Abstract
Mate competition provides the opportunity for sexual selection which often acts strongly on males, but also the opportunity for sexual conflict that can alter natural selection on females. Recent attention has focused on the potential of sexual conflict to weaken selection on females if male sexual attention, and hence harm, is disproportionately directed towards high- over low-quality females, thereby reducing the fitness difference between these females. However, sexual conflict could instead strengthen selection on females if low-quality females are more sensitive to male harm than high-quality females, thereby magnifying fitness differences between them. We quantify the effects of male exposure on low- versus high-quality females in Drosophila melanogaster in each of two environments (‘simple’ and ‘complex’) that are known to alter behavioural interactions. We show that the effects of male harm are greater for low- compared to high-quality females in the complex but not the simple environment, consistent with mate competition strengthening selection on females in the former but not in the latter environment.
Keywords: mate competition, natural selection, sexual conflict, sexual selection
1. Introduction
Mate competition generates strong and persistent sexual selection on males that underlies the evolution of sexual displays and weapons [1]. However, the opportunity for mate competition also permits interlocus sexual conflict that can favour traits in males that enhance reproductive success relative to other males but which sometimes harm females as a by-product [2–4]. Much attention has been given to the prevalence of interlocus sexual conflict [5] and, recently, to its potential to weaken natural selection on females. Long et al. [6] recognized that a ‘cost of attractiveness' can arise if males preferentially direct their sexual attention towards intrinsically higher quality (e.g. more fecund) females. The increased male harm these females experience can reduce or even eliminate their intrinsic advantage over otherwise lower quality females that attract less harm, and the population-level consequence is a weakening of natural selection on females. Recent experiments have implicated this in hampering adaptation and purging [7–10].
While sexual conflict can weaken natural selection through females under some conditions, there may be other scenarios under which sexual conflict could strengthen selection on females and hence promote adaptation and purging. The effect of male harm on selection depends on two factors [6]: (i) the extent to which males preferentially direct their efforts towards high-quality females; and (ii) the extent to which high- versus low-quality females are affected by male harassment. Though more attention has been given to the former, the latter may also be important, particularly when males are less able to bias their sexual attention. If low-quality females are more sensitive to the harmful effects of males than are high-quality females, then differences in relative fitness among females could be magnified by male harassment, thereby strengthening natural selection. To gain insight into this possibility, we manipulate the opportunity for male harm (low versus high exposure to males) and quantify the effects on the fitness of low- and high-quality females. If high-quality females are more resistant to male harm, their fitness should be less affected by increased exposure to harmful males compared to that of low-quality females.
We quantify the effects of male harm on low- and high-quality D. melanogaster females when mate competition occurs in two environments that differ in size and structural complexity: the ‘simple’ mating environment is a standard high-density fly vial, whereas the ‘complex’ mating environment is a larger (approx. 1.65 l) cage that includes multiple food patches and additional structural features. From previous work, we know that in the complex relative to the simple environment, sexual interactions are less frequent and less biased towards high-quality females, and male harm is reduced [10]. The opportunity for males to bias their harassment resulted in a weakening of selection on females in the simple environment (consistent with other studies [6]), but had the opposite effect in the complex environment (i.e. strengthening selection). The previous study made no attempt to assess differences in sensitivity to male-induced harm of low- versus high-quality females. Here, we examine, in each environment, how the fitness of low- versus high-quality females is affected by differential exposure to males.
2. Material and methods
The flies came from a population of D. melanogaster taken from generation 55 of an evolution experiment testing the effects of mate competition on adaptation to novel larval environments. This is the same population used in the male harm study reported in [10]. Its life cycle includes a 6-day mating phase followed by 24 h of egg-laying. Information concerning its origin and maintenance are in [9,10].
We tested the effects of female quality and mating environment on male-induced harm using a three-way factorial design. Low- versus high-quality females were obtained via a density manipulation and we quantified the survival and subsequent fecundity of these females under low versus high exposure to males in either a simple or a complex mating environment during their normal 6-day mating phase. The experiment was performed in two fully balanced blocks. Low- and high-quality females were obtained by collecting adults from vials in which approximately 400 or approximately 100 eggs, respectively, had been added using a pipetting technique [11]. The simple environment was a standard Drosophila culture vial (25 × 95 mm) containing 10 ml of cornmeal–yeast medium with abundant live yeast sprinkled on top, while the complex environment was a 1.65 l Ziploc® container that had pipe cleaners protruding from the lid and which held five cups of cornmeal–yeast medium with abundant live yeast on top (two 1 oz plastic cups and three 3 oz waxed paper cups, the latter having plastic dividers in the surface of the food). We refer to these as ‘simple’ and ‘complex’ environments for simplicity, noting that they also differ in volume and the availability of food and egg-laying sites. These are the same mating environments used in [9,10,12] (see [10,12] for pictures).
