Abstract
Bacteriophages (phages) are bacterial viruses that parasitize bacteria. They are highly prevalent in nature, with an estimated 1031 viral particles in the whole biosphere, and they outnumber bacteria by at least 10-fold. Hence, phages represent important drivers of bacterial evolution, although our knowledge of the role played by phages in the mammalian gut is still embryonic. Several pathogens owe their virulence to the integrated phages (prophages) they harbor, which encode diverse virulence factors such as toxins. Clostridioides (Clostridium) difficile is an important opportunistic pathogen and several phages infecting this species have been described over the last decade. However, their exact contribution to the biology and virulence of this pathogen remains elusive. Current data have shown that C. difficile phages can alter virulence-associated phenotypes, in particular toxin production, by interfering with bacterial regulatory circuits through crosstalk with phage proteins for example. One phage has also been found to encode a complete binary toxin locus. Multiple regulatory genes have also been identified in phage genomes, suggesting that their impact on the host can be complex and often subtle. In this minireview, the current state of knowledge, major findings, and pending questions regarding C. difficile phages will be presented. In addition, with the apparent role played by phages in the success of fecal microbiota transplantation and the perspective of phage therapy for treatment of recurrent C. difficile infection, it has become even more crucial to understand what C. difficile phages do in the gut, how they impact their host, and how they influence the epidemiology and evolution of this clinically important pathogen.
Keywords: Clostridium difficile, Clostridioides difficile, bacteriophages, prophages, toxins, virulence, lysogenic conversion
Bacteriophages
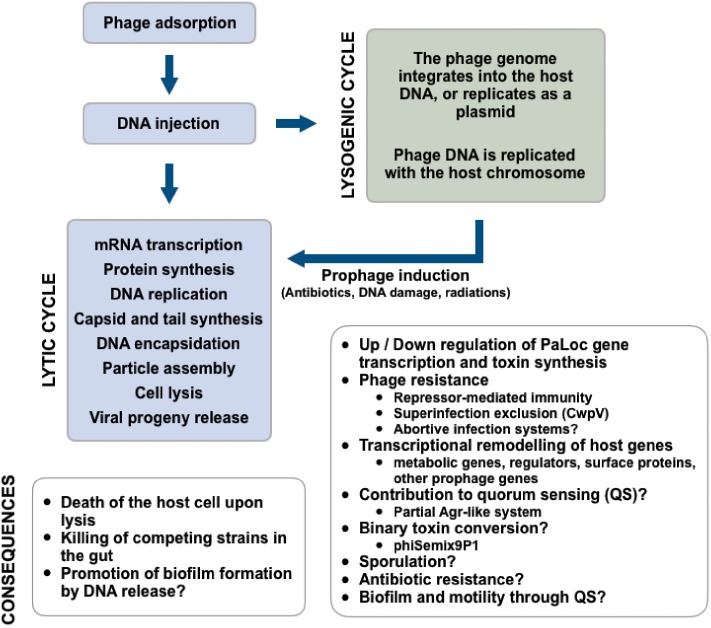
Bacteriophages (phages) are bacterial viruses that infect bacteria. At ∼1031 viral particles, they represent the most abundant biological entities in the biosphere and almost all bacteria are susceptible to phage attacks. Phages are therefore important drivers of bacterial evolution (Brüssow and Hendrix, 2002). Depending on the phages’ replication strategy, their impact on bacterial populations can be drastically different. The two most frequent mechanisms of phage replication are the lytic cycle, and the lysogenic cycle (Figure 1). Phages that replicate only via the lytic pathway are referred to as “virulent” and inevitably lead to death of the host upon infection. Those phages that can replicate either by the lytic or the lysogenic cycle are said to be “temperate.” When temperate phages become integrated into the host genome (i.e., prophages) their host is said to be lysogenic.
FIGURE 1.
Summary of the replication strategies and multiple impacts of phages infecting C. difficile.
Bacteriophages Infecting C. Difficile
Most phages infecting Clostridioides (Clostridium) difficile were isolated following induction of prophages (Shan et al., 2012; Hargreaves and Clokie, 2014; Sekulovic et al., 2014). However, free phages have also been isolated directly from fecal supernatants of patients infected with C. difficile, hence prophage induction occurs in vivo (Meessen-Pinard et al., 2012). In the 1980s and 1990s, C. difficile phages were studied for their potential as strain typing tools (Sell et al., 1983; Dei, 1989; Mahony et al., 1991). Now their potential for phage therapy applications is being explored (Nale et al., 2016b, 2018). At the time of writing this manuscript, at least 24 complete phage genomes were available in public databases (Table 1). Most of them are members of the Myoviridae family of the order Caudovirales (i.e., phages with contractile tails), and six phages are members of the Siphoviridae family (i.e., phages with long non-contractile tails) (Ackermann and Prangishvili, 2012). Functional data describing the lytic cycle of C. difficile phages are quite scarce. The few one-step growth curve experiments published so far suggest highly variable latency periods (from ∼30 min to 2 h), (Goh et al., 2005b; Sekulovic et al., 2011) and burst sizes (i.e., virions released per infected cell), with as few as 5 phages/cell for phage ϕC2 (Goh et al., 2005b), to 122 phages/cell for phage 56 (Mahony et al., 1985). In addition, most phages have relatively narrow host ranges (Goh et al., 2005b; Sekulovic et al., 2011, 2014; Rashid et al., 2016), which is directly related to the availability of a suitable host receptor, the presence of endogenous prophages conferring resistance, and the presence of antiphage systems including clustered regularly interspaced short palindromic repeat sequences (CRISPRs) (Hargreaves et al., 2014a; Boudry et al., 2015), the recently described superinfection exclusion system CwpV (Sekulovic et al., 2015), and possibly others. Of particular interest, the receptor(s) used by C. difficile phages to infect their host remain(s) to be clearly identified. In other Gram-positive bacteria, different cell surface components are used as phage receptors, from single proteins to polysaccharides or teichoic acids. As examples, the Bacillus subtilis YueB (São-José et al., 2006) and Lactococcus lactis Pip (Babu et al., 1995) proteins are, respectively, used by phages SPP1 and c2 to infect their host. Different polysaccharides composing the pellicle are used as receptors by lactococcal phages such as p2 (Bebeacua et al., 2013). Of note, recent data with Diffocins, i.e., phage tail-like bacteriocins that resemble Myoviridae phage tails and that kill their host by puncturing the cell, point to a central role of the surface layer protein A (SlpA) as a general phage receptor used by Diffocins and Myoviridae phages (Gebhart et al., 2015; Kirk et al., 2017). Detailed molecular interactions between phages and the C. difficile surface need to be further investigated, in particular regarding Siphoviridae phages, and considering the potential of phages as therapeutic agents.
