Abstract
Accumulating evidence points at similarities between substance use disorders (SUD) and gambling disorder on the behavioral and neural level. In SUD, attenuation of striatal D2/3-receptor availability is a consistent finding, at least for stimulating substances. For gambling disorder, no clear association with striatal D2/3-receptor availability has been unveiled so far. With its presumably negligible dopaminergic toxicity, possible differences in receptor availability in gambling disorder might constitute a vulnerability marker. Spontaneous eye blink rate (sEBR) is discussed as a potential proxy measure for striatal dopamine D2/3-receptor availability. Here we examined sEBR in 21 male problem gamblers and 20 healthy control participants. In addition, participants completed a screening questionnaire for overall psychopathology and self-reported measures of alcohol and nicotine consumption. We found no significant difference in sEBR between gamblers and controls. However, in gamblers, sEBR was negatively associated with gambling severity and positively associated with psychopathology. A final exploratory analysis revealed that healthy controls with low sEBR displayed higher alcohol and nicotine consumption than healthy participants with high sEBR. Although the exact association between dopamine transmission and sEBR is still debated, our findings reveal that sEBR is sensitive to inter-individual differences in gambling disorder severity in problem gamblers.
Introduction
Placing something valuable at risk with the hope of gaining something of greater value is a popular recreational activity among adults, and is referred to as gambling1. Approximately five percent of the population encounter sublinical problematic gambling and around one percent display pathological gambling2,3. Gambling disorder has been classified as an addiction disorder in the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013). This classification is based on accumulating evidence revealing similarities between pathological gambling and substance use disorders (SUDs) in both behavioral observations and underlying neural mechanisms4–6. Individuals meeting at least one of the DSM V criteria are commonly referred to as problem gamblers, whereas individuals meeting four or more are referred to as pathological gamblers.
A consistent finding in individuals suffering from addiction to stimulating drugs or alcohol is a dysregulation of dopaminergic transmission within striatal target sites7. Patients suffering from SUDs show reduced dopamine D2/3-receptor availability in the striatum8–12, constituting a candidate risk factor for the development of SUDs13. In monkeys and rodents, attenuated striatal dopamine D2/3-receptor availability prior to drug exposure is related to a more rapid acquisition of drug self-administration14,15. Reduced D2/3-receptor modulation of corticostriatal pathways may translate into a higher risk of escalating drug abuse via increased impulsivity15–17. D2/3-receptor function is also tightly coupled to learning from negative outcomes18,19, a process recently shown to be impaired in cocaine addicts compared with healthy controls20.
In gambling disorder, initial evidence for alterations in dopaminergic transmission came from studies that reported increased dihydroxtyphenylacetic (DOPAC) levels in cerebrospinal fluid of gambling addicts, and increased dopamine levels in blood samples of gamblers compared with controls during gambling21,22. In addition, dopaminergic medication in Parkinson’s disease patients can induce problem gambling as a side-effect23. More recent positron-emission-tomography (PET) studies tested direct associations between gambling disorder and central dopamine transmission. Van Holst et al.24 found increased striatal dopamine synthesis capacity, as assessed with [18F]fluoro-levo-dihydroxyphenylalanine ([18F]DOPA) PET, in gamblers compared with controls. Gamblers also showed higher amphetamine induced striatal dopamine release compared with controls25. Striatal D2/3-receptor availability was shown to be associated with mood related impulsivity and gambling severity in pathological gamblers, but no differences in striatal D2/3-receptor availability between gamblers and controls in general were observed so far26–28.
Spontaneous eye blink rate (sEBR) is discussed as a potential non-invasive proxy measure of striatal dopamine transmission29. Initial evidence linking sEBRs to dopamine transmission came from observations in several neurological and psychiatric disorders that relate to alterations in central dopamine regulation such as Parkinson’s disease, schizophrenia and psychosis30–33. In line with reduced D2/3-receptor availability, cocaine users34 and chronic cannabis consumers35 display reduced sEBRs compared to healthy controls. Several pharmacological studies observed a reduction in sEBR following dopamine antagonist administration, and an increase after dopamine agonist administration36–39. Complementing these findings, a recent PET study in monkeys found a strong positive correlation between sEBR and D2/3-receptor availability in ventral and parts of dorsal striatum40. Consistent with the idea that sEBR measures trait-like differences in dopaminergic transmission, it has good reliability (Cronbach’s Alpha: 0.79–0.85, see Kruis et al.41). However, two recent studies report opposing findings. Dang et al.42 found no significant correlation between sEBR and D2/3-receptor availability in midbrain and striatum in humans. In addition, they observed no significant impact of bromocriptine, a dopamine agonist, on participants’ sEBRs. Noteworthy, their subject sample was quite heterogeneous regarding age and body weight, and between 3 and 32 months separated subjects’ sEBR assessment from PET imaging. Quite recently, Sescousse et al.43 report a negative correlation between sEBR and striatal dopamine synthesis capacity in a mixed sample of healthy controls and gamblers. Thus, it is still debated to what extent, and with which specific aspect of dopaminergic transmission sEBR is associated with. A recent review suggested that current evidence is most supportive of a link between D2/3-receptor availability and sEBR44.
