Abstract
In the present study, 40 pigment-producing microbes were isolated from various soil sources. Among these, a novel water-soluble yellow pigment-producing fungal isolate (MBYP1) was identified as Aspergillus sp. through ITS gene sequencing. The maximum pigment yield (UA430nm, 12.45 ± 0.5 g/l) was obtained when strain MBYP1 was cultured under optimum conditions (28 °C and pH 5.5 under static condition). Subsequently, the pigment was purified through gel chromatography and high-performance liquid chromatography (HPLC). Characterization of purified pigment through UV–Vis and liquid chromatography–mass spectrometry (LC–MS) reveal maximum absorbance at 430 nm and molecular mass of 301 m/z, respectively. Further, the pigment exhibited a maximum dyeing capacity of up to 80% irrespective of mordant. Toxicity evaluation of purified pigment with zebra fish model system reported an IC50 value of 710 µg/mL. Pigment antioxidant ability was established by DPPH (35.7 µg/mL) and phosphomolybdenum assay (226.61 mg/g) thus ascertaining improvised light fastness of dyed fabric. Moreover, lack of antimicrobial activity (up to 40 µg/mL) improves pigment bio-degradability. In collective, the novel yellow pigment from Aspergillus sp. MBYP1 strain was found to be an eco-friendly alternative to synthetic dye for potential applications in textile industries.
Keywords: Yellow pigment, Aspergillus sp., Antioxidant, Textile dyeing, Zebrafish toxicity
Introduction
Colors play a vital role in increasing the esthetic appeal of food items, textile cloths, cosmetics and pharmaceuticals (Venil et al. 2014). The production volume of dyes and pigments has been estimated to increase at a compounded annual growth rate (CAGR) of 6% between 2017 and 2022. The growing demand for dyes, especially in the textile market, is estimated to increase from USD 7.34 billion in 2017 to a USD 9.82 billion by 2022. Nevertheless, due to their adverse effects such as carcinogenicity, hypersensitivity and other toxicological effects, many of the synthetic pigments are banned in the worldwide. Therefore, World Health Organization (WHO), European Food Standards Authority (EFSA) and Food and Drug Administration (FDA) have established stringent regulations and recommended safe dosage for food, drug and cosmetic applications (Tuli et al. 2015). Moreover, since past two decades, the synthetic pigments for food, textile, and cosmetic industries were found to be less stable as well as expensive when compared with the natural pigments (Joshi et al. 2003). Hence, there is a global shift in the demand for eco-friendly products and natural colorants.
The vast majority of natural pigments are generally obtained from plants and microorganisms; however, the plant-derived pigments have many drawbacks such as seasonal variation (availability throughout the year), larger cultivable area, high man power consumption and complexity of extraction. Microorganisms such as bacteria and fungi offer a readily available choice of natural pigments (Indra Arulselvi et al. 2014). Some of the greater potentials of the microorganisms include diverse color compound production, ease of environmental acceptability (bio-degradable) and renewabilty, widely encouraged for its developmental strategies. Microorganisms such as Monascus purpureus, Serratia marcescens, Penicillium atrovenetum, Rhodotorula glutinis, Phaffia rhodozyma, Paecilomyces sp., Bacillus sp., and Achromobacter sp., have the ability to produce various types of pigments such as carotenoid, violacein, naphthaquinone, polyketides, flavin, melanin, monascins and prodigiosin (Dufosse 2006). In addition, the pigments also display several biological advantages such as antimicrobial, antioxidant and anticancer activities (Tuli et al. 2015).
Only limited research studies are available on microbial exploration for pigment production, especially in Indian scenario, which really points towards investigating microbial pigments in greater detail. In textile industries, a number of safety issues arise from the disposal of synthetic dyes due to their adverse environmental effects. Most of the dyes escaped during the dyeing process from the textile industries amount up to 2,00,000 tons per year (Ogugbue and Sawidis 2011). The processed dyes persist in the environment because of their high stability to light, temperature, and detergent, thereby resulting in reduced photosynthetic activity due to lower light penetration, oxygen deficiency, and altered life cycle in aquatic organisms. In the textile industries, recurrent antimicrobial agent usage in clothes led to retarded biological degradation of the dyes (O’Neill et al. 1999). On the other hand, the microbial pigments extracted and studied have been limited to research and developmental stage, since the pigment production (yield and color intensity) has not been effective to meet the industrial necessities (Tuli et al. 2015). Thereupon, isolation of high-yield and color-intensified pigment-producing microorganisms are necessary for the current scenario. Among the different microbial sources, the fungi have been metabolically active in the production of various types of compounds, including pigments (Dufosse et al. 2014). Thus the present study has been focused to achieve novel, highly intense and greater pigment yielding microbial strains from various locations of Tamil Nadu.
Materials and methods
Isolation of pigment-producing microbial strains
Soil samples were collected from different eco-climatic regions such as decomposed land, Western Ghats, tilted soils and fertile field of Coimbatore, Tamil Nadu, India. Samples were collected by scraping off the soil surface (up to 2–5 cm depth). Collected samples were aseptically transferred in a sterile container. The pigment-producing bacterial and fungal strains were isolated by plating on nutrient agar (NA) and potato dextrose agar (PDA) medium, respectively (Indra Arulselvi et al. 2014).