Flies were collected from the low- and high-density rearing vials using light CO2 (males were collected from the low density vials only) and then stored separately by sex (35 flies/vial) for 3 days prior to use, matching the life cycle of the population. Thirty replicates were created for each of the six treatment combinations (i.e. two male exposures × two female qualities × two mating environments). During the 6-day mating phase, for a given low-exposure replicate, 35 females were held together with 35 males in a vial with fresh medium for 5 h on days 1, 4 and 6; males were absent for the rest of the time and females were kept in their appropriate environment (i.e. vial or cage). Between exposure bouts, males were stored in vials with fresh medium. In high-exposure replicates, the 35 females from a given replicate experienced the same three bouts of 5 h exposure to 35 males in a vial with fresh medium on days 1, 4 and 6, but outside of these periods, they were held with 35 males in their appropriate environment. This ensured that all females were handled in the same way and received the same CO2 exposure. Immediately after the third male exposure, females were scored for survival and then 10 randomly chosen survivors from each replicate were placed as pairs in five vials with 10 ml fresh medium to lay eggs for 24 h, after which they were discarded. The number of adult offspring emerging from these vials was subsequently counted.
Female fitness was calculated as the product of survival in a given replicate and average number of adults produced by the 10 females. Variation in fitness was analysed using a general linear model with female quality, mating environment and male exposure as fixed effects, along with the two- and three-way interactions among these. Block was also included as a fixed effect given only two levels. The three-way interaction was significant so the effects of male exposure and female quality were subsequently tested separately by environment via two-way versions of the above model (i.e. dropping the environment term and related interactions). Given a significant male exposure × female quality interaction in one environment, a post hoc comparison of the effect of male exposure was performed separately for low- and high-quality females in this environment, using a Bonferroni correction for two comparisons.
3. Results
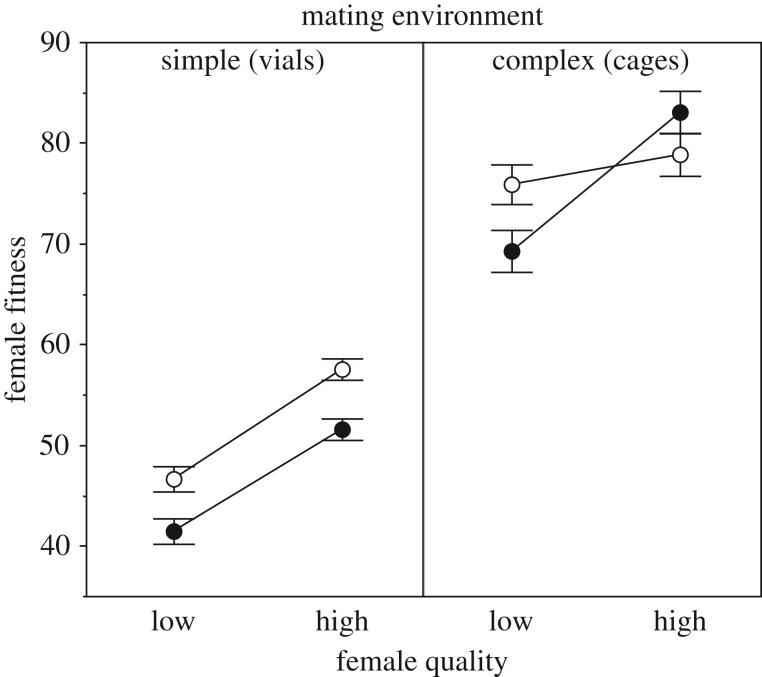
The fitness of high-quality females was greater than that of low-quality females overall (figure 1), confirming that our density manipulation worked (‘female quality’ effect: F1,229 = 63.53, p < 0.0001). Increased exposure to males was also harmful overall (‘exposure’ effect: F1,229 = 8.20, p = 0.0046). However, the effect of male exposure also varied by female quality and mating environment, generating a significant three-way interaction (F1,229 = 5.99, p = 0.0151). When tested separately by environment, the effect of male exposure did not differ between low- versus high-quality females in the simple environment (exposure × quality interaction, table 1), with increased male exposure reducing female fitness by 11.1% and 10.4%, respectively. However, in the complex environment, the effect of male exposure differed significantly between low- and high-quality females (figure 1 and table 1) such that the fitness of low-quality females decreased significantly with greater male exposure (t57 = −2.31, Padjusted = 0.049; 8.6% decrease), while the fitness of high-quality females was not affected (t58 = 1.40, Padjusted = 0.334; 5.3% increase).
Figure 1.
Mean (±1s.e.) fitness of low- and high-quality females under low (open circles) and high (filled circles) exposure to males in a simple or complex environment.
Table 1.
Results of a general linear model, fit separately by environment, testing effects on female fitness.
| mating environment | term | F1,114 | p-value |
|---|---|---|---|
| simple (vials) | male exposure | 22.76 | <0.0001 |
| female quality | 80.78 | <0.0001 | |
| male exposure × female quality | 0.11 | 0.7401 | |
| block | 0.01 | 0.9089 | |
| complex (cages) | male exposure | 0.33 | 0.5670 |
| female quality | 16.51 | <0.0001 | |
| male exposure × female quality | 7.02 | 0.0092 | |
| block | 3.28 | 0.0730 |
4. Discussion
Differential sensitivity to male-induced harm among females can have important consequences for selection and hence the net effect of mate competition. We tested for differential sensitivity of low- and high-quality females by manipulating exposure to males in different mating environments. In the simple environment, increased male exposure reduced female fitness, demonstrating male harm consistent with numerous previous studies in this species [2,10,13,14]. The magnitude of this harm, however, did not differ between high- and low-quality females. By contrast, in the complex environment, increased exposure to males reduced the fitness of low- but not high-quality females.