A key advantage of sEBR over other methods is that it is affordable and easily obtainable. In the light of the putative link of sEBR and D2/3-receptor availability, it is of considerable clinical interest to explore alterations of sEBR in behavioral addictions such as gambling disorder. Here, we utilize sEBR to examine potentially D2/3-receptor availability modulated group differences between problem gamblers and healthy control participants. According to the PET studies that showed a negative correlation between D2/3-receptor availability in gamblers and impulsivity, and gambling severity, respectively26–28, we hypothesize to observe a negative association between gambling severity and sEBR. As psychopathology is known to be related to aberrant central dopamine transmission45–47 and recently was shown to correlate with sEBR33, we utilize the SCL-90-R questionnaire48 as a screening test, and to control for overall psychopathology.
Results
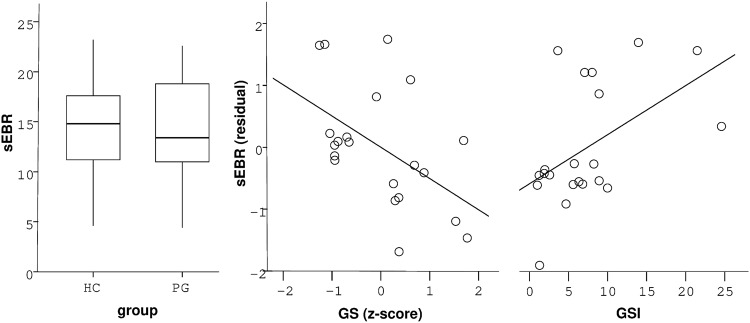
SEBRs did not differ significantly between healthy controls (HC) and gamblers (G) (mean +/− SEM HC: 14.6 ± 1.1; G: 14.4 ± 1.4; F(1,39) = 0.02, p = 0.89, Fig. 1a). The Bayes factor quantifying the evidence for the null hypothesis similarly showed moderate evidence in favor of no group difference (BF01 = 3.24). Including only pathological gamblers (DSM-5 > = 5), revealed a similar effect (PG: 13.4 ± 1.7; F(1,30) = 0.41, p = 0.53). In gamblers however, stepwise multiple regression analysis (adj. R² = 0.31, F(2,18) = 5.43, p = 0.01, Table 1, final model) revealed a negative correlation of sEBR with gambling severity (mean [95% CI]: β = −0.53 [−5.61 −0.69], p = 0.02, Fig. 1b) and a positive correlation with overall psychopathology (GSI) (β = 0.54 [0.1 0.78], p = 0.01, Fig. 1c). Age showed no significant effect on sEBR and was thus removed from the model.
Figure 1.
(a) SEBR did not differ in problem gamblers (PG) compared with healthy controls (HC). Vertical lines within boxplots represent the median, 25th, and 75th percentile, respectively. Whiskers represent the range. (b) Multiple regression analysis revealed a negative correlation of gambling severity (GS, z-score) with sEBR in problem gamblers. (c) Overall psychopathology (GSI) was positively correlated with sEBR in gamblers.
Table 1.
Regression results (gamblers only): Gambling severity (GS) and psychopathology (GSI) predicted sEBR in gamblers according to stepwise regression (final model).
| initial model | final model | alternative model | |
|---|---|---|---|
| model statistics | adj. R² = 0.27 F(3,17) = 3.42, p = 0.04 | adj. R² = 0.31, F(2,18) = 5.43, p = 0.01 | adj. R² = 0.11 F(2,18) = 2.22, p = 0.14 |
| GS | β = −0.54 [−5.88 −0.45] p = 0.03 | β = −0.53 [−5.61 −0.69] p = 0.02 | β = −0.47 [−5.68 0.09] p = 0.06 |
| GSI | β = 0.55 [0.05 0.85] p = 0.03 | β = 0.54 [0.1 0.78] p = 0.01 | — |
| age | β = 0.008 [−0.35 0.36] p = 0.97 | — | — |
| BDI-II | — | — | β = 0.31 [−0.08 0.34] p = 0.2 |
Age (initial model) was no significant predictor of sEBR. Depressive symptoms (BDI-II) instead of GSI scores did not explain significant variance in gamblers’ sEBR (alternative model). Values in brackets represent 95% confidence intervals.
