Abstract
In semi-arid protected areas, artificial waterholes ensure that water is locally available to animals for extended periods. However, artificial waterholes may limit animal movement, which contributes towards habitat deterioration. Challenges of artificial water provisioning worsen in the presence of ecosystem engineers like African elephants Loxodonta africana, capable of transforming environments. Camera traps were used to monitor elephant visitation at 21 artificial waterholes in the Kruger National Park, South Africa. We also assessed if water quality parameters influenced elephant preference for certain waterholes. There were no significant correlations between elephant abundance and water physicochemical properties. However, there was a strong negative correlation between elephant abundance and levels of Escherichia coli in water. Our findings suggest that elephants avoid drinking water with high levels of faecal microbial loads. Whereas most studies addressing animal management in protected areas consider waterholes as homogeneous units, we posit that water quality could also determine local landscape use and movement patterns of key species like elephants, a finding with relevant implications in reserve management practices.
Keywords: camera trap, elephant abundance, E. coli, protected areas, water quality
1. Introduction
Surface water availability is a fundamental constraint of herbivore distribution in semi-arid savannah regions [1,2]. Artificial water provisioning has become a common practice for managing herbivore populations, especially in water-scarce protected areas, aiming to increase populations while limiting their spatial dispersion [3,4]. Yet, this practice is controversial, because its perceived benefits are counteracted by an increase in inter- and intraspecific conflicts [2,5], and by exacerbated piosphere effects as a result of increased animal densities around waterholes [2,3]. The effects of artificial water provisioning are more evident in the presence of high African elephant (Loxodonta africana) numbers, because elephants can modify landscapes and displace other species [6].
At broad scales, elephants prefer landscapes with high levels of heterogeneity, which provide good foraging opportunities [2,6]. However, at finer scales, water availability becomes the most important driver of elephant abundance [7]. A perpetual water supply provided by artificial waterholes may discourage seasonal elephant movements [8,9], that in turn promotes landscape homogenization [3]. This decreases the patchiness of the savannah, making the landscape unsuitable for both elephants and the ecosystem at large [2].
Water quality varies spatially across landscapes and can influence animal distribution; however, despite the numerous studies that link water supply and elephants as drivers of landscape homogenization [6], few studies address whether differences between individual waterholes would influence elephant abundance. At Hwange National Park (Zimbabwe), elephants preferred waterholes with high levels of sodium [10], although this pattern seemed to weaken at higher elephant densities [11]. Some studies even suggest that the annual migration of herbivores in the Serengeti may be triggered by declining water quality [12]. Therefore, water physicochemical differences among waterholes could be important in determining elephant distribution and landscape use. Furthermore, faecal microbial load in water could be an additional factor affecting habitat selection [13].
The objective of this study was to examine the possible water characteristics that drive elephant waterhole preference in the Kruger National Park (KNP). We hypothesized that physicochemical water traits and microbial contaminants would influence elephant abundance at waterholes. Elucidating the factors that affect waterhole preference is paramount to our knowledge of managing elephants in protected areas.
2. Material and methods
(a). Study site
The KNP is located in the north-eastern Lowveld region of South Africa (S 24°0′41″, E 31°29′7″). The climate is subtropical, with wet hot summers and dry mild winters. We sampled 21 artificial water points at four different regions (figure 1) during the dry season (July 2016), amidst one of the worst droughts on record [14]. We selected sampling locations to ensure an even latitudinal distribution within the KNP. Selected waterholes were more than 3 km away from other permanent water sources, which is the average distance that elephants normally travel per day to drink [15]. Artificial waterholes are fed from boreholes using solar panel-powered pumps. Water was either channelled directly into ground level pans or first pumped into 2.5 m tall open-top concrete reservoirs that replenish a nearby pan (figure 1). As adult elephants can drink from pans and reservoirs, we sampled both types of waterholes at the same location, to also test for differences in water quality between waterhole types (which otherwise were located in the same area, i.e. 50 m apart). We sampled eight pairs of reservoir + pan and included another five standalone pans to test for broader scale effects of water quality.
