Cytarabine and anthracyclines represent the core therapeutic drugs for acute myeloid leukemia (AML). Despite the paucity of therapeutic innovations, large population-based registries have demonstrated incremental survival improvements over the decades, especially among younger patients1,2, potentially attributable to intensified chemotherapy, improved supportive care, or improved risk stratification and selection for allogeneic stem cell transplantation (SCT). However, it is difficult to ascertain the relative contribution of each to improvements in patient outcome.
The Australasian Leukemia and Lymphoma Group (ALLG) has conducted a series of randomized clinical trials in adult AML, leading to the stepwise incorporation of etoposide3 and high-dose cytarabine intensification in the induction phase4. Subsequently, both AMLM7 (1995–2000)5 and AMLM12 (2003–2013)6 trials, spanning an 18-year treatment period, used an identical induction protocol (ICE: idarubicin 9 mg/m2 days 1–3; cytarabine 3 g/m2 twice a day on days 1, 3, 5, 7; etoposide 75 mg/m2 days 1–7) and shared a common consolidation control arm (IcE: idarubicin days 1–2; cytarabine 100 mg/m2 days 1–5; etoposide days 1–5 × 2 cycles) as part of a 1:1 randomization with an investigational regimen in the post-remission phase. In AMLM7, the investigational arm included a second round of ICE, shown to be non-superior to standard IcE5. In AMLM12, anthracycline intensification incorporating an extra day of idarubicin in each of the two consolidation cycles was explored and shown to significantly improve leukemia-free survival6.
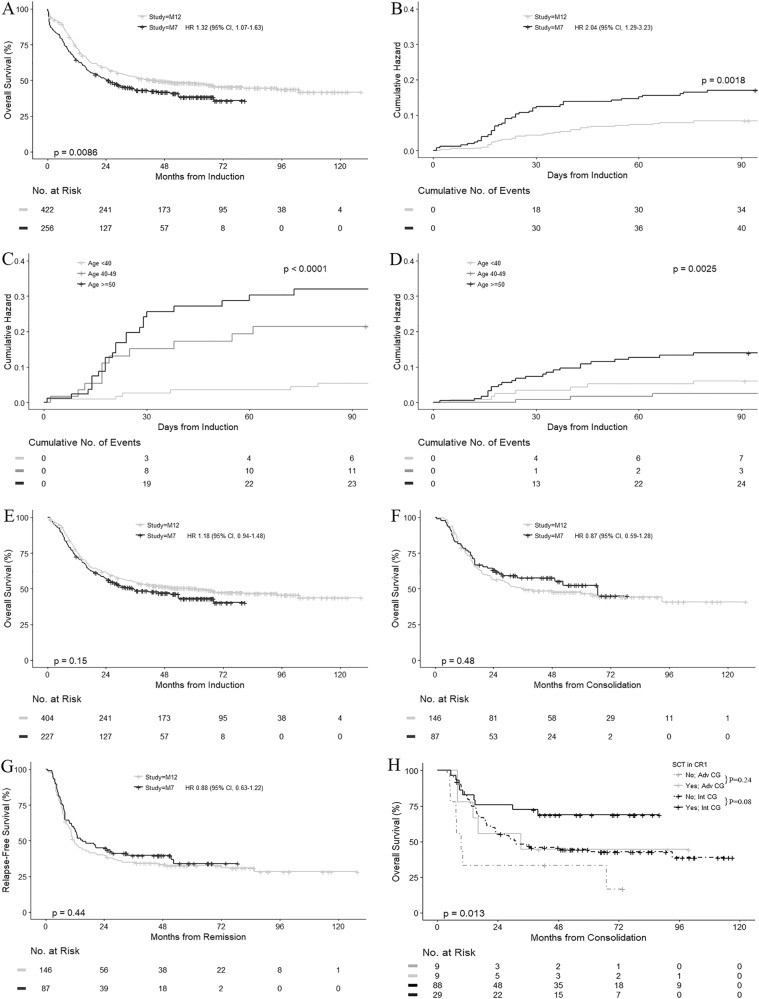
The overall survival (OS) in AMLM12 was superior to AMLM7 (median 44.3 vs. 24.8 months, p = 0.009) (Fig. 1a). To explore reasons for the differences in OS, we compared between each study era: (1) early induction outcomes following the identical ICE induction regimen; (2) post-remission outcomes in patients receiving the common standard IcE consolidation arm; and (3) survival following disease relapse.
Fig. 1.