Screening of pigment-producing microbial strains
The isolated microorganisms were further screened to obtain the exclusive pigment-producing strains. In brief, all the pigmented isolates were subjected to mass production in their respective growth medium. Further, the pigments were extracted based on extracellular and intracellular nature with organic solvents. The intracellular pigments were extracted following the previously reported method by Balraj et al. (2014). The extracellular pigment was separated by filtration (Whatman filter paper No. 2) and subsequent liquid–liquid extraction (Lu et al. 2018). Amongst the various screened colonies, the suitable pigment-producing fungal isolate MBYP1 was selected for further characterization.
Molecular identification of pigment-producing fungi
The selected isolate MBYP1 was identified through molecular characterization techniques with universal fungal primers ITS 1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS 4 (5′-TCCTCCGCTTTATTGATATGC-3′). The amplicon thus obtained was gel purified and sequenced at Eurofins Laboratory Pvt, Ltd (Bengaluru, India). The obtained sequence data were aligned and compared with known search sequences with BLASTn (NCBI). The phylogenetic tree was constructed by data matrix following neighbor-joining method using MEGA 6.1 software (Rajeendran et al. 2017).
Optimization and recovery of pigments
To optimize the pigment-producing ability of fungal strain MBYP1, overnight grown culture was inoculated in 200 mL of PDB medium and effect of various parameters such as temperature (25–31 °C), pH (4–7), agitation (80–200 rpm) and incubation period (1–10 days) on pigment production was determined (Gunasekaran and Poorniammal 2008). Followed by incubation, the culture-free supernatant was separated using organic solvents (chloroform:ethanol:water; 1:1:1). The extracted pigments were further purified through column chromatography (Silica gel 100–200 mesh) and the absorbance of the collected fractions was measured with a spectrophotometer (Shimadzu UV 3600, UV–Vis–NIR spectrophotometer, Japan). The pigment purity was analyzed through high-performance liquid chromatography (C18, 25 cm × 4.6 mm diameter 4 µm analytical column) (Shimadzu, Japan) with 20 µL injection volume. The mobile phase was a combination of a gradient solvent system (methanol, water, ethyl acetate; 6:3:1) with a 1.0 mL/min flow rate (Lu et al. 2010).
Antioxidant potential of purified pigment
The antioxidant potential of the purified pigment was evaluated through radical-scavenging activity 1-diphenyl-2-picrylhydrazyl (DPPH) and phosphomolybdenum assay.
DPPH assay
The antioxidant property of the extract was established through hydrogen-donating or radical-scavenging capability utilizing DPPH according to Blios (1958) method. Sample extracts, as well as standards (BHT and ascorbic acid) at different concentrations (10–100 µg), were taken and the final volume was adjusted to 100 µL with methanol. Further, 5 mL of a 0.1 mM DPPH solution (w/v in methanol) was added to the samples and incubated in dark for 20 min at 27 °C. The absorbance of the sample was measured at 517 nm against the blank (methanol) and 0.1 mM DPPH in methanol as a negative control (Blois 1958).
Phosphomolybdenum assay
The phosphomolybdenum assay was carried out according to Prieto et al. (1999) to determine the antioxidant activity of the extracted samples. The sample aliquots (10–40 µg), standards (ascorbic acid in 1 mM dimethyl sulfoxide) and blank (MilliQ water) were added with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). These were incubated at 95 °C for 90 min and then allowed to cool at room temperature. The absorbance values were read at 695 nm against the reagent blank.
Evaluation of dyeing ability on textile cloths
Dyeing efficiency of the pigment was assessed by conventional dyeing (acid bath and alkaline bath) and hydrothermal (autoclave) analysis using with and without mordant (Morales-Oyervides et al. 2017). The dying nature of pigment was optimized under different pH (5, 6, 7, 8 and 9), temperature (50, 60, 70, 80, 90 and 100 °C) and time (10, 20, 30, 60 and 90 min). The pigment uptake by fabrics was evaluated through reduction of dyeing solutions absorbance value measured spectroscopically. The decolorising action of soap solution, organic solvents (chloroform, ethyl acetate, acetone, ethanol, and methanol) on dyed fabrics, was determined through the absorbance measurement of the post-wash solution at 430 nm. Furthermore, the dyed fabrics were additionally subjected to different light sources such as sunlight (8 h for 3 days) and UV light (30 min) to determine the capacity of lightfastness.
Color value measurement of pigments
The dyed cloth color values (L*, a*, b* and c*) were evaluated calorimetrically (CR 300) with CIELAB color system (Hunter associate Laboratory Inc.,). The L* signifies run from top to bottom, wherein L* value of 100 represents bright (white) and 0 corresponds to dark (black). The positive a* value indicates red color and negative a* value implies green color. Likewise, a positive b* value represents yellow color and negative b* value denotes blue color. The C* value corresponds to chroma or purity of color, whereas hue angle represents, 0 (red), 90 (yellow), 180 (green) and 270 (blue) (Morales-Oyervides et al. 2017).