Individual GSI scores were highly correlated with BDI-II scores (adj. R² = 0.78, F(1,19) = 71.31, p = 7.44 * 10−8). To exclude that the impact of psychopathology on gamblers’ sEBRs was exclusively driven by depressive symptoms, we computed another regression model that included BDI-II scores instead of participants GSI scores and gambling severity. This model did not explain a significant amount of variance of individual sEBRs (adj. R² = 0.09, F(2,18) = 2.0, p = 0.16, Table 1, alternative model) and BDI-II scores were not significantly associated with sEBRs (β = 0.31 [−0.09 0.38], p = 0.2).
In a further exploratory analysis (see Statistical analyses), we tested whether sEBR in healthy controls was associated with substance use (alcohol and nicotine consumption). For this purpose, we computed a compound score of alcohol and nicotine consumption and separated our control participants into a low sEBR and a high sEBR group according to a median split. Control participants with a low sEBR showed higher nicotine and alcohol consumption than participants with a high sEBR (F(1,18) = 5.92, p = 0.03, Fig. 2). In contrast to gamblers, GSI scores were not correlated with sEBRs in control participants (R² = 0.13, p = 0.11).
Figure 2.
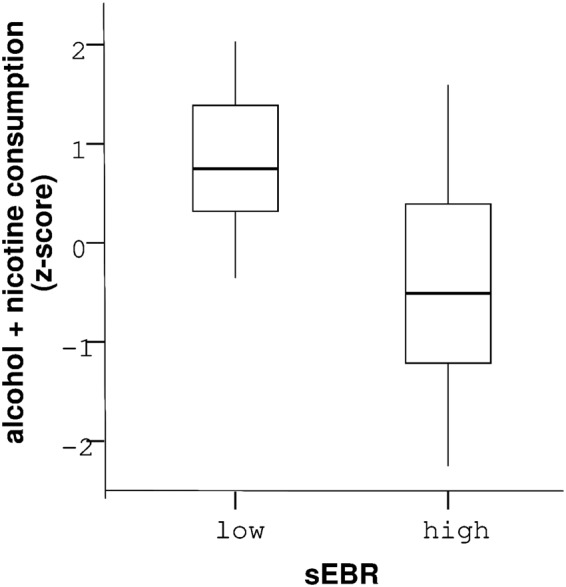
Healthy controls with low sEBR consumed more alcohol and nicotine (z-standardized) than control participants with high sEBR. Vertical lines within boxplots represent the median, 25th, and 75th percentile, respectively. Whiskers represent the range of standardized consumption values.
Discussion
We tested for an association between problematic gambling behavior and spontaneous eye blink rate (sEBR) as a potential marker of striatal D2/3-receptor availability. We observed no differences in sEBR between problem gamblers and healthy controls. However, in gamblers, gambling severity was negatively associated with sEBR, suggesting a potential modulatory effect of striatal D2/3-receptor availability on the escalation of gambling behavior. Overall psychopathology, as assessed with the SCL-90-R questionnaire, was positively linked to sEBR in gamblers. An exploratory analysis revealed a negative association between sEBR and alcohol/nicotine use in healthy controls.
Our observation of similar sEBRs in gamblers and matched controls supports recent findings from direct assessments of striatal D2/3-receptor availability in pathological gamblers via PET27,28. Reduced striatal D2/3-receptor availability might therefore not constitute a risk factor for gambling disorder, in contrast to substance-based addictions, where reduced D2/3-receptor availability is a consistent finding in both animals14,15, and humans8,49. Likewise, reduced sEBR has been related to recreational cocaine use in humans34. Drug consumption presumably causes a higher subsequent increase in striatal dopamine levels than engagement in gambling. Thus, gambling may not suffice to compensate for reduced striatal D2/3-receptor availability as it is assumed for drug use. Noteworthy, recurrent abuse of stimulating substances can cause a decrease in striatal D2/3-receptor availability23,50,51. This aggravates the differentiation between cause and consequence regarding altered dopamine signaling in addiction disorders at least in cross-sectional studies in humans.
We found that in problem and pathological gamblers, gambling severity was negatively associated with sEBR. As a potential marker of striatal D2/3-receptor availability, this may indicate that gamblers with reduced D2/3-receptor availability are at higher risk for developing more severe gambling behavior. Interestingly, Clark et al.27 observed a negative association between D2/3-receptor binding potential in striatum and mood-related impulsivity in gamblers. Thus, lower striatal D2/3-receptor availability may be linked to the escalation of gambling behavior, possibly due to increased impulsivity in mood-intense states. This is further supported by a longitudinal study showing a clear link between an impulsive, negative-emotional personality and the development of gambling disorder52. Interestingly, Boileau et al.23 found a positive correlation between gambling severity and D3-receptor availability, as assessed via [11C]-(+)-PHNO binding potential, in substantia nigra (SN). They also revealed that [11C]-(+)-PHNO binding potential in SN negatively correlated with amphetamine induced dopamine release in striatum and that gamblers showed stronger amphetamine induced striatal dopamine release than controls25. This seems in line with recent evidence for a hyperdopaminergic state within striatum of pathological gamblers24. Taken together, our observed negative correlation of (presumably) striatal D2/3-receptor availability with gambling severity may at least partly translate into a positive association between striatal dopaminergic tone and severity of problematic gambling.