Table 1.
List and relevant characteristics of C. difficile phages for which a complete genome sequence is available in GenBank.
| Phage | Family∗ | Genome size (bp) | Accession No. | Date of Release | Relevant characteristics/observations | Reference |
|---|---|---|---|---|---|---|
| phiCD119 | M | 53,325 | AY855346.1 | 2006 | • First C. difficile phage genome to be sequenced • The phage repressor RepR was shown to repress transcription of the five PaLoc genes through binding to the tcdR promoter element |
Govind et al., 2006, 2009 |
| phiC2 | M | 56,538 | DQ466086.1 | 2007 | • Increased TcdB production in certain lysogens carrying phiC2, although transcription of the tcdB gene remained unaffected • Transcription of the tcdA gene was increased or reduced in some lysogens, although the level of TcdA toxin remained unaffected •phiC2 was shown to promote transduction of erythromycin resistance through transfer of the erm(B) gene carried on Tn6215 |
Goh et al., 2005a, 2007, 2013 |
| phiCD27 | M | 50,930 | NC_011398.1 | 2008 | • The phage’s endolysin gene was cloned and expressed in Escherichia coli and Lactococcus lactis. The specificity of the lytic activity of the CD27L endolysin toward C. difficile was demonstrated using a panel of 30 C. difficile isolates + other commensal bacteria • phiCD27 significantly reduced the burden of C. difficile and toxin production in prophylactic assays using in vitro batch fermentation and human colon models |
Mayer et al., 2008; Meader et al., 2010, 2013 |
| phiCD6356 | S | 37,664 | NC_015262.1 | 2010 | • First C. difficile Siphoviridae phage genome to be sequenced | Horgan et al., 2010 |
| phiCD38-2 | S | 41,090 | HM568888.1 | 2011 | • Increased toxin production and PaLoc gene transcription in ribotype 027 lysogens carrying the phiCD38-2 prophage. The impact of phiCD38-2 on toxin production was strain-dependent. • First demonstration of a C. difficile prophage genome maintained as an extrachromosomal plasmid • RNAseq analysis of a R20291-lysogen carrying phiCD38-2 led to the discovery of the antiphage activity of the CwpV phase-variable surface protein |
Sekulovic et al., 2011, 2015 |
| phiMMP02 phiMMP04 |
M M |
48,396 31,674 |
JX145341.1 JX145342.1 |
2012 2012 |
• Free phage particles were isolated from filter-sterilized fecal supernatants from C. difficile infected patients, demonstrating that prophage induction occurs in vivo, during infection | Meessen-Pinard et al., 2012; Boudry et al., 2015 |
| phiCDHM1 | M | 54,279 | NC_024144.1 | 2013 | • The phage genome encodes three homologs of the Staphylococcus aureus Agr quorum sensing (QS) system, namely agrD (pre-peptide of an autoinducing peptide, AIP), agrB (processes the pre-AIP), and agrC (histidine kinase) • Possibly affects QS-mediated phenotypes, although no AgrA-like response regulator could be identified in phiCDHM1 • A cocktail containing phiCDHM1, phiCDHM2, phiCDHM5, and phiCDHM6 was shown to reduce C. difficile burden in vitro and in colonization experiments in hamsters and in a Galleria mellonella larva C. difficile infection model. The phage cocktail also prevented biofilm formation in vitro |
Hargreaves et al., 2014b; Nale et al., 2016a,b |
| phiCDHM13 | M | 33,596 | HG796225.1 | 2013 | Hargreaves et al., 2014a | |
| phiCDHM14 | M | 32,651 | LK985321.1 | 2014 | Hargreaves et al., 2014a | |
| phiCDHM19 | M | 54,295 | LK985322.1 | 2014 | Hargreaves et al., 2014a | |
| phiCD211 phiCDIF1296T |
S S |
131,326 131,326 |
NC_029048.1 CP011970.1 |
2014 2015 |
• phiCD211 and phiCDIF1296T are identical phages • Large phage genome maintained as an extrachromosomal plasmid in lysogens • The genome encodes several genes potentially affecting various phenotypes in C. difficile, including antimicrobial resistance, spore germination, and CRISPR-mediated resistance • Encodes a CRISPR-cas3 gene in addition to a CRISPR array |
Boudry et al., 2015; Wittmann et al., 2015; Garneau et al., 2018 |
| phiCD24-1 | S | 44,129 | LN681534.1 | 2015 | Boudry et al., 2015 | |
| phiCD111 | S | 41,560 | LN681535.1 | 2015 | Sekulovic et al., 2014; Boudry et al., 2015 | |
| phiCD146 | S | 41,507 | LN681536.1 | 2015 | Sekulovic et al., 2014; Boudry et al., 2015 | |
| phiMMP01 phiMMP03 |
M M |
44,461 52,261 |
LN681541.1 LN681542.1 |
2015 2015 |
• Free phage particles were isolated from filter-sterilized fecal supernatants from C. difficile infected patients, demonstrating that prophage induction occurs in vivo, during infection | Meessen-Pinard et al., 2012; Boudry et al., 2015 |
| phiCD481-1 | M | 32,846 | LN681538.1 | 2015 | Sekulovic et al., 2014; Boudry et al., 2015 | |
| phiCD505 | M | 49,316 | LN681539.1 | 2015 | Sekulovic et al., 2014; Boudry et al., 2015 | |
| phiCD506 | M | 33,274 | LN681540.1 | 2015 | Sekulovic et al., 2014; Boudry et al., 2015 | |
| phiCDHM11 | M | 32,000 | HG798901.1 | 2015 | Hargreaves and Clokie, 2015 | |
| phiCDKM9 | M | 49,822 | KX228399 | 2016 | Rashid et al., 2016 | |
| phiCDKM15 | M | 50,605 | KX228400 | 2016 | Rashid et al., 2016 | |
| phiSemix9P1 | N∖ A | 56,606 | KX905163.1 | 2017 | • The phage genome encodes a complete and functional binary toxin locus (CdtLoc) | Riedel et al., 2017 |
∗M, Myoviridae; S, Siphoviridae.