Overall survival, relapse-free survival and cumulative hazard curves. a Overall survival comparing the AMLM7 and AMLM12 study cohorts. b Cumulative hazard of early deaths in the AMLM7 and AMLM12 study cohorts. c Cumulative hazard of early deaths in the AMLM7 cohort stratified by age groups. d Cumulative hazard of early deaths in the AMLM12 cohort stratified by age groups. e Overall survival by landmark analysis at 30 days in the AMLM7 and AMLM12 study cohorts. f Overall survival of patients receiving the common IcE consolidation chemotherapy. g Relapse-free survival in patients receiving the common IcE consolidation chemotherapy. h Overall survival in the AMLM12 study cohort receiving standard IcE consolidation chemotherapy, restricted to patients with >90 days of remission duration, stratified by allogeneic SCT in first remission and cytogenetic risk. Pairwise comparisons using log-rank test, with p-values adjusted by Benamini & Hochberg (BH) method
Cytogenetic risk was classified in both studies in accordance with the revised MRC classification7. Patients with favorable-risk karyotype were excluded from the AMLM7 cohort as this subgroup was excluded in AMLM12. OS was calculated from the start of treatment and relapse-free survival from the date of first remission (CR1). Kaplan–Meier survival curves were compared using log-rank statistics, hazard ratios by Cox proportional hazard model, and univariate/multivariate analyses by logistic regression model. All tests were two-sided and considered significant where p < 0.05. R statistical software version 3.4.4 (R foundation for statistical computing, Vienna, Austria) was used.
Table 1 summarizes patient and treatment characteristics. The AMLM7 cohort was younger (median 43 vs. 48 years, p < 0.001), less commonly received granulocyte colony-stimulating factor (G-CSF), less commonly received allogeneic SCT and more commonly received autologous SCT. Complete remission (CR) rates (75% in AMLM7 and 76% in AMLM12) were similar after the first cycle of ICE. Median follow-up duration was 51.5 and 71.5 months for survivors in AMLM7 and AMLM12, respectively.
Table 1.
Patient characteristics and outcomes in the entire AMLM7 and AMLM12 cohorts
| AMLM7 (n = 256) | AMLM12 (n = 422) | p-valuea | |
|---|---|---|---|
| Age, median years (IQR) | 43.4 (31.6–51.6) | 48.1 (38.4–55.2) | <0.001 |
| Male gender (%) | 55.1 | 57.1 | 0.6 |
| ECOG PS > 0 (%) | 50.0 | 54.9 | 0.2 |
| Cytogenetics, n (%) | 0.2 | ||
| Intermediate | 201 (78.5) | 338 (80.1) | |
| Adverse | 39 (15.2) | 70 (16.6) | |
| Unknown | 16 (6.3) | 14 (3.3) | |
| WCC (x 10 9 /L), median (IQR) | 10.6 (3.3–43.5) | 10.0 (2.9–33.7) | 0.3 |
| >40 × 109/L (%) | 26.2 | 21.6 | 0.2 |
| >100 × 109/L (%) | 10.5 | 6.6 | 0.082 |
| Febrile (%) | 30.1 | 29.1 | 0.8 |
| Bleeding (%) | 22.3 | 12.8 | 0.002 |
| DIC (%) | 4.7 | 3.1 | 0.3 |
| G-CSF (%) | 80.1 | 96.7 | <.001 |
| >1 induction cycle, n (%) | 19 (7.4) | 41 (9.7) | 0.3 |
| CR—after 1 cycle (%) | 75.4 | 76.3 | 0.8 |
| CR—total (%) | 79.7 | 82.5 | 0.4 |
| Randomized to standard IcE, n (%) | 87 (34.0) | 146 (34.6) | 0.9 |
| Received 2 cycles of standard IcE | 81 (93.1) | 125 (85.6) | 0.7 |
| Early mortality, n (%) | |||
| 30-day mortality | 30 (11.7) | 18 (4.3) | <0.001 |
| 60-day mortality | 36 (14.1) | 30 (7.1) | 0.005 |
| 90-day mortality | 40 (15.6) | 34 (8.1) | 0.003 |
| Allogeneic SCT, n (%) | 76 (29.7) | 215 (51.3) | <0.001 |
| Transplant in first CR | 32 (12.5) | 120 (28.4) | <0.001 |
| Transplant other status | 44 (17.2) | 95 (22.5) | 0.12 |
| Autologous SCT, n (%) | 25 (9.8) | 7 (1.7) | <0.001 |
aFisher’s exact test for categorical variables; Mann-Whitney U tests for continuous variables
CR complete remission, DIC disseminated intravascular coagulation, ECOG PS Eastern Cooperative Oncology Group performance status, G-CSF granulocyte colony stimulating factor, IQR interquartile range, SCT stem cell transplantation, WCC white cell counts
We initially focused on early induction outcomes among 678 patients receiving the ICE induction protocol (n = 256 in AMLM7 and n = 422 in AMLM12). Early deaths (at 30 days) were more common in AMLM7 (11.7%) than AMLM12 (4.3%) (p = < 0.001) (Fig. 1b), and strongly linked to increasing age: with higher mortality occurring in patients >40 years in AMLM7 (Fig. 1c) but only >50 years in AMLM12 (Fig. 1d). Although OS appeared improved in the AMLM12 study (Supplementary Figure S1A), this difference in OS was attenuated if OS was compared using a 30-day landmark analysis (Fig. 1e), confirming the initial divergence of the survival curves seen in Fig. 1a. Higher 30-day mortality (odds ratio 3.46, 95% confidence interval, 1.83–6.69) was observed in the AMLM7 vs. the AMLM12 study eras (Supplementary Table S1). In addition to these inter-study differences, there also appeared a trend for reduced intra-study early deaths in the later half of the AMLM12 recruiting period (2007–2010), compared to the first half (2003–2006) (Supplementary Figure S1A-B).
