Abstract
All Lepidoptera produce two sperm types: normal, nucleated ‘eupyrene’ sperm and anucleate ‘apyrene’ sperm. One hypothesis for the evolution of apyrene sperm suggests that they act to reduce female remating rate. Apyrene sperm require less resources to produce than do eupyrene sperm, and could delay remating by females by acting as a ‘cheap filler’, packing the spermatheca and thereby reducing receptivity. This would reduce the risk of sperm competition, giving a potential adaptive advantage to the male producing these sperm. This leads to the prediction that the probability of a female remating should correlate with the number of stored apyrene sperm, which has previously been supported by experiments using the green-veined white butterfly, Pieris napi. We repeated this experiment using the Indian meal moth, Plodia interpunctella. We find that in this species, eupyrene, not apyrene sperm number is the best predictor of female remating probability, indicating that the ‘cheap filler’ hypothesis for the function of apyrene sperm is not well supported in Pl. interpunctella.
Keywords: sperm competition, sperm heteromorphy, apyrene sperm, female receptiveness, Lepidoptera
1. Introduction
Many invertebrates produce more than one type of sperm, most notably the Lepidoptera, all of which produce anucleate ‘apyrene’ sperm as well as normal, nucleated ‘eupyrene’ sperm [1–3]. Apyrene sperm are incapable of fertilization but nonetheless often make up a large proportion of the entire ejaculate. They are unlikely simply to be aberrant forms of ‘normal’ sperm because they have distinct structure and developmental timing [4] and they are highly active, migrating from the spermatophore within the bursa copulatrix to the spermatheca (the female's sperm storage organ) before the eupyrene sperm [2]. Many explanations for the function of apyrene sperm have been proposed [1], including providing resources to the eupyrene sperm or to the female, and assisting in the movement of eupyrene sperm. The best supported explanations for the evolution of these sperm, however, focus on the idea that they act to increase a male's fertilization success by decreasing the risk of sperm competition [5]. This could result from them enhancing the removal or displacement of competitor sperm already within the spermatheca, or by their acting as a ‘cheap filler’, packing the female's spermatheca with low-cost sperm and reducing the probability of her remating.
Evidence to date mostly supports the cheap filler hypothesis [1,2], although the hypothesis has been tested directly in only one species to date: Cook & Wedell [6] examined the prediction that the amount of apyrene sperm present in a female's spermatheca should covary with her receptivity to remating. Once-mated females of the green-veined white butterfly, Pieris napi, were given the opportunity to remate. Directly after remating, or immediately after the end of the allotted time if remating did not occur, the sperm numbers in the spermatheca were counted: because it takes some time after mating for newly inseminated sperm to migrate to the spermatheca, this gives a measure of sperm numbers remaining from the original mating. There was no difference in the numbers of eupyrene sperm present in the spermathecae of females who chose to remate and those who did not, but the females who were receptive to remating had considerably fewer apyrene sperm present than unreceptive females. Further work on this species has confirmed the importance of apyrene sperm number in delaying female remating [7,8].
Here, we report a similar experiment to that reported by Cook & Wedell [6] but carried out using Plodia interpunctella, a polyandrous pyralid moth which is an important laboratory model for evolutionary biologists [9–14]. Previously reported patterns of apyrene sperm allocation in Pl. interpunctella both support and contradict the ‘cheap filler’ hypothesis. Remating probability in female Pl. interpunctella mated with previously mated males was negatively correlated with the number of apyrene, but not eupyrene sperm that a male produced on his first mating [12], and Pl. interpunctella males evolving under increased intensity of sperm competition had peak allocation of apyrene sperm in their first ejaculate, potentially caused by strong selection on males to protect their investment [13]. These indirectly support the cheap filler hypothesis, but it has also been shown that male Pl. interpunctella adjust the numbers of eupyrene, but not apyrene sperm in an ejaculate on the basis of a female's mating history, delivering more eupyrene sperm to females who have previously received a large ejaculate [9]: the opposite pattern to that predicted if apyrene sperm functioned to reduce sperm competition. The picture regarding the function of apyrene sperm in this system is thus confused, and we report the findings of a direct test of the hypothesis that stored apyrene sperm should reduce female remating probability.