High Prevalence of Prophages in C. Difficile Genomes
Over 1,300 C. difficile genomes have been fully sequenced and are available in public repositories, but thousands of additional genomes have also been sequenced and are available through collaborative research (Garneau et al., 2018). Although uncommon, as many as 5–6 different prophages were identified in a single C. difficile genome (Amy et al., 2018; Ramírez-Vargas et al., 2018). However, between 1 and 3 prophages are more frequently observed, in addition to genomic “islands” containing phage-related genes. Recent studies highlighted the prevalence of large phage genomes that reside as extrachromosomal DNA in C. difficile (Garneau et al., 2018; Ramírez-Vargas et al., 2018). For example, the large phiCD211/phiCDIF1297T and related phages, with genomes of ≥131-kb, have been detected in 5% of 2,584 C. difficile genomes analyzed, spanning 21 different multi-locus sequence types (MLST) (Wittmann et al., 2015; Garneau et al., 2018). Ten other large phage genomes (∼128–135-kb), including phiCD5763, phiCD5774, and phiCD2955, were recently described in C. difficile isolates from around the world and representing seven different MLST sequence types (Ramírez-Vargas et al., 2018). Comparative genomic analyses underlined the important genetic variability among large phages, and they could eventually be used as genetic markers to subtype and monitor specific strains during epidemiological studies, as suggested for Salmonella enterica (Mottawea et al., 2018).
It is worth mentioning that extrachromosomal phage genomes can be difficult to differentiate from large plasmids containing phage genes. A study by Amy et al. (2018) reported the characterization of a large plasmid in C. difficile strain DLL3026. This 46-kb plasmid, called pDLL3026, and several other plasmids of similar size identified in other isolates, harbor a significant number of phage structural genes coding for head and tail morphogenesis, recombinases/integrases and phage regulators. The presence of partition genes like parM and parR and DNA similarity with plasmids led the authors to conclude that these were plasmids. However, the presence of partition genes like parA has also been reported in other phages, including phiCD6356 (Horgan et al., 2010), ϕCD38-2 (Sekulovic et al., 2011), and phiSemix9P1 (Riedel et al., 2017), the latter two known to be maintained as extrachromosomal DNAs in lysogenic cells. Large phage genomes such as phiCD211/phiCDIF1296T and phiCD5763 also seem to be frequently found as extrachromosomal DNA, and ParM homologs were identified in some of them (Garneau et al., 2018; Ramírez-Vargas et al., 2018). Therefore, in the absence of functional data to assess the inducibility and production of infectious particles from these large “plasmids,” it is hard to conclude on their exact nature.
The identification of complete prophages in bacterial genomes has been greatly improved, thanks to the development of tools such as PHAST and PHASTER (Arndt et al., 2016, 2017). But the task is more challenging with decaying prophage remnants that have lost many of the conserved phage components such as structural genes. Yet, these remnants could still influence their host even if they can’t replicate or produce complete infectious particles. Diffocins are a good example: these phage tail-like particles resemble Myoviridae phage tails, but lack a capsid and genetic material (Gebhart et al., 2012). They kill their host following induction and lysis of the cell, and also kill other competing cells around, but they can’t produce infectious particles. The functional role of Diffocins remains to be clarified, but they possibly provide a competitive advantage to C. difficile strains carrying them by killing surrounding competitors (Kirk et al., 2017).
The Consequences of Prophage Induction
The role of prophages in the physiology and virulence of C. difficile is a topic of great interest, considering their prevalence and diversity, and the historical role prophages played in the virulence of other bacterial pathogens (Brüssow et al., 2004; Fortier and Sekulovic, 2013). Prophage stability is critical because of the direct consequence on the viability of the host itself and susceptible surrounding strains/species that can be re-infected. Induction can occur spontaneously, but is promoted by common antibiotics and various environmental stresses (Rokney et al., 2008; Meessen-Pinard et al., 2012; Shan et al., 2012). Of note, prophage induction triggered by antibiotics promote horizontal gene transfer and spreading of antibiotic resistance genes in mice (Modi et al., 2013). In vitro, phage ϕC2 was shown to mediate transduction of the Tn6215 transposon between C. difficile strains (Goh et al., 2013). Differences in abundance and diversity of gut phages has also been associated with diseases and could be the result of prophage induction (Norman et al., 2015; Manrique et al., 2016). Hence, better understanding the role of prophage induction in complex ecosystems such as the gut is of great interest.
Induction of prophages and phage-related elements can also have other important physiological roles in C. difficile. For example, excision of the phage-related mobile element called skinCd is important during the sporulation process (Haraldsen and Sonenshein, 2003; Saujet et al., 2014). The skinCd is a putative prophage remnant similar to the one identified in B. subtilis (skinBs) that interrupts the coding sequence of SigK, a sporulation-associated alternative sigma factor. Excision of the skinCd element at a specific time point during the sporulation process restores the coding sequence of the gene, allowing expression of sigK (Saujet et al., 2014; Fimlaid and Shen, 2015). Control of the excision of the skin element remains unclear, but a putative site-specific recombinase similar to SpoIVCA, encoded by cd1231 and located within skinCd, is suspected to be involved. Some crosstalk between prophages has been reported (Lemire et al., 2011; Sekulovic and Fortier, 2015), including between their recombinases (Singh et al., 2014). Therefore, other phage-encoded recombinases could possibly participate in skinCd excision as well, and thus influence sporulation (Saujet et al., 2014).
Prophages Influence Toxin Production in C. Difficile
The main virulence factors of C. difficile are the large TcdA and TcdB exotoxins. They are encoded on a 19.6-kb pathogenicity locus, the PaLoc (Rupnik et al., 2009). The PaLoc is thought to originate from an ancient prophage, since it shares a number of features with phages, in particular the tcdE gene encoding a phage-like holin involved in toxin secretion (Govind and Dupuy, 2012; Govind et al., 2015; Monot et al., 2015). Prophage induction per se has not been directly associated with toxin release or synthesis in C. difficile, as opposed to Shiga toxin-encoding phages in Escherichia coli (Kimmitt et al., 1999; Zhang et al., 2000) but some prophages interfere with toxin synthesis. For example, phage ϕCD119 was shown to express the RepR repressor, capable to bind a DNA region in the promoter of tcdR in the PaLoc, resulting in repression of toxin genes (Govind et al., 2009). On the contrary, phage ϕCD38-2 was shown to increase transcription of all five PaLoc genes by a yet unknown mechanism, resulting in more toxins produced in vitro. However, the impact of ϕCD38-2 on toxin synthesis was strain-dependent (Sekulovic et al., 2011) and similar observations were reported with other C. difficile phages (Goh et al., 2005a), suggesting that the influence of a prophage on its host partly depends on the genetic background.
