Abstract
NACs are one of the largest transcription factor families in plants and are involved in the response to abiotic stress. BoNAC019, a homologue of AtNAC019, was isolated from cabbage (Brassica oleracea). BoNAC019 was localized in the nucleus and functioned as a transcriptional activator. The expression of BoNAC019 was induced by dehydration, salt, abscisic acid (ABA), and H2O2 treatments. BoNAC019 overexpressing plants were generated to explore the function of BoNAC019 in response to drought stress. Overexpression (OE) of BoNAC019 reduced drought tolerance with lower survival rate, higher water loss rate, lower proline content and ABA content. The seed germination and root length assays of BoNAC019-OE plants showed decreased sensitivity to ABA. Under drought condition, antioxidant enzymes and anthocyanin content decreased in BoNAC019 -OE plants, resulting in the accumulation of more reactive oxygen species (ROS), which cause damage to plants. Several stress-responsive genes, antioxidant enzymatic genes, anthocyanin biosynthetic genes and ABA signaling genes were down-regulated under drought condition while the ABA catabolism genes were induced in BoNAC019-OE plants under both normal and drought conditions. Our results demonstrated that BoNAC019 might participated in regulating drought tolerance by inducing ABA catabolism genes and decreasing ABA content.
Introduction
Drought stress induces lots of changes in plants and limits plant growth, development, and productivity1–3. A set of strategies were evolved to cope with drought stress in plants, including shortening the life cycle, reducing water loss, adjusting osmotic content, and altering gene expression and cellular metabolism4–6. Transcription factors play important roles in different biological processes7–9.
NACs are one of the largest transcription factor families in plants, it has been reported in many species. There are 117 NAC genes in Arabidopsis, 151 in rice, and 152 each in soybean and tobacco10–12. The C-terminal region of NAC proteins is transcriptional region, and the N-terminal region is highly conserved, and can be divided into five subdomains (A–E)13–15.
NAC transcription factors play key roles in complex drought signaling processes16,17. AtNAC019, AtNAC055, and AtNAC072 were stress-responsive NAC genes, the expressions of these genes were induced by drought treatment. Overexpressing AtNAC019, AtNAC055, and AtNAC072 improved drought tolerance and up-regulated the expression of ERD1 (early responsive to drought 1)16,18. OsNAC1 was reported as a stress responsive NAC gene, overexpressing OsNAC1 enhanced drought tolerance in transgenic rice, and lots of stress-responsive genes were induced in OsNAC1 overexpressing plants. OsERD1 was verified as the target gene of OsNAC117. Overexpressing OsNAC3 in rice showed improved tolerance to heat and drought stresses in transgenic plants. Moreover, OsNAC3 directly regulated the expression of five ROS-associated genes19. RhNAC3 was reported as a novel rose NAC and induced by dehydration. Overexpressing RhNAC3 improved drought tolerance in transgenic Arabidopsis, and many genes respond to stress were induced in overexpressing lines20.
Abiotic acid (ABA) is the most important phytohormone for plants to resist abiotic stresses, especially for drought stress. When plants suffered from drought stress, ABA content is significantly increased and might result in complex changes, such as stomatal closure, inducing the expression of numerous stress responsive genes and eventually leading to physiological responses21,22. In last twenty years, ABA synthesis and signaling genes has been widely studied in different species. The synthesis gene NCED (9-cis-epoxycarotenoid dioxygenase) was cloned from crops and shown to help plants to resist drought stress23,24. For ABA signaling, the receptors named PYRs (pyrabactin resistances) were reported in 2009. Overexpressing these genes have been verified to improve drought stress resistance in Arabidopsis25,26. Overexpression of CsATAF1 enhanced the hypersensitivity to ABA and drought tolerance by directly regulating the expressions of CsDREB2C and CsABI527.
Adverse environmental conditions lead to the accumulation of ROS in plants28,29. Enzymatic antioxidants and non-enzymatic antioxidants, including ascorbate, glutathione (GSH), carotenoids, tocopherols, and flavonoids are defense systems for scavenging ROS in plants30,31.
