Abstract
Hearing is one of the most important sense organs for man. Hearing loss is often associated with delayed speech and language development in young children. Early identification and intervention improves the chance a child gets to lesser delays in development and improving the overall quality of life. To find out the prevalence of hearing loss in neonates in the rural taluka of Maval, Pune, Maharashtra, India. Prospective Non Randomized Clinical Study. The study was carried out between April 2012 and April 2015. A total of 8192 babies were screened across various centers around the Maval area. The babies who had some high risk factors were 1683 in number and babies who had no high risk factors i.e. well babies were 6509. In our study, the overall prevalence of hearing loss in neonates in Maval taluka of Maharashtra was found to be 3.54 per 1000 live births, in normal born neonates (well babies) was 1.689 per 1000 births, in high risk babies was 10.69 per 1000 high risk births. The prevalence of low birth weight neonates, hyperbilirubinemia neonates and neonates with craniofacial abnormalities developing hearing impairment was found to be 5.9, 3.56 and 1.18 per 1000 high risk births respectively. India is the second most populated country in the world with nearly a fifth of the world’s population. There is a need for the universal neonatal screening for deafness for earlier detection of deafness and rehabilitation.
Level of Evidence: Level IV.
Keywords: Neonatal screening, Deafness, Otoacoustic emission, Brainstem evoked response audiometry
Introduction
Hearing is one of the most important sense organs for man important for normal speech and language acquisition. Hearing loss is often associated with delayed speech and language development. This often cause children with hearing impairment to fall behind their peers in aspects of literacy, academics and overall social wellbeing. This indirectly leads to lower educational and employment levels in adults. It is estimated that 50% of children with hearing impairment in school dropout at the age of 13 years. The rural areas of India lack in health care facilities as compared to the urban areas. In a recent survey, 4 out of every 1000 children born in India were found to have severe, to profound hearing loss. The incidence of hearing loss could be alarmingly in rural areas where neither study is done to detect the incidence/prevalence of hearing loss in infants nor audiological rehabilitation is provided. This could be either due to lack of awareness among parents, health care professionals and the society as a whole. With no dedicated National Health Program to detect neonatal hearing loss, it becomes all the more important to us to detect these unfortunate infants with congenital hearing loss in our rural area of Maval.
Neonatal hearing loss has a prevalence that is more than twice that of other newborn disorders amenable to screening such as congenital hypothyroidism and phenylketonuria [1, 2].
World Problem Statement
World Health Organization (WHO) estimates that 5.3% of the world’s population, 360 million, suffers from disabling hearing loss with 91% adults and 9% children. Disabling Hearing Loss is defined as hearing loss greater than 40 dB in the better hearing ear in adults (15 years or older) and greater than 30 dB in the better hearing ear in children (0–14 years). The prevalence of hearing loss in children is highest in South Asia, Asia Pacific and Sub-Saharan Africa. India is one of the countries in South Asia [3].
Problem Statement in India
As per National Sample Survey Organization survey, currently there are 291 persons per one lakh population who are suffering from severe to profound hearing loss [4]. Out of this population, a large percentage is children between the ages of 0–14 years.
Congenital bilateral hearing impairment occurs in approximately in 1–5 per 1000 live births and when permanent unilateral hearing loss is included, the incidence increases to 8 per 1000 live births [5, 6]. Studies done in India using different hearing screening protocols have estimated the neonatal hearing loss to vary between 1 and 8 per 1000 babies screened [7–9]. Early identification and intervention for hearing loss by 6 months of age provides better prognosis in language development, academic success, social integration and successful participation in the society [5].
The statistics indicate a huge number of children suffer from hearing loss which must be detected at an early age to integrate the children in society. The Joint Committee on Infant Hearing (JCIH) was established with such points in mind. The JCIH was established in late 1969 and composed of representatives from audiology, otolaryngology, pediatrics, and nursing. It was the American Speech Language Hearing Association (ASHA), the then American Academy of Ophthalmology and Otolaryngology (AAOO) and the American Academy of Pediatrics (AAP) who were the founders. The Committee was charged with a two-fold responsibility: first, to make recommendations concerning the early identification of children with, or at-risk for hearing loss and second, newborn hearing screening. The first statement issued by the JCIH in 1971 [10], stated that mass hearing screening could not be justified at that time because of lack of appropriate test procedures. The statement encouraged ongoing research and acknowledged the need to detect hearing loss early in life.