The ICE induction protocol was identical except for the first 44 patients in the AMLM7 study who received idarubicin 12 mg/m2. Thirty-day mortality was not higher in this initial subgroup (4/44; 9.1%). The remaining patients in AMLM7 and all patients in AMLM12 received the identical induction and consolidation regimen in the control arm. Hence, the reduction in early deaths in AMLM12 was not related to differences in chemotherapy intensity, and was likely attributable to improved supportive care practices in the AMLM12 study. Aspects of supportive care were explored where data were available (Supplementary Table S2).
Antifungal prophylaxis was documented in 75/87 (86%) patients in AMLM7, all using fluconazole/itraconazole, and in 397/414 (96%) patients in AMLM12, using fluconazole/itraconazole (71%) or posaconazole/voriconazole (38%). Many patients received >1 antifungal agent at different time points. AMLM12 patients who received mold-active antifungal prophylaxis had a trend for reduced incidence of documented fungal infections (4.7 vs. 10.2%, p = 0.06), similar 30-day mortality (3.4 vs. 3.8%) and potentially lower 90-day mortality (4.0 vs. 9.1%, p = 0.07), consistent with previously demonstrated benefits of posaconazole over fluconazole or itraconazole8. Strict definitions of possible/probable/definite invasive fungal infections were not available in the database and so could not be compared. The availability of mold-active antifungal agent in the later era may have contributed to the improved outcomes observed for patients recruited to the second half of the AMLM12 study era (Supplementary Figure S1C).
Although the use of G-CSF (80% in AMLM7, 97% in AMLM12) and palifermin (0 vs. 18%) were more frequent in the later AMLM12 cohort, published randomized trials did not demonstrate significant impact of either agent on induction deaths9,10. Around one-third of patients received prophylactic fluoroquinolones in both cohorts, with similar fever days and documented infections. Transfusion support was also similar for both red blood cells and platelets. The leading causes of death for both cohorts, where documented, were infection (59% in AMLM7 and 39% in AMLM12) and multiorgan failure (28% in AMLM7 and 50% in M12).
After examining early induction outcomes, we next compared post-remission outcomes in 233 patients (n = 87 in AMLM7 and n = 146 in AMLM12) randomized to the IcE consolidation control arm common to both studies. Despite older age and more patients with ECOG > 0 in the AMLM12 cohort (Supplementary Table S3), there was no significant difference in either OS (median 36.4 months in AMLM12 vs. 66.7 months in AMLM7, p = 0.48) (Fig. 1f) or relapse-free survival (median 11.8 months vs. 15.1 months, p = 0.44) (Fig. 1g). Although significantly more patients underwent CR1 allogeneic SCT in AMLM12 (28 vs. 10%), long-term post-remission survival outcomes were identical, despite matching for baseline characteristics, censoring for SCT, or treating SCT as a competing risk (Supplementary Figures S2–S3). Allogeneic SCT in CR1, however, was associated with improved survival in patients with intermediate (n = 117), but not adverse cytogenetic risk (n = 18) in the AMLM12 cohort (Fig. 1h). In AMLM7, SCT frequency was too low to enable meaningful interpretation of its impact (Supplementary Figure S4).