2. Methods
Full details of the methods are given in the electronic supplementary material, which also has a description of the data analysis. The males used in the initial matings were from a series of selection lines which were set up to examine the effect of strong or weak sexual selection in Pl. interpunctella [14]. We found no indication of an effect of selection line (see full analysis in the electronic supplementary material), and for clarity, we report our results here without the variables associated with the selection background for these males.
Final instar caterpillars were isolated by sex to ensure virginity. Within 24 h of eclosion, males were placed with a female and matings allowed to occur for an hour. Following mating, the male was frozen for later measurement. After 48 h, giving the female time to process the spermatophore, the females were given the opportunity to mate with a recently eclosed virgin male using the same mating protocol as the first mating assay. Upon termination of copulation, or after 1 h if mating did not occur, the male and female were immediately frozen, meaning that sperm migration from the new spermatophore to the spermatheca was not allowed to occur. Wing length of both male and female animals was measured as a proxy for body size, the spermatheca was dissected out of the female moths and the sperm of both types counted using a modification of the protocol in [9].
The relationship between the amount of stored sperm and the probability of remating was examined by fitting generalized linear models to the data with whether the female remated coded as a binary response variable.
3. Results
In total, we were able to acquire sperm counts from 54 females (two were excluded because there was no sperm present in the spermatheca), of whom 21 remated and 33 did not. The mean number of eupyrene sperm present was 215 (σ = 255) and the mean number of apyrene sperm was 1198 (σ = 1510). Nine individuals had apyrene sperm present but no detectable eupyrene sperm.
The numbers of eupyrene and apyrene sperm that were present in the spermatheca 48 h after mating were correlated (figure 1: correlation analysis of log + 1-transformed numbers: r = 0.550, t = 4.75, d.f. = 52, p < 0.001), with high numbers of eupyrene sperm being associated with high numbers of apyrene sperm. Neither the size of the male originally mated with the female nor female size predicted the numbers of either eupyrene or apyrene sperm remaining in the spermatheca (all tests partial F-tests comparing models with and without the explanatory variable in question. Eupyrene sperm numbers: male size F1,51 = 0.138, p = 0.711, female size F1,52 = 1.204, p = 0.278. Apyrene sperm numbers: male size F1,52 = 1.30, p = 0.259, female size F1,51 = 0.087, p = 0.769).
Figure 1.
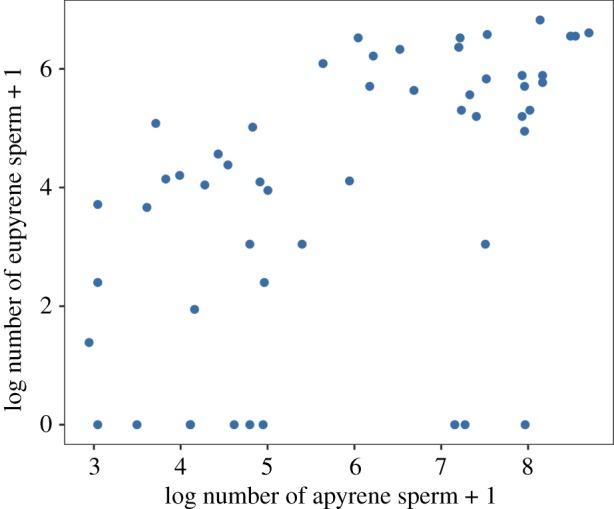
Correlation between the number of apyrene and the number of eupyrene sperm present in the spermatheca of each female. (Online version in colour.)
The number of eupyrene sperm present in the spermatheca was the only explanatory variable retained in the minimal adequate model for remating probability (likelihood ratio (LR) test, χ12 = 9.00, p = 0.0027). Females with low numbers of eupyrene sperm were much more likely to remate (figure 2). The number of apyrene sperm was not a significant predictor of remating probability (LR test, χ12 = 0.79, p = 0.37).
Figure 2.