The first attempts at hearing screening were behavioural observation techniques in mid 1960s using the auropalpebral response, startle response and limb and head movements to judge a response to high frequency narrow band noise at about 90–100 dB SPL. The drawbacks of this method were that the method was time consuming identified only infants with bilateral severe to profound high frequency hearing loss. The method also did not provide ear and frequency specific information and had a high false negative rate [11].
The 1971 statement given by JCIH defined the first high-risk factors for hearing loss and recommended following infants with these high risk factors: history of hereditary childhood hearing impairment, congenital perinatal infection such as rubella or other nonbacterial fetal infection like cytomegalovirus, and herpes; cran1ofacial anomalies, birth weight less than 1500 g and a bilirubin level greater than 20. In 1982, bacterial meningitis and severe asphyxia were added. Additional risk indicators were added between 1982 and 1994.
JCIH in 1994 [12] endorsed universal detection of hearing loss in newborns and infants and stated that all infants with hearing loss be identified before 3 months of age and receive intervention by 6 months. In 2000, the focus was given on quality of the care provided for each infant. JCIH endorsed the development of early hearing detection and identification with use of state or universal hearing screening protocols. JCIH in 2000 provided guidelines for early hearing detection and identification programs. Recently JCIH in 2007 issued another position statement which updated the principles from the 2000 statement. The changes made were-updating the definition of targeted hearing loss, issue separate protocols for well-baby nursery and NICU babies, tests included in diagnostic audiological evaluation, medical evaluation, early intervention and monitoring and surveillance of developmental milestones. The position statement made clear each role and responsibility of each team member in the early detection and intervention program and also the protocol to be followed.
The effectiveness and need for universal hearing screening in neonates has previously been well proven [12, 13]. Although hearing screening programs using different screening protocols have been setup in some centers, procedures for systematic identification and rehabilitation on a large scale are yet to be tested and implemented in the Indian setting.
This study was undertaken with the primary objective of detecting hearing impairment in Maval Taluka with two stage sequential screening protocol with optoacoustic emission (OAE) and brainstem evoked response audiometry (BERA).
Materials and Methods
This descriptive study was carried out between April 2012 and April 2015 around the Maval area. The project was approved by the MIMER ethical committee. Various centers which catered to neonates were selected. A two tier screening protocol was followed.
All normal new born babies delivered in MIMER Medical College, Nursing homes around Talegaon, PHC and private hospitals were screened by the Audiologist using TEOAE between 24 and 72 h after birth. Newborns admitted in the Neonatal Intensive Care unit (NICU) were screened prior to discharge from NICU (once their general condition was stable). A quiet area was chosen for screening. The OAE probe was introduced in the neonate’s ear after examination of the externa ear for debris, wax etc. The results were saved on the device and later transferred to a computer for analysis. Mothers of all babies were counselled regarding the benefits of hearing screening, procedure of the test, need for follow up and further tests if neonate failed the screening test, and the interventions available if hearing loss was confirmed. The first screening test was done in the post-natal wards or NICU after obtaining informed consent from the mother. Parents of babies who failed (“refer”) screening test once were counselled and asked to return after 2 weeks for second screening. These babies underwent a second testing in a quiet room. Those who passed on the second screening were discharged from the study while those who failed second time were called after 2 weeks for ABR testing.
A detailed case history, which included questions relating to mother’s history, pre, peri and post-natal birth history and family history was obtained. In addition to this, a detailed history regarding the high risk factors was also taken. The high risk factors taken into consideration, on the basis of JCIH, 1994 position statement were—family history of hearing loss, maternal infections like TORCH, craniofacial anomalies, birth weight less than 1500 g, hyperbilirubinemia at serum level requiring exchange transfusion, ototoxic medications taken by the mother, bacterial meningitis, APGAR scores of 0–4 at 1 min or 0–6 at 5 min, mechanical ventilation lasting for 5 days or longer and syndromes associated with sensorineural or conductive hearing loss. The neonate underwent an examination by the ENT surgeon for outer, middle ear anomalies. Next step of the protocol was TEOAE screening using LABAT Ecolab-Screener. The protocol set was as follows:
Type of stimuli: clicks
SNR: 3–6 dB
Frequency: 1–4 kHz.
The Auditory Brainstem Response (ABR) was carried out using LABAT Epic Plus-ABR system, either under sedated sleep or natural sleep. The following protocol was used.
Type of stimuli: clicks
Intensity: varying intensity (threshold estimation)
Polarity: alternating
Number of clicks: 2000
Time window: 15 ms
Filter settings: 30–3000 Hz
Electrode montage: FPz—M1–M2.
