Abstract
Engineered nanomaterials (ENMs), or small anthropogenic particles approximately < 100 nm in size and of various shapes and compositions, are increasingly incorporated into commercial products and used for industrial and medical purposes. There is an exposure risk to both the population at large and individuals in the workplace with inhalation exposures to ENMs being a primary concern. Further, there is increasing evidence to suggest that certain ENMs may represent a significant health risk, and many of these ENMs exhibit distinct similarities with other particles and fibers that are known to induce adverse health effects, such as asbestos, silica, and particulate matter (PM). Evidence regarding the importance of lysosomal membrane permeabilization (LMP) and release of cathepsins in ENM toxicity has been accumulating. The aim of this review was to describe our current understanding of the mechanisms leading to ENM-associated pathologies, including LMP and the role of cathepsins with a focus on inflammation. In addition, anti-cathepsin agents, some of which have been tested in clinical trials and may prove useful for ameliorating the harmful effects of ENM exposure are examined.
Keywords: Engineered nanoparticles, cathepsins, inflammasome, anti-cathepsin agents, lysosomal membrane permeabilization
Engineered nanomaterials: overview
To understand the potential effects of engineered nanomaterial (ENM) exposures, it is helpful to examine the various applications in which these substances are used. The most commonly cited uses of ENMs include biomedical, industry (e.g. paint), and cosmetics (e.g. sunscreen). While ENMs are increasingly applied to a wide range of industrial pursuits, the biomedical applications are perhaps one of the most promising (Zhao and Castranova, 2011). Metal oxide nanomaterials (both natural and engineered) are being tested for use in drug release systems, medical diagnostics, and sunscreens; metal oxide nanomaterials are highly reactive, with photocatalytic properties in some cases (Zhao and Castranova, 2011; EPA, 2014). ENMs known as quantum dots that are comprised of cadmium selenide, cadmium telluride, and zinc selenide with various possible metal structures are utilized in medical imaging applications, while engineered dendrimers (highly branched polymers), composite nanomaterials (synthesized with more than one type), and engineered silver (Ag) nanomaterials (composed of many Ag atoms) are being utilized or proposed for use in medical applications such as drug delivery, cancer detection, and antimicrobial applications (EPA, 2012). ENMs of different types and composition are being applied to focused delivery of chemotherapeutic agents to cancer sites, bypassing healthy tissue that would otherwise be affected. ENMs have also shown promise as targeting delivery agents that regulate lipid metabolism and inflammation to treat atherosclerosis (Zhang et al., 2015a), and the application of ENMs to drug delivery systems for multi-modal chemotherapeutic agents are anticipated to greatly enhance the efficacy of cancer drug protocols and outcomes (Flynn and Wei 2005). Further, because of their unique characteristics ENMs are increasingly being applied towards improved imaging for biomedical diagnostics, leading to earlier detection and improved prognoses of various cancers and other diseases.
While the field of study focusing on ENMs has gained tremendous ground in recent years, there is a distinct imbalance in information between potential applications of ENMs and possible adverse health effects. Further, there has been discussion regarding the most appropriate methods for determining the safety of certain ENMs (Alaraby et al., 2016; Hornos Carneiro and Barbosa, 2016; Hartmann et al., 2015; Kermanizadeh et al., 2016). Therefore, it is important to obtain a better understanding of mechanisms leading to toxicity so that anticipated benefits continue to outweigh risks. To address this paucity of information, intense efforts to understand ENM toxicity have been ongoing in recent years. ENM-activation of inflammatory pathways is one of the most studied areas of concern because of the known role for inflammation in many, if not most, chronic and degenerative health diseases (Stephenson et al. 2016, Maisch et al. 2005, Sethi et al. 2012, Kundu and Surh 2008). In addition to inflammation, concerns regarding oxidative stress, pulmonary toxicity, fibrosis, reproductive system, malignant transformations, granuloma formation, and genetic alterations have been raised (Liu et al., 2012; Shvedova et al. 2008; Farcas et al, 2016; Schramm et al, 2016; Snyder-Talkington et al, 2016; Chakraborty et al, 2017).
Engineered nanomaterials: A human health issue
There is an apparent risk to humans from occupational inhalation exposures to ENMs and exposure through consumer products such as food and cosmetics; however, there has been insufficient time to conduct adequate epidemiology studies. Nonetheless, investigations on workers heavily exposed to titanium dioxide (TiO2), a nanoparticle (NP) that is commonly used in commercial products, have repeatedly demonstrated its safety (Warheit and Donner 2015). In contrast, in vivo and in vitro studies consistently suggested that certain types of bioactive nanomaterials represent a significant risk to human health (Bonner et al. 2013, Xia et al. 2013), and many ENMs share similar properties to particles already found in the environment with known adverse human health effects (Peters et al. 2011). For example, carbon nanotubes (CNT) have been characterized as having asbestos-like properties and exposure to the mesothelial lining in mice led to development of mesothelioma—a known outcome of asbestos exposure in some people (Takagi et al., 2012, Sargent et al., 2014, Suzui et al., 2016; Lemen, 2016). Similar to ENMs, differences in toxicity between different types of natural particles and fibers, such as silica and asbestos are thought to arise from various physicochemical properties, including size, morphology, and composition (Nemmar et al. 2013). It stands to reason, therefore, that many advances toward an increased understanding of the underlying mechanisms associated with environmental particle and fiber exposures, such as asbestos, may also apply to ENMs (Bunderson-Schelvan et al. 2016). In addition, it has been shown in mice that multi-walled carbon nanotubes (MWCNTs), a specific type of ENM, are distributed throughout the body and accumulate over time after an inhalation exposure, including the parietal pleura, respiratory musculature, liver, kidneys, heart, and brain (Mercer et al., 2013); gold nanoparticles have also been shown to extensively redistribute after exposure (Khlebtsov and Dykman, 2011; Hornos Carneiro and Barbosa, 2016).
While inhalation exposures are the most likely (Oberdörster et al., 2015), environmental exposures from water and food are increasingly of concern (Hendren et al., 2013). Little is known regarding the potential health adverse effects specifically resulting from environmental exposure to ENMs; however, studies demonstrated that silver nanoparticles (Ag-NPs) are bioavailable in estuarine waters (Khan et al. 2012; Gagne et al, 2013) and the same is likely true for other ENMs. ENMs may be released into the environment through both point and non-point sources and remain in suspension or react with other materials (EPA, 2012). ENMs are readily transported over a greater distance than larger particles of the same material; transport in the environment may be affected by surface chemistry, size, as well as biological and abiotic processes (EPA 2014). Therefore, there is little question that environmental exposures to ENMs might occur over time and need to be addressed, and future adverse outcomes associated with ENMs may be linked to the quantity of internalized ENMs. Translocation of ENMs to secondary organs such as liver, kidneys, and heart is known to occur at different rates from inhalation, intratracheal or intranasal instillation, and pharyngeal aspiration exposures (Kermanizadeh et al., 2015; 2016). In general, ENMs are insoluble, persisting in biological fluids for extended periods; however, the toxicity of a few NP is known to be affected by their dissolution in culture media or biological fluids, particularly metal and metal oxide NP (Lai, 2015). Further, it has been recognized that the absorption, distribution, metabolism, and excretion (ADME, which characterizes the disposition of a compound within a biological system), as well as toxicity of silica NP are largely unknown, despite their successful use as effective drug carriers that may, in fact, result in shape-dependent renal damage (Li et al., 2015). Similar data characterizing ADME properties of most ENMs currently in use are also lacking. Ultimately, human health outcomes might reflect a combination of ENM bioactivity and ADME. As such, therapeutic strategies aimed at treating ENM-related diseases may be beneficial for limiting future costs to human health. Here, possible mechanisms underlying pathological outcomes following ENM exposure are examined here as well as potential treatment strategies with a focus on anti-cathepsin agents are presented
Potential risk associated with ENM exposure
Strategies for developing risk assessment protocols for ENMs in commercial products, workplace areas, and the environment are under intense discussion (Cuddy et al. 2015). Currently, there is a significant amount of variation in the types of ENMs being developed (Table 2), as their physical traits are easily manipulated in terms of size, shape, and composition. This has resulted in a significant challenge to toxicologists and policy makers regarding the safety of these compounds. Consideration of multiple lines of evidence is necessary due to the complex nature and broad range of physicochemical characteristics for existing ENMs, such as specific surface area, surface texture, zeta-potential, particle morphology, aspect ratio, presence of metals, and particle dissolution rate (Simko et al. 2014). Further, ENM-enabled (or containing) products are likely to cause most exposures, and there is a great deal of uncertainty regarding release of ENMs from these products and consequent health impacts along an ENM’s lifecycle.
Table 2.