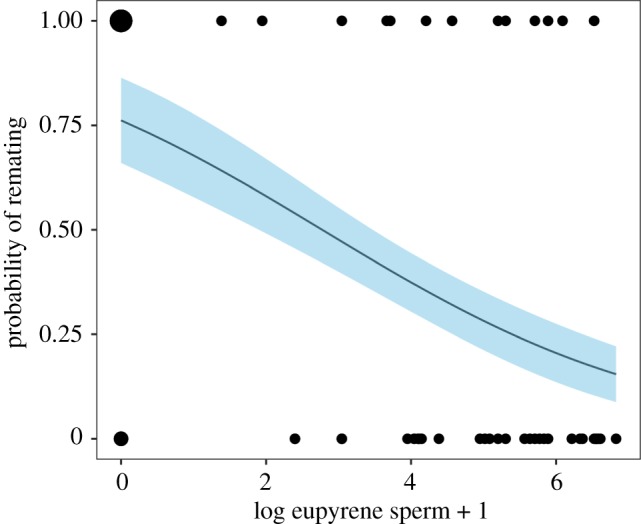
The probability of remating declines with increasing numbers of eupyrene sperm in the spermatheca. The line shows the probability of remating as predicted by the fitted model, and the shaded area represents±1 standard error for the predicted probability. Points represent individual females from the experiment. The larger plot symbols represent eight individuals who did remate and had no eupyrene sperm present and three who did not and had no eupyrene sperm. (Online version in colour.)
The strong correlation between the number of eupyrene and apyrene sperm suggests that multi-collinearity could be a problem with the model fit. The variance inflation factor for these two variables is 1.43, which is well below the value of 10 which is sometimes quoted as indicating serious issues in this respect [15]. Nonetheless, we refitted a further model for remating with the number of eupyrene sperm removed as an explanatory variable. In this case, the main effect of the number of apyrene sperm was retained as the only significant predictor in the minimal adequate model (LR test, χ21 = 4.33, p = 0.0373), although the effect of apyrene sperm in this case is considerably weaker than that for eupyrene sperm.
4. Discussion
These results contrast with those previously reported for Pi. napi [6], where the number of apyrene sperm strongly predicted the likelihood of female remating providing support for the ‘cheap filler’ hypothesis. In Pl. interpunctella, however, we find that it is the number of eupyrene sperm, rather than apyrene sperm, which are most associated with remating probability. The number of apyrene sperm is associated with remating probability, but this correlation is far weaker and might well only arise because of the correlation between eupyrene and apyrene sperm numbers found in this case. This finding appears to be at odds with some previous work on the roles of eupyrene and apyrene sperm in Pl. interpunctella. In particular, the finding that the number of apyrene sperm in the first ejaculate produced by males of this species was negatively related to remating probability of females who were subsequently mated to them [12] suggests that apyrene sperm should be important in determining female receptiveness to remating. There are a number of possible explanations for this discrepancy. One might lie in the somewhat indirect nature of the test used in [12], which relies on the assumption that subsequent ejaculates can be predicted from the first, and also on the assumption that numbers of apyrene sperm in the ejaculate correspond with the numbers of apyrene sperm which are stored in the spermatheca. It is also notable that the timing varied between these two experiments: remating rate in the present study was measured 2 days after the original mating, but 1 day afterwards in [12]. If apyrene sperm are only effective in reducing remating rate for a short time in Pl. interpunctella, this could explain the difference in results.
The cheap filler hypothesis has been regarded as the best explanation for the evolution and maintenance of sperm dimorphism in the Lepidoptera for some years [1,2,16]. This has recently been challenged following the observation that males of the monandrous butterfly species Byasa alcinous produce a high proportion of apyrene sperm, and that these sperm are transferred in the ejaculate and stored in the spermatheca, as happens in polyandrous species [16]. The data presented here also challenge this idea, suggesting that in some polyandrous species, apyrene sperm are not so closely associated with remating. We suggest that a better knowledge of how apyrene sperm numbers vary between species that vary in their degree of polyandry would be a valuable next step in understanding the evolutionary factors behind sperm heteromorphy.
Supplementary Material
Acknowledgements
We thank A. Laughton and J. Westcoatman for access to the original stock population. We are grateful to Zen Lewis and two anonymous reviewers for constructive and helpful comments which have significantly improved this paper.
Data accessibility
R code for the full analysis is available in the online supplementary material. Data are available from the Dryad digital repository at: http://dx.doi.org/10.5061/dryad.sb6sd80 [17].
Authors' contributions
R.J.K. and J.M.P. conceived the experiment, J.M.P. and D.-M.J.T. carried out the laboratory work. D.-M.J.T. and R.J.K. carried out further analysis, all contributed to writing the manuscript. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
J.M.P. was supported by a QMUL studentship.