Overall psychopathology, as assessed via the GSI of the SCL-90-R screening, correlated positively with sEBR as a potential indicator of striatal dopamine transmission in gamblers, but not in healthy controls. Psychological aberrations such as psychoticism have previously been related to heightened sEBR33,53 and reduced striatal D2/3-receptor availability46. The consistent association of schizophrenia and increased striatal dopamine function54–56 together with a continuum model of psychosis57 further support the hypothesis that a psychosis-prone personality has a dopaminergic basis58. In healthy controls, the range of the GSI score was significantly lower than in problem and pathological gamblers with a maximum score of 1.1 compared to 2.46, respectively. This complements earlier observations of an association between mental health disorders and pathological gambling59–61, and may at least partly explain the absence of a correlation between GSI scores and sEBR in control participants. Note, however, that the GSI score is a coarse measure that incorporates a variety of different psychological aberrations, warranting caution in the interpretation of these findings.
In an exploratory analysis, we found that healthy controls displaying relatively low sEBR (i.e. potentially low striatal D2/3-receptor availability) consumed more alcohol and nicotine than participants with relatively high sEBR. This is in accordance with findings from PET studies showing reduced D2/3-receptor availability in alcohol and nicotine addiction12,62–65. Notably, alcohol and nicotine consumption both increase extracellular dopamine in striatum63,66,67 that likely leads to a downregulation of striatal dopamine signaling following chronic intake12,64,65. Thus, this finding might partly reflect a consequence of recurrent consumption, and should be further explored in larger samples.
Several limitations of the present study need to be acknowledged. First, the assumption of a positive correlation between sEBR and D2/3-receptor availability in striatum in humans is still debated. In a recent publication, Sescousse et al.43, report a negative relation between sEBR and dopamine synthesis capacity as assessed with [18 F]DOPA PET in a mixed sample of gamblers and control participants. Dang et al.42 found no significant correlation between D2/3-receptor availability and sEBR in humans. Notably, their sample of 20 subjects was quite heterogeneous in age (20–50 y) and body weight (<60–120 kg). As there is growing evidence that body weight is associated with D2/3-receptor availability68, this might have influenced their findings. Furthermore, PET imaging and sEBR assessment were separated on average by 17 months (3–32 months). Hence, more work is needed to clarify the exact relationship between sEBR and dopamine transmission in humans. Second, this was a cross-sectional study. Thus, we cannot exclude that higher sEBR in gamblers exhibiting more severe gambling may be a consequence of gambling history and corresponding adaptations in the dopaminergic system similar to observations in substance-based addiction. Third, only male participants were tested, limiting the generalization of the findings. Fourth, our sample of gamblers consisted predominantly of slot-machine and sports betting gamblers, thus limiting our conclusions to this particular subgroup of gamblers. Finally, the sample size was insufficient to examine potential differences between gambling subtypes that have for example been proposed in the “pathways model”69.
In light of the potential link between sEBR and striatal D2/3-receptor availability, our findings in gamblers and healthy controls indicate that attenuated striatal D2/3-receptor availability is not necessarily a risk factor for developing gambling disorder as postulated for substance-based addictions. Rather, attenuated striatal D2/3-receptor availability might aggravate engagement in gambling in problematic gamblers. One endophenotype of lower striatal D2/3-receptor availability is impulsivity17. Pathological gamblers suffering from relatively low striatal D2/3-receptor availability may be prone to an increased escalation of gambling behavior through attenuated cognitive control, heightened cue-reactivity and/or steeper delay discounting, specifically in emotionally-demanding states15,59,70–72.
Given that sEBR is an affordable and easily obtainable measure with a putative link to D2/3-receptor availability, it might be worthwhile to explore its applicability in clinical practice. For example, it would be of interest to explore sEBR as a potential predictor of treatment outcome in addiction and/or examine interindividual differences in sEBR changes post-treatment.