The impedance was kept as low as possible and the electrical activity was kept at a minimum. The least intensity at which a replicable and robust wave V was seen was considered as the threshold. The babies who were detected with hearing loss with ABR were considered for hearing rehabilitation either with hearing aids or cochlear implants.
The data collected was tabulated and analyzed.
Results
The study was carried out between April 2012 and April 2015. The aim of the study was to find out the incidence of hearing loss in neonates in rural areas of Maval taluka of Maharashtra. A total of 8192 babies were screened across various centers around the Maval area. The babies who had some high risk factors were 1683 in number and babies who had no high risk factors i.e. well babies were 6509.
In the well-baby group, 4926 passed in the first tier of screening. The 1583 who were referred in the first phase underwent another screening after two weeks. At the second visit, 1454 passed the screening, 105 failed the screening and 24 were lost to follow up. In the high risk group, 1406 passed in the first tier of screening. The 277 who were referred in the first phase underwent another screening after two weeks. At the second visit, 184 passed the screening, 72 failed the screening and 21 were lost to follow up.
A total of 18 out of 72 referred neonates for ABR were detected with hearing impairment, whereas 54 out of 72 neonates were having hearing sensitivity within normal limits on ABR. Among the well-baby group, 105 well babies who were referred for ABR 11 were detected with hearing impairment, 67 had hearing sensitivity within normal limits and 27 were lost to follow up. Among the 18 high risk babies detected with hearing loss, 10 had low birth weight, 6 had hyperbilirubinemia and 2 had craniofacial abnormalities (Table 1 and Fig. 1).
Table 1.
Table showing the number of babies screened and referred at both stages
| No. of babies screened | Babies referred on 1st screen | Babies who underwent 2nd screen | Babies referred on 2nd screen | Babies who underwent ABR | Babies with confirmed hearing loss | |
|---|---|---|---|---|---|---|
| Normal | 6509 | 1583 | 1559 | 105 | 78 | 11 |
| High risk | 1683 | 277 | 256 | 72 | 72 | 18 |
| Total | 8192 | 1860 | 1815 | 177 | 150 | 29 |
Fig. 1.
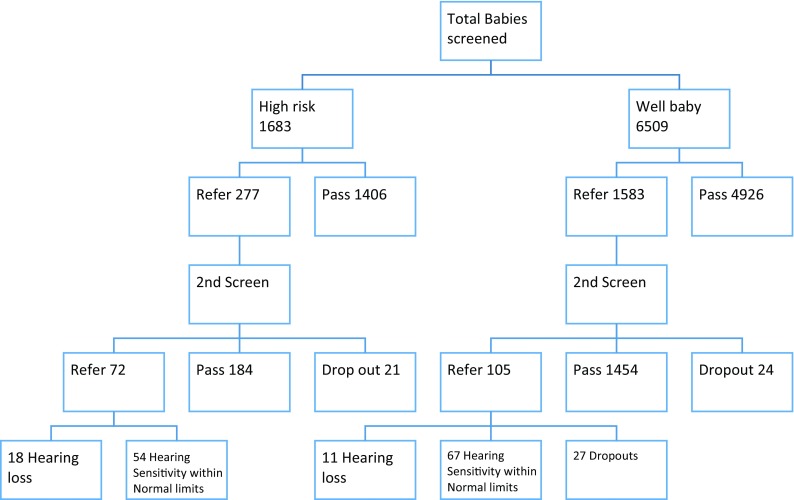
Flow chart depicting the screening protocol and results obtained
In our study, the overall prevalence of hearing loss in neonates in Maval taluka of Maharashtra was found to be 3.54 per 1000 live births, in normal born neonates (well babies) was 1.689 per 1000 births, in high risk babies was 10.69 per 1000 high risk births. The prevalence of low birth weight neonates, hyperbilirubinemia neonates and neonates with craniofacial abnormalities developing hearing impairment was found to be 5.9, 3.56 and 1.18 per 1000 high risk births respectively.
Discussion
Many universal screening programs use a two-step protocol consisting of OAE and Automated ABR (AABR). In developed nations, testing with either OAE or AABR is mandatory, or at least encouraged, prior to discharge from maternity hospital. In the US and UK, it is mandatory for the newborn to undergo hearing screening either before leaving the hospital or shortly after discharge from hospital. However, the scenario in developing nations is different. There are often barriers like expensive equipment, scarcity of trained and skilled personnel, and shortage of skilled maternal and newborn health workers especially in rural areas [14]. Additionally, the births may not take place in a hospital setting where the screening resources are available. Many infants are also lost for follow up [15].