Among the entire AMLM7 and AMLM12 cohorts, 114 patients and 207 patients, respectively, experienced disease relapse associated with limited OS (median 6.5 months vs. 7.9 months) (Supplementary Figure S5A). Data on salvage therapy and outcome were not available, but more patients in AMLM12 (47%) than AMLM7 (38%) underwent subsequent allogeneic SCT (p = 0.10), where survival was significantly improved, compared to patients not transplanted (Supplementary Figure S5B).
The goal of high-dose cytarabine-based induction is the rapid achievement of high-quality CR from the initial chemotherapy and reduce the likelihood of re-induction therapy, sparing patients the risk of additional complications. ICE results in a very high first-cycle CR rate (~76% excluding favorable-risk AML), compared to 59 and 71% after 1 and 2 cycles of 7 + 3 (90 mg/m2 daunorubicin)11. Our analysis of randomized clinical trials from two different eras demonstrates that ICE induction has become more tolerable over time, especially in those <50 years, likely from multi-faceted improvements in supportive care. Interestingly, a volume effect was evident in the AMLM12 (but not in the AMLM7 study), where patients in the top five recruiting centers had better survival outcome (Supplementary Figure S6); similar effects have been observed by others12. Early deaths from ICE induction in AMLM12 were 2.1 and 7.0% in patients <50 and ≥50 years. This compares favorably to other AML studies conducted in a similar era: 5.5% induction deaths in an ECOG study (2002–2008)11, and 5.5 and 10.1% in patients <46 and ≥46 years in the EORTC-GIMEMA AML-12 study (1999–2008)13.
Although a crude comparison between the AMLM12 and AMLM7 studies indicated a significant improvement in OS, potentially attributable to chemotherapy intensification in CR1, our analyses demonstrate that a substantial component of this benefit may also be linked to non-chemotherapy related factors. By examining deaths occurring during the induction and consolidation phases separately, we find that the major effect on survival was related to reduced treatment-related mortality during the induction phase of AMLM12 associated with improvements in supportive care practices, although the precise factors could not be fully determined. In the post-remission setting, there was no major difference in relapse-free or OS, despite the increased incidence of allogeneic SCT in the more recent AMLM12 study. In conclusion, these findings demonstrate the complexities in making interpretations between identical treatment regimens delivered in sequential eras and highlights the importance of ensuring major practice changing decisions are based on prospectively conducted randomized controlled trials.
Electronic supplementary material
Acknowledgements
Contributors to the Australasian Leukemia & Lymphoma Group AMLM12 trial - Royal Melbourne Hospital: J. Szer, A. Grigg, A. Roberts; Westmead Hospital: W. Benson, M. Hertzberg, J. Koutts, K. Bradstock, A. Johnston, C. Turtle, I. Kerridge, F. Kwok; Calvary Mater Newcastle: A. Enno, P. Rowlings, S. Deveridge, M. Seldon, A. Enjeti, M Walsh, M. Seldon; Princess Alexandra Hospital: P. Marlton, A. Mills, D. Gill, P. Mollee, P. Wood, M. Gandhi, H. Middleton, S. Mapp, S. Hanlon, R. Bird, A. Henden, J. Seymour, M. Wolf, M. Prince, H. Januszewicz, D. Ritchie, W. Rasheed, S. Harrison; Box Hill Hospital: A. Schwarer, A. Wei, J. McKendrick; Royal Adelaide Hospital: I. Lewis, P. Bardy, P. Giri, N. Horvath, C-H. Hui, N. Patton, F. Szabo, B. To; Canberra Hospital: A. McDonald, I. Prosser, J. D’rozario, M. Pidcock, P. Crispin, D. Tallaulikar; Royal North Shore Hospital, C. Arthur, C. Ward, L. Coyle, K. Fay, N. Mackinley, M. Greenwood, S. Mulligan, W. Stevenson; Sir Charles Gairdner Hospital: G. Cull, D. Joske, D. Auguston, S. Ward, P. Crawford; Geelong Hospital: P. Campbell, R. Bell, R. McLennan; Alfred Hospital, A. Wei, A. Spencer, S. Avery, S. Patil; Fremantle Hospital: A. McQuillan, M. Leahy, F. Cordingley, J. Cooney, T. Calogero; Queen Elizabeth Hospital, U. Hahn, W. Jaksic, M. Kee, P. Bardy, S. Mcrae; Royal Perth Hospital, P. Cannell, B. Carnley, J. Cooney; Gosford Hospital: C. Tiley, M. Dean, B. Wylie; Royal