Figure 1.
(a) Map of KNP showing the location of the 21 sampling sites, at four regions. Black dot, pan + reservoir. White dot, pan only. Inset: Location of KNP in southern Africa. (b) Elephant drinking from a reservoir. (c) Example of a tandem of pan (foreground) and concrete reservoir (background).
(b). Elephant abundance
The average daily number of elephants visiting each waterhole was calculated for 5 days using pictures taken with camera traps (Bushnell Nature view HD, 12 megapixels). We assumed that animals drank once per day at a waterhole and recorded each animal visitation as a once-off daily occurrence. Only adult elephants (greater than 2 m in height and with developed tusks), i.e. those able to drink from the reservoirs (‘adults’, henceforth), were counted. Absolute elephant abundance around waterholes was not determined at the time of the study due to logistical constraints. However, we used the previous year's (June 2015) georeferenced elephant census data [16] to determine elephant abundance within a 3 km radius around the sampled waterholes.
(c). Water analyses
Water pH, conductivity and total dissolved solids was measured directly from the waterholes. Additional samples were collected on day 1 and analysed in the laboratory to measure concentrations of calcium, chloride, magnesium and total water hardness (CaCO3), as well as water turbidity (NTU), chemical oxygen demand (COD) and the amount of Escherichia coli (CFU 100 ml−1).
(d). Statistical analyses
All variables [17] were log-transformed to normalize value distributions. Linear mixed models were used to test for the effect of water quality on animal abundance. We first performed a Pearson correlation test to determine and discard variable pairs with a Pearson r correlation value higher than 0.5. Accordingly, the explanatory variables included were local elephant abundance within a radius of 3 km, water hardness, COD, NTU and CFU, as well as interactions between CFU and the two other metrics of microbial contamination. ‘Region’ and ‘sampling site’ (nested within ‘region’) were considered as random factors to account for non-independence of pans and reservoirs at the same location, and for differences among regions. Akaike's information criterion (AIC) values were used to rank all potential candidate models, and we used Akaike weights to assess the relative importance of each explanatory variable. Full details of methods and analyses are provided in the electronic supplementary material.
3. Results
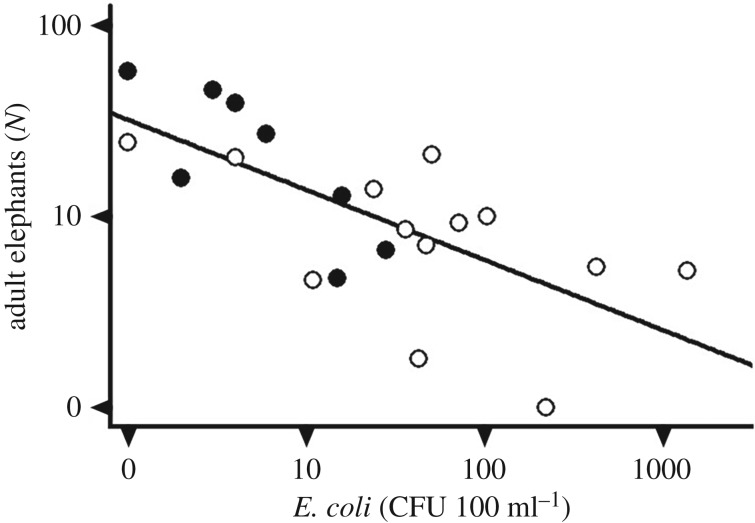
A total of 1421 drinking occurrences were recorded. At four pans and one reservoir, we only obtained complete datasets for 4 days because elephants displaced the cameras. Nevertheless, the average number of elephants visiting the waterholes per day was fairly constant among all sampling points (coefficient of variation = 7.8%). There were significantly more adults drinking from the reservoirs (mean ± s.d. = 25.61 ± 18.31, n = 39) compared to pans (mean ± s.d. = 10.76 ± 7.17, n = 38) across the eight paired sampling points (F1,14 = 14.13, p < 0.001). All the most likely models explaining how elephant abundance was related to water parameters (ΔAIC < 7 from best model) included CFU, either alone or in combination with one of the other variables, and no interactions (table 1). Therefore, count differences in adults drinking from waterholes were primarily related to differences in bacterial loads (F1,19 = 7.94, p = 0.01, all sampling points combined; figure 2). CFU accounted for approximately 57% of variance in adult numbers among the eight pairs of pans and reservoirs, whereas uncontrolled variation among pairs of waterholes accounted for an additional 20% (table 1; 50% and 20%, respectively, considering all 21 sampling sites; electronic supplementary material, table S1).