A complete binary toxin locus (CdtLoc) has been recently identified in the genome of phage phiSemix9P1 (Riedel et al., 2017). The binary toxin, normally located on a 6.2-kb chromosomal locus called CdtLoc comprises 2 genes coding for the toxin components, cdtA and cdtB, as well as a regulator encoded by cdtR (Carman et al., 2011; Gerding et al., 2014). Of note, all three genes from the CdtLoc were shown to be transcribed from the phiSemix9P1 prophage, suggesting that it is functional, although no toxin assays have been performed (Riedel et al., 2017). The CdtLoc is present only in a subset of C. difficile isolates, including the epidemic ribotype 027 isolates (Bauer et al., 2011), and studies suggest that CDT contributes to virulence by promoting adhesion to epithelial cells (Schwan et al., 2009, 2014; Gerding et al., 2014). phiSemix9P1 has limited DNA homology with another C. difficile phage, ϕCD505, and the large pCDBI1 plasmid, suggesting that it is genetically unique. The identification of a CDT-encoding phage is intriguing but it might represent a rare isolated case, since it has never been observed in other C. difficile phages, including the numerous prophages identified in the course of genome sequencing projects. Nevertheless, it further supports the evolutionary role of phages in toxin conversion of C. difficile (Riedel et al., 2017).
Prophage Gene Expression During Lysogeny
During active phage replication, the transcriptional program of the host is profoundly restructured and metabolic resources are redirected toward phage replication. However, during the lysogenic cycle, prophages are generally quiescent and minimal gene transcription is observed from the prophage itself. Only a few gene products are required to establish and maintain lysogeny (Ainsworth et al., 2013), the CI repressor from the E. coli phage Lambda being the most well-characterized gene expressed during lysogeny (Oppenheim et al., 2005; Rokney et al., 2008).
Very little is known about transcriptional reprogramming during phage infection or lysogeny in C. difficile. In fact, only one study has looked at global gene expression during lysogeny (Sekulovic and Fortier, 2015). In that study, the ϕCD38-2 prophage was introduced into the epidemic strain R20291 and mRNA levels were assessed by RNAseq. It is important to mention that the prophage was maintained as a circular extrachromosomal DNA, so the host genome integrity was unaffected. On a genome-wide scale, the expression of 39 genes was significantly altered by the introduction of the prophage, including genes from the phi027 prophage already present in the host. This further supports the existence of some crosstalk between prophages (Sekulovic and Fortier, 2015). Two-thirds of the differentially expressed genes were downregulated twofold to threefold, and half of the differentially expressed genes were related to sugar uptake and metabolism, suggesting a possible impact on growth kinetics. Of note, the cwpV gene encoding a conserved surface protein was induced 20-fold in the lysogen. Transcription of cwpV is dependent on the configuration of a genetic switch located between the promoter and the gene. Recombination of the switch, catalyzed by the host-encoded RecV recombinase, turns transcription of cwpV ON or OFF in a phase-variable manner (Reynolds et al., 2011; Fagan and Fairweather, 2014). Only ∼5% of bacterial cells in culture express the CwpV protein at their surface, but in the R20291 lysogen carrying ϕCD38-2, this proportion increased to 95%, hence explaining the higher mRNA levels observed. The exact mechanism by which ϕCD38-2 influences phase variation remains unknown (Sekulovic and Fortier, 2015). CwpV is a large conserved cell wall protein suspected to contribute to cell adhesion and biofilm formation, and possibly immune evasion (Reynolds et al., 2011). The location of the protein at the cell surface and its apparent link with lysogeny suggested that it could play some role in phage infection. It turned out that CwpV has strong antiphage activity against several C. difficile phages of the Siphoviridae and Myoviridae families when overexpressed from a plasmid or from a “locked-ON” strain. Current data suggest that CwpV functions as a superinfection exclusion system (Sekulovic et al., 2015) that blocks phage DNA injection. The biological relevance of such an antiphage system seems obvious in the context of the gut microbiota. CwpV-ON strains would be protected from lytic phage attacks, which are expected to be relatively frequent in the gut due to high phage and bacterial densities (Manrique et al., 2016, 2017). Higher numbers of CwpV-ON cells could also contribute to colonization of the gut through increased bacterial adhesion and biofilm formation. Maybe of greater concern, however, is the fact that cwpV-expressing cells are naturally occurring in vitro due to phase variation and these cells are resistant to phage infection. Hence, looking at future phage therapy perspectives (Nale et al., 2016b), naturally occurring CwpV-positive cells in the gut could potentially compromise the efficacy of therapeutic phages. Further in vivo assays will be required to clarify the biological role and consequences of CwpV expression.
Other Impacts of Prophages on their Host
Several phage genomes carry cargo genes unrelated to the phage replication cycle, and their expression is often independent from the phage circuitry and occurs during lysogeny. The genes often code for virulence factors, including toxins, superantigens, and hydrolytic enzymes (Brüssow et al., 2004; Fortier and Sekulovic, 2013). Certain prophage genes can also provide phage immunity via superinfection exclusion (Mahony et al., 2008; Labrie et al., 2010).
The genomes of many C. difficile prophages encode genes that are suspected to influence their host. For example, the large phiCD211-like phages encode putative multidrug resistance genes, spore proteases, and multiple regulators that could interfere with host regulation (Garneau et al., 2018). A CRISPR array with a cas3 gene was also identified, suggesting that phiCD211-like phages possibly participate in CRISPR interference. The presence of CRISPR arrays has been reported in other C. difficile phages, including the two prophages from strain 630 (Hargreaves et al., 2014a; Boudry et al., 2015) as well as the phi027 prophage present in the epidemic strain R20291 and most R027 isolates (Sekulovic and Fortier, 2015). Transcriptomic analyses by RNAseq showed that these CRISPR arrays are transcribed and thus, possibly contribute to C. difficile resistance to invading DNA (Boudry et al., 2015; Sekulovic and Fortier, 2015).
