YAP and TAZ are two transcriptional co-activators regulated by the Hippo pathway kinases (MST1,2 and LATS1,2), cytoskeletal tension, and several other signaling pathways [1–3]. As such, YAP/TAZ are emerging as key regulators of cell growth and tissue homeostasis, both in lower organisms and in mammals [3]. In particular, YAP/TAZ are activated in somatic stem cells to support self-renewal and pluripotency and, when ectopically expressed, can reprogram terminally differentiated cells into a stem cell/progenitor like state [4, 5].
Although a systematic genetic analysis is still missing, there is evidence implicating YAP/TAZ in the regulation of the haematopoietic system, both in physiological and in pathological conditions.
In cord blood-derived human haematopoietic stem cells (HSCs), TEAD1, the obligatory partner of YAP/TAZ, regulates differentiation in early B cells [3]. TAZ, but not YAP1, has been implicated in the lineage choice during naive T cell differentiation, where it functions as a coactivator of RORγt favoring pro-inflammatory TH17 differentiation over the immunosuppressive Treg fate [6]. This may also account for the reported role of the MST kinases (Yap/Taz inhibitors) in limiting autoimmune responses [7, 8].
While in solid tumors, YAP/TAZ are emerging as either potent oncogenes or downstream targets of oncogenic pathways, their role in haematopoietic malignancies appears to be context dependent. In multiple myeloma (MM) and leukemias, YAP seems to exert a tumor suppressive function by regulating the Abl1-dependent DNA damage response, which leads to apoptosis in cancer cells. This explains why deletion or downregulation of YAP/TAZ are frequently observed in MM and leukemias [9]. On the other hand, TEADs have been proposed to reinforce transcriptional activation of oncogenic programs and enhancer reprogramming during B cells transformation [10] and upstream Hippo pathway components are lost in leukemias and lymphomas [11, 12], thus arguing for a pro-oncogenic role of YAP/TAZ in some hematological malignancies.
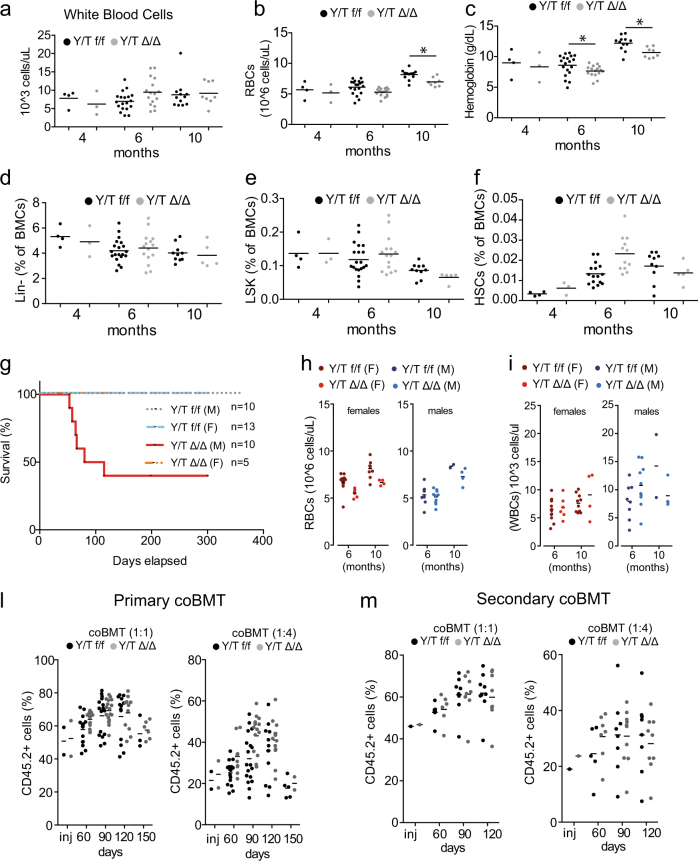
To analyze the role of Yap and Taz in the adult haematopoietic system, we crossed mice double homozygotes for conditional knockout alleles of both Yap and Taz (Yapflox/flox/Tazflox/flox mice) with Mx1-CRE mice (Supplementary Figure 1). Conditional activation of the Mx1-CRE led to an efficient and stable deletion of both alleles, thus ruling out counter selection of Yap/Taz floxed cells upon prolonged haematopoiesis (Supplementary Figure 2a, b). Blood analysis revealed no difference in the number of circulating white blood cells (WBCs) over time (Fig. 1a). Similarly, FACS analyzes of WBCs lineages confirmed the lack of alterations both in circulating and in bone marrow cells (BMCs) (Supplementary Figure 2). Red blood cells (RBCs) were largely unaffected by Yap/Taz loss, with the exception of a mild, decrease in RBCs count and hemoglobin concentration detected starting from 6 months post deletion (Fig. 1b, c). This was not paralleled by a statistically significant decrease in circulating erythroid progenitors (Ter119+ cells, Supplementary Figure 2g), arguing against a general defect in RBCs differentiation.
Fig. 1.