Top 11 nanomaterial classes (Committee to Develop a Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials, 2012) and effects.
| Engineered Nanomaterial | Induces LMP? | Possible Associated Pathologies | Study Model | References |
|---|---|---|---|---|
| Ceramic Nanoparticles | Possibly, shown to be bioactive | Wear and tear of joint prostheses | Raw264.7 cells Human Mθ |
Zhang, 2011 Lucarelli 2004 |
| Carbon Nanotubes | Yes | Apoptosis | Hepatocytes | Zhu 2016 |
| Yes | Lung Disease |
Raw267.7 cells Murine Mθ |
Tahara 2012 Jessop 2017 Yang 2014 |
|
| Nanoporous Materials | Unlikely |
Tanaka 2010 Korhonen 2016 |
||
| Graphene | Possible, lysosomes are increased when used as a drug carrier | Unknown | HepG2 cells | Yang 2016b |
| Metal Nanoparticles | Possible, when exposed to UV light (WO3/Pt) | Cardiovascular Disease | THP-1 cells | Clark 2016 Xu 2015 |
| Yes (AgNPs) | Unknown | 4T1 breast cancer cells | Jimeno-Romero 2017 | |
| Nanoscale Encapsulation | Unlikely | |||
| Fullerenes | Possible, shown to cause mitochondrial damage | Unknown | Isolated mitochondria | Yang 2016a |
| Dendrimers | Possible, may cause endosomal rupture | Unknown | Numerical simulation | Mukherjee 2013 |
| Nanostructured Metals | Unknown | |||
| Nanowires | Likely | Cell death | Human Mθ | Müller 2010 |
| Quantum Dots | Likely | Reproductive toxicity Pulmonary inflammation |
Invertebrate Human lung fibroblasts |
Yan 2016 Stan 2015 |
Mθ, macrophage; LMP, lysosomal membrane permeabilization
Recently, a significant amount of progress has been made toward identifying the mechanisms associated with ENMs and natural particle toxicity. Inflammation resulting from permeabilization of the lysosomal (alternatively referred to as late endosome) membrane followed by activation of the NLRP3 inflammasome appears to play a critical role (Bunderson-Schelvan et al. 2016). A key event following ENM exposure is release of cathepsins from phago-lysosome vesicles associated with ENM-damaged membranes into the cytoplasm and extracellular milieu. Cathepsins are a family of protein-degrading enzymes that serve a function in a variety of physiological processes. Investigators have increasingly been interested in the role that intracellular cathepsins play in both normal and pathological processes; in fact, drugs targeting cathepsins K and S are in various stages of clinical development.
Particle-induced lysosomal membrane permeability (LMP)
The lysosome is a key component of autophagic and endosomal pathways, which are responsible for sequestering pathogens as well as organelles and proteins including microorganisms, ENMs, amyloid plaques, cholesterol crystals, damaged or dead cells that have become damaged or marked for degradation. This process is triggered by acidic activation of proteolytic digestive enzymes primarily in phagocytic cells such as monocytes and macrophages (Hullin-Matsuda et al. 2014). Autophagosomes possess a double-membrane that fuses with lysosomes to form an autolysosome, which facilitates the breakdown of encapsulated materials that subsequently may be employed for cell survival (Suzuki et al. 2016). Various investigators suggested that nanomaterial-induced autophagy occurs in response to the body perceiving the nanomaterials as foreign, similar to that of bacteria or other pathogens (Peynshaert et al., 2014; Neibert and Maysinger, 2012; Luo et al., 2013). However, autophagy was also found to play both a protective and pro-death role following NP exposures, depending upon the specific particle (Zhou et al., 2013), with autophagic clearance apparently dependent on the charge of the NP (Song et al., 2015). A primary mechanism of ENM-induced inflammation involves the destabilization and pore formation of the lysosomal membrane, termed lysosomal membrane permeability (LMP) (Figure 1). Many diseases associated with LMP provide evidence of autophagy dysfunction, perhaps by preventing the fusion of autophagosomes and lysosomes (Settembre et al. 2008). This process is not completely understood and not unique to ENM (Stern et al. 2012). Since the lysosome organelle is present in all nucleated cells (Alroy et al. 2014), a digestion process related to normal immune system activity is necessary for foreign body removal and antigen presentation. Some particles initiate acute inflammation by destabilizing the lysosomal membrane, which releases the catalytic enzyme contents of the lysosome, triggering inflammatory signaling and potentially cell death (Aits and Jaattela 2014). The release of cathepsin B and possibly cathepsin C (Kono et al. 2012) from the lysosome was reported to initiate NLRP3 inflammasome activation, which subsequently activates Caspase 1 and in the presence of NF-κB co-stimulation, cleaves pro-IL-1β and releases mature IL-1β, inducing an acute inflammatory signal (Figure 1) (Dostert et al. 2008). This acute inflammation might become sustained and result in a chronic inflammatory condition that manifests in various pathologies depending upon the inflammatory area (Bunderson-Schelvan et al. 2016). Recently, Hughes et al (2016) demonstrated increased proteolytic activity from intracellular Caspase 1, extracellular Caspase 1, and cathepsin S in response to silica, alum, and polystyrene particulate exposures suggesting viable markers for lysosomal rupture and acute inflammation. Regarding ENMs, the lungs, skin, and digestive tract are the primary areas of interest, but with the inclusion of ENMs as drug delivery systems, any part of the body is potentially susceptible to LMP-induced inflammation (Donaldson and Seaton 2012, Oberdorster et al. 2007). Further, ENM physicochemical characteristics influence their ability to initiate LMP, and it is not known whether certain ENMs induce LMP (Table 2). In particular, ENM toxicity was noted to be dependent upon cell type, dose, and aspect ratio, with models of longer nanotubes resulting in elevated frequency of cell death, increased changes in morphology, greater tumor necrosis factor alpha release, higher LMP incidence, and enhanced endoplasmic reticulum stress than seen with shorter nanotubes (Wang et al., 2015).
Figure 1.
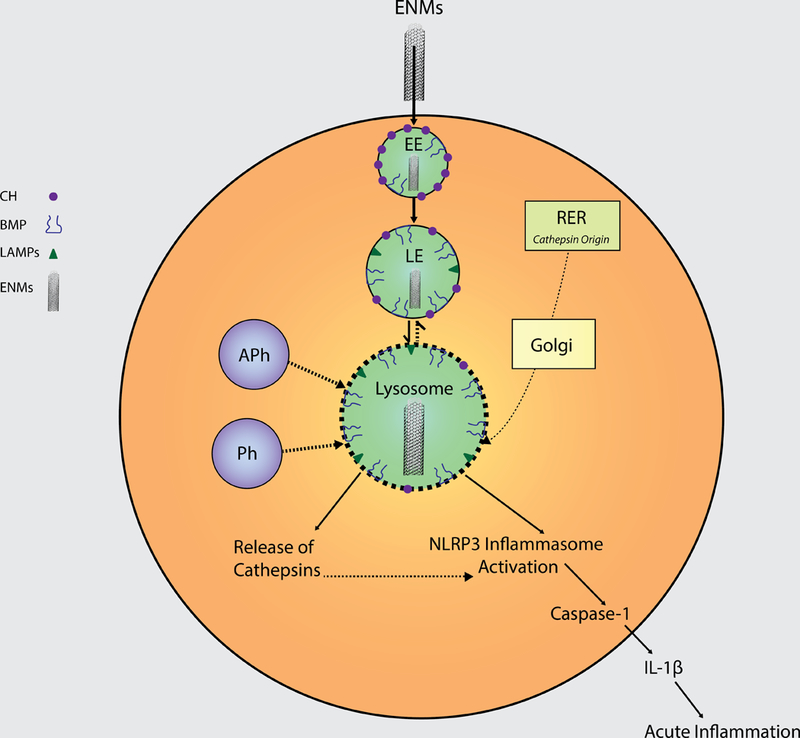
Exposure to engineered nanomaterials (ENMs, represented here as multi-walled carbon nanotubes) results in their uptake by early endosomes (EE) and subsequent transport to the lysosome via the late endosome (LE) in a potentially iterative process that results in an increase in permeability of the lysosomal membrane. Lysosomes are more susceptible to permeabilization than the LE and EE, attributable to lower membrane cholesterol (CH, purple dot) content and higher bis (monoglycero) phosphate (BMP, lipid symbol) in the lysosome than those of the LE and EE, which have progressively higher amounts of CH in their membranes, respectively. Intra-lysosomal cathepsins originate in the rough endoplasmic reticulum (RER) and are processed by the Golgi apparatus for activation and recognition by the lysosome. The increased permeability of the lysosome causes an aberrant release of these cathepsins, a process that is modulated by autophagosomes (APh) and phagosomes (Ph). This, in turn, affects many aspects of normal cell physiology, including activation of the NLRP3 inflammasome and the subsequent cascade of acute inflammation that has been associated with various inflammatory pathological conditions. EE, early endosomes; LE, late endosomes; IL-1β, interleukin-1 beta; ENMs, engineered nanomaterials; CH, cholesterol; BMP, bis (monoglycero) phosphate; LAMPS, lysosomal membrane proteins
The lysosomal membrane is composed of lipids and proteins (lysosomal membrane associated proteins - LAMP’s and kinase/phosphatase enzymes involved in lipid modification) that are involved in a lipid-sorting process where cholesterol is depleted with lower pH and negatively charged bis (monoacylglycero) phosphate (BMP) is subsequently increased (Hullin-Matsuda et al. 2014). BMP is resistant to lysosomal phospholipases and predominantly located on the interior membrane (Hullin-Matsuda et al. 2014), and membrane-stabilizing cholesterol is almost completely absent from lysosomal membranes (Schulze et al. 2009). There is some evidence that cathepsins, specifically B and C, regulate LMP, at least with respect to soluble initiators (Brojatsch et al. 2014), but the exact mechanism underlying ENMs initiating this process is not completely understood (Bunderson-Schelvan et al. 2016). Other potential initiators/mediators of ENM-induced LMP are sphingosine kinases, sphingosine, ceramidase, ceramide, LAMP2, sphingomyelin, and sphingomyelinase (Bunderson-Schelvan et al. 2016). For example, excess sphingosine, in the absence of sphingosine kinase, might alter membrane lipids by neutralizing the negative charge on BMP, subsequently displacing membrane proteins resulting in a permeable lysosomal membrane (Schulze et al. 2009). Unfortunately, there is no experimental evidence that high aspect ratio ENMs initiate this process.