Phage phiCDHM1 and other predicted C. difficile prophages encode homologs of an Agr-like quorum sensing (QS) system (Hargreaves et al., 2014b). QS is used to coordinate specific phenotypes at the whole population level in function of cell density. QS has been implicated in virulence of several pathogens, by coordinating toxin secretion, biofilm production, motility, and sporulation (Novick and Geisinger, 2008; Antunes et al., 2010; Rutherford and Bassler, 2012). The Agr system of Staphylococcus aureus is the most well-characterized QS system in Gram-positive bacteria, and regulates the expression of hundreds of genes, including exotoxins and surface proteins. It is encoded by an operon of four genes, agrD-agrB-agrC-agrA (Novick and Geisinger, 2008). At least two types of QS systems have been described in C. difficile; one is similar to the S. aureus Agr, while the other is related to the luxS/AI-2 from Vibrio harveyi (Stabler et al., 2009). Both systems control the expression of C. difficile toxins in function of cell density (Lee and Song, 2005; Martin et al., 2013; Darkoh et al., 2015). During lysogeny, the agrB and agrC genes are expressed from the phiCDHM1 prophage, suggesting that these components of the QS system are active. However, in the absence of an agrA homolog in phiCDHM1, it is impossible to conclude if the system is functional or not and whether it participates in some way to QS. We can speculate that AgrB and AgrC contribute to autoinducer secretion and signal detection, but no response would be elicited due to the absence of an associated response regulator. Alternatively, these phage-encoded genes could partly complement another Agr system from the host (Hargreaves et al., 2014b). QS likely affects multiple phenotypes in C. difficile (Martin et al., 2013) so it will be interesting to establish whether phage-encoded QS genes influence virulence-associated phenotypes such as toxin production, sporulation, or biofilm formation. QS signals detected by the phage-encoded AgrC could also lead to prophage induction, as observed with soil bacteria (Ghosh et al., 2009). This could be a means for the prophage to “determine” the best moment to initiate a replication cycle that will ensure its successful propagation into the bacterial population. It is therefore reasonable to hypothesize that during infection of the gut, high cell densities would trigger prophage induction, hence promoting phage dissemination and possibly horizontal gene transfer. Further research on the impact of QS on prophage stability would be necessary.
Conclusion and Perspectives
The contribution of phages to the evolution and virulence of C. difficile remains to be clarified (Fortier and Sekulovic, 2013). So far, prophages seem to impact C. difficile’s lifestyle and biology in subtle ways, depending on the genetic background of the host. Studying phage–host interactions requires extensive knowledge of the biology of the phage and the host. Unfortunately, many phage genes have no homologs in databases or have no assigned function. Therefore, one way to investigate the impact of a prophage on its host is to introduce a given temperate phage into a susceptible bacterial host to create a new lysogen and to study various phenotypes in comparison with the parental strain lacking that prophage. However, bacterial genomes often carry multiple prophages and phenotypes can sometimes result from the cumulative effects of more than one prophages, like reported for the DNAses secreted by Streptococcus pyogenes SF370 (Euler et al., 2016). In addition, natural lysogens have been carrying prophages for extensive periods of time and as such, the prophages’ regulatory circuits are often seamlessly integrated into the host network (Ehrbar and Hardt, 2005). Therefore, a better alternative is to remove parts or whole prophages from their natural lysogen to study their impact. Such “prophage-cured” strains can then be compared with the lysogenic parental strain. Curing lysogens from their prophages can be quite challenging depending on the host and the availability of molecular tools. Reports of successful curing using extensive screening for spontaneous prophage-cured mutants, or using allelic exchange with counter selection methods have been published in Gram-negative (e.g., E. coli) and Gram-positive bacteria (e.g., S. pyogenes, S. aureus). These studies have shed light on the role of individual prophages as well as their combined contribution to virulence of their host (Bae et al., 2006; Wang et al., 2010; Euler et al., 2016). Of note, curing of one of the two prophages from C. difficile strain 630 has been recently reported, and involved the use of the CRISPR technology (Hong et al., 2018). This first example of prophage curing in C. difficile paves the way for additional studies on the role of prophages in this pathogen, in particular in epidemic strains such as the R20291 that carries the conserved phi027 prophage (Stabler et al., 2009). Better understanding how phages interact with C. difficile at the molecular level will be essential, especially for future phage therapy applications. Hence research focusing on identifying the cell receptor(s) and the phages’ receptor binding protein and how these two influence the phages’ host range will be crucial. In addition, understanding how phages affect C. difficile and whole bacterial populations in complex ecosystems such as the gut microbiota will be determinant as well. For instance, transfer of certain phages from donors to recipients seems to contribute to the success of fecal microbiota transplantation to treat recurrent C. difficile infections (Zuo et al., 2017). Studying the interplay between the virome and the microbiome in health and disease is thus of high relevance. In conclusion, there is a lot more to discover about C. difficile phages and the newly developed molecular tools and the availability of bacterial genome sequences will certainly foster research in this domain.
Author Contributions
L-CF collected the ideas, concepts, and interpretations, as well as wrote the manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by a discovery grant from the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2015-06334). L-CF is a member of the Centre de Recherche du CHUS.