Table 1.
Set of best-ranked models, according to AIC values, examining variations in adult elephant numbers drinking from waterholes in the KNP. Each line of the table represents a model that includes the variables that have estimated values. The estimates of the effect of each variable are averaged across the five models with AIC values differing less than seven units from the best model. The relative importance of each variable is calculated according to Akaike weights (across all possible 52 models). The results of this table correspond to the eight pairs of reservoir + pan; the results of the models including all 21 waterholes are in electronic supplementary material, table S1. CFU, bacteria abundance; COD, chemical oxygen demand; NTU, water turbidity.
| (intercept) | CFU | COD | NTU | elephant abundance | water hardness | CFU × COD | CFU × NTU | d.f. | AIC | ΔAIC | weight | R2 | R2 conditional | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.653 | −0.525 | 4 | 23.198 | — | 0.710 | 0.572 | 0.771 | |||||||
| 1.559 | −0.630 | 0.201 | 5 | 27.444 | 4.246 | 0.085 | 0.673 | 0.779 | ||||||
| 1.392 | −0.518 | 0.182 | 5 | 28.108 | 4.910 | 0.061 | 0.589 | 0.784 | ||||||
| 1.718 | −0.526 | −0.027 | 5 | 28.627 | 5.430 | 0.047 | 0.546 | 0.781 | ||||||
| 1.730 | −0.514 | −0.058 | 5 | 28.651 | 5.453 | 0.046 | 0.556 | 0.764 | ||||||
| averaged estimates (s.e.) | 1.635 (±0.237) | −0.534 (±0.103) | −0.003 (±0.052) | 0.018 (±0.069) | 0.012 (±0.069) | −0.001 (±0.054) | ||||||||
| variable importance | 0.981 | 0.062 | 0.107 | 0.073 | 0.058 | 0.003 | 0.01 |
Figure 2.
Average number of adult elephants visiting a waterhole per day as a function of E. coli abundance in water. Axes are in logarithmic scale. Black dots, reservoirs. White dots, pans. CFU, colony forming units.
4. Discussion
Elephant visitation was unevenly distributed between pans and reservoirs, indicating preference for certain waterholes. Our results strongly suggest that this preference is related to water quality, as indicated by the response to E. coli concentrations, i.e. contamination by faecal microbes. Given that the study took place during a severe drought in the region [14], this further suggests that microbial contaminants are a significant determinant of waterhole preference for elephants even when water options are limited [18].
Previous studies reported lower levels of bacterial loads in water filtered from wells dug by elephants compared to nearby open water sources [13,19], but to our knowledge, this study is the first to suggest that elephants are able to discern water quality among waterholes, and that given the chance, they select cleaner water. Most elephants drank from the reservoir at sites that had both a pan and a reservoir, even if drinking from the reservoir appeared to be ergonomically costly (figure 1). Adults even poured water from the reservoir to the ground for calves that were unable to reach the reservoir water (M Ndlovu 2016, personal communication). The lower bacterial loads that reservoirs have compared to pans is probably due to the fact that other animal species also drank from the pans, some even bathed and defaecated in the water (M Ndlovu 2016, personal communication), whereas only adult elephants (and giraffes Giraffa camelopardalis, on occasion) were able to access the reservoir water. Elephants are known to avoid certain waterholes and dig for alternate water sources when water quality is poor [13,19]; hence, it is not surprising that in this study, elephants displayed a preference for waterholes with low microbial contaminants.