There are several possibilities for how ENMs induce LMP, although none have been proven. One possibility is that ENM physically shears, pokes, or tears the lysosomal membrane. This would be particularly applicable to ENMs that possess a spiny or rigid structure such as some MWCNTs (Palomaki et al. 2011). Another possibility is that the ENMs ionize or solubilize once inside the acidic environment, which would be particularly relevant to metal oxides including zinc (Zn), copper (Cu), and nickel (Ni) oxides and Ag ENMs (Bunderson-Schelvan et al. 2016, Hamilton et al. 2014). This may also explain the bioactivity of some MWCNT contaminated with metals such as Ni or iron (Fe) (Hamilton et al. 2012). There is some evidence that the amount of Ni contamination on MWCNT is positively correlated with the toxicity/bioactivity of the CNT, indicating that dissolution of metal contaminants of MWCNTs in the acidic environment of the lysosome is at least partially responsible for some of the effects (Hamilton et al. 2012). Still another possibility is that ENMs interact directly with the protein/lipid BMP matrix that composes the internal lysosomal membrane. The protein corona, or innate proteins that have adsorbed to the ENM surface (Kharazian et al., 2016), provides a barrier between ENMs and biological systems, likely affecting ENM recognition and uptake. However, the combination of an acidic environment and degradative enzymes within the phagolysosome was reported to strip off the protein corona (Ma et al., 2015), leaving the raw ENMs to come in physical contact with the interior of the phagolysosome (Bunderson-Schelvan et al. 2016). In addition, formation of a protein corona on ENMs demonstrates that the particles interact and bind proteins outside of the lysosome; therefore, it is possible that this may occur inside the protein/lipid-rich organelle, leading to disruption of the lysosomal membrane (Donaldson et al. 2010; Mahon et al. 2012). It is important to understand that the lysosome was not evolutionally developed to deal with ENM-particle processing, and LMP might simply be a way for the cell to create a distress signal that results in acute inflammation and may have evolved in response to inhaled xenobiotic particles.
One logical approach to eliminate ENM-induced LMP may involve manipulation of the cholesterol content of the lysosomal membrane, creating a hyper-stable particle-resistant lysosome as elevated cholesterol content was found to reduce LMP (Appelqvist et al. 2011). This approach has worked with other similar ENMs in the same model. However, the problem with manipulating the lysosome is that it may mimic any of the 51 identified lysosomal storage diseases (LSD) such as Niemann-Pick or Wolman disease and is apparently an unnatural state for the proper operation of the lysosome (Alroy et al. 2014; Alroy and Lyons 2014; Schulz and Sandhoff 2011). Most LSDs result from inadequate or absent enzyme function somewhere along the endosomal/lysosomal pathway. Typically, this occurs from an inherited dysfunction of specific enzymes, but it may also be acquired from certain drugs or plant ingestions (Alroy and Lyons 2014). Patients suffering from adverse health effects attributed to ENM exposure might be responding to conditions of acute stress and inflammation; therefore, a therapeutic intervention for ENM-induced LMP should probably deal with bioactive catalytic enzymes that create or exacerbate inflammation. Of the several cathepsin enzymes released during LMP, cathepsin S is the only one reported to operate at neutral pH (Turk et al. 2012). Cathepsins, in general, are reliable candidates for therapeutic intervention due to their ability to function in a variety of organs and situations and react with a variety of substrates (Reiser et al. 2010). Cathepsin S is currently being studied for its involvement in a number of diseases and inflammatory conditions from cancer to rheumatoid arthritis to chronic pain (Fonovic and Turk 2014). There are numerous cathepsin S inhibitors available and some are already approved for human therapeutic applications (Payne et al. 2014; Petzoldt et al. 2014). Similarly, cathepsin K inhibitors are in various stages of clinical trials (Brömme and Lecaille 2009; Helali et al. 2013).
Cathepsins
Opinions regarding the role of cathepsins within physiological and pathological pathways have dramatically changed since their early description as being primarily responsible for protein turnover. Currently, cathepsins are identified as players in specific biological functions, depending upon type and location. Cathepsins were found to play a role in regulating many key physiological processes, such as generating epitopes for antigen presentation within the immune system (Sadegh-Nasseri and Kim 2015). Cathepsins have garnered a great deal of interest for their apparent role in many disease states, including cardiovascular-related diseases (Platt and Shockey 2015; Zhao et al. 2015), neurodegenerative diseases (Chandra et al. 2015; Bae et al. 2015), cancer (Gomez-Auli et al. 2015; Loser and Pietzsch 2015), and chronic lung disease (Lecaille et al. 2016). Turk et al. (2012) best described cathepsin activity in living organisms as “a delicate balance of expression, targeting, zymogen activation, inhibition by protein inhibitors, and degradation.” Cathepsins B, H, L, C, and X are postulated to be ubiquitously expressed in human tissues as part of normal protein recycling pathways (Turk et al. 2012). Less widely expressed cathepsins, including cathepsin K, W, F, and S are expressed in a more tissue-specific manner—suggesting these proteins serve in a narrower set of cellular functions (Turk et al. 2012).
Cathepsins are small proteins that are primarily monomeric and cleaved into disulfide-linked heavy and light chains during posttranslational modification (Reiser et al. 2010) and that contain unique binding sites. Cathepsins are generally activated in the late endosomes in order to begin proteolytic processing. Once cathepsins become activated, these proteins might be recruited from late endosomes or lysosomes and then secreted into the extracellular space (Reiser et al. 2010). Cathepsins specific to the lysosome or phagolysosome (fused phagosome and lysosome) are D, L, S, C, B, and H (Guha and Padh 2008). Lysosomal cathepsins are activated by low pH (< 5) generated by an ATPase proton-pump mechanism that releases H+ into the lysosomal organelle (Saftig and Klumperman 2009). Cathepsin S is unique among the lysosomal cathepsins because it is active at neutral and acidic pH and operates outside of the lysosomal structure in the cell cytoplasm or completely independent of the cell (Repnik et al. 2014). Cathepsins are not the only proteolytic enzymes in the lysosome, which also includes glycosidases, lipases, nucleases, phosphatases, and sulfatases. All lysosomal enzymes including cathepsins originate in the rough endoplasmic reticulum and processed through the Golgi apparatus where they receive a terminal mannose-6-phosphate that serves as a recognition marker in the lysosome (Alroy et al. 2014).
Cathepsins are classified according to their active site amino acid as being serine, aspartic, or cysteine cathepsins. The cysteine cathepsin group constitutes the largest family, containing cathepsins B, C, F, H, K, L, O, S, W, V and Z (Reiser et al. 2010). The cysteine cathepsins are synthesized as pre-proenzymes that are directed towards the endoplasmic reticulum where the short N-terminal pre-sequences are cleaved by signal peptidases (Novinec et al. 2014). The proenzyme is then directed to the endolysosomal cell compartments through the mannose-6-phosphate receptor pathway, where the acidic environment cleaves the propeptide region, thereby activating the enzyme. The reducing, acidic environment found in the endolysosomal compartments provides optimal conditions for maintaining the cysteine cathepsins in their active state. In fact, under conditions of neutral pH, most cathepsins are quickly and irreversibly inactivated (Turk et al. 1995); as previously indicated, one exception is cathepsin S, which is moderately stable even under neutral pH conditions (Wilkinson et al. 2015). Endogenously, inhibitors known as cystatins play a key role in monitoring and controlling cathepsin activity.
Under pathological conditions, the cysteine cathepsins may be secreted extracellularly, where they are known to degrade extracellular proteins in a way that may contribute to tissue injury and disease (Figure 2) (Turk et al. 1995). Degradation of the extracellular matrix following cathepsin activation of matrix-metalloproteinases exerts a destabilizing effect within key signaling pathways and represents a key mechanism by which the cathepsins may contribute to disease development (Christensen and Shastri 2015). The extracellular matrix is a complex network of proteoglycans, collagens, elastin, and other molecules that are highly dynamic and serve as a scaffold to anchor cells, thereby forming tissues and organs. Understandably, the extracellular matrix varies according to its location and is continually being remodeled based upon the physiological needs of the organism or under pathological conditions (Theocharis et al. 2014). The complex relationship between proteases such as cathepsins and functional proteins within the extracellular matrix, such as proteoglycans, has been the focus of a great deal of research (Panwar et al. 2013; Theocharis et al. 2014; Repnik et al. 2015).
Figure 2.