References
- Ackermann H.-W., Prangishvili D. (2012). Prokaryote viruses studied by electron microscopy. Arch. Virol. 157 1843–1849. 10.1007/s00705-012-1383-y [DOI] [PubMed] [Google Scholar]
- Ainsworth S., Zomer A., Mahony J., van Sinderen D. (2013). Lytic infection of Lactococcus lactis by bacteriophages Tuc2009 and c2 triggers alternative transcriptional host responses. Appl. Environ. Microbiol. 79 4786–4798. 10.1128/AEM.01197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy J., Bulach D., Knight D., Riley T., Johanesen P., Lyras D. (2018). Identification of large cryptic plasmids in Clostridioides (Clostridium) difficile. Plasmid 9 25–38. 10.1016/j.plasmid.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Antunes L. C. M., Ferreira R. B. R., Buckner M. M. C., Finlay B. B. (2010). Quorum sensing in bacterial virulence. Microbiology 156 2271–2282. 10.1099/mic.0.038794-0 [DOI] [PubMed] [Google Scholar]
- Arndt D., Grant J. R., Marcu A., Sajed T., Pon A., Liang Y., et al. (2016). PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44 W16–W21. 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt D., Marcu A., Liang Y., Wishart D. S. (2017). PHAST, PHASTER and PHASTEST: tools for finding prophage in bacterial genomes. Brief. Bioinformatics 4:354. 10.1093/bib/bbx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu K. S., Spence W. S., Monteville M. R., Geller B. L. (1995). Characterization of a cloned gene (pip) from Lactococcus lactis required for phage infection. Dev. Biol. Stand. 85 569–575. [PubMed] [Google Scholar]
- Bae T., Baba T., Hiramatsu K., Schneewind O. (2006). Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62 1035–1047. 10.1111/j.1365-2958.2006.05441.x [DOI] [PubMed] [Google Scholar]
- Bauer M. P., Notermans D. W., van Benthem B. H. B., Brazier J. S., Wilcox M. H., Rupnik M., et al. (2011). Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377 63–73. 10.1016/S0140-6736(10)61266-4 [DOI] [PubMed] [Google Scholar]
- Bebeacua C., Tremblay D., Farenc C., Chapot-Chartier M.-P., Sadovskaya I., van Heel M., et al. (2013). Structure, adsorption to host, and infection mechanism of virulent lactococcal phage p2. J. Virol. 87 12302–12312. 10.1128/JVI.02033-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry P., Semenova E., Sekulovic O., Fortier L.-C., Semenov E., Monot M., et al. (2015). Function of the CRISPR-cas system of the human pathogen: Clostridium difficile. mBio 6 e1112–e1115. 10.1128/mBio.01112-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Canchaya C., Hardt W.-D. (2004). Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68 560–602. 10.1128/MMBR.68.3.560-602.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Hendrix R. W. (2002). Phage genomics: small is beautiful. Cell 108 13–16. [DOI] [PubMed] [Google Scholar]
- Carman R. J., Stevens A. L., Lyerly M. W., Hiltonsmith M. F., Stiles B. G., Wilkins T. D. (2011). Clostridium difficile binary toxin (CDT) and diarrhea. Anaerobe 17 161–165. 10.1016/j.anaerobe.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Darkoh C., DuPont H. L., Norris S. J., Kaplan H. B. (2015). Toxin synthesis by Clostridium difficile is regulated through quorum signaling. mBio 6:e02569. 10.1128/mBio.02569-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei R. (1989). Observations on phage-typing of Clostridium difficile: preliminary evaluation of a phage panel. Eur. J. Epidemiol. 5 351–354. [DOI] [PubMed] [Google Scholar]
- Ehrbar K., Hardt W.-D. (2005). Bacteriophage-encoded type III effectors in Salmonella enterica subspecies 1 serovar Typhimurium. Infect. Genet. Evol. 5 1–9. 10.1016/j.meegid.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Euler C. W., Juncosa B., Ryan P. A., Deutsch D. R., McShan W. M., Fischetti V. A. (2016). Targeted curing of all lysogenic bacteriophage from Streptococcus pyogenes using a novel counter-selection technique. PLoS One 11:e0146408. 10.1371/journal.pone.0146408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan R. P., Fairweather N. F. (2014). Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 12 211–222. 10.1038/nrmicro3213 [DOI] [PubMed] [Google Scholar]
- Fimlaid K. A., Shen A. (2015). Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 24C, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier L.-C., Sekulovic O. (2013). Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 4 354–365. 10.4161/viru.24498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau J. R., Sekulovic O., Dupuy B., Soutourina O., Monot M., Fortier L.-C. (2018). High Prevalence and Genetic Diversity of Large phiCD211 (phiCDIF1296T)-Like Prophages in Clostridioides difficile. Appl. Environ. Microbiol. 84:e02164-17. 10.1128/AEM.02164-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart D., Lok S., Clare S., Tomas M., Stares M., Scholl D., et al. (2015). A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 6:e02368-14. 10.1128/mBio.02368-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart D., Williams S. R., Bishop-Lilly K. A., Govoni G. R., Willner K. M., Butani A., et al. (2012). Novel high-molecular-weight, R-type bacteriocins of Clostridium difficile. J. Bacteriol. 194 6240–6247. 10.1128/JB.01272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Johnson S., Rupnik M., Aktories K. (2014). Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes 5 15–27. 10.4161/gmic.26854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Roy K., Williamson K. E., Srinivasiah S., Wommack K. E., Radosevich M. (2009). Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl. Environ. Microbiol. 75 7142–7152. 10.1128/AEM.00950-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S., Chang B. J., Riley T. V. (2005a). Effect of phage infection on toxin production by Clostridium difficile. J. Med. Microbiol. 54 129–135. 10.1099/jmm.0.45821-0 [DOI] [PubMed] [Google Scholar]
- Goh S., Riley T. V., Chang B. J. (2005b). Isolation and characterization of temperate bacteriophages of Clostridium difficile. Appl. Environ. Microbiol. 71 1079–1083. 10.1128/AEM.71.2.1079-1083.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S., Hussain H., Chang B. J., Emmett W., Riley T. V., Mullany P. (2013). Phage C2 Mediates Transduction of Tn6215, Encoding Erythromycin Resistance, between Clostridium difficile Strains. mBio 4:e00840-13. 10.1128/mbio.00840-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S., Ong P. F., Song K. P., Riley T. V., Chang B. J. (2007). The complete genome sequence of Clostridium difficile phage phiC2 and comparisons to phiCD119 and inducible prophages of CD630. Microbiology 153 676–685. 10.1099/mic.0.2006/002436-0 [DOI] [PubMed] [Google Scholar]