The Government of India initiated the National Programme for Prevention and Control of Deafness (NPPCD) in 2006 [16]. It was initially started as a pilot project and was implemented in 25 districts in 10 states and 1 union territory. The project aims to cover three levels of prevention and care: primary, secondary and tertiary ear care. It aims at preventing avoidable hearing loss which is due to disease or injury, early identification and treatment either medically or by rehabilitation. The programme has four main components-manpower training and development; building infrastructure at district hospitals, community health centers and primary health centers; early detection and management of hearing and speech impaired cases at different levels of health care system and fourth component is the creation of awareness among levels of health care system. In developing nations particularly where limited resources are available, use of low cost calibrated mechanical noisemakers to conduct hearing screening was studied [17]. They trained six health workers who were supervised by a qualified audiologist to observe behavioral responses of neonates using calibrated noisemakers. Twenty out of 425 neonates with confirmed severe to profound hearing loss by ABR testing were included. The mechanical calibrated noisemakers of 50, 60, 70 and 80 dB (A) were used to elicit behavioral responses. Neonates were in state of light sleep when the test was performed. The observer was blinded to the stimulus. The tester presented the stimulus at a distance of 1 m from the testing ear which was not in the visual field of observer. The stimuli was presented 3 times with a 1 min interval. The tester noted the time at which the stimulus was presented. The observer noted the response and the time at which it was observed. A qualified audiologist observed if the health worker correctly identified the response. The authors found that the sensitivity and specificity was high for 70 and 80 dB (A) noisemakers with least false positive referrals. The authors, thus concluded that in controlled settings, health workers with primary education can be trained by qualified audiologists to use calibrated noisemakers to conduct screening for severe to profound hearing loss.
Our study carried out between April 2012 and April 2015 in the Maval taluka of Maharashtra included total of 8192 babies with 1683 neonates having high risk factors and 6509 well babies. This is the first study in the state of Maharashtra for screening of deafness in neonates. The overall prevalence of hearing loss in neonates of Maval taluka in our study was found to be 3.54 per 1000 live births; in normal born neonates (well babies) was 1.689 per 1000 births and in high risk babies was 10.69 per 1000 high risk births. The prevalence of low birth weight neonates, hyperbilirubinemia neonates and neonates with craniofacial abnormalities developing hearing impairment was found to be 5.9, 3.56 and 1.18 per 1000 high risk births respectively.
Tests used for screening newborns for hearing loss include Otoacoustic Emissions (OAE) and Automated Auditory Brainstem Evoked Response (aABR). While OAE is cheap, quick, simple and reliable with a sensitivity of 100% and specificity of 99% [18–20], ABR has the additional advantage of identifying neonates with auditory neuropathy.
The advent of Otoacoustic Emissions (OAE) and Auditory Brainstem Evoked Response (ABR) has provided noninvasive recordings of physiologic activity underlying normal auditory function and both are easily performed in neonates and infants. Otoacoustic emissions (OAEs) are sounds given off by the inner ear when the cochlea is stimulated by a sound. When sound stimulates the cochlea, the outer hair cells vibrate. The vibration produces a nearly inaudible sound that echoes back into the middle ear. The sound can be measured with a small probe inserted into the ear canal. The auditory brainstem response (ABR) test gives information about the inner ear (cochlea) and brain pathways for hearing.
Various studies are published which are constrained to a particular center usually tertiary care centers [7, 15, 21–23]. These studies follow different protocols, screening of at risk versus well baby clinic, difference in times between re screening and different use of instruments and tests.
Nagapoornima et al. [20], studied a total of 1769 neonates from a total of 8192 out of which 6509 were not at risk babies and 1683 were at risk babies. The babies underwent Transient evoked Oto Acoustic Emissions (OAE) as the first level of screening by 6 weeks. The neonates who failed at the first screening underwent re screening within 3 weeks of first screening. If neonates failed a second screening, they underwent an Auditory Brainstem Response and behavioral audiometry to confirm the hearing loss. Results in the study reported an incidence of 10 per 1769 infants screened which is 5.65 per 1000 screened. Out of 1769 infants, 279 were at risk neonates 3 out of which were detected to have hearing loss. This puts the incidence to 10.75 per 1000 neonates. Out of the 1490 who are not at risk, 7 had hearing loss, which makes the incidence 4.70 per 1000 screened. The authors extrapolated the findings and found an incidence of 5.60 per 1000 neonates in a tertiary care center. The study also showed that hearing screening of only high risk neonates can miss the detection up to 70% of children with hearing impairment.