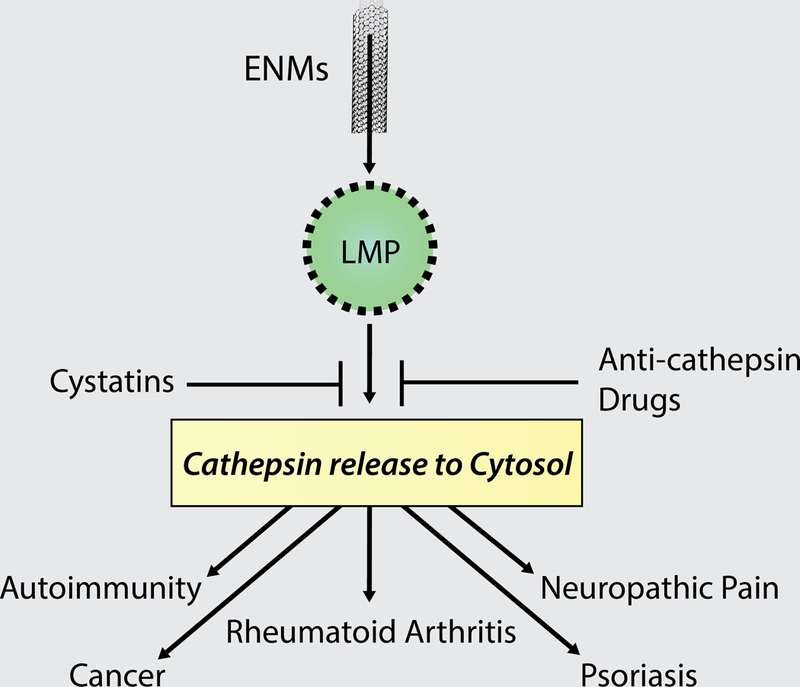
Many of the intra-lysosomal cathepsins have been directly associated with a variety of inflammation-related diseases (Table 1), and an emerging group of therapeutics/inhibitors have been developed for the study or treatment of cathepsin-related disorders. It is possible, therefore, that anti-cathepsin agents can be designed for the treatment of diseases associated with ENM exposure, since the mechanism of lysosomal membrane permeabilization is similar to other endogenous inflammatory agents (cholesterol crystals, amyloid plaques, uric acid crystals, etc.).
Cathepsin, LMP, and inflammation
Several cathepsins have been associated with inflammatory diseases, while links to LMP were correlated with cathepsins B, C, D, and S (Hughes et al. 2016; Jacobson et al. 2013; Hornung et al. 2008). Cathepsin B has been associated with ischemic cell death resulting from LMP in cerebral ischemia/reperfusion injured rats, an effect that is attenuated by CA074-me, a cathepsin B inhibitor that protects against lysosomal rupture (Xu et al. 2016). Cathepsin B and S activities are also elevated in unstable carotid plaques, which contribute to the inflammatory development of atherosclerosis (Abd-Elrahman et al. 2016), and cathepsin B activity is increased in patients with active arthritis (Däbritz et al. 2016). Cathepsin B was also identified as a key regulator of lysosomal biogenesis (Qi et al. 2016). In a model of oxygen-glucose deprivation/reperfusion-induced apoptosis resulting in LMP, cytosolic levels of cathepsin D were significantly raised, resulting in caspase-dependent apoptosis in astrocytes (Liu et al. 2016). Extracellular cathepsin S and intracellular Caspase 1 were suggested to be regulators of the innate immune response, resulting in release of IL-1β. In particular, their proteolytic activities were associated with LMP following particle exposures (Hughes et al. 2016). Cathepsin S was also correlated with autoimmune responses as well as acute and chronic inflammation. Overexpression of cathepsin S was associated with adverse effects on the immune system (Turk et al. 2012) consistent with some ENM exposures (Kononenko et al. 2015). Excess cathepsin S expression and activity was also correlated with several inflammatory conditions such as rheumatoid arthritis, osteoarthritis, atherosclerosis, acute and chronic lung disease, psoriasis, type II diabetes, and certain forms of heart disease (Fonovic and Turk 2014). The absence of this enzyme was noted in cystic fibrosis patients (Wilkinson et al. 2015). Cathepsin S might be released from phagocytic cells during inflammatory conditions, in the presence of lipopolysaccharides (LPS), and in wound healing (Guha and Padh 2008). Cathepsin S-deficient mice with defective MHC activity improperly process antigens and was accompanied by unusually enlarged endosomal morphology (Guha and Padh 2008). Because of its role in MHC class antigen presentation, inhibition of cathepsin S is thought to be immunosuppressive (Costantino et al. 2009) and potential side effects have been a barrier against anti-cathepsin S drug development. While epidemiological studies have not directly shown a relationship between ENM exposures and many of these cathepsin-associated diseases, ENMs have been linked to adverse pulmonary effects (Morimoto et al. 2013), a potential for cardiovascular disturbances (Meng et al. 2012), and exacerbation of brain pathologies in diabetic rats (Lafuente et al. 2012). Further, there are few studies examining ENM biopersistence over the long term. However, carbon encapsulated FeNP were shown to be present in the lung and liver one year after intravenous administration in mice (Herrmann et al., 2016); while no adverse effects were observed (i.e. inflammation, fibrosis, necrosis, or carcinogenesis). This study demonstrates the potential for ENMs to persist in biological organisms. Further, potential bioaccumulation of ENM in aquatic organisms and environments (Gagne et al, 2013; Wang et al., 2014) as well as contamination of drinking water under certain conditions (Troester et al., 2016) may result in prolonged exposure conditions, which might produce persistent phagolysosomal membrane damage and promote disease.
Cathepsin inhibitors and anti-cathepsin agents
Cathepsin inhibitors represent a promising area of new drug development (Table 1). Endogenously, cathepsin inhibitors are categorized into three distinct families, including the stefins, cystatins, and kininogens, along with several uncategorized proteins with cystatin-like sequences (Ochieng and Chaudhuri 2010). Cystatins are thought to help sequester unwanted cathepsin activity (Ochieng and Chaudhuri 2010) and aberrant regulation of cystatin expression levels may indirectly contribute to cathepsin-associated diseases. Cystatin levels were found to decline when tumors are approaching end-stage or metastatic categories (Ochieng and Chaudhuri 2010). Further, fetuin A, a cystatin-like protein, was shown to stimulate tumor cell growth both in vitro and in vivo (Kundranda et al. 2005). Cystatin C is upregulated in patients with dementia, serving a neuroprotective role through pathways that are dependent on inhibition of the cysteine cathepsins (Gauthier et al. 2011). High cathepsin S and low cystatin C levels were correlated with the presence of atherosclerosis in human studies, and it was proposed that cystatin C may be employed as a biomarker for this disease (Lv et al. 2012). Experimentally, cystatin C was observed to be protective against neurodegeneration (Gauthier et al. 2011) and it was proposed as a potential anti-cancer agent (Kos et al. 2014).
Table 1.
Inhibitors against LMP-related cathepsins and associated diseases
| LMP Cathepsins | Active Site Amino Acid | Inhibitor | Associated Diseases/References/Study Details |
||
|---|---|---|---|---|---|
| Experimental Reagent | Drug Candidate | ||||
| Cathepsin B | Cysteine | Ca-074 (CA-074-Me) E64d |
PADK | Cancer Lung Disease, RA, OA Neurodegenerative Diseases |
Appelqvist 2013, Review; Gondi 2004, mice injected via tail vein and evaluated for 72 h; Reiser 2010, Review; Sevenich 2010, knockout mice; Tummalapalli 2007, in vitro; Vasiljeva 2006, in vitro |
| Cathepsin C (Dipeptidyl Peptidase I) | Cysteine | Semi-carbazides Non-peptidic Cyanamides Dipeptide-derived Nitriles |
Semi-carbazides Non-peptidic Cyanamides Dipeptide-derived Nitriles |
Immune Disorders Lung Diseases |
Laine 2010, Review; Reiser 2010, Review |
| Cathepsin D | Aspartic Acid | Pepstatin A Hydroxyethyl-amine isosteres |
Pepstatin A derivatives Hydroxyethyl-amine isosteres |
Cancer Alzheimer’s Disease |
McConnell 2006, in vitro; Yan 1999, in vitro |
| Cathepsin H | Cysteine | Cystatins α2-macroglobulin |
Cancer, Lung Diseases | Gocheva 2006, knockout mice and 2010, in vitro and in vivo; Reiser 2010, Review | |
| Cathepsin K | Cysteine | Odanacatib MIV-711 Balicatib Relicatib ONO-5334 SAR114137 |
Atherosclerosis, Cancer,Metabolic Syndrome Lung Disease, RA, OA, Osteoporosis |
Buhling 2004, mice and humans; Dejica 2008, humans; Duong 2016, Review; Fonovic 2014, Review; Gocheva 2006, knockout mice and 2010, in vitro and in vivo; Lutgens 2006, knockout mice; Schurigt 2008, transgenic mice; Svelander 2009, daily dose (25 mg/kg) in mice for 14 days; Yang 2008, humans | |
| Cathepsin L | Cysteine | Z-FY-CHO PADK | Atherosclerosis Cancer Metabolic Syndrome Lung Disease Immune Disorders RA, osteoarthritis |
Gocheva 2006, knockout mice and 2010 in vitro and in vivo; Honey 2002, in vitro and in vivo; Hsieh 2002, in vitro; Huang 2003, diabetic mice; Kitamoto 2007, knockout mice; Maehr 2005, diabetic mice; Nakagawa 1999, cathepsin null mice; Reiser 2010, Review | |
| Cathepsin S | Cysteine | Cystatin C Paecilopeptin LHVS |
RWJ-445380 VBY-036 VBY-891 CRA-028129 SAR114137 |
Atherosclerosis Cancer Metabolic Syndrome, lung disease, OA Immune Disorders Rheumatoid Arthritis Neuropathic Pain Psoriasis Abdominal Aortic Aneurism |
Fonovic 2014, Review; Gocheva 2006, knockout mice and 2010, in vitro and in vivo; Hsieh 2002, in vitro; Nakagawa 1999, cathepsin null mice; Payne 2014, humans; Sukhova 2003, LDL deficient mice; Reiser 2010, Review |
RA, rheumatoid arthritis; OA, osteoarthritis; LHVS morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl; PADK, Z-phe-ala-diazomethylketone
In addition to the cystatins, morpholinurea-leucine- homopenylalanine-vinylsulfone phenyl (LHVS) is a cathepsin S inhibitor that was shown to exert neuroprotective effects in a murine model of traumatic brain injury (Xu et al. 2013). LHVS has been utilized to impede antigen presentation in a mouse model as a potential therapy for autoimmune disease (Fujii et al. 2012), specifically multiple sclerosis (Allan and Yates 2015). Cathepsin S inhibitors are currently in development for treatment of numerous pathologies, including neuropathic pain, cancer, rheumatoid arthritis, autoimmune disease, and psoriasis (Reiser et al. 2010; Fonovic and Turk 2014).