Toxicity assay on zebrafish embryo model
Toxicity of the pigment was evaluated on zebrafish (Danio rerio) embryo model. Briefly, female and male zebra fishes (1:2) were selected for spawning. The embryos were collected in 24-well plates (five embryos per well) containing 2 mL growth medium with different pigment concentrations (10–1000 µg/mL). Reactive yellow 42 (RY42) (Sigma-Aldrich, US; S472301) was used as a standard synthetic dye. The pigment-induced deformity and mortality on the embryos were monitored under a light microscope up to 96 h with 24-h interval (Walton et al. 2014).
Characterization of yellow pigments
UV–Vis spectroscopy
Spectral information is a useful tool for distinguishing and identifying various colored and non-colored compounds. The absorbance maxima of ethanol extract containing pigments were evaluated by UV–Vis spectrophotometer (Shimadzu UV-3600, UV–Vis–NIR spectrophotometer, Japan) with a scanning range of 200–800 nm.
LC–MS/MS analysis
Purified pigments were analyzed further to determine its molecular mass by Waters Xevo G2 Q-TOF mass spectrometer with Waters Acquity H class ultraperformance liquid chromatography (UPLC) system via electrospray ionization (ESI) interface. Chromatography was performed on UPLC equipped with an Acquity BEH C18 column (50 mm × 2.1 mm i.d., 1.7 m particle size) (Waters, Milford, MA, USA) with a conditioned autosampler at 4 °C. The mobile phase consisted of methanol (solvent A) and water (solvent B) with flow rate of 0.4 mL/min. The ESI source was operated in the negative ionization mode with a full scan analysis ranging from 50 to 1000 m/z.
Statistical analysis
Statistical analyses were carried out using GraphPad software (version 5.1). One-way ANOVA followed by Tukey’s multiple comparison tests was performed to evaluate the significant difference. Data were presented as mean ± SEM and the statistical acceptance level was p < 0.05. All the experiments were performed in triplicates.
Results and discussion
Screening of pigment-producing microbial strains
A total of 56 fungal and bacterial colonies was isolated from different soil sources and amongst these 40 were found to be pigmented (Table 1). Based on high color intensity, ten pigmented colonies were chosen and amidst these, an extracellular pigment-producing fungal isolate MBYP1 was selected for further morphological and molecular characterization.
Table 1.
Soil sampling site from the diverse environmental region
| S. No | Types of area | Geographic coordinate system | No. of pigmented microbes (40) |
|---|---|---|---|
| 1 | Western Ghats | 11°01′13.3″N 76°50′30.0″E | 11 |
| 2 | Decomposed land | 11°01′51.9″N 76°53′07.3″E | 15 |
| 3 | Tilted soils | 10°15′08.8″N 78°14′47.6″E | 6 |
| 4 | Fertile field | 10°15′08.8″N 78°14′47.6″E | 8 |
The MBYP1 isolate produced yellow-colored pigment with fluffy sporulation on PDA agar. They exhibited compact, biseriate, and densely columnar conidia. Moreover, they are globose shaped and vary in appearance from light yellow to hyaline. The MBYP1 isolate DNA was amplified with ITS 1 and 4 primers for molecular characterization. The 550-bp-sized gene product was sequenced and subsequent homology analysis through BLASTn revealed maximum (96%) similarity with Aspergillus terreus (GenBank Accession number KX198128). The obtained sequence MBYP1 strain was deposited to GenBank with an accession number MG515236. Furthermore, the phylogenetic tree for the MBYP1 strain was constructed through Mega 6.1 software as depicted in Fig. 1.
Fig. 1.
Phylogenetic evaluation of strain MBYP1 by neighbor-joining method: The ITS gene region constituting 550 bp from the strain MBYPI was sequenced and the organism was identified as Aspergillus sp. MBYP1 (MG515236). Algorithms with bootstrap values (expressed as percentage of 1000 replications) are indicated on the branch and bar substitutions signifies per nucleotide location
Optimization and recovery of pigments
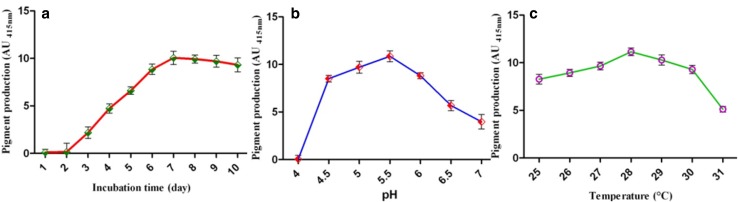
In general, microbial metabolism is greatly influenced by various parameters such as temperature, pH, agitations and incubation time. The different temperature conditions examined revealed that maximum biomass (8.831 ± 0.2 g/L) and pigment (UA430nm, 11.15 ± 0.08 g/L) yield were obtained at 28 °C (Fig. 2a). Similarly, different pH conditions investigated further demonstrated that high biomass (8.001 ± 0.6 g/L) and pigment yield (UA430nm, 10.85 ± 0.3 g/L) were obtained at pH 5.5 (Fig. 2b). This was also clearly evident from previous reports that suggest acidic pH as highly favorable for increased biomass and pigment production by fungal species. (Bae et al. 2000; Kumar et al. 2015). Moreover, Akilandeswari and Pradeep (2017) reported that maximum biomass and pigment yield were achieved at 28 °C and pH 5.0. Conversely, Geweely (2011) reported that high biomass and pigmentation were accomplished at pH 7 and 30 °C for A. nidulans. Nevertheless, 28 °C with pH 5.5 was selected as the optimum condition for biomass and pigment production by the MBYP1 strain.