John et al. [7] screened 500 neonates with automated distortion product otoacoustic emission (aDPOAE) followed by automated Auditory Brainstem Response (aABR) on neonates who failed a repeat DPOAE. On the first screening, 32 failed the screening. On repeat OAE only 8 failed the test, out of which 3 did not pass the aABR test. The authors thus concluded that the incidence of moderate to moderately severe sensorineural hearing loss in their study was 0.6%.
A two tier centralized screening programme which consisted of otoacoustic emission as first screen followed by auditory brainstem response for those who failed the first screen was initiated for all newborns in Cochin. A total of 10,165 babies were screened using this program which brought together twenty major hospitals with maternity units. The incidence of hearing loss in the high risk group was found to be 10.3 per 1000 and 0.98 per 1000 in the well-baby group [8].
A similar programme was started at Ludhiana [21]. The screening initially was done by using TOAE after 48 h of birth. Neonates who failed the initial screen were screened again after 1 month and those who failed this screening were referred to Audiologist. Among the 1000 neonates who were screened initially, 6% failed the initial screen. Four failed the next screening out of the 42 who reported for the rescreening out of which 3 had risk factors. Two babies had severe sensorineural hearing loss and two had moderate to severe hearing loss.
In spite of the individual regional studies of neonatal screening for deafness, till now, there is an absence of large scale studies which target hearing screening of neonates.
Auditory neuropathy is defined as an abnormal or absent auditory brainstem response but intact OAE or cochlear microphonics. In our screening protocol like most other universal screening programs [13, 16–21], OAE was performed first and ABR was performed only when OAE failed twice. Neonates who undergo automated ABR with OAE can be screened successfully for auditory neuropathy. The screening protocols [24, 25], in which ABR is done in patients with passed OAE in NICU can detect early auditory neuropathy. We did not detect cases of auditory neuropathy in our study.
Conclusion
In our study, the overall prevalence of hearing loss in neonates in Maval taluka of Maharashtra was found to be 3.54 per 1000 live births, in normal born neonates (well babies) was 1.689 per 1000 births, in high risk babies was 10.69 per 1000 high risk births.
India is the second most populated country in the world with nearly a fifth of the world’s population. Rural areas lack in medical and health care facilities as compared to urban areas. There is a need for the implementation of the National Health Programme for neonatal deafness screening for earlier detection and rehabilitation of these unfortunate neonates so as to allow less delay in development and to improve the overall quality of life.
Funding
ICMR funded.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 helsinski declaration and its later ammendments or comparable ethical standards. Institutional Ethics Committee has approved the study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Augustine AM, Jana AK, Kuruvilla KA, Danda S, Lepcha A, Ebenzer J, Paul RR, Tyagi A, Balraj A. Neonatal hearing screening-experience from a tertiary care hospital in Southern India. Indian J Pediatr. 2014;51:179–183. doi: 10.1007/s13312-014-0380-5. [DOI] [PubMed] [Google Scholar]
- 2.Bickel H, Bachmann C, Beckers R, et al. Neonatal mass screening for metabolic disorders. Eur J Pediatr. 1981;137:133. [Google Scholar]
- 3.De Capua B, De Felice C, Costantini D, Bagnoli F, Passali D. Newborn hearing screening by transient evoked otoacoustic emissions: analysis of response as a function of risk factors. Acta Otorhinolaryngol Ital. 2003;23:16–20. [PubMed] [Google Scholar]
- 4.Directorate General of Health Services. Ministry of Health and Family Welfare, National Program for Prevention and Control of Deafness, Project Proposal. New Delhi (2006). https://mohfw.gov.in/sites/default/files/51892751619025258383Operational%20Guidlines%20for%2012th%20Plan_0.pdf
- 5.Fisher DA, Dussault JH, Foley TP, Klein AH, LaFranchi S, Larsen PR, et al. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979;94:700–705. doi: 10.1016/S0022-3476(79)80133-X. [DOI] [PubMed] [Google Scholar]