Analysis of peripheral adult haematopoiesis upon Yap/Taz loss. a–c Peripheral blood analysis of Yapflox/flox/Tazflox/flox (Y/T f/f) or YapΔ/Δ/TazΔ/Δ (Y/T Δ/Δ) mice. a WBCs, b RBCs, and c hemoglobin at 4, 6, and 10 months post recombination. d–f FACS analysis of BMCs: d Lineage-negative cells, e LSK progenitors, and f HSCs (Lin-, Kit+, Sca+, CD48−, CD150+, and CD34− cells). g Kaplan–Meier survival analysis (M: males, F: females). h, i Peripheral blood analysis: h RBCs and i WBCs. l, m coBMT experiments: l Chimerism assessed at different time points post-BMT. inj = donor cells mix. m Chimerism of the secondary coBMTs, as in l
FACS analyses revealed no difference in the frequency of early haematopoietic progenitor cells (Lin- and LSK) or in HSCs (Fig. 1d–f). Survival analysis showed a partially penetrant lethality of YapΔ/Δ/TazΔ/Δ males (Fig. 1g). Importantly, these mice did not show any hematological defect (Fig. 2h, i and Supplementary Figure 3), or signs of progressive cachexia or anemia, which are typically associated with pan-cytopenia of the haematopoietic system. While the cause of lethality of YapΔ/Δ/TazΔ/Δ males is still under investigation, it is most probably related to the activity of the Mx1-CRE in the liver.
Fig. 2.
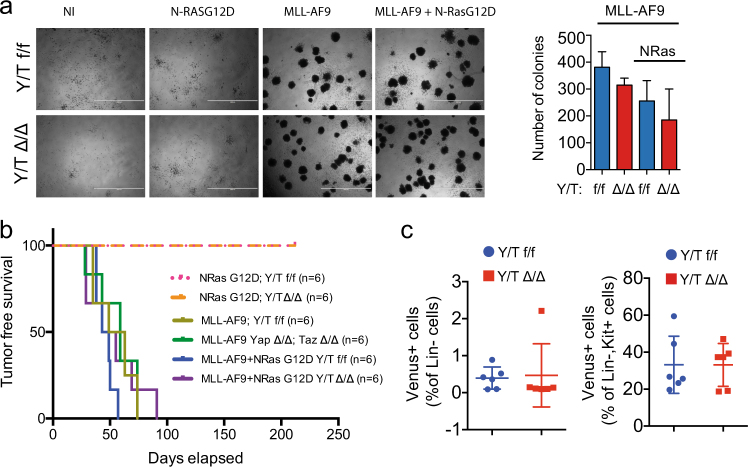
Yap/Taz do not contribute to MLL-AF9-driven leukemia. Modeling of MLL-AF9 and RAS-driven AMLs by viral transduction of haematopoietic progenitors from Yapflox/flox/Tazflox/flox (Y/T f/f) or YapΔ/Δ/TazΔ/Δ (Y/T Δ/Δ) mice. a Colony forming unit (CFU) assay (n = 6). b Kaplan–Meier analysis of mice xenografted (time = 0) as indicated. c Peripheral blood analysis of mice transplanted with HPCs transduced with MLL-AF9 cells (n = 6). Left: leukemic progenitors; right: leukemic stem cells
To test the role of Yap and Taz during enforced haematopoiesis, we performed competitive adoptive transfer experiments of BMCs (coBMT). Cells derived either from YapΔ/Δ/TazΔ/Δ mice or from Yapflox/flox/Tazflox/flox control (CD45.2+) were mixed with wild-type BMCs (CD45.1+) at different ratios (i.e., 1:1 and 1:4) and transplanted in lethally irradiated C57/Bl6 CD45.1 mice. Peripheral blood analysis showed that YapΔ/Δ/TazΔ/Δ and Yapflox/flox/Tazflox/flox BMCs reconstituted lethally irradiated mice equally well (Fig. 1l and Supplementary Figure 4).
Moreover, YapΔ/Δ/TazΔ/Δ BMCs gave rise to differentiated cells of the myeloid and lymphoid lineages with the same efficiency of Yapflox/flox/Tazflox/flox BMCs (Supplementary Figures 4 and 5). Coherently with these results, YapΔ/Δ/TazΔ/Δ and Yapflox/flox/Tazflox/flox BMCs contributed equally well to the stem cell and progenitors compartments, when transplanted in competition (Supplementary Figure 4c). Secondary coBMT experiments confirmed the haematopoietic proficiency of YapΔ/Δ/TazΔ/Δ BMCs (Fig. 1m). Overall, these results suggest that Yap/Taz are dispensable for adult haematopoiesis and HSCs self-renewal. This is in line with similar studies conducted in other somatic tissues [3, 13] and reinforces the notion that YAP/TAZ might be more relevant in proliferative responses elicited by either regenerative or pathological cell growth. Obviously, our observations do not rule out a role for YAP/TAZ in lineage-restricted functions or their relevance in particular settings: an example is provided by the recent report showing how Taz regulates T-cell-mediated inflammatory responses [6]. Along the same line, Yap loss in myeloid cells potentiated the production of IFN-β and mediated immune responses to viral infections [14]. Thus, we believe our observations will be the ground for further investigation focused on more specialized haematopoietic functions.