Fig. 2.
Optimization of fungal pigment production: effect of different growth parameters on fungal pigment production; a temperature, b pH and c incubation time
The MPYP1 strain showed no significant difference in mycelia dry weight beyond 5 days of incubation. However, the increase in pigment production was observed up to 7 days of incubation. The pigmentation yield (UA430nm, 12.45 ± 0.5 g/L) (Fig. 2c) was achieved under static conditions, whereas constant shaking at 120 rpm resulted in drastically reduced pigment yield (UA430nm, 1.57 ± 0.6 g/L). These results were found to correlate with the previous studies of Akilandeswari and Pradeep (2017); whereas, Geweely (2011) has reported maximum biomass and pigment production within 4 days of incubation period. Therefore, the incubation period of 7 days was selected as optimum based on the present investigation and previous literature evidence to achieve a high yield (Gunasekaran and Poorniammal 2008; Bae et al. 2000).
The yellow pigment was highly soluble in water as well as organic polar solvents such as acetone, methanol, and ethanol. The solubility of extracted pigment in water was analyzed by recording its absorbance at 430 nm. No significant changes in absorbance were observed and were recorded between the pigments filtrates (0.714) and control pigment (0.731). Therefore, this establishes the solubility of the pigment. Nonetheless, the widely reported pigments belong to carotenoid group (zeaxanthin and xanthomonadins) that is extremely hydrophobic in nature and thus insoluble in water (Sajilata et al. 2008). The pigment purification was achieved through non-complex and rapid extraction techniques that have been optimized based on the system polarity. The pigment was extracted through a simple liquid–liquid separation with water, chloroform and ethanol (1:1:1) unlike the previous reports (Ahmad et al. 2012) wherein water-soluble pigments were usually extracted by a rotary evaporator (long-time process). Chloroform being a non-polar and high-density solvent resolves non-polar compounds at the bottom of separating funnel with the crude pigment. Subsequent water addition facilitates precipitation of the pigment from the chloroform mixture. Thereafter, the introduction of ethanol results in attraction of these pigments due to the polar nature of the solvent. However, the exact molecular mechanism behind this strategy could be determined after the elucidation of the pigment structure.
Furthermore, the extracted pigments were purified by a silica gel column and its purity was confirmed through HPLC analysis. HPLC chromatogram shows a single peak at 6.776 RT (Fig. 3). The retention time of yellow pigment (RT—6.776) from MBYP1 differs from previously reported yellow pigments such as zeaxanthin (7.409), ankaflavin and monascins, due to the difference in polarity (Singh et al. 2013). Retention time for any compound under study will differ based on the column matrix and mobile phase used for analysis (Khoo et al. 2011; Young and Lowe 2018). Further analytical studies are necessary to reveal the structural details.
Fig. 3.
HPLC characterization: HPLC analysis of purified yellow pigment analyzed in silica gel column reveals single peak (RT 6.776) justifying pigment purity
Reduction of photo-fading by antioxidants
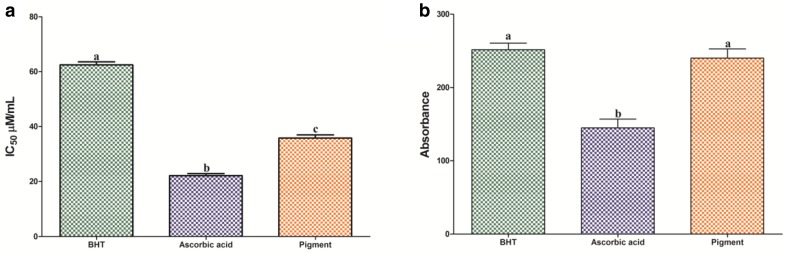
As per the report of Thiagarajan and Nalankilli (2013), two factors, namely UV and visible light, are highly liable for color fading on fabrics induced via unimolecular decomposition and photo oxidations, respectively. In addition, reactive oxygen species (ROS) produced by irradiated dyed fabrics give rise to photo-fading thereby destroying dyes. With this connection, our study aimed to evaluate the antioxidant capacity against standard free radicals such as DPPH and phosphomolybdenum. Most of the microbial pigments have the efficiency to scavenge the free radical, through its polyene chain and OH/NH functional groups. DPPH is a stable free radical mostly used to determine the antioxidant capacity. The antioxidant potential of pigment extract (35.7 µg/mL) and ascorbic acid (22.1 µg/mL) results confirmed the radical-scavenging activity of microbial pigment was better than that for the reference standard antioxidant BHT (62.4 µg/mL) (Fig. 4a). The free radical-scavenging activity of yellow pigment (842.18 ± 161.24 µg/mL) extracted from Streptomyces strain Eri12 shows 200 times lower than standard antioxidant ascorbic acids (4.81 µg/mL) which suggested that our pigment has better activity (Zhong et al. 2011). The prior investigation also highlighted the antioxidant ability of carotenoid derivatives, which has an activity at a concentration of 51.38 µg/mL (Balraj et al. 2014). The total antioxidant activity (TOC) results revealed that (226.61 mg/g) the fungal pigment had a maximum percentage of activity. Moreover, antioxidant activities of fungal pigment were higher