High-quality females may be less sensitive to male-induced harm because they are physiologically more tolerant of damage resulting from sexual interactions, and/or better able to repair such damage. They may also be better at hiding, escaping and/or resisting males (possibly due to their larger size), exposing them to less harm. Determining the relative contribution of these mechanisms would require further study. The lack of an effect of female quality on resistance to harm in the simple environment is consistent with behavioural avoidance mechanisms that are less effective in the small, high-density vials. Nevertheless, it is also possible that an advantage of high- over low-quality females (physiological and/or behavioural) was also present in vials but was offset by males exerting more harassment effort in replicates with high-quality females.
Using the same population, a previous study showed that sexual interactions were more frequent and more biased towards high-quality females in the simple relative to the complex environment, generating a cost of sexual attractiveness for females in the former but not the latter environment [10]. Our current results indicate that low-quality females are more strongly affected by male exposure than are high-quality females in the complex environment but not the simple one. Taken together, the results of these two studies point towards males weakening selection on females in the simple environment and strengthening it in the complex environment. This notion is supported by evolution experiments showing that the effect of mate competition on adaptation and purging is more beneficial in a complex environment [9,12,15]. From our current and other recent results [6,10], it is apparent that the opportunity for mate competition can shape selection in a variety of ways and on both sexes, and that the environment in which mating occurs may be an important factor mediating this.
Acknowledgements
We thank P. Chen for help in the laboratory.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9v017mr [16].
Authors' contributions
H.D.R. and A.F.A. conceived the project and A.M., L.Y. and T.S.B. conducted the experiment. A.M., L.Y. and H.D.R. analysed the data and A.M. and H.D.R wrote the paper with input from the others. All authors agree to be held accountable for the contents of this work and approve the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
Funding was provided by NSERC Canada.
References
- 1.Andersson M. 1994. Sexual selection, 624 p Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Rice WR. 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234. ( 10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- 3.Holland B, Rice WR. 1998. Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 5.Arnqvist G, Rowe L. 2005. Sexual conflict, p. 330 Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Long TA, Pischedda A, Stewart AD, Rice WR. 2009. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 7, e1000254 ( 10.1371/journal.pbio.1000254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbuthnott D, Rundle HD. 2012. Sexual selection is ineffectual or inhibits the purging of deleterious mutations in Drosophila melanogaster. Evolution 66, 2127–2137. ( 10.1111/j.1558-5646.2012.01584.x) [DOI] [PubMed] [Google Scholar]
- 8.Chenoweth SF, Appleton NC, Allen SL, Rundle HD. 2015. Genomic evidence that sexual selection impedes adaptation to a novel environment. Curr. Biol. 25, 1860–1866. ( 10.1016/j.cub.2015.05.034) [DOI] [PubMed] [Google Scholar]
- 9.Yun L, Chen PJ, Kwok KE, Angell CS, Rundle HD, Agrawal AF. 2018. Competition for mates and the improvement of nonsexual fitness. Proc. Natl Acad. Sci. USA 115, 6762–6767. ( 10.1073/pnas.1805435115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun L, Chen PJ, Singh A, Agrawal AF, Rundle HD. 2017. The physical environment mediates male harm and its effect on selection in females. Proc. R. Soc. B 284, 20170424 ( 10.1098/rspb.2017.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun L, Agrawal AF. 2014. Variation in the strength of inbreeding depression across environments: effects of stress and density dependence. Evolution 68, 3599–3606. ( 10.1111/evo.12527) [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Agrawal AF, Rundle HD. 2017. Environmental complexity and the purging of deleterious alleles. Evolution 71, 2714–2720. ( 10.1111/evo.13334) [DOI] [PubMed] [Google Scholar]
- 13.Fowler K, Partridge L. 1989. A cost of mating in female fruit-flies. Nature 338, 760–761. ( 10.1038/338760a0) [DOI] [Google Scholar]
- 14.Edward DA, Fricke C, Gerrard DT, Chapman T. 2011. Quantifying the life-history response to increased male exposure in female Drosophila melanogaster. Evolution 65, 564–573. ( 10.1111/j.1558-5646.2010.01151.x) [DOI] [PubMed] [Google Scholar]
- 15.Colpitts J, Williscroft D, Sekhon HS, Rundle HD. 2017. The purging of deleterious mutations in simple and complex mating environments. Biol. Lett. 13, 20170518 ( 10.1098/rsbl.2017.0518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacPherson A, Yun L, Barrera TS, Agrawal AF, Rundle HD. 2018. Data from: The effects of male harm vary with female quality and environmental complexity in Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.9v017mr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- MacPherson A, Yun L, Barrera TS, Agrawal AF, Rundle HD. 2018. Data from: The effects of male harm vary with female quality and environmental complexity in Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.9v017mr) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9v017mr [16].