Anthocyanins are water soluble flavonoid pigments in plants. A variety of stress factors affect anthocyanin biosynthesis and accumulation in plants32,33. Drought conditions promote anthocyanin synthesis to improve drought resistance by scavenging ROS34,35. Chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), Phenylalanine ammonia-lyase (PAL), cinnamic, leucoanthocyanidin dioxygenase (LDOX), acid 4-hydroxylase (C4H), dihydroflavonol 4-reductase (DFR), chalcone isomerase (CHI), and UDP -glucose: flavonoid 3-O-glucosyltransferase (UFGT) are important enzymes in the anthocyanin biosynthetic pathway. The MYB (TT2, PAP1, PAP2, MYB113, and MYB114) and bHLH (TT8, GL3, and EGL3) transcription factors interacted with the WD40 protein (TTG1) to regulate these anthocyanin biosynthetic genes36–40. Several NAC genes have been identified to participate in anthocyanin biosynthesis. Under a high light stress condition, AtNAC078 positively regulated anthocyanin production41, while JUB1/ANAC042 and AtNAC032 negatively regulated anthocyanin biosynthesis33,42.
Brassica oleracea is one of the most important vegetables of the Brassica species, and there are 271 NAC genes in the Chinese cabbage genome (http://brassicadb.org/brad/index.php)43,44. However, the functions of NAC transcription factors in response to abiotic stress and anthocyanin biosynthesis have not been reported in cabbage.
The expression of BoNAC019 was induced by abiotic stress treatments. BoNAC019-OE reduced drought tolerance in Arabidopsis, with higher water loss rates and higher MDA and proline contents. Overexpression of BoNAC019 accumulated more ROS and decreased antioxidant enzyme activities. QPCR experiments showed that the expressions of many stress responsive genes decreased in the OE lines.
Overexpression of BoNAC019 also reduced anthocyanin accumulation under drought conditions. Compared with the WT plants, anthocyanin content was much lower in BoNAC019-OE plants, and the expressions of anthocyanin biosynthetic genes decreased in BoNAC019-OE plants. These results showed that BoNAC019 negatively regulated the tolerance to drought stress and anthocyanin biosynthesis.
Methods and Materials
Cloning and sequence analysis of BoNAC019
According to the BoNAC019 cDNA sequence, the BoNAC019 gene was cloned and the primers were showed in supporting information Table S1. DNAman software was used to analyze the homology between BoNAC019 and the NAC proteins of other species, and the Neighbor-Joining (NJ) algorithm in MEGA program (ver. 5.0) was used to construct the phylogenetic tree.
Subcellular localization of the BoNAC019 protein
The pCAMBIA 1302 vector (Addgene, Cambridge, MA, USA) was used to analyze the subcellular location of BoNAC019. The fusion constructs (BoNAC019-GFP) and empty vector (GFP) were transformed by particle bombardment. The confocal microscopy (Nikon Inc., Melville, NY, USA) was used to observe these epidermal cells of onion with and without fluorescence after a 26 h incubation in the dark at 26 °C.
Transactivation assay of BoNAC019
The PCR products of BoNAC019 (GAL4BD - BoNAC019-FL1–361), the N-terminus of BoNAC019 (GAL4-BD- BoNAC019-N1–171) and the C-terminus of BoNAC019 (GAL4-BD- BoNAC019-C171–361) were fused into the GAL4-BD vector (Table S1). The vectors were transferred into Arabidopsis protoplasts20. The luciferase activity was measured by luminometer.
Growth conditions for the plant materials and qPCR analysis
The cabbage line studied was ‘Zhonggan-11’. The cabbage seedlings were planted under a 16 h light/8 h dark cycle were subjected to different stress treatments. Four-week-old cabbage seedlings were transferred into Hoagland nutrient solution containing 150 mM NaCl, 10% PEG, 10% H2O2, or 100 μM ABA for the indicated times. Leaves were collected at the designated time points after different stress treatments.
The qPCR was performed according to our laboratory’s own method described previously27. Three biological replicates were performed for each sample. The primer sequences utilized are listed in Supplemental Table 1.
Plant transformation and evaluation of drought stress tolerance
To investigate the function of BoNAC019, overexpressing Arabidopsis plants were generated. The BoNAC019 cDNA was fused into pBI121 (Table S1). The Agrobacterium tumefaciens strain GV3101 contained these constructs were transformed them into Arabidopsis Col-0 plants using the floral dip method45. Transgenic plant seeds were selected in MS containing kanamycin (80 mg/L). The positive plants were screened by PCR and qPCR.
Before the drought treatment, forty T3 transgenic plants and WT plants were grown for four weeks at 22 °C/16 °C and under a 16 h/8 h light/dark cycle. For drought assay, these seedlings were treated without watering for three weeks. The mock control (CK) were the seedlings treated with water. These drought treatments experiments were repeated three times.
Physiological index of transgenic plants and WT plants
The water loss rate and MDA content in leaves were measured according to Mao et al.46 and Zhang et al.47. Proline