than the standard antioxidants such as ascorbic acids 169.48 mg/g and BHT 264.03 mg/g (Fig. 4b). Safafar et al. (2015) reported the percentage of total antioxidant capacity is 8.95 mg GAE/g, which is expressed as equivalent to gallic acids. Previously, Gulani et al. (2012) also evaluated the total antioxidant ability of bacterial pigment prodigiosin shows 22.05 µg/mL activity. These studies confirmed the yellow pigment had the better antioxidant capacity, compared to other microbial pigments. In previous studies described by Thiagarajan et al. (2013), the dyed fabrics were coated with an antioxidant compound to improve the light fastness properties. Moreover, another study highlighted coated cotton fabrics with vitamin E in cosmetotextile application as a skin protection and anti-aging product (Ghaheh et al. 2017). As reported earlier, coating antioxidant compounds will enhance the light fastness properties of dyed fabrics. The fungal pigment employed in the present study naturally possesses antioxidant properties as established through DPPH and phosphomolybdenum assay, and thereby evade the need for an additional coating with antioxidants. Hence, to ascertain this inherent property signifies the antioxidant characteristics of reported novel fungal pigment, the assay was performed. On another hand, the antimicrobial experiment results were revealed that the fungal pigments did not show any antimicrobial activities against human and plant pathogens (results not given).
Fig. 4.
Evaluation of antioxidant potential: the antioxidant potential of extracted fungal pigment was evaluated by a DPPH scavenging activity and b phosphomolybdenum assays. The values are mean of triplicates (n = 3) ± standard deviations. Mean values followed by different letters were significantly different according to Tukey’s test at p < 0.05
Evaluation of dyeing ability on textile cloths
The textile cloth dyeing through hydrothermal method was found to be ideal (> 80% of total dye uptake) compared to other methods such as dye bath (acid and alkaline bath). Tables 2 and 3 represents color value analysis and fastness (wash and light) character of the dyed clothes through the hydrothermal method. The yellow pigment tested for dyeing ability on to textile cloths demonstrated maximum color uptake at 90 °C (60 min) with pH of 6.0 (Fig. 5), whereas lower temperatures resulted in reduced dyeing efficiency. The corresponding color loss in lesser temperature (< 90 °C) dyed textile clothes substantiates for the above-mentioned condition (90 °C for 60 min with a pH of 6.0) as optimum.
Table 2.
Color value measurement of dyed samples by CIELAB
| Fabrics | L* | a* | b* | c* | h |
|---|---|---|---|---|---|
| Cotton | 69.8 | 4.1 | 51.0 | 37.7 | 96.2 |
| Silk | 64.2 | 15.1 | 48.8 | 25.2 | 82.0 |
| Cellulose | 73.6 | 5.7 | 55. 1 | 34.4 | 98.2 |
| Cotton* | 68.4 | 11.3 | 44.3 | 41.6 | 93.7 |
*Cotton dyed with synthetic pigment (RY) by hydrothermal dyeing
Table 3.
Fastness properties of the dyed fabrics with pigments
| Fastness properties | Wash fastness (rating)* | Light fastness (rating)* |
|---|---|---|
| Cotton | 4 | 4–5 |
| Silk | 4–5 | 4–5 |
| Cellulose | 3 | 4 |
| Cotton* | 3–4 | 3 |
Gray scale rating: (1–2) poor, 3—fair, 4—good and 5—excellent. *Cotton dyed with synthetic pigment (RY) by hydrothermal dyeing
Fig. 5.
Evaluation of textile dyeing feasibility: The fungal pigment treated onto textile fabric and cellulose membrane by the hydrothermal method; a untreated fabric, b pigment treated fabric, c untreated cellulose membrane and d pigment treated cellulose membrane
In comparison with synthetic dyes, natural colorants have drawbacks in terms of its narrow shade range and poor fastness capability. However, these could be overcome with mordant that plays a vital role in the dyeing process with natural colorants (Shahid and Mohammad 2013). Nevertheless, in the present study, no significant difference was observed in the presence of mordant with respect to its dyeing efficiency. Therewith, it could be ascertained that the yellow pigment does not necessitate the need of mordant. Previous reports by Couto (2009) show that these processed dyes (synthetic) remain persistent in the environment resulting in decreased light penetration, photosynthetic activity thereby leading to oxygen deficiency, which in turn affects the aquatic life systems. Contrastingly, the microbial pigments are bio-degradable compared to their synthetic counterparts (Daroit et al. 2007). Recently, usage of natural pigments with antimicrobial activity has become a growing trend to impart additional properties for textile clothing (Singh et al. 2005). Nonetheless, these have negative effects on normal microbial flora persistent in an aquatic environment that facilitates the dye degradation (O’Neill et al. 1999). Moreover, excessive human usage could also lead to reduced immune levels as these reduce the normal skin flora.