This differential preference for certain waterholes according to water quality has implications for the management of elephants and artificial water provisioning in protected areas [20]. Two-thirds of the artificial waterholes in the KNP have been closed in the last few decades, and additional removals are imminent [21]. Therefore, manipulating the function of artificial waterholes in order to modify elephant movement and distribution should also consider elephant preferences. Changes in the design of waterhole pans, i.e. slightly raised edges with narrow troughs, to prevent animals from entering, bathing or defaecating in the water, could also improve water quality and in turn substantially affect elephant water preference. Finally, assessing water quality and elephant preference at several waterholes and across greater spatial–temporal scales, as well as to extend the analyses to other species, could produce a more detailed understanding of the patterns observed in this study.
Supplementary Material
Acknowledgements
We are grateful to SANParks and OTS for logistical support rendered during this study. SANParks provided the elephant census data. The comments of five anonymous reviewers greatly improved this manuscript.
Ethics
The University of the Free State granted Research and Animal ethics approval for this study (Ref: UFS-AED2016/0006). Permits for research in KNP were issued by the South African National Parks (Ref: NDLM1329).
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fq92325 [17].
Authors' contributions
M.N. conceived, designed and executed the study, and analysed data. A.P.-R., E.D., M.T., A.C. and L.M. contributed to experimental design, data collection and analysis. All authors contributed to the preparation and revision of this manuscript, gave final approval for publication and agree to be accountable for the content.
Competing interests
We declare we have no competing interests.
Funding
The study was funded through the NRF incentive funding for rated researchers awarded to M.N.
References
- 1.Gaylard A, Owen-Smith N, Redfern J. 2003. Surface water availability: implications for heterogeneity and ecosystem processes. In The Kruger experience: ecology and management of savanna heterogeneity (eds du Toit JT, Biggs H, Rodgers K), pp. 171–188. Washington, DC: Island Press. [Google Scholar]
- 2.Smit IPJ, Grant CC, Devereux BJ. 2007. Do artificial waterholes influence the way herbivores use the landscape? Herbivore distribution patterns around rivers and artificial surface water sources in a large African savanna park. Biol. Conserv. 136, 85–99. ( 10.1016/j.biocon.2006.11.009) [DOI] [Google Scholar]
- 3.Shannon G, Matthews WS, Page BR, Parker GE, Smith RJ. 2009. The effects of artificial water availability on large herbivore ranging patterns in savanna habitats: a new approach based on modelling elephant path distributions. Divers. Distrib. 15, 776–783. ( 10.1111/j.1472-4642.2009.00581.x) [DOI] [Google Scholar]
- 4.Smit IPJ, Grant CC. 2009. Managing surface-water in a large semi-arid savanna park: effects on grazer distribution patterns. J. Nat. Conserv. 17, 61–71. ( 10.1016/j.jnc.2009.01.001) [DOI] [Google Scholar]
- 5.Hayward MW, Hayward MD. 2012. Waterhole use by African fauna. S. Afr. J. Wildl. Res. 42, 117–127. ( 10.3957/056.042.0209) [DOI] [Google Scholar]
- 6.Guldemond RAR, Purdon A, van Aarde RJ. 2017. A systematic review of elephant impact across Africa. PLoS ONE 12, e0178935 ( 10.1371/journal.pone.0178935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Knegt HJ, et al. 2011. The spatial scaling of habitat selection by African elephants. J. Anim. Ecol. 80, 270–281. ( 10.1111/j.1365-2656.2010.01764.x) [DOI] [PubMed] [Google Scholar]