While anti-cathepsin agent development focused on the discovery of selective substrates and small-molecule inhibitors, this field has benefited from discovery of important regulatory molecules that served as models for subsequent drug design. In addition to the cystatins discussed above, glycosaminoglycans facilitate autocatalysis of the cathepsin proenzyme, and in some cases, modulate that activity. As such, they are known to play a crucial role in the binding between cysteine cathepsins and their protein substrates (Aguda et al. 2014). In particular, negatively charged glycosaminoglycans are known to modulate the activity of cathepsin S (Sage et al. 2013), which was associated with autoimmune diseases (Stoeckle et al. 2012; Baugh et al. 2011), cancer (Zhang et al. 2015b), and atherosclerosis (Figueiredo et al. 2015). As a primary component of the extracellular matrix, the glycosaminoglycans are covalently-linked negatively-charged polysaccharides that are highly variable and able to interact with many of the other components within the matrix as well as growth factors, cytokines, and chemokines (Theocharis et al. 2010). Drugs aimed at modulating the interaction between glycosaminoglycans and their respective proteases, such as the cathepsins, display potential for treatment of many diseases associated with aberrant cathepsin activity, including potential pathologies related to ENM exposures (Figure 2).
Concluding Remarks
In summary, the use of ENMs is rapidly increasing and ENMs have many useful purposes. However, our ability to screen these materials for human health risk and develop regulatory mechanisms for protecting the public has lagged behind their release into the market. These products were found not only in specific commercial products and applications, but increasingly as contaminants in the environment. As such, exposure risks are becoming a significant concern. In addition, studies describing serious pathological outcomes in animal models following exposure to certain types of ENMs suggest that over time there may be human exposure cases resulting in disease. Therefore, preemptive discussions regarding possible treatment options are timely. The most likely mechanism of ENM-induced pathologies may be attributed to phagolysosomal membrane permeabilization. This results in the aberrant release of cathepsins that may contribute to disease development, as well as activation of the NLRP3 inflammasome and an increase in release of inflammatory mediators. There is a significant body of evidence regarding the utilization of anti-cathepsin agents, both in clinical and lab settings. Therefore, there is great potential to capitalize on this information to preemptively prepare for an expected rise in ENM-associated illnesses. Further, there is a great deal of interest in re-purposing drugs that are currently approved by the FDA—potentially saving time and money that would otherwise be required for development of new pharmaceuticals. As such, future research aimed at inhibiting the pathological effects of LMP-associated cathepsin release may provide prevention or treatment strategies that minimize the harmful effects of ENM exposure.
Acknowledgements
We would like to thank Charles M. Raffety for his assistance creating the figures.
References
- Abd-Elrahman I, Meir K, Kosuge H, Ben-Nun Y, Weiss Sadan T, Rubinstein C, Samet Y, McConnell MV, Blum G.2016. Characterizing cathepsin activity and macrophage subtypes in excised human carotid plaques. Stroke 47: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Aguda AH, Panwar P, Du X, Nguyen NT, Brayer GD, Brömme D. 2014. Structural basis of collagen fiber degradation by cathepsin K. Proc Natl Acad Sci USA 111: 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aits S, Jaattela M. 2013. Lysosomal cell death at a glance. J Cell Sci 126: 1905–1912. [DOI] [PubMed] [Google Scholar]
- Alaraby M, Annangi B, Marcos R, Hernández A.2016. Drosophila melanogaster as a suitable in vivo model to determine potential side effects of nanomaterials: A review. J Toxicol Environ Health B 19: 65–104. [DOI] [PubMed] [Google Scholar]
- Allan ER, Yates RM. 2015. Redundancy between cysteine cathepsins in murine experimental autoimmune encephalomyelitis. PLoS One 10: e0128945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy J, Lyons JA.2014. Lysosomal storage diseases. J Inborn Errors Metab Screen 2014: 1 – 20. [Google Scholar]
- Alroy J, Garganta C, Wiederschain G. 2014Secondary biochemical and morphological consequences in lysosomal storage diseases. Biochemistry 79: 619–636. [DOI] [PubMed] [Google Scholar]
- Appelqvist H, Nilsson C, Garner B, Brown AJ, Kågedal K, Ollinger K. 2011. Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am J Pathol 178: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelqvist H, Jäättelä M. 2009. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta 1793: 746–754. [DOI] [PubMed] [Google Scholar]
- Bae EJ, Yang NY, Lee C, Kim S, Lee HJ, Lee SJ. 2015. Haploinsufficiency of cathepsin D leads to lysosomal dysfunction and promotes cell-to-cell transmission of α-synuclein aggregates. Cell Death Dis 6: e1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh M, Black D, Westwood P, Kinghorn E, McGregor K, Bruin J, Hamilton W, Dempster M, Claxton C, Cai J, Bennett J, Long C, McKinnon H, Vink P, Hoed L den, Gorecka M, Vora K, Grant E, Percival MD, Boots AM, van Lierop MJ. 2011. Therapeutic dosing of an orally active, selective cathepsin S inhibitor suppresses disease in models of autoimmunity. J Autoimmun 36: 201–209. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdörster G, Harkema JR, Bramble LA, Kavanagh TJ, Botta D, Nel A, Pinkerton KE.2013. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ Health Perspect 121: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brojatsch J, Lima H, Kar AK, Jacobson LS, Muehlbauer SM, Chandran K, Diaz-Griffero F. 2014. A proteolytic cascade controls lysosome rupture and necrotic cell death mediated by lysosome-destabilizing adjuvants. PLoS One 9: e95032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brömme D, Lecaille F. 2009. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin Invest Drugs 18: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühling F, Röcken C, Brasch F, Hartig R, Yasuda Y, Saftig P, Brömme D D, Welte T. 2004. Pivotal role of cathepsin K in lung fibrosis. Am J Pathol 164: 2203–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunderson-Schelvan M, Hamilton RF Jr, Trout KL, Jessop F, Gulumian M, Holian A.2016 Approaching a unified theory for particle-induced inflammation In Biological Effects of Fibrous and Particulate Substances (Otsuki T, Ed), (2016) Springer; Japan: pp. 51–76 [Google Scholar]
- Chakraborty S, Castranova V, Perez MK, Piedimonte G 2017. Nanoparticles-induced apoptosis of human airway epithelium is mediated by proNGF/p75ntr signaling. J Toxicol Environ Health A 80: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G, Bagh MB, Peng S, Saha A, Sarkar C, Moralle M, Zhang Z, Mukherjee AB. 2015. Cln1 gene disruption in mice reveals a common pathogenic link between two of the most lethal childhood neurodegenerative lysosomal storage disorders. Human Mol Genet 24: 5416–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Shastri VP. 2015. Matrix-metallopreoteinase-9 is cleaved and activated by cathepsin K. BMC Res Notes 8: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Petty HR.2016. WO3/Pt nanoparticles promote light-induced lipid peroxidation and lysosomal instability within tumor cells. Nanotechnology 27: 075103. [DOI] [PubMed] [Google Scholar]
- Committee to Develop a Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials; National Research Council2012. A research strategy for environmental, health, and safety aspects of engineered nanomaterials. Washington (DC): National Academies Press (US); 2012 Background; Available from: https://www.ncbi.nlm.nih.gov/books/NBK189513/ [PubMed] [Google Scholar]
- Costantino CM, Ploegh HL, Hafler DA. 2009. Cathepsin S regulates class II MHC processing in human CD4+ HLA-DR+ T cells. J Immunol 183: 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy MF, Poda AR AR, Moser RD, Weiss CA CA, Cairns C3, Steevens JA. 2016. A weight-of-evidence approach to identify nanomaterials in consumer products: A case study of nanoparticles in commercial sunscreens. J Expo Sci Environ Epidemiol 26: 26–34. [DOI] [PubMed] [Google Scholar]
- Däbritz J, Weinhage T, Varga G, Wirth T, Ehrchen JM, Barczyk-Kahlert K, Roth J, Schwarz T, Foell D. 2016. Activation-dependent cell death of human monocytes is a novel mechanism of fine-tuning inflammation and autoimmunity. Eur J Immumol 46: 1997–2007. [DOI] [PubMed] [Google Scholar]
- Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR.2008. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol 173: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Poland CA, Schins RP. 2010. Possible genotoxic mechanisms of nanoparticles: Criteria for improved test strategies. Nanotoxicology 4: 414–420. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Seaton A. 2012. A short history of the toxicology of inhaled particles. Part Fibre Toxicol 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong le T, Leung AT, Langdahl B.2016. Cathepsin k inhibition: A new mechanism for the treatment of osteoporosis. Calcif Tissue Int 98: 381–397. [DOI] [PubMed] [Google Scholar]
- EPA, United States Environmental Protection Agency. 2015. Emerging contaminant—nanomaterials Solid Waste and Emergency Response: (2015P). EPA 505-F-11–009. [Google Scholar]