Toxicity assay on zebrafish embryo model
The toxicity study on fungal pigments was performed on zebrafish embryos, an environmentally relevant aquatic vertebrate model. Embryos were assessed for its viability and developmental progression wherein no changes were observed below the concentration of 710 µg/mL at different time periods (24, 48, 72 and 96 hpf) (Fig. 6). However, the delayed hatching was observed at 72 hpf when exposed with 845 µg/mL of pigment. At 96 hpf, we also observed tail bent and spinal curvature at above 845 µg/mL treated embryos. Conversely, the synthetic pigment RY showed the greatest effect of delayed hatching and mortality at concentrations above 135 µg/mL and 175 µg/mL, respectively. Likewise, Shen et al. (2015) reported that synthetic dyes such as basic violet 14; direct red 28 shows deformities in zebrafish embryos exposed at a concentration of 60.63 and 476.84 µg/mL. Similarly, Jaja-Chimedza et al. (2017) also reported the natural pigment of lutein and canthaxanthin caused teratogenicity effect after exposure to 15 µM on zebrafish embryos. Moreover, the study also reports keto-variant pigment induced severe deformity even at lower concentrations (~ 5 µM) in zebrafish embryos. Hence, the present study proves that the obtained pigment (< 710 µg/mL) could be applied for textile dyeing as a synthetic agent alternative.
Fig. 6.
In vivo zebra fish cytotoxicity assay: zebra fish embryos were treated with the fungal pigment to analyze developmental effects; a, d (control); b, e (fungal pigment) and c, f (synthetic pigment—RY42) treated zebrafish embryos
Characterization of pigment
UV–Vis spectroscopy
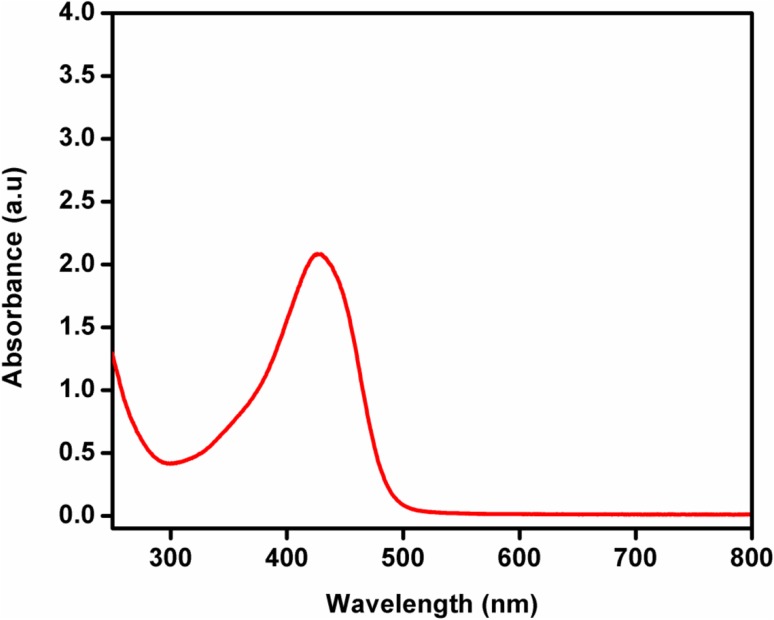
The UV–Vis spectroscopy showed maximum absorbance for the extracted pigment at 430 nm (Fig. 7). The primary reason for stronger absorptions by pigment at longer wavelength was due to electron conjugation effects (Ahmad et al. 2012). Pigments belonging to carotenoids (zeaxanthin—yellow pigment) exhibit three characteristic peaks within the absorption spectrum ranging from 400 to 500 nm (Kohler 1995). Thereby, it could be ascertained that the extracted pigment does not fall under the carotenoid group of pigments.
Fig. 7.
UV–Vis spectroscopic characterization: The yellow pigment extracted from strain MBYP1 was analyzed by UV–Vis spectroscopy wherein the maximum absorbance was observed at 430 nm
LC–MS analysis
Complete pigment profiling was performed with liquid chromatography followed by mass spectrometry (LC–MS). Single prominent peaks occurred at 7.36 min on the mass chromatogram and its corresponding molecular weight was determined as 301 m/z [M + H] through electrospray mass spectrometry (Fig. 8). However, no structural similarity was observed with prior reported compounds upon analysis by Mass Frontier 5.0 (HighChem, Ltd.) software. This was quite evident from previously reported yellow pigments such as monascine, ankaflavin and zeaxanthin that had mass values of 358 m/z, 386 m/z and 568.88 m/z [M + H], respectively (Blanc et al. 1994; Prasain et al. 2005). Hence, we report a novel yellow pigment extracted from Aspergillus sp., and further characterization through NMR (1D and 2D), elemental analysis and IR spectrum is necessary to decipher its structural properties.