Methods
Subjects
21 male problem gamblers (#DSM-5 criteria >= 1), and 20 healthy male control subjects participated in this study. All gamblers reported regular gambling, and suffered from losing money while gambling. 12 gamblers fulfilled DSM-5 criteria (#criteria >= 4) of gambling disorder. Gamblers and healthy controls were matched for age, educational background, socioeconomic status, alcohol and nicotine consumption. Severity of gambling disorder was assessed by the ‘Kurzfragebogen zum Glücksspielverhalten’ (KFG)73, and a German adaptation of the South Oaks Gambling Screen (SOGS)74. Both questionnaires are validated screening tools for quantifying gambling disorder severity, and show good reliability (Cronbach’s Alpha: KFG: 0.79; SOGS: 0.97). Comprehensive demographic information is provided in Table 2. Participants were recruited via advertisements on local internet bulletin boards. Prior to enrollment in the study, phone interviews were conducted, and only gamblers who reported gambling on a regular basis, suffered from monetary loss, and fulfilled at least one of the DSM-5 criteria of pathological gambling were invited to participate. Gamblers were mainly engaged in slot machine gambling (67%), and sports betting (57%). A fraction also pursued (online) poker (14%), and roulette (14%). Eligible participants were interviewed by a psychologist to exclude a history of neurological or psychiatric disorders, current medication, and substance abuse other than nicotine and alcohol. All study procedures were approved by the local Institutional Review Board (Hamburg Board of Physicians). We confirm that all research was performed in accordance with relevant guidelines and regulations, and participants provided informed written consent prior to their participation. Participants received 10 EUR per hour as a compensation for participation.
Table 2.
Sample description [mean ± standard deviation (min-max)]: Gamblers did not differ regarding age, income, years of education (YOE), alcohol (AUDIT) and nicotine consumption (#cigarettes), and eye blink rate (sEBR).
| n | healthy controls | Gamblers | F/U | p |
|---|---|---|---|---|
| 20 | 21 | — | — | |
| age | 26.4 ± 6.39 (19–45) | 26.0 ± 6.66 (18–42) | F = 0.04 | 0.85 |
| income | 1028.15 ± 575.05 (0–2000) | 1375.1 ± 819.51 (300–2700) | F = 2.44 | 0.13 |
| YOE | 11.75 ± 1.37 (9–14) | 11.71 ± 1.82 (9–15) | F = 0.01 | 0.94 |
| BDI-II | 8.7 ± 8.3 (0–28) | 15.1 ± 11.87 (2–42) | F = 3.96 | 0.05 |
| GSI | 0.32 ± 0.34 (0–1.1) | 0.73 ± 0.62 (1–2.46) | F = 6.7 | 0.01 |
| DSM-5 | 0.1 ± 0.38 (0–1) | 5.1 ± 2.28 (1–8) | U = 1 | 1.1 * 10−8 |
| KFG | 1.45 ± 4.07 (0–18) | 25.29 ± 14.54 (6–54) | U = 9 | 7.6 * 10−8 |
| SOGS | 0.4 ± 1.0 (0–4) | 8.48 ± 4.61 (3–17) | U = 5.5 | 3.7 * 10−8 |
| GS | −0.77 ± 0.21 (−0.85–0.08) | 0.73 ± 0.86 (−0.38–2.3) | U = 217 | 8.2 * 10−8 |
| AUDIT | 6.75 ± 4.8 (0–15) | 5.95 ± 6.98 (0–23) | F = 0.43 | 0.52 |
| #cigarettes | 9.25 ± 8.75 (0–30) | 5.95 ± 6.76 (0–19) | U = 167.5 | 0.25 |
| sEBR | 14.6 ± 5.02 (4.6–23.2) | 14.37 ± 5.08 (4.4–22.6) | F = 0.14 | 0.7 |
Gamblers displayed higher gambling severity (DSM-5, KFG, SOGS, sum of z-scores of KFG & SOGS (GS)), higher psychoticism (GSI), and a tendency for more depressive symptoms (BDI-II). Tests for group differences were based on ANOVA (F) for normally distributed variables, and Mann-Whitney U Tests otherwise.
General procedure
Participants entered the lab in the afternoon around 2 pm. After they gave written informed consent, participants started with a five minutes sEBR assessment. Notably, sEBRs are stable throughout the day and rise in the evening53. Subsequently, they completed our lab’s standard questionnaire battery on the computer. On a separate testing day, participants performed two reward-based learning task in an fMRI setting. These data will be reported elsewhere.
Psychological assessment
Following the sEBR assessment, participants completed questionnaires assessing gambling disorder severity (DSM-5 criteria, KFG, SOGS). In addition, participants completed the Symptom Check-List-90-R (SCL-90-R)48 that constitutes a screening tool for capturing current psychological pathology. As depressive symptoms are a common co-morbidity in pathological gambling3 participants also completed the Beck Depression Inventory (BDI-II)75. Within our standard questionnaire battery, subjects were also screened for any past or current psychiatric or neurological disease. We quantified nicotine consumption as self-reported number of cigarettes smoked per day. Alcohol use was measured via the Alcohol Use Disorders Identification Test (AUDIT)76.