- Govind R., Dupuy B. (2012). Secretion of Clostridium difficile toxins A and B requires the holin-like protein TcdE. PLoS Pathog. 8:e1002727. 10.1371/journal.ppat.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind R., Fitzwater L., Nichols R. (2015). Observations on the Role of TcdE Isoforms in Clostridium difficile Toxin Secretion. J. Bacteriol. 197 2600–2609. 10.1128/JB.00224-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind R., Fralick J. A., Rolfe R. D. (2006). Genomic organization and molecular characterization of Clostridium difficile bacteriophage PhiCD119. J. Bacteriol. 188 2568–2577. 10.1128/JB.188.7.2568-2577.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind R., Vediyappan G., Rolfe R. D., Dupuy B., Fralick J. A. (2009). Bacteriophage-mediated toxin gene regulation in Clostridium difficile. J. Virol. 83 12037–12045. 10.1128/JVI.01256-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen J. D., Sonenshein A. L. (2003). Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Mol. Microbiol. 48 811–821. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. R., Clokie M. R. J. (2014). Clostridium difficile phages: still difficult? Front. Microbiol. 5:184 10.3389/fmicb.2014.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K. R., Clokie M. R. J. (2015). A Taxonomic Review of Clostridium difficile Phages and Proposal of a Novel Genus, “Phimmp04likevirus”. Viruses 7 2534–2541. 10.3390/v7052534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K. R., Flores C. O., Lawley T. D., Clokie M. R. J. (2014a). Abundant and diverse clustered regularly interspaced short palindromic repeat spacers in Clostridium difficile strains and prophages target multiple phage types within this pathogen. mBio 5:e01045-13. 10.1128/mBio.01045-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K. R., Kropinski A. M., Clokie M. R. J. (2014b). What does the talking?: quorum sensing signaling genes discovered in a bacteriophage genome. PLoS One 9:e85131. 10.1371/journal.pone.0085131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Zhang J., Cui G., Wang L., Wang Y. (2018). Multiplexed CRISPR-Cpf1-mediated genome editing in Clostridium difficile toward the understanding of pathogenesis of C. difficile Infection. ACS Synth. Biol. 7 1588–1600. 10.1021/acssynbio.8b00087 [DOI] [PubMed] [Google Scholar]
- Horgan M., O’Sullivan O., Coffey A., Fitzgerald G. F., van Sinderen D., McAuliffe O., et al. (2010). Genome analysis of the Clostridium difficile phage PhiCD6356, a temperate phage of the Siphoviridae family. Gene 462 34–43. 10.1016/j.gene.2010.04.010 [DOI] [PubMed] [Google Scholar]
- Kimmitt P. T., Harwood C. R., Barer M. R. (1999). Induction of type 2 Shiga toxin synthesis in Escherichia coli O157 by 4-quinolones. Lancet 353 1588–1589. [DOI] [PubMed] [Google Scholar]
- Kirk J. A., Gebhart D., Buckley A. M., Lok S., Scholl D., Douce G. R., et al. (2017). New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Science Transl. Med. 9:eaah6813. 10.1126/scitranslmed.aah6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie S. J., Samson J. E., Moineau S. (2010). Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8 317–327. 10.1038/nrmicro2315 [DOI] [PubMed] [Google Scholar]
- Lee A. S. Y., Song K. P. (2005). LuxS/autoinducer-2 quorum sensing molecule regulates transcriptional virulence gene expression in Clostridium difficile. Biochem. Biophys. Res. Commun. 335 659–666. 10.1016/j.bbrc.2005.07.131 [DOI] [PubMed] [Google Scholar]
- Lemire S., Figueroa-Bossi N., Bossi L. (2011). Bacteriophage crosstalk: coordination of prophage induction by trans-acting antirepressors. PLoS Genet. 7:e1002149. 10.1371/journal.pgen.1002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony D. E., Bell P. D., Easterbrook K. B. (1985). Two bacteriophages of Clostridium difficile. J. Clin. Microbiol. 21 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony D. E., Clow J., Atkinson L., Vakharia N., Schlech W. F. (1991). Development and application of a multiple typing system for Clostridium difficile. Appl. Environ. Microbiol. 57 1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J., McGrath S., Fitzgerald G. F., van Sinderen D. (2008). Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl. Environ. Microbiol. 74 6206–6215. 10.1128/AEM.01053-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique P., Bolduc B., Walk S. T., van der Oost J., de Vos W. M., Young M. J. (2016). Healthy human gut phageome. Proc. Natl. Acad. Sci. U.S.A. 113 10400–10405. 10.1073/pnas.1601060113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique P., Dills M., Young M. J. (2017). The human gut phage community and its implications for health and disease. Viruses 9 141. 10.3390/v9060141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. J., Clare S., Goulding D., Faulds-Pain A., Barquist L., Browne H. P., et al. (2013). The agr locus regulates virulence and colonization genes in Clostridium difficile 027. J. Bacteriol. 195 3672–3681. 10.1128/JB.00473-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. J., Narbad A., Gasson M. J. (2008). Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J. Bacteriol. 190 6734–6740. 10.1128/JB.00686-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader E., Mayer M. J., Gasson M. J., Steverding D., Carding S. R., Narbad A. (2010). Bacteriophage treatment significantly reduces viable Clostridium difficile and prevents toxin production in an in vitro model system. Anaerobe 16 549–554. 10.1016/j.anaerobe.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Meader E., Mayer M. J., Steverding D., Carding S. R., Narbad A. (2013). Evaluation of bacteriophage therapy to control Clostridium difficile and toxin production in an in vitro human colon model system. Anaerobe 22 25–30. 10.1016/j.anaerobe.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Meessen-Pinard M., Sekulovic O., Fortier L.-C. C. (2012). Evidence of in vivo prophage induction during Clostridium difficile infection. Appl. Environ. Microbiol. 78 7662–7670. 10.1128/AEM.02275-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S. R., Lee H. H., Spina C. S., Collins J. J. (2013). Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499 219–222. 10.1038/nature12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monot M., Eckert C., Lemire A., Hamiot A., Dubois T., Tessier C., et al. (2015). Clostridium difficile: new insights into the evolution of the pathogenicity locus. Sci. Rep. 5:15023. 10.1038/srep15023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W., Duceppe M.-O., Dupras A. A., Usongo V., Jeukens J., Freschi L., et al. (2018). Salmonella enterica prophage sequence profiles reflect genome diversity and can be used for high discrimination subtyping. Front. Microbiol. 9:836. 10.3389/fmicb.2018.00836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nale J., Redgwell T., Millard A., Clokie M. (2018). Efficacy of an optimised bacteriophage cocktail to clear Clostridium difficile in a batch fermentation model. Antibiotics 7:13. 10.3390/antibiotics7010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nale J. Y., Chutia M., Carr P., Hickenbotham P. T., Clokie M. R. J. (2016a). Biofilm and wax moth (Galleria mellonella) models reveal new insights into the therapeutic potential of Clostridium difficile bacteriophages. Front. Microbiol. 7:1383. 10.3389/fmicb.2016.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nale J. Y., Spencer J., Hargreaves K. R., Buckley A. M., Trzepiński P., Douce G. R., et al. (2016b). Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob. Agents Chemother. 60 968–981. 10.1128/AAC.01774-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J. M., Handley S. A., Baldridge M. T., Droit L., Liu C. Y., Keller B. C., et al. (2015). Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160 447–460. 10.1016/j.cell.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Geisinger E. (2008). Quorum sensing in staphylococci. Annu. Rev. Genet. 42 541–564. 10.1146/annurev.genet.42.110807.091640 [DOI] [PubMed] [Google Scholar]