Hobart Hospital: R. Harrup, R. Kimber, J. Daley, R. Lowenthal; St Vincent’s Hospital Sydney, K. Fay, S. Milliken, J. Moore, D. Yeung, T. Dodds; Woollongong Hospital: K. Cartwright, P. Presgrave, P. Warburton; Concord Hospital, J. Trottman, L. Y. Kwan; Nepean Hospital: J. Taper, S. Fuller; Mater Brisbane Hospital: L. Catley, M. Ashish; Townsville Hospital: I. Irving. Contributors to the Australasian Leukemia & Lymphoma Group AMLM7 trial—Westmead Hospital: J. Koutts, W. Benson, K. Bradstock, D. Gottlieb, M. Hertzberg, P. Castaldi; Royal Prince Alfred Hospital: D. Joshua, H. Kronenberg, J. Gibson, G. Young, H. Iland; Wesley Medical Center: P. Eliadis, J. Bashford, I. Bunce, S. Fanning, J. Morton; Calvary Mater Newcastle, S. Deveridge, A. Enno, M. Seldon, A. Spencer, I. Kerridge; Royal Brisbane Hospital, S. Durrant, A. Gillett, A. Nicol, I. Bunce; Princess Alexandra Hospital: D. Gill, P. Marlton, G. Cull; Liverpool Hospital: D. Rosenfeld, P. Motum, J. Gallo, I. Dunlop; Monash Medical Center: J. Catalano, E. Gan; P. Cannell, R. Herrmann, R. Baker; St George Hospital: T. Brighton, L. Y. Kwan; Royal North Shore Hospital: C. Arthur, D. Ma, K. Fay, R. McKinley, C. Ward; Alfred Hospital: A. Schwarer, P. Elliott, A. Spencer; Mater Brisbane Hospital: K. Taylor, S. Wright; Royal Melbourne Hospital, J. Szer, A. Grigg, P. Bardy, A. Roberts; Fremantle Hospital: F. Cordingley, M. Leahy, M. Webb; Royal Hobart Hospital: R. Lowenthal, R. Kimber, D. Jupe; Royal Adelaide Hospital: N. Horvath, J. Ho, T. Hughes, J. Dart; Peter MacCallum Cancer Center: H. Januszewicz, M. Wolf; Sir Charles Gairdner Hospital: S. Rule, D. Joske; Canberra Hospital: I. Prosser, M. Webb, I. Pidcock; Medical Oncology Clinic of Rosebank, Johannesburg, South Africa: B. Rapoport; Pretoria Academic Hospital, Pretoria, South Africa: C. Falkson; Austin Hospital: C. Smith; Repatriation Hospital Concord: I. Cunningham; Prince of Wales Hospital: R. Lindeman, P. Rowlings; Queen Elizabeth Hospital: J. Norman. Hematology Society of Australia and New Zealand Young Investigator Award for IST.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41408-018-0121-4).
References
- 1.Derolf A. R., et al. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood 2009, 113, 3666–3672. [DOI] [PubMed]
- 2.Pulte D, Gondos A, Brenner H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica. 2008;93:594. doi: 10.3324/haematol.12304. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JF, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group. Blood. 1990;75:27–32. [PubMed] [Google Scholar]
- 4.Bishop JF, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- 5.Bradstock KF, et al. A randomized trial of high-versus conventional-dose cytarabine in consolidation chemotherapy for adult de novo acute myeloid leukemia in first remission after induction therapy containing high-dose cytarabine. Blood. 2005;105:481–488. doi: 10.1182/blood-2004-01-0326. [DOI] [PubMed] [Google Scholar]
- 6.Bradstock KF, et al. Idarubicin dose escalation during consolidation therapy for adult acute myeloid leukemia. J. Clin. Oncol. 2017;35:1678–1685. doi: 10.1200/JCO.2016.70.6374. [DOI] [PubMed] [Google Scholar]
- 7.Grimwade D, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 9.Bradstock K, et al. Effects of glycosylated recombinant human granulocyte colony-stimulating factor after high-dose cytarabine-based induction chemotherapy for adult acute myeloid leukaemia. Leukemia. 2001;15:1331–1338. doi: 10.1038/sj.leu.2402218. [DOI] [PubMed] [Google Scholar]
- 10.Bradstock KF, et al. A randomized trial of prophylactic palifermin on gastrointestinal toxicity after intensive induction therapy for acute myeloid leukaemia. Br. J. Haematol. 2014;167:618–625. doi: 10.1111/bjh.13086. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez HF, et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri S, et al. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood. 2015;125:3359–3360. doi: 10.1182/blood-2015-01-625764. [DOI] [PubMed] [Google Scholar]
- 13.Willemze R, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J. Clin. Oncol. 2014;32:219–228. doi: 10.1200/JCO.2013.51.8571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.