- 8.Loarie SR, van Aarde RJ, Pimm SL. 2009. Fences and artificial water affect African savannah elephant movement patterns. Biol. Conserv. 142, 3086–3098. ( 10.1016/j.biocon.2009.08.008) [DOI] [Google Scholar]
- 9.Purdon A, van Aarde RJ. 2017. Water provisioning in Kruger National Park alters elephant spatial utilisation patterns. J. Arid. Environ. 141, 45–51. ( 10.1016/j.jaridenv.2017.01.014) [DOI] [Google Scholar]
- 10.Weir JS. 1972. Spatial distribution of elephants in an African National Park in relation to environmental sodium. Oikos 23, 1–13. ( 10.2307/3543921) [DOI] [Google Scholar]
- 11.Chamaillé-Jammes S, Fritz H, Holdo RM. 2007. Spatial relationship between elephant and sodium concentration of water disappears as density increases in Hwange National Park, Zimbabwe. J. Trop. Ecol. 23, 725–728. ( 10.1017/S0266467407004531) [DOI] [Google Scholar]
- 12.Gereta E, Wolanski E. 1998. Wildlife-water quality interactions in the Serengeti National Park, Tanzania. Afr. J. Ecol. 36, 1–14. ( 10.1046/j.1365-2028.1998.102-89102.x) [DOI] [Google Scholar]
- 13.Stommel C, Hofer H, Grobbel M, East ML. 2016. Large mammals in Ruaha National Park, Tanzania, dig for water when water stops flowing and water bacterial load increases. Mamm. Biol. 81, 21–30. ( 10.1016/j.mambio.2015.08.005) [DOI] [Google Scholar]
- 14.Bureau for Food and Agricultural Policy (BFAP). 2016. Policy Brief on the 2015/2016 drought See www.bfap.co.za.
- 15.Thomas B, Holland JD, Minot EO. 2012. Seasonal home ranges of elephants (Loxodonta africana) and their movements between Sabi Sand Reserve and Kruger National Park. Afr. J. Ecol. 50, 131–139. ( 10.1111/j.1365-2028.2011.01300.x) [DOI] [Google Scholar]
- 16.Ferreira SM, Greaver C, Simms C. 2017. Elephant population growth in Kruger National Park, South Africa, under a landscape management approach. Koedoe 59, a1427 ( 10.4102/koedoe.v59i1.1427) [DOI] [Google Scholar]
- 17.Ndlovu M, Pérez-Rodríguez A, Devereux E, Thomas M, Colina A, Molaba L. 2018. Data from: Water for African elephants (Loxodonta africana): faecal microbial loads affect use of artificial waterholes Dryad Digital Repository. ( 10.5061/dryad.fq92325) [DOI] [PMC free article] [PubMed]
- 18.Thrash I, Theron GK, Bothma J. 1993. Herbivore dung deposit counts around drinking troughs in Kruger National Park. Koedoe 36, 87–93. ( 10.4102/koedoe.v36i1.365) [DOI] [Google Scholar]
- 19.Ramey EM, Ramey RR, Brown LM, Kelley ST. 2013. Desert-dwelling African elephants (Loxodonta africana) in Namibia dig wells to purify drinking water. Pachyderm 53, 66–72. [Google Scholar]
- 20.Smit IPJ, Grant CC, Whyte IJ. 2007. Elephants and water provision: what are the management links? Divers. Distrib. 13, 666–669. ( 10.1111/j.1472-4642.2007.00403.x) [DOI] [Google Scholar]
- 21.Sutherland K, Ndlovu M, Pérez-Rodríguez A. 2018. Use of artificial waterholes by animals in the southern region of the Kruger National Park. South Africa. Afr. J. Wildl. Res. 48, 1–4. ( 10.3957/056.048.023003) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ndlovu M, Pérez-Rodríguez A, Devereux E, Thomas M, Colina A, Molaba L. 2018. Data from: Water for African elephants (Loxodonta africana): faecal microbial loads affect use of artificial waterholes Dryad Digital Repository. ( 10.5061/dryad.fq92325) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.fq92325 [17].