- EPA, United States Environmental Protection Agency. 2014. Technical Fact Sheet—Nanomaterials Solid Waste and Emergency Response (5106P): EPA 505-F-14–002. [Google Scholar]
- Farcas MT, Kisin ER, Menas AL, Gutkin DW, Star A,, Reiner RS, Yanamala N, Savolainen K Shvedova AA. 2016. Pulmonary exposure to cellulose nanocrystals caused deleterious effects reproductive system in male mice. J Toxicol Environ Health A 79: 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo JL, Aikawa M, Zheng C, Aaron J, Lax L, Libby P, Lima Filho JL de, Gruener S, Fingerle J, Haap W W, Hartmann G, Aikawa E. 2015Selective cathepsin S inhibition attenuates atherosclerosis in apolipoprotein E-deficient mice with chronic renal disease. Am J Pathol 185: 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn T, Wei C. 2005. The pathway to commercialization for nanomedicine. Nanomedicine 1: 47–51. [DOI] [PubMed] [Google Scholar]
- Fonovic M, Turk B. 2014. Cysteine cathepsins and their potential in clinical therapy and biomarker discovery. Proteom Clin Appl 8: 416–426. [DOI] [PubMed] [Google Scholar]
- Fujii H, Ivison SM, Shimizu H, Kajiwara R, Kariminia A, Yan M, Dutz JP, Schultz KR. 2012. Inhibition of cathepsin S reduces allogeneic T cell priming but not graft-versus-host disease against minor histocompatibility antigens. Biol Blood Marrow Transplant 18: 546–556. [DOI] [PubMed] [Google Scholar]
- Gagne F, Auclair J, Fortier M, Bruneau A, Fournier M, Turcotte P Pilote M, Gagnon C. 2013. Bioavailability and immunotoxicity of silver nanoparticles to the freshwater mussel Elliptio complanta. J Toxicol Environ Health A 76: 767–777. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Kaur G, Mi W, Tizon B, Levy E. 2011. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci 3: 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. 2006. Distinct roles for cystine cathepsien genes in multistage tumorigenesis. Genes Dev 20: 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA.2010. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24: 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Auli, Hillebrand LE, Biniossek ML, Peters C, Reinheckel T, Schilling O. 2016. Impact of cathepsin B on the interstitial fluid proteome of murine breast cancers. Biochimie 122: 88–98. [DOI] [PubMed] [Google Scholar]
- Gondi CS, Lakka SS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Tung CH, Weissleder R, Rao JS.2004. Adenovirus-mediated expression of antisense urokinase plasminogen activator receptor and antisense cathepsin B inhibits tumor growth, invasion, and angiogenesis in gliomas. Cancer Res 64: 4069–4077. [DOI] [PubMed] [Google Scholar]
- Guha S, Padh H.2008. Cathepsins: Fundamental effectors of endolysosomal proteolysis. Indian J Biochem Biophys 45: 75–90 [PubMed] [Google Scholar]
- Hamilton RF, Buford M, Xiang C, Wu N, Holian A.2012. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal Toxicol 24: 995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Buckingham S, Holian A 2014. The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution. Int J Mol Sci 15: 6815–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann NB, Jensen KA, Baun A, Rasmussen K, Rauscher H, Tantra R, Cupi D, Gilliland D, Pianella F, Riego Sintes JM. 2015. Techniques and protocols for dispersing nanoparticle powders in aqueous media-is there a rationale for harmonization? J Toxicol Environ Health B 18: 299–326. [DOI] [PubMed] [Google Scholar]
- Helali AM, Iti FM, Mohamed IN. 2013. Cathepsin K inhibitors: A novel target but promising approach in the treatment of osteoporosis. Curr Drug Targets 14: 1591–600. [DOI] [PubMed] [Google Scholar]
- Hendren CO, Lowry M, Grieger KD, Money ES, Johnston JM, Wiesner MR, Beaulieu SM.2013. Modeling approaches for characterizing and evaluating environmental exposure to engineered nanomaterials in support of risk-based decision making. Environ Sci Technol (2013): 47, 1190–1205. [DOI] [PubMed] [Google Scholar]
- Herrmann IK, Beck-Schimmer B, Schumacher CM, Gschwind S, Kaech A, Ziegler U, Clavien PA, Gunther D, Stark WJ, Schlegel AA. 2016. In vivo risk evaluation of carbon-coated iron carbide nanoparticles based on short- and long-term exposure scenarios. Nanomedicine 11: 783–796. [DOI] [PubMed] [Google Scholar]
- Honey K, Benlagha K, Beers C, Forbush K, Teyton L, Kleijmeer MJ, Rudensky AY, Bendelac A.2002. Thymocyte expression of cathepsin L is essential for NKT cell development. Nat Immumol 3: 1069–1074. [DOI] [PubMed] [Google Scholar]
- Hornos Carneiro MF, Barbosa F.2016. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J Toxicol Environ Health B 19: 129–148. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, deRoos P, Honey K, Beers C, Rudensky AY.2002. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol 168: 2618–2625. [DOI] [PubMed] [Google Scholar]
- Huang X, Vaag A, Carlsson E, Hansson M, Ahrén B, Groop L. 2003. Impaired cathepsin L gene expression in skeletal muscle is associated with type 2 diabetes. Diabetes 52: 2411–2418. [DOI] [PubMed] [Google Scholar]
- Hughes CS, Colhoun LM, Bains BK, Kilgour JD, Burden RE, Burrows JF, Lavelle EC, Gilmore BF, Scott CJ.2016. Extracellular cathepsin S and intracellular caspase 1 activation are surrogate biomarkers of particulate-induced lysosomal disruption in macrophages. Part Fibre Toxicol 13: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullin-Matsuda F, Taguchi T T, Greimel P P, Kobayashi T. 2014. Lipid compartmentalization in the endosome system. Semin Cell Dev Biol 31: 48–56. [DOI] [PubMed] [Google Scholar]
- Jacobson LS, Lima H, Goldberg MF, Gocheva V, Tsiperson V, Sutterwala FS, Joyce JA, Gapp BV, Blomen VA, Chandran K, Brummelkamp TR, Diaz-Griffero F, Brojatsch J.2013. Cathepsin-mediated necrosis controls the adaptive immune response by Th2 (T helper type 2)-associated adjuvants. J Biol Chem 288: 7481–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop F, Hamilton RF Jr, Rhoderick JF, Fletcher P, Holian A.2017. Phagolysosome acidification is required for silica and engineered nanoparticle-induced lysosome membrane permeabilization and resultant NLRP3 inflammasome activity. Toxicol Appl Pharmacol 318: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno-Romero A, Bilbao E, Izagirre U, Cajaraville MP, Marigómez I, Soto M. 2017. Digestive cell lysosomes as main targets for Ag accumulation and toxicity in marine mussels, Mytilus galloprovincialis, exposed to maltose-stabilised Ag nanoparticles of different sizes. Nanotoxicology [Epub ahead of print, 2017]. doi: 10.1080/17435390.2017.1279358. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A, Balharry D, Wallin H, Loft S, Møller P.2015. Nanomaterial translocation--the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs--a review. Crit Rev Toxicol 45: 837–872. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A, Gosens I, MacCalman L, Johnston H, Danielsen PH, Jacobsen NR, Lenz AG, Fernandes T, Schins RP, Cassee FR, Wallin H, Kreyling W, Stoeger T, Loft S, Møller P, Tran L, Stone V. 2016. A multilaboratory toxicological assessment of a panel of 10 engineered nanomaterials to human health--ENPRA project--the highlights, limitations, and current and future challenges. J Toxicol Environ Health B: 19: 1–28. [DOI] [PubMed] [Google Scholar]
- Kharazian B, Hadipour NL, Ejtehadi MR.2016. Understanding the nanoparticle-protein corona complexes using computational and experimental methods. Int J Biochem Cell Biol 75: 162–174. [DOI] [PubMed] [Google Scholar]
- Khlebtsov N, Dykman L. 2011. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 40: 1647–1671. [DOI] [PubMed] [Google Scholar]
- Khan FR, Misra SK, García-Alonso J, Smith BD, Strekopytov S, Rainbow PS, Luoma SN, Valsami-Jones E.2012. Bioaccumulation dynamics and modeling in an estuarine invertebrate following aqueous exposure to nanosized and dissolved silver. Environ Sci Technol 46: 7621–7628. [DOI] [PubMed] [Google Scholar]
- Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. 2007. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation 115: 2065–2075. [DOI] [PubMed] [Google Scholar]
- Kono H, Orlowski GM, Patel Z, Rock KL.2012. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol 189: 3734–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko V, Narat M, Drobne D.2015. Nanoparticle interaction with the immune system. Arh Hiq Rada Toksikol 66: 97–108. [DOI] [PubMed] [Google Scholar]
- Korhonen E, Rönkkö S, Hillebrand S, Riikonen J, Xu W, Järvinen K, Lehto VP, Kauppinen A. 2016. Cytotoxicity assessment of porous silicon microparticles for ocular drug delivery. Eur J Pharm Biopharm 100: 1–8. [DOI] [PubMed] [Google Scholar]
- Kos J, Mitrović A, Mirković B. 2014. The current stage of cathepsin B inhibitors as potential anticancer agents. Future Med Chem 6: 1355–1371. [DOI] [PubMed] [Google Scholar]
- Kundranda MN, Henderson M, Carter KJ, Gorden L, Binhazim A, Ray S, Baptiste T, Shokrani M, Leite-Browning ML, Jahnen-Dechent W, Matrisian LM, Ochieng J.2005. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res 65: 499–506. [PubMed] [Google Scholar]
- Kundu JK, Surh YJ.2008. Inflammation: Gearing the journey to cancer. Mutat Res 659: 15–30. [DOI] [PubMed] [Google Scholar]
- Lafuente JV, Sharma A, Patnaik R, Muresanu DF, Sharma HS.2012. Diabetes exacerbates nanoparticles induced brain pathology. CNS Neurol Disord Drug Targets 11: 26–39. [DOI] [PubMed] [Google Scholar]
- Lai DY.2015. Approach to using mechanism-based structure activity relationship (SAR) analysis to assess human health hazard potential of nanomaterials. Food Chem Toxicol 85: 120–126. [DOI] [PubMed] [Google Scholar]