SEBR assessment
Spontaneous eye blink rates (SEBRs) were assessed via electromyography (EMG), utilizing a MP100 system running under the software Acqknowledge 3.9.1 (Biopac Systems, Goleta, California). Data was recorded via three Ag-AgCL electrodes with a sample rate of 1000 Hz, and an online bandpass filter of 28–500 Hz. One reference electrode was placed on the middle of the participant’s forehead and two electrodes were fixed below the lower lash line of the left eye, one of the electrodes centrically and the other one 2–3 mm in peripheral proximity. Participants sat in front of a computer screen and were instructed to move as little as possible while staring at a fixation cross at approximately 0.5 m distance for 5 minutes. A duration of 5 minutes has been shown to suffice for assessing stable mean sEBR values77. They were not explicitly told that sEBR was assessed and subjects were monitored during sEBR assessment to ensure that they were fixating the screen as instructed.
Individual sEBRs were computed using Matlab 2012b (MathWorks, Natick, MA) via the ‘findpeaks’ function in a sliding window approach of 10 seconds to the acquired raw data. Blinks were defined as peaks exceeding the data’s mean within the moving window by six standard deviations. SEBRs were then calculated as average number of blinks per minute.
Statistical analyses
All reported results were computed with PASW-SPSS-Statistics 17.0 (IBM Corporation, Somers, NY, USA). We utilized an ANOVA model to assess differences in sEBR between pathological gamblers and healthy controls. In addition, we calculated the Bayes factor in favor of the null hypothesis (BF01) via the JASP software package (Version 0.8.6, University of Amsterdam). Stepwise (backward elimination) multiple regression analysis was used to test the association between sEBR and gambling severity in gamblers. Gambling severity (GS) was computed as the mean of the two z-standardized gambling questionnaire scores (SOGS + KFG). Results were similar if only one score was used in the regression model. To control for overall psychopathology, the global severity index (GSI) of the SCL-90-R served as an additional predictor. We further controlled for age-related effects. In a second regression model, we controlled for individual depressive symptoms via individual BDI-II scores instead of GSI scores. We did not include both predictors in a single model due to their high correlation (R² = 0.78, p = 7.44 * 10−8).
In addition, we ran an exploratory analysis to test an association between sEBR and substance use in healthy controls based on the consistent finding in animals and humans, that low D2/3-receptor availability is a risk factor for developing SUD12,13,78. An individual substance use score was calculated as the sum of the z-standardized AUDIT questionnaire score and the z-standardized number of cigarettes smoked per day. Controls were separated into a low and a high sEBR group via a median split to test if the low sEBR group consumed more alcohol and /or nicotine according to the substance use score than the high sEBR group. Additionally, we computed a correlation analysis between sEBR and GSI scores in healthy controls.
Gaussianity, heteroscedasticity and absence of multicollinearity were tested for the respective analyses.
Author Contributions
J.P. and A.W. designed research. A.W. performed research. D.M. and A.W. analyzed data. K.C. and D.G. contributed analytical tools. All authors contributed to writing of the paper. Deutsche Forschungsgemeinschaft (PE 1627/5-1 to J.P.) funded this research.
Data Availability
All data will be made available upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David Mathar and Antonius Wiehler contributed equally.