- Oppenheim A. B., Kobiler O., Stavans J., Court D. L., Adhya S. (2005). Switches in bacteriophage lambda development. Annu. Rev. Genet. 39 409–429. 10.1146/annurev.genet.39.073003.113656 [DOI] [PubMed] [Google Scholar]
- Ramírez-Vargas G., Goh S., Rodríguez C. (2018). The novel phages phiCD5763 and phiCD2955 represent two groups of big plasmidial siphoviridae phages of Clostridium difficile. Front. Microbiol. 9:26. 10.3389/fmicb.2018.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid S. J., Barylski J., Hargreaves K. R., Millard A. A., Vinner G. K., Clokie M. R. J. (2016). Two novel myoviruses from the north of iraq reveal insights into Clostridium difficile phage diversity and biology. Viruses 8:310. 10.3390/v8110310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. B., Emerson J. E., de la Riva L., Fagan R. P., Fairweather N. F. (2011). The Clostridium difficile cell wall protein CwpV is antigenically variable between strains, but exhibits conserved aggregation-promoting function. PLoS Pathog. 7:e1002024. 10.1371/journal.ppat.1002024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel T., Wittmann J., Bunk B., Schober I., Spröer C., Gronow S., et al. (2017). A Clostridioides difficile bacteriophage genome encodes functional binary toxin-associated genes. J. Biotechnol. 250 23–28. 10.1016/j.jbiotec.2017.02.017 [DOI] [PubMed] [Google Scholar]
- Rokney A., Kobiler O., Amir A., Court D. L., Stavans J., Adhya S., et al. (2008). Host responses influence on the induction of lambda prophage. Mol. Microbiol. 68 29–36. 10.1111/j.1365-2958.2008.06119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M., Wilcox M. H., Gerding D. N. (2009). Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7 526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- Rutherford S. T., Bassler B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. 10.1101/cshperspect.a012427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- São-José C., Lhuillier S., Lurz R., Melki R., Lepault J., Santos M. A., et al. (2006). The ectodomain of the viral receptor YueB forms a fiber that triggers ejection of bacteriophage SPP1 DNA. J. Biol. Chem. 281 11464–11470. 10.1074/jbc.M513625200 [DOI] [PubMed] [Google Scholar]
- Saujet L., Pereira F. C., Henriques A. O., Martin-Verstraete I. (2014). The regulatory network controlling spore formation in Clostridium difficile. FEMS Microbiol. Lett. 358 1–10. 10.1111/1574-6968.12540 [DOI] [PubMed] [Google Scholar]
- Schwan C., Kruppke A. S., Nölke T., Schumacher L., Koch-Nolte F., Kudryashev M., et al. (2014). Clostridium difficile toxin CDT hijacks microtubule organization and reroutes vesicle traffic to increase pathogen adherence. Proc. Nat. Acad. Sci. U.S.A. 111 2313–2318. 10.1073/pnas.1311589111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan C., Stecher B., Tzivelekidis T., van Ham M., Rohde M., Hardt W.-D., et al. (2009). Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 5:e1000626. 10.1371/journal.ppat.1000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovic O., Fortier L.-C. C. (2015). Global transcriptional response of Clostridium difficile carrying the phiCD38-2 prophage. Appl. Environ. Microbiol. 81 1364–1374. 10.1128/AEM.03656-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovic O., Garneau J. R., Néron A., Fortier L.-C. C. (2014). Characterization of temperate phages infecting Clostridium difficile isolates of human and animal origins. Appl. Environ. Microbiol. 80 2555–2563. 10.1128/AEM.00237-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovic O., Meessen-Pinard M., Fortier L.-C. (2011). Prophage-stimulated toxin production in Clostridium difficile NAP1/027 lysogens. J. Bacteriol. 193 2726–2734. 10.1128/JB.00787-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovic O., Ospina-Bedoya M., Fivian-Hughes A. S., Fairweather N. F., Fortier L.-C. (2015). The Clostridium difficile cell wall protein CwpV confers phase-variable phage resistance. Mol. Microbiol. 98 329–342. 10.1111/mmi.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell T. L., Schaberg D. R., Fekety F. R. (1983). Bacteriophage and bacteriocin typing scheme for Clostridium difficile. J. Clin. Microbiol. 17 1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Patel K. V., Hickenbotham P. T., Nale J. Y., Hargreaves K. R., Clokie M. R. J. (2012). Prophage carriage and diversity within clinically relevant strains of Clostridium difficile. Appl. Environ. Microbiol. 78 6027–6034. 10.1128/AEM.01311-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Rockenbach K., Dedrick R. M., VanDemark A. P., Hatfull G. F. (2014). Cross-talk between Diverse Serine Integrases. J. Mol. Biol. 426 318–331. 10.1016/j.jmb.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler R. A., He M., Dawson L., Martin M., Valiente E., Corton C., et al. (2009). Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. 10.1186/gb-2009-10-9-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kim Y., Ma Q., Hong S. H., Pokusaeva K., Sturino J. M., et al. (2010). Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 1:147. 10.1038/ncomms1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann J., Riedel T., Bunk B., Spröer C., Gronow S., Overmann J. (2015). Complete Genome Sequence of the Novel Temperate Clostridium difficile Phage phiCDIF1296T. Genome Announc. 3:e00839-15. 10.1128/genomeA.00839-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., McDaniel A. D., Wolf L. E., Keusch G. T., Waldor M. K., Acheson D. W. (2000). Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181 664–670. 10.1086/315239 [DOI] [PubMed] [Google Scholar]
- Zuo T., Wong S. H., Lam K., Lui R., Cheung K., Tang W., et al. (2017). Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 67 634–643. 10.1136/gutjnl-2017-313952 [DOI] [PMC free article] [PubMed] [Google Scholar]