Fig. 8.
LC–MS characterization: the yellow pigment extracted from strain MBYP1 was analyzed through LC–MS and a the chromatogram with sharp peak at 7.36 min was observed and b electrospray mass spectra recorded during the LC–MS analysis that detected signal of molecular ion at 301 m/z
Conclusion
The present work describes the isolation of a yellow pigment-producing fungal strain MBYP1 from natural sources. The morphological and molecular characterization studies identify the organisms to be Aspergillus sp. MBYP1. Further, the strain was optimized for maximum pigment production and a strategy for its simple purification was also deduced. The pigment was then assessed for free radical-scavenging property that assists in the prevention of photo-fade. Moreover, the water-soluble property and antioxidant potentials may find potential applications in the food and pharmaceuticals industries. The hydrothermal method for textile clothing without mordant addition was also optimized and revealed better utility in comparison with synthetic dyes. These results imply that the pigment could be utilized as an alternative to synthetic dyes, hence resulting in an eco-friendly approach for textile applications. Additionally, further studies are necessary for deducing the pigment structural.
Acknowledgements
The authors are sincerely grateful to the Defense Research and Development Organization (DRDO), for financial support through Phase II project DRDO-BU CLS, Coimbatore, India. We also acknowledge DFRL, Mysore for great support.
Author contributions
PP and KK designed the experiment. SP performed the experiments, analyzed the data and prepared the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ahmad WA, Ahmad WY, Zakaria ZA, Yusof NZ. Application of bacterial pigments as colorant. Berlin: Springer; 2012. Application of bacterial pigments as colorant; pp. 57–74. [Google Scholar]
- Akilandeswari P, Pradeep BV. Aspergillus terreus KMBF1501 a potential pigment producer under submerged fermentation. Int J Pharm Pharm Sci. 2017;9:38–43. doi: 10.22159/ijpps.2017v9i4.16176. [DOI] [Google Scholar]
- Bae JT, Sinha J, Park JP, Song CH, Yun JW. Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica. J Microbiol Biotechnol. 2000;10(4):482–487. [Google Scholar]
- Balraj J, Pannerselvam K, Jayaraman A. Isolation of pigmented marine bacteria Exiguobacterium sp. from peninsular region of India and a study on biological activity of purified pigment. IJSTR. 2014;3(3):375–384. [Google Scholar]
- Blanc PJ, Loret MO, Santerre AL, Pareilleux A, Prome D, Prome JC, Laussac JP, Goma G. Pigments of monascus. J Food Sci. 1994;59(4):862–865. doi: 10.1111/j.1365-2621.1994.tb08145.x. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Couto SR. Dye removal by immobilised fungi. Biotechnol Adv. 2009;27(3):227–235. doi: 10.1016/j.biotechadv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Daroit DJ, Silveira ST, Hertz PF, Brandelli A. Production of extracellular β-glucosidase by Monascus purpureus on different growth substrates. Process Biochem. 2007;42(5):904–908. doi: 10.1016/j.procbio.2007.01.012. [DOI] [Google Scholar]
- Dufosse L, Fouillaud M, Caro Y, Mapari SA, Sutthiwong N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr Opin Biotechnol. 2014;26:56–61. doi: 10.1016/j.copbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Dufossé L. Microbial production of food grade pigments. Food Technol Biotechnol. 2006;44(3):313–323. [Google Scholar]
- Geweely NS. Investigation of the optimum condition and antimicrobial activities of pigments from four potent pigment-producing fungal species. J Life Sci. 2011;5(9):697–711. [Google Scholar]
- Ghaheh FS, Khoddami A, Alihosseini F, Jing S, Ribeiro A, Cavaco-Paulo A, Silva C. Antioxidant cosmetotextiles: cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017;59:46–51. doi: 10.1016/j.procbio.2017.04.020. [DOI] [Google Scholar]
- Gulani C, Bhattacharya S, Das A. Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Malays J Microbiol. 2012;8(2):116–122. [Google Scholar]
- Gunasekaran S, Poorniammal R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr J Biotechnol. 2008;7(12):1894–1898. doi: 10.5897/AJB2008.000-5037. [DOI] [Google Scholar]
- Indra Arulselvi P, Umamaheswari S, Ranandkumar Sharma G, Karthik C, Jayakrishna C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J Food Process Technol. 2014;5(292):2. [Google Scholar]
- Jaja-Chimedza A, Sanchez K, Gantar M, Gibbs P, Schmale M, Berry JP. Carotenoid glycosides from cyanobacteria are teratogenic in the zebrafish (Danio rerio) embryo model. Chemosphere. 2017;174:478–489. doi: 10.1016/j.chemosphere.2017.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi VK, Attri D, Bala A, Bhushan S. Microbial pigments. Indian J Biotech. 2003;2:362–369. [Google Scholar]
- Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules. 2011;16(2):1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler BE. Electronic structure of carotenoids. ChemInform. 1995;26(32):1–12. [Google Scholar]
- Kumar A, Vishwakarma HS, Singh J, Dwivedi S, Kumar M. Microbial pigments: production and their applications in various industries. Int J Pharm Chem Biol Sci. 2015;5:203–212. [Google Scholar]
- Lu M, Zhao C, Zhou P, Yu L. Analysis and identification of astaxanthin and its carotenoid precursors from Xanthophyllomyces dendrorhous by high-performance liquid chromatography. Naturforsch C. 2010;65(7–8):489–494. doi: 10.1515/znc-2010-7-812. [DOI] [PubMed] [Google Scholar]
- Lu F, Liu L, Huang Y, Zhang X, Wang Z. Production of Monascus pigments as extracellular crystals by cell suspension culture. Appl Microbial biotechnol. 2018;102(2):677–687. doi: 10.1007/s00253-017-8646-1. [DOI] [PubMed] [Google Scholar]
- Morales-Oyervides L, Oliveira J, Sousa-Gallagher M, Méndez-Zavala A, Montañez JC. Assessment of the dyeing properties of the pigments produced by Talaromyces spp. J Fungi. 2017;3(3):38. doi: 10.3390/jof3030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill C, Hawkes FR, Hawkes DL, Lourenço ND, Pinheiro HM, Delée W. Colour in textile effluents–sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol. 1999;74(11):1009–1018. doi: 10.1002/(SICI)1097-4660(199911)74:11<1009::AID-JCTB153>3.0.CO;2-N. [DOI] [Google Scholar]
- Ogugbue CJ, Sawidis T (2011) Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnol Res int 1–11 [DOI] [PMC free article] [PubMed]
- Prasain JK, Moore R, Hurst JS, Barnes S, van Kuijk FJ. Electrospray tandem mass spectrometric analysis of zeaxanthin and its oxidation products. J Mass spectrom. 2005;40(7):916–923. doi: 10.1002/jms.868. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rajeendran A, Nulit R, Yien CY, Ibrahim MH, Kalhori N (2017) Isolation and molecular identification of Colletotrichum gloeosporioides from infected peanut seeds. IJPSS 19:1–8
- Safafar H, Van Wagenen J, Møller P, Jacobsen C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs. 2015;13(12):7339–7356. doi: 10.3390/md13127069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajilata MG, Singhal RS, Kamat MY. The carotenoid pigment zeaxanthin—a review. Compr Rev Food Sci Food saf. 2008;7(1):29–49. doi: 10.1111/j.1541-4337.2007.00028.x. [DOI] [Google Scholar]
- Shahid M, Mohammad F. Recent advancements in natural dye applications: a review. J Clean Prod. 2013;53:310–331. doi: 10.1016/j.jclepro.2013.03.031. [DOI] [Google Scholar]
- Shen B, Liu HC, Ou WB, Eilers G, Zhou SM, Meng FG, Li CQ, Li YQ. Toxicity induced by basic violet 14, direct red 28 and acid red 26 in zebrafish larvae. J Appl Toxicol. 2015;35(12):1473–1480. doi: 10.1002/jat.3134. [DOI] [PubMed] [Google Scholar]
- Singh R, Jain A, Panwar S, Gupta D, Khare SK. Antimicrobial activity of some natural dyes. Dyes Pigments. 2005;66(2):99–102. doi: 10.1016/j.dyepig.2004.09.005. [DOI] [Google Scholar]
- Singh D, Puri M, Wilkens S, Mathur AS, Tuli DK, Barrow CJ. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand. Mar Bioresour Technol. 2013;143:308–314. doi: 10.1016/j.biortech.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P, Nalankilli G. Improving light fastness of reactive dyed cotton fabric with antioxidant and UV absorbers. Indian J Fiber Text Res. 2013;48:161–164. [Google Scholar]
- Tuli HS, Chaudhary P, Beniwal V, Sharma AK. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2015;52(8):4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venil CK, Aruldass CA, Dufossé L, Zakaria ZA, Ahmad WA. Current perspective on bacterial pigments: emerging sustainable compounds with coloring and biological properties for the industry—an incisive evaluation. RSC Adv. 2014;4(74):39523–39529. doi: 10.1039/C4RA06162D. [DOI] [Google Scholar]
- Walton K, Gantar M, Gibbs PD, Schmale MC, Berry JP. Indole alkaloids from Fischerella inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Toxins. 2014;6(12):3568–3581. doi: 10.3390/toxins6123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ, Lowe GL. Carotenoids—antioxidant properties. Antioxidants. 2018;7:28–31. doi: 10.3390/antiox7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong K, Gao XL, Xu ZJ, Gao H, Fan S, Yamaguchi I, Li LH, Chen RJ. Antioxidant activity of a novel Streptomyces strain Eri 12 isolated from the Rhizosphere of Rhizoma curcumae longae. Curr Res Bacteriol. 2011;4(2):63–72. doi: 10.3923/crb.2011.63.72. [DOI] [Google Scholar]