References
- 1.Swallow JG, Wilkinson GS. 2002. The long and short of sperm polymorphisms in insects. Biol. Rev. 77, 153–182. ( 10.1017/S1464793101005851) [DOI] [PubMed] [Google Scholar]
- 2.Wedell N. 2005. Female receptivity in butterflies and moths. J. Exp. Biol. 208, 3433–3440. ( 10.1242/jeb.01774) [DOI] [PubMed] [Google Scholar]
- 3.Gage MJG. 1994. Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc. R. Soc. Lond. B 258, 247–254. ( 10.1098/rspb.1994.0169) [DOI] [Google Scholar]
- 4.Friedländer M. 1997. Control of the eupyrene–apyrene sperm dimorphism in Lepidoptera. J. Insect Physiol. 43, 1085–1092. ( 10.1016/S0022-1910(97)00044-9) [DOI] [PubMed] [Google Scholar]
- 5.Silberglied RE, Shepherd JG, Dickinson JL. 1984. Eunuchs: the role of apyrene sperm in Lepidoptera? Am. Nat. 123, 255–265. ( 10.1086/284200) [DOI] [Google Scholar]
- 6.Cook PA, Wedell N. 1999. Non-fertile sperm delay female remating. Nature 397, 486. [Google Scholar]
- 7.Wedell N. 2001. Female remating in butterflies: interaction between female genotype and nonfertile sperm. J. Evol. Biol. 14, 746–754. ( 10.1046/j.1420-9101.2001.00327.x) [DOI] [Google Scholar]
- 8.Wedell N, Wiklund C, Bergström J. 2009. Coevolution of non-fertile sperm and female receptivity in a butterfly. Biol. Lett. 5, 678–681. ( 10.1098/rsbl.2009.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook P, Gage M. 1995. Effects of risks of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera, Pyralidae). Behav. Ecol. Sociobiol. 36, 261–268. ( 10.1007/BF00165835) [DOI] [Google Scholar]
- 10.Gage M, Cook P. 1994. Sperm size or numbers: effects of nutritional stress upon eupyrene and apyrene sperm production strategies in the moth Plodia interpunctella (Lepidoptera, Pyralidae). Funct. Ecol. 8, 594–599. ( 10.2307/2389920) [DOI] [Google Scholar]
- 11.Lewis Z, Wedell N, Hunt J. 2011. Evidence for strong intralocus sexual conflict in the Indian meal moth, Plodia interpunctella. Evolution 65, 2085–2097. ( 10.1111/j.1558-5646.2011.01267.x) [DOI] [PubMed] [Google Scholar]
- 12.Lewis Z, Lizé A, Wedell N. 2013. The interplay between different stages of reproduction in males of the moth Plodia interpunctella. Anim. Behav. 86, 917–922. ( 10.1016/j.anbehav.2013.08.006) [DOI] [Google Scholar]
- 13.Ingleby FC, Lewis Z, Wedell N. 2010. Level of sperm competition promotes evolution of male ejaculate allocation patterns in a moth. Anim. Behav. 80, 37–43. ( 10.1016/j.anbehav.2010.03.022) [DOI] [Google Scholar]
- 14.Parrett JM, Knell RJ. 2018. The effect of sexual selection on adaptation and extinction under increasing temperatures. Proc. R. Soc. B 285, 20180303 ( 10.1098/rspb.2018.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Konagaya T, Mutoh N, Suzuki M, Rutowski RL, Watanabe M. 2015. Estimates of female lifetime fecundity and changes in the number and types of sperm stored with age and time since mating in the monandrous swallowtail butterfly, Battus philenor (Lepidoptera: Papilionidae) in the Arizona desert. Appl. Entomol. Zool. 50, 311–316. ( 10.1007/s13355-015-0336-9) [DOI] [Google Scholar]
- 17.Thorburn D-MJ, Knell RJ, Parrett JM. 2018. Data from: Sperm morph and remating frequency in the Indian meal moth, Plodia interpunctella Dryad Digital Repository. ( 10.5061/dryad.sb6sd80) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Thorburn D-MJ, Knell RJ, Parrett JM. 2018. Data from: Sperm morph and remating frequency in the Indian meal moth, Plodia interpunctella Dryad Digital Repository. ( 10.5061/dryad.sb6sd80) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
R code for the full analysis is available in the online supplementary material. Data are available from the Dryad digital repository at: http://dx.doi.org/10.5061/dryad.sb6sd80 [17].


