW, Wallace HJ, Jr, Ellison RR, et al. : Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep 57:485-488, 1973 [PubMed] [Google Scholar]
- 9.Kayser S, Döhner K, Krauter J, et al. : The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 117:2137-2145, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Schoch C, Kern W, Schnittger S, et al. : Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): An analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia 18:120-125, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Dombret H, Gardin C: An update of current treatments for adult acute myeloid leukemia. Blood 127:53-61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer RJ, Davis RB, Schiffer CA, et al. : Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med 331:896-903, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Burnett AK, Milligan D, Goldstone A, et al. : The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: The results of the LRF AML14 trial. Br J Haematol 145:318-332, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Stone RM, Mandrekar SJ, Sanford BL, et al. : Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454-464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hills RK, Castaigne S, Appelbaum FR, et al. : Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15:986-996, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim WS, Tardi PG, Dos Santos N, et al. : Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res 34:1214-1223, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Kim HP, Gerhard B, Harasym TO, et al. : Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol 39:741-750, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Mayer LD, Harasym TO, Tardi PG, et al. : Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther 5:1854-1863, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Tardi P, Johnstone S, Harasym N, et al. : In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res 33:129-139, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Lancet JE, Cortes JE, Hogge DE, et al. : Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 123:3239-3246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115, 1975 [PubMed] [Google Scholar]
- 22. doi: 10.1200/JCO.2003.04.036. Cheson BD, Bennett JM, Kopecky KJ, et al: Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21:4642-4649, 2003 [Erratum: J Clin Oncol 22:576, 2004] [DOI] [PubMed] [Google Scholar]
- 23.Lichtman MA: A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells Mol Dis 50:119-130, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Feldman EJ, Kolitz JE, Trang JM, et al. : Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res 36:1283-1289, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Feldman EJ, Lancet JE, Kolitz JE, et al. : First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol 29:979-985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leith CP, Kopecky KJ, Chen IM, et al. : Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood 94:1086-1099, 1999 [PubMed] [Google Scholar]
- 27.Juliusson G, Antunovic P, Derolf A, et al. : Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113:4179-4187, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Stone RM, Mazzola E, Neuberg D, et al. : Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol 33:1252-1257, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Willemze R, Suciu S, Meloni G, et al. : High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: Results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol 32:219-228, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Holowiecki J, Grosicki S, Giebel S, et al. : Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J Clin Oncol 30:2441-2448, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Araki D, Wood BL, Othus M, et al. : Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol 34:329-336, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon MJ, Tardi P, Loriaux MM, et al. : CPX-351 exhibits potent and direct ex vivo cytotoxicity against AML blasts with enhanced efficacy for cells harboring the FLT3-ITD mutation. Leuk Res 53:39-49, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Walter MJ, Shen D, Ding L, et al. : Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 366:1090-1098, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsley RC, Mar BG, Mazzola E, et al. : Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125:1367-1376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]