- Laine DI, Busch-Petersen J.2010. Inhibitors of cathepsin C (dipeptidyl peptidase I). Expert Opin Ther Pat 20: 497–506. [DOI] [PubMed] [Google Scholar]
- Lecaille F, Lalmanach G, Andrault PM. 2016. Antimicrobial proteins and peptides in human lung diseases: A friend and foe partnership with host proteases. Biochimie 122: 151–168. [DOI] [PubMed] [Google Scholar]
- Lemen RA 2016. Mesothelioma from asbestos exposures: Epidemiological patterns and impact in the United States. J Toxicol Environ Health B 19: 250–265. [DOI] [PubMed] [Google Scholar]
- Li L, Liu T, Fu C, Tan L, Meng X, Liu H. 2015. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomedicine 11: 1915–1924. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang L, Tian H, Ma Q.2016. Cathepsin D is involved in the oxygen and glucose deprivation/reperfusion-induced apoptosis of astrocytes. Int J Mol Med 38: 1257–1263. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Sun B, Chen C. 2013. Understanding the toxicity of carbon nanotubes. ACC Chem Res 46: 702–713. [DOI] [PubMed] [Google Scholar]
- Löser R, Pietzsch J. 2015. Cysteine cathepsins: Their role in tumor progression and recent trends in the development of imaging probes. Front Chem 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli M, Gatti AM, Savarino G, Quattroni P, Martinelli L, Monari E, Boraschi D.2004. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur Cytokine Network 15: 339–346. [PubMed] [Google Scholar]
- Luo YH<, Wu SB, Wei YH, Chen YC, Tsai MH, Ho CC, Lin SY, Yang CS, Lin P. 2013. Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem Res Toxicol 26: 662–673. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, P Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. 2006. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation 113: 98–107. [DOI] [PubMed] [Google Scholar]
- Lv BJ, Lindholt JS, Cheng X, Wang J, Shi GP. 2012. Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: A randomized population-based study. PLoS ONE 7: e41813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Bai J, Jiang X. 2015. Monitoring of the enzymatic degradation of protein corona and evaluating the accompanying cytotoxicity of nanoparticles. ACS Appl Mater Interfaces 7: 17614–17622. [DOI] [PubMed] [Google Scholar]
- Maehr R, Mintern JD, Herman AE, Lennon-Duménil AM, Mathis D, Benoist C, Ploegh HL.2005. Cathepsin L is essential for onset of autoimmune diabetes in NOD mice. J Clin Invest 115: 2934–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon E, Salvati A, Baldelli Bombelli F, Lynch I, Dawson KA.2012. Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J Control Release 161: 164–174. [DOI] [PubMed] [Google Scholar]
- Maisch B, Richter A, Sandmöller A, Portig I, Pankuweit S; 2005. BMBF-Heart Failure Network. Inflammatory dilated cardiomyopathy (DCMI). Herz 30: 535–544. [DOI] [PubMed] [Google Scholar]
- McConnell RM, Green AW, Trana CJ, McConnell MS, Lindley JF, Sayyar K, Godwin WE, Hatfield SE.2006. New cathepsin d inhibitors with hydroxyethylamine isosteres: Preparation and characterization. Med Chem 2: 27–38. [DOI] [PubMed] [Google Scholar]
- Meng J, Yang XD, Jia L, Liang XJ, Wang C. 2012. Impacts of nanoparticles on cardiovascular diseases: Modulating metabolism and function of endothelial cells. Curr Drug Metab 13: 1123–1129. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. 2013. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Horie M, Kobayashi N, Shinohara N, Shimada M.2013. Inhalation toxicity assessment of carbon-based nanoparticles. ACC Chem Res 46: 770–781. [DOI] [PubMed] [Google Scholar]
- Mukherjee SP, Byrne HJ. 2013. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: experimental observation and numerical simulation. Nanomedicine 9: 202–211. [DOI] [PubMed] [Google Scholar]
- Müller KH, Kulkarni J, Motskin M, Goode A, P Winship JN, Skepper, Ryan MP, Porter AE.2010. pH-dependent toxicity of high aspect ratio ZnO nanowires in macrophages due to intracellular dissolution. ACS Nano 4: 6767–6779. [DOI] [PubMed] [Google Scholar]
- Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, Rosloniec EF, Elliott EA, Rudensky AY.1999. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 10: 207–217. [DOI] [PubMed] [Google Scholar]
- Neibert KD, Maysinger D. Mechanisms of cellular adaptation to quantum dots--the role of glutathione and transcription factor EB. Nanotoxicology 6: 249–262. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. 2013. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed Res Int 2013: 279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novinec M, Lenarčič B, Turk B. 2014. Cysteine cathepsin activity regulation by glycosaminoglycans. Biomed Res Int 2014: 309718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Castranova V, Asgharian B, Sayre P.2015. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and dosimetry. J Toxicol Environ Health B 18: 121–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Stone V, Donaldson K.2007. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 1: 2–25. [Google Scholar]
- Ochieng J, Chaudhuri G. 2010. Cystatin superfamily. J Health Care Poor Underserved 21: 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomäki J, Välimäki E, Sund, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. 2011. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5: 6861–6870. [DOI] [PubMed] [Google Scholar]
- Panwar P, Xin D, Sharma V, Lamour G, Castor M, Li H, Brömme D. 2013. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J Biol Chem 288: 5940–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CD, Deeg MA, Chan M, Tan LH, LaBell ES, Shen T, DeBrota DJ. 2014. Pharmacokinetics and pharmacodynamics of the cathepsin S inhibitor, LY3000328, in healthy subjects. Br J Clin Pharmacol 78: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Rückerl R, Cyrys J.2011. Lessons from air pollution epidemiology for studies of engineered nanomaterials. J Occup Environ Med 53: S8–S13. [DOI] [PubMed] [Google Scholar]
- Petzoldt C, Bley O, Byard SJ, Andert D, Baumgartner B, Nagel N, Tappertzhofen C, Feth M.2014. An example of how to handle amorphous fractions in API during early pharmaceutical development: SAR114137--a successful approach. Eur J Pharm Biopharm 86: 337–350. [DOI] [PubMed] [Google Scholar]
- Peynshaert K, Manshian BB, Joris F, Braeckmans K, Smedt SC De, Demeester J, Soenen SJ. 2014. Exploiting intrinsic nanoparticle toxicity: the pros and cons of nanoparticle-induced autophagy in biomedical research. Chem Rev 114: 7581–7609. [DOI] [PubMed] [Google Scholar]
- Platt MO, Shockey WA. 2016. Endothelial cells and cathepsins: Biochemical and biomechanical regulation. Biochimie 122: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Man SM, Malireddi RK, Karki R, Lupfer C, Gurung P, Neale G, Guy CS, Lamkanfi M, Kanneganti TD. 2016. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J Exp Med 213: 2081–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Adair B, Reinheckel T. 2010. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 120: 3421–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repnik U, Hafner Česen M, Turk B. 2014. Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion 19: 49–57. [DOI] [PubMed] [Google Scholar]
- Repnik U, Starr AE, Overall CM, Turk B. 2015. Cysteine cathepsins activate ELR chemokines and inactivate non-ELR chemokines. J Biol Chem 290: 13800–13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S, Kim A. 2015. Exogenous antigens bind MHC class II first, and are processed by cathepsins later. Mol Immunol 68: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J. 2009. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635. [DOI] [PubMed] [Google Scholar]
- Sage J, Mallèvre F, Barbarin-Costes F, Samsonov SA, Gehrcke JP, Pisabarro MT, Perrier E, Schnebert S, Roget A, Livache T, Nizard C, Lalmanach G, Lecaille F. 2013. Binding of chondroitin 4-sulfate to cathepsin S regulates its enzymatic activity. Biochemistry 52: 6487–6498. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S, Endo M, Fluharty KL, Castranova V, Reynolds SH. 2014. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm F, Lange M, Hoppmann P, Heutelbeck A 2016. Cytotoxicity of carbon nanohorns in different human cells of the respiratory system. J Toxicol Environ Health A 79: 1085–1093. [DOI] [PubMed] [Google Scholar]
- Schulze H, Kolter T, Sandhoff K. 2009. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta 1793: 674–683. [DOI] [PubMed] [Google Scholar]
- Schulze H, Sandhoff K. 2011. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol 3: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurigt U, Hummel KM, Petrow PK, Gajda M, Stöckigt R, Middel P, Zwerina J, Janik T, Bernhardt R, Schüler S, Scharnweber D, Beckmann F, Saftig P, Kollias G, Schett G, Wiederanders B, Bräuer R. 2008. Cathepsin K deficiency partially inhibits, but does not prevent, bone destruction in human tumor necrosis factor-transgenic mice. Arthritis Rheum 58: 422–434. [DOI] [PubMed] [Google Scholar]
- Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. 2012. Multifaceted link between cancer and inflammation. Biosci Rep 32: 1–15. [DOI] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, Pablo R de, Tacchetti C, Rubinsztein DC, Ballabio A. 2008. A block of autophagy in lysosomal storage disorders. Human Mol Genet 17: 119–129. [DOI] [PubMed] [Google Scholar]
- Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Müller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, Peters C, Reinheckel T. 2010. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc Natl Acad Sci USA 107: 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, Jin J, Yin J, Stone S, Chen BT, Deye G, Maynard A, Castranova V, Baron PA, Kagan VE. 2008. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol 295: L552–L565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkó M, Nosske D, Kreyling WG. 2014. Metrics, dose, and dose concept: the need for a proper dose concept in the risk assessment of nanoparticles. Int J Environ Res Public Health 11: 4026–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Popp L, Yang J, Kuma A, Ganoli VS, Segatori L. 2015. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J Nanobiotechnol 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington B, Dong C, Porter DW,Ducatman B, Wolfarth MG, Andrew M, Battelli L, Raese R, Castranova V, Guo NL Qian Y. 2016. Multiwalled carbon nanotube-induced pulmonary inflammation and fibrotic responses and genomic changes following aspiration exposure in mice: a 1-year postexposure study. J Toxicol Environ Health A 79: 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan MS, Sima C, Cinteza LO, Dinischiotu A. 2015. Silicon-based quantum dots induce inflammation in human lung cells and disrupt extracellular matrix homeostasis. FEBS J 282 : 2914–2929. [DOI] [PubMed] [Google Scholar]
- Stephenson E, K Savvatis K, Mohiddin SA, Marelli-Berg FM. 2016. T cell immunity in myocardial inflammation: Pathogenic role and therapeutic manipulation. Br J Pharmacol doi: 10.1111/bph.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ST, Adiseshaiah PP, Crist RM.2012. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckle C, Quecke P, Rückrich T, Burster T, Reich M, Weber E, Kalbacher H, Driessen C, Melms A, Tolosa E. 2012. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. J Autoimmun 38: 332–343. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. 2003. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest 111: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzui M, Futakuchi M, Fukamachi K, Numano T, Abdelgied M, Takahashi S, Ohnishi M, Omori T, Tsuruoka S, Hirose A, Kanno J, Sakamoto Y, Alexander DB, Alexander WT, Jiegou X, Tsuda H.2016. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci 107: 9241–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Osawa T, Fujioka Y, Noda NN.2016. Structural biology of the core autophagy machinery. Curr Opin Struct Biol 43: 10–17. [DOI] [PubMed] [Google Scholar]
- Svelander L, Erlandsson-Harris H, Astner L, Grabowska U, Klareskog L, Lindstrom E, Hewitt E.2009. Inhibition of cathepsin K reduces bone erosion, cartilage degradation and inflammation evoked by collagen-induced arthritis in mice. Eur J Pharmacol 613: 155–162. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Nakamura M, Yang M, Zhang M, Iijima S, Yudasaka M. 2012. Lysosomal membrane destabilization induced by high accumulation of single-walled carbon nanohorns in murine macrophage RAW 264.7. Biomaterials 33: 2762–2729. [DOI] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. 2012. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci 103: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Godin B, Bhavane R, Nieves-Alicea R, Gu J, Liu X, Chiappini C, Fakhoury JR, Amra S, Ewing A, Li Q, Fidler IJ, Ferrari M.2010. In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice. Int J Pharm 402: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. 2010. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 277: 3904–3923. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Gialeli C, Bouris P, Giannopoulou E, Skandalis SS, Aletras AJ, Lozzo RV, Karamanos NK. 2014. Cell-matrix interactions: focus on proteoglycan-proteinase interplays and pharmacological targeting in cancer. FEBS J 281: 5023–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troester M, Brauch HJ, Hofmann T. 2016. Vulnerability of drinking water supplies to engineered nanoparticles. Water Res 96: 255–279. [DOI] [PubMed] [Google Scholar]
- Tummalapalli P, Spomar D, Gondi CS, Olivero WC, Gujrati M, Dinh DH, Rao JS. 2007. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int J Oncol 31: 1039–1050. [PMC free article] [PubMed] [Google Scholar]
- Turk B, Bieth JG, Björk I, Dolenc I, Turk D, Cimerman N, Kos J, Colic A, Stoka V, Turk V.1995. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol Chem Hoppe Seyler 376: 225–230. [DOI] [PubMed] [Google Scholar]
- Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D.2012. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim Biophys Acta 1824: 68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva O, Papazoglou A, Krüger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T. 2006. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res 66: 5242–5250 [DOI] [PubMed] [Google Scholar]
- Wang H, Ho KT, Scheckel KG, Wu F, Cantwell MG, Katz DR, Horowitz DB, Boothman WS, Burgess RM. 2014. Toxicity, bioaccumulation, and biotransformation of silver nanoparticles in marine organisms. Environ Sci Technol 48: 13711–13717. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kaur G, Zysk A, Liapis V, Hay S, Santos A, Losic D, Evdokiou A. 2015. Systematic in vitro nanotoxicity study on anodic alumina nanotubes with engineered aspect ratio: understanding nanotoxicity by a nanomaterial model. Biomaterials 46: 117–130. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Donner EM. 2015. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles: Recognizing hazard and exposure issues. Food Chem Toxicol 85: 138–147. [DOI] [PubMed] [Google Scholar]
- Wilkinson RD, Williams R, Scott CJ, Burden RE.2015. Cathepsin S : Therapeutic, diagnostic, and prognostic potential. Biol Chem: 396: 867–882. [DOI] [PubMed] [Google Scholar]
- Xia T, Hamilton RF, Bonner JC, Crandall ED, Elder A, Fazlollahi F, Girtsman TA, Kim K, Mitra S, Ntim SA, Orr G, Tagmount M, Taylor AJ, Telesca D, Tolic A, Vulpe CD, Walker AJ, Wang X, Witzmann FA, Wu N, Xie Y, Zink JI, Nel A, Holian A. 2013. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ Health Perspect 121: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang H, Ding K, Lu X, Li T, Wang J, Wang C, Wang J. 2013. Inhibition of cathepsin S produces neuroprotective effects after traumatic brain injury in mice. Mediators Inflamm 2013: 187873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang L, Bai R, Zhang T, Chen C. 2015. Silver nanoparticles impede phorbol myristate acetate-induced monocyte-macrophage differentiation and autophagy. Nanoscale 7: 16100–16109. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang J, Song X, Wei R, He F, Peng G, Luo B. 2016. Protective mechanisms of CA074-me (other than cathepsin-B inhibition) against programmed necrosis induced by global cerebral ischemia/reperfusion injury in rats. Brain Res Bull 120: 97–105. [DOI] [PubMed] [Google Scholar]
- Yan SQ, Xing R, Zhou YF, Li KL, Su YY, Qiu JF, Zhang YH, Zhang KQ, He Y, Lu XP, Xu SQ.2016. Reproductive toxicity and gender differences induced by cadmium telluride quantum dots in an invertebrate model organism. Sci Rep 6: 34182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, Darakhshan F, Guerre-Millo M, Clement K, Gelb BD, Dolgnov G, Shi GP.2008. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol 28: 2202–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhang M, Tahara Y, Chechetka S, Miyako E, Iijima S, Yudasaka M.2014. Lysosomal membrane permeabilization: Carbon nanohorn-induced reactive oxygen species generation and toxicity by this neglected mechanism. Toxicol Appl Pharmacol 280: 117–126. [DOI] [PubMed] [Google Scholar]
- Yang LY, Gao JL, Gao T, Dong P, Ma L, Jiang FL, Liu Y.2016a. Toxicity of polyhydroxylated fullerene to mitochondria. J Hazard Mater 301: 119–126. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhong X, Li Q, Zhang X, Wang Y, Yang K, Zhang LW. 2016. Potentiation of drug-induced phospholipidosis in vitro through PEGlyated graphene oxide as the nanocarrier. Toxicol Sci [Epub ahead of print, 2016b]. doi: 10.1093/toxsci/kfw233. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Zheng YF, Quin L. 2011, A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomedicine 7: 975–982. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, Farokhzad OC, Fisher EA. 2015a. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv Health Mater 4: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang H, Xu J. 2015b. Cathepsin S as a cancer target. Neoplasma 62: 16–26. [DOI] [PubMed] [Google Scholar]
- Zhao J, Castranova V. 2011, Toxicology of nanomaterials used in nanomedicine. J Toxicol Environ Health B 14: 593–632. [DOI] [PubMed] [Google Scholar]
- Zhao G, Li Y, Cui L, Li X, Jin Z, Han X, Fang E, Gao Y, Zhou D, Jiang H, Jin X, Piao G, Li X, Yang G, Jin J, Zhu E, Piao M, Piao L, Yuan K, Lei Y, Ding D, Jin C, Nan Y, Cheng X. 2015. Increased circulating cathepsin K in patients with chronic heart failure. PLoS One 10: e0136093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Miao Y, Zhang Y, Liu L, Lin J, Yang JY, Xie Y, Wen L. 2013. Induction of cyto-protective autophagy by paramontroseite VO2 nanocrystals. Nanotechnology 24: 165102. [DOI] [PubMed] [Google Scholar]
- Zhu W, von dem Bussche A, Yi X, Qiu Y, Wang Z, Weston P, Hurt RH, Kane AB, Gao H. 2016. Nanomechanical mechanism for lipid bilayer damage induced by carbon nanotubes confined in intracellular vesicles. Proc Natl Acad Sci 113: 12374–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]