- 6.Jewel J, Varghese P, Singh T, Varghese A. Newborn hearing screening—experience at a tertiary hospital in northwest India. Int J Otolaryngol Head Neck Surg. 2013;2(5):211–214. doi: 10.4236/ijohns.2013.25044. [DOI] [Google Scholar]
- 7.John M, Balraj A, Kurien M. Neonatal screening for hearing loss: pilot study from a tertiary care center. Indian J Otolaryngol Head Neck Surg. 2009;61:23–26. doi: 10.1007/s12070-009-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint Committee on Infant Hearing 1994 position statement. AAO-HNS Bull. 1994;12:13. [PubMed] [Google Scholar]
- 9.Joint Committee on Infant Hearing 1994 position statement. ASHA. 1994;36(12):38–41. [PubMed] [Google Scholar]
- 10.Joint Committee on Infant Hearing Joint Committee on Infant Hearing (JICH) 1994 position statement. Pediatrics. 1994;95:152. [PubMed] [Google Scholar]
- 11.Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 12.Joint Committee on Infant Hearing. American Academy of Audiology. American Academy of Pediatrics. American Speech-Language-Hearing Association. Directors of Speech and Hearing Programs in State Health and Welfare Agencies Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2000;2000(106):798–817. doi: 10.1542/peds.106.4.798. [DOI] [PubMed] [Google Scholar]
- 13.Judith A, Mason MS, Kenneth R, Herrmann MD. Universal infant hearing screening by automated auditory brainstem response measurement. Pediatrics. 1998;101:221–228. doi: 10.1542/peds.101.2.221. [DOI] [PubMed] [Google Scholar]
- 14.National Sample Survey Organization (2003) Disabled persons in India, NSS 58th round (July–December 2002) Report no. 485 (58/26/1). New Delhi: National Sample Survey Organization, Ministry of Statistics and Programme Implementation, Government of India
- 15.Paul AK. Early identification of hearing loss and centralized newborn hearing screening facility—the Cochin experience. Indian Pediatr. 2011;48:355–359. doi: 10.1007/s13312-011-0067-0. [DOI] [PubMed] [Google Scholar]
- 16.Rai N, Thakur N. Universal screening of newborns to detect hearing impairment—is it necessary? Int J Pediatr Otorhinolaryngol. 2013;77:1036–1041. doi: 10.1016/j.ijporl.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Ramesh A, Jagdish C, Nagapoorinima M, Suman Rao PN, Ramakrishnan AG, Thomas GC, Dominic M, Swarnarekha A. Low cost calibrated mechanical noise maker for hearing screening of neonates in resource constrained settings. Indian J Med Res. 2012;135:170–176. [PMC free article] [PubMed] [Google Scholar]
- 18.Maxon AB, White KR, Behrens TR, Vohr BR. Referral rates and cost efficiency in a universal newborn hearing screening program using transient evoked optoacoustic emissions. J Am Acad Audiol. 1995;6:271–277. [PubMed] [Google Scholar]
- 19.Maxon AB, White KR, Vohr BR, Behrens TR. Using transient evoked otoacoustic emissions for neonatal hearing screening. Br J Audiol. 1993;27:149–153. doi: 10.3109/03005369309077906. [DOI] [PubMed] [Google Scholar]
- 20.Nagapoornima P, Ramesh A, Rao S, Patricia PL, Gore M, et al. Universal hearing screening. Indian J Pediatr. 2007;74:545–549. doi: 10.1007/s12098-007-0105-z. [DOI] [PubMed] [Google Scholar]
- 21.Sanders R, Durieux-Smith A, Hyde M, Jacobson J, Kileny P, Murnane O. Incidence of hearing loss in high risk and intensive care nursery infants. J Otolaryngol. 1985;14:28–33. [PubMed] [Google Scholar]
- 22.World Health Organization. Fact sheet. Deafness and hearing impairment. http://www.who.int/mediacentre/factsheets/fs300/en/index.html. Accessed on 10 Jan 2009
- 23.World Health Organization. State of hearing and ear care in the South East Asia Region. WHO Regional Office for South East Asia. WHO-SEARO. SEA/Deaf/9. http://www.searo.who.int/LinkFiles/Publications_HEARING_&_EAR_CARE.pdf. Accessed on 10 Jan 2009
- 24.Berg AL, Spitzer JB, Towers HM, Bartosiewicz C, Diamond BE. Newborn hearing screening in the NICU: profile of failed auditory brainstem response/passed otoacoustic emission. Pediatrics. 2005;116:933–938. doi: 10.1542/peds.2004-2806. [DOI] [PubMed] [Google Scholar]
- 25.Kirkim G, Serbetcioglu B, Erdag TK, Ceryan K. The frequency of auditory neuropathy detected by universal newborn hearing screening program. Int J Pediatr Otorhinolaryngol. 2008;72:1461–1469. doi: 10.1016/j.ijporl.2008.06.010. [DOI] [PubMed] [Google Scholar]