To directly assess the role of YAP/TAZ in acute myeloid leukemia (AML) development, we took advantage of an established orthotopic mouse model of AML, based on the retroviral transduction of hematopoietic progenitor cells (HPCs) with the MLL-AF9 oncogene, alone or in combination with activated mutant NRAS (N-RASG12D) (Supplementary Figure 6a). As expected, N-RASG12D transduction did not provide any growth advantage in Yapflox/flox/Tazflox/flox cells; similarly no growth advantage was detected in YapΔ/Δ/TazΔ/Δ HPCs transduced with N-RASG12D (Supplementary Figure 6b). MLL-AF9 conferred substantial growth advantage both alone or in combination with N-RASG12D, with similar extent both in Yapflox/flox/Tazflox/flox and in YapΔ/Δ/TazΔ/Δ HPCs (Supplementary Figure 6b).
In line with these results, MLL-AF9 strongly stimulated the development of AML progenitors, with similar efficiency both in Yapflox/flox/Tazflox/flox and in YapΔ/Δ/TazΔ/Δ cells, while no CFU-stimulating activity was detected by N-RASG12D transduction in either control or Yap/Taz-deleted HPCs (Fig. 2a).
N-RASG12D HPCs did not give rise to AML once transplanted, regardless of their genotype, thus suggesting that Yap/Taz are not limiting factors (i.e., tumor suppressive) in RAS-driven transformation of HPCs (Fig. 2b). MLL-AF9 gave rise to AML with 100% penetrance and similar latency in both control and Yap/Taz-deleted HPCs (Fig. 2b). Accordingly, whole body leukemic cells dissemination was unaffected by Yap/Taz deletion (Supplementary Figure 6c, d) and leukemic progenitors and leukemic stem cells frequencies were similar in leukemias derived from either control or Yap/Taz-deleted cells (Fig. 2c). Overall, these results suggest that (i) Yap/Taz are dispensable for cell transformation and dissemination in MLL-AF9-driven leukemia and that (ii) Yap/Taz are not tumor suppressive in either N-RASG12D or MLL-AF9-driven AMLs. This suggests that the proposed gatekeeper function of YAP/TAZ [9] may be only relevant in selective settings, when specific genetic lesions may engage YAP/TAZ-regulated tumor suppressive mechanism(s).
Considering the growing interest in pharmacologically targeting YAP/TAZ to eradicate a variety of solid tumors, Yap/Taz dispensability in haematopoiesis has direct implications on the safety of such approach in terms of unwanted side effects, potentially detrimental for systemic haematopoiesis, which are frequently associated with cancer treatments. The hematological safety is further supported by the observation that deletion of Yap/Taz, despite their role as tumor suppressors in multiple myeloma and leukemias [9], does not predispose to spontaneous blood malignancies. The partial lethality of YapΔ/Δ/TazΔ/Δ males, which is of non-hematological origin, is a cautionary note, which should be considered when Yap/Taz inhibitors will be evaluated in preclinical and clinical studies.
Electronic supplementary material
Acknowledgements
We thank Prof. S. Piccolo, Dr. L. Azzolin, and Dr. J. Zuber for kindly sharing reagents. This work was supported by grants from the Italian Health Ministry (RF-2011-02346976), and the Italian Association for Cancer Research-AIRC to BA (2015-16768) and to SC (IG grant 13135).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41375-018-0111-3) contains supplementary material, which is available to authorized users.
References
- 1.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 2.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–14. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 3.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panciera T, Azzolin L, Fujimura A, Di Biagio D, Frasson C, Bresolin S, et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–37. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–38. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18:800–12. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
- 7.Du X, Shi H, Li J, Dong Y, Liang J, Ye J, et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J Immunol. 2014;192:1525–35. doi: 10.4049/jimmunol.1301060. [DOI] [PubMed] [Google Scholar]
- 8.Salojin KV, Hamman BD, Chang WC, Jhaver KG, Al-Shami A, Crisostomo J, et al. Genetic deletion of Mst1 alters T cell function and protects against autoimmunity. PLoS ONE. 2014;9:e98151. doi: 10.1371/journal.pone.0098151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Zhang Z, Kashiwagi M, Yoshida T, Joshi I, Jena N, et al. Superenhancer reprogramming drives a B-cell-epithelial transition and high-risk leukemia. Genes Dev. 2016;30:1971–90. doi: 10.1101/gad.283762.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TS, Lee DH, Kim SK, Shin SY, Seo EJ, Lim DS. Mammalian sterile 20-like kinase 1 suppresses lymphoma development by promoting faithful chromosome segregation. Cancer Res. 2012;72:5386–95. doi: 10.1158/0008-5472.CAN-11-3956. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann EM, Campo E, Wright G, Lenz G, Salaverria I, Jares P, et al. Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood. 2010;116:953–61. doi: 10.1182/blood-2010-01-263806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–70. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Xie F, Chu F, Zhang Z, Yang B, Dai T, et al. YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKvarepsilon-mediated phosphorylation. Nat Immunol. 2017;18:733–43. doi: 10.1038/ni.3744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.