References
- 1.Potenza MN. Should addictive disorders include non‐substance‐related conditions? Addiction. 2006;101:142–51. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer HJ, Hall MN, Bilt JV. Estimating the prevalence of disordered gambling behavior in the United States and Canada: A research synthesis. Am J Public Health. 1999;89:1369–75. doi: 10.2105/AJPH.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiat. 2005;66:564–74. doi: 10.4088/JCP.v66n0504. [DOI] [PubMed] [Google Scholar]
- 4.Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Roy Soc B. 2008;363:3181–89. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann NY Acad Sci. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genauck A, et al. Reduced loss aversion in pathological gambling and alcohol dependence is associated with differential alterations in amygdala and prefrontal functioning. Sci Rep. 2017;7:16306. doi: 10.1038/s41598-017-16433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutt DJ, et al. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015;16:305–12. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 10.Heinz A, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–89. doi: 10.1176/ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 11.Martinez D, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacol. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 12.Fehr C, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165:507–14. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, et al. High Levels of Dopamine D2 Receptors in Unaffected Members of Alcoholic Families. Possible Protective Factors. Arch Gen Psychiat. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 14.Nader MA, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–56. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 15.Dalley JW, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckholtz JW, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. P Natl Acad Sci USA. 2007;104:16311–16. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathar D, et al. The role of dopamine in positive and negative prediction error utilization during incidental learning - Insights from Positron Emission Tomography, Parkinson’s disease and Huntington’s disease. Cortex. 2017;90:149–62. doi: 10.1016/j.cortex.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Parvaz MA, et al. Impaired neural response to negative prediction errors in cocaine addiction. J Neursosci. 2015;35:1872–79. doi: 10.1523/JNEUROSCI.2777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergh C, Eklund T, Södersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–75. doi: 10.1017/S0033291796003789. [DOI] [PubMed] [Google Scholar]
- 22.Meyer G, et al. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29:1272–80. doi: 10.1016/j.psyneuen.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Voon V, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–49. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 24. van Holst, R.J. et al. Increased Striatal Dopamine Synthesis Capacity in Gambling Addiction. Biol Psychiatry, 83, 1036–43. [DOI] [PMC free article] [PubMed]
- 25.Boileau I, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatr. 2014;19:1305–13. doi: 10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- 26.Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand J Psychol. 2011;52:28–34. doi: 10.1111/j.1467-9450.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 27.Clark L, et al. Striatal dopamine D2/D3 receptor binding in pathological gambling is correlated with mood-related impulsivity. Neuroimage. 2012;63:40–46. doi: 10.1016/j.neuroimage.2012.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boileau I, et al. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108:953–63. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- 29.Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–53. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- 30.Freed WJ, et al. Eye-blink rates and platelet monoamine oxidase activity in chronic schizophrenic patients. Biol Psychiatry. 1980;15:329–32. [PubMed] [Google Scholar]
- 31.Mackert A, Flechtner KM, Woyth C, Frick K. Increased blink rates in schizophrenics. Influences of neuroleptics and psychopathology. Schizophr Res. 1991;4:41–47. doi: 10.1016/0920-9964(91)90008-F. [DOI] [PubMed] [Google Scholar]
- 32.Deuschl G, Goddemeier C. Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. J Neurol Neurosurg Psychiatry. 1998;64:320–24. doi: 10.1136/jnnp.64.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colzato LS, Slagter HA, van den Wildenberg WPM, Hommel B. Closing one’s eyes to reality: evidence for a dopaminergic basis of psychoticism from spontaneous eye blink rates. Pers Individ Dif. 2009;46:377–80. doi: 10.1016/j.paid.2008.10.017. [DOI] [Google Scholar]
- 34.Colzato LS, van den Wildenberg WPM, Hommel B. Reduced spontaneous eye blink rates in recreational cocaine users: evidence for dopaminergic hypoactivity. Plos One. 2008;3:e3461. doi: 10.1371/journal.pone.0003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowal MA, Colzato LS, Hommel B. Decreased Spontaneous Eye blink rates in chronic cannabis users: evidence for striatal cannabinoid-dopamine interactions. Plos One. 2011;6:e26662. doi: 10.1371/journal.pone.0026662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blin O, Masson G, Azulay JP, Fondarai J, Serratrice G. Apomorphine-induced blinking and yawning in healthy volunteers. Br J Clin Pharmacol. 1990;30:769–73. doi: 10.1111/j.1365-2125.1990.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsworth JD, et al. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J Pharmacol Exp Ther. 1991;259:595–600. [PubMed] [Google Scholar]
- 38.Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J Pharmacol Exp Ther. 1996;279:1211–19. [PubMed] [Google Scholar]
- 39.Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci. 2011;31:11256–67. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groman SM, et al. In the blink of an eye: relating positive-feedback sensitivity to striatal dopamine D2-like receptors through blink rate. J Neurosci. 2014;34:14443–54. doi: 10.1523/JNEUROSCI.3037-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruis A, Slagter HA, Bachhuber DRW, Davidson RJ, Lutz A. Effects of meditation practice on spontaneous eyeblink rate. Psychophysiology. 2016;53:749–58. doi: 10.1111/psyp.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dang, L.C. et al. Spontaneous eye blink rate (EBR) is uncorrelated with dopamine D2 receptor availability and unmodulated by dopamine agonism in healthy adults. eNeuro, 4, 10.1523/ENEURO.0211-17.2017 (2017). [DOI] [PMC free article] [PubMed]
- 43.Sescousse G, et al. Spontaneous eye blink rate and dopamine synthesis capacity: preliminary evidence for an absence of positive correlation. Eur J Neurosci. 2018;47:1081–86. doi: 10.1111/ejn.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jongkees BJ, Colzato LS. Spontaneous eye blink rate as predictor of dopamine-related cognitive function – A review. Neurosci Biobehav Rev. 2016;71:58–82. doi: 10.1016/j.neubiorev.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Maas JW, et al. Studies of catecholamine metabolism in schizophrenia/psychosis–I. Neuropsychopharmacol. 1993;8:97–109. doi: 10.1038/npp.1993.11. [DOI] [PubMed] [Google Scholar]
- 46.Gray NS, Pickering A, Gray JA. Psychoticism and dopamine D2 binding in the basal ganglia using single photon emission tomography. Pers Individ Dif. 1994;17:431–34. doi: 10.1016/0191-8869(94)90289-5. [DOI] [Google Scholar]
- 47.Gray JA. Dopamine release in the nucleus accumbens: the perspective from aberrations of consciousness in schizophrenia. Neuropsychologia. 1995;33:1143–53. doi: 10.1016/0028-3932(95)00054-7. [DOI] [PubMed] [Google Scholar]
- 48.Derogatis, L.R. & Unger, R. Symptom Checklist-90-Revised. The Corsini Encyclopedia of Psychology. (John Wiley & Sons, Inc., 2010).
- 49.Martinez D, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–77. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow ND, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- 51.Groman SM, et al. D. Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–52. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slutske, W. S., Caspi, A., Moffitt, T. E. & Poulton, R. Personality and Problem Gambling. A Prospective Study of a Birth Cohort of Young Adults. Arch Gen Psychiatry, 62, 769–75. [DOI] [PubMed]
- 53.Barbato, G., Della Monica, C., Costanzo, A. & De Padova, V. Dopamine activation in Neuroticism as measured by spontaneous eye blink rate. Physiology & Behavior, 105, 332–36. [DOI] [PubMed]
- 54.Gray JA, Feldon J, Rawlins JNP, Hemsley DR, Smith AD. The neuropsychology of schizophrenia. Behav Brain Sci. 1991;14:1–20. doi: 10.1017/S0140525X00065055. [DOI] [Google Scholar]
- 55.Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia–all roads lead to dopamine. European Neuropsychopharmacol. 2007;17:101–107. doi: 10.1016/j.euroneuro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Eyles D, Feldon J, Meyer U. Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl Psychiatry. 2012;2:e81. doi: 10.1038/tp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21:1125–41. doi: 10.1016/S0272-7358(01)00103-9. [DOI] [PubMed] [Google Scholar]
- 58. Ettinger, U., Corr, P. J., Mofidi, A., Williams, S. C. R. & Kumari, V. Dopaminergic basis of the psychosis-prone personality investigated with functional magnetic resonance imaging of procedural learning. Front Hum Neurosci, 7, 10.3389/fnhum.2013.00130 (2013). [DOI] [PMC free article] [PubMed]
- 59.Cunningham-Williams RM, Cottler LB, Compton WM, Spitznagel EL. Taking chances: problem gamblers and mental health disorders–results from the St. Louis Epidemiologic Catchment Area Study. Am J Public Health. 1998;88:1093–96. doi: 10.2105/AJPH.88.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant JE, Levine L, Kim D, Potenza MN. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162:2184–88. doi: 10.1176/appi.ajp.162.11.2184. [DOI] [PubMed] [Google Scholar]
- 61.Desai RA, Potenza MN. A cross-sectional study of problem and pathological gambling in patients with schizophrenia/schizoaffective disorder. J Clin Psychiatry. 2009;70:1250–57. doi: 10.4088/JCP.m04359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinz A, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–20. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 63.Brody AL, et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–18. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- 64.Martinez D, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boileau I, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 67.Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- 68.Horstmann A, Fenske WK, Hankir MK. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes Rev. 2015;16:821–30. doi: 10.1111/obr.12303. [DOI] [PubMed] [Google Scholar]
- 69.Blaszczynski A, Nower L. A pathways model of problem and pathological gambling. Addiction. 2002;97:487–99. doi: 10.1046/j.1360-0443.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 70.Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–39. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Miedl SF, Peters J, Büchel C. Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Arch Gen Psychiat. 2012;69:177–86. doi: 10.1001/archgenpsychiatry.2011.1552. [DOI] [PubMed] [Google Scholar]
- 72.Miedl SF, Büchel C, Peters J. Cue-induced craving increases impulsivity via changes in striatal value signals in problem gamblers. J Neurosci. 2014;34:4750–55. doi: 10.1523/JNEUROSCI.5020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petry, J. Psychotherapie der Glücksspielsucht. (Weinheim: Beltz, 1996).
- 74.Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–88. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- 75.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 76.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 77.Zaman ML, Doughty MJ. Some methodological issues in the assessment of the spontaneous eyeblink frequency in man. Opthal Physl Opt. 1997;17:421–32. doi: 10.1111/j.1475-1313.1997.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 78.Hietala J, et al. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology. 1994;116:285–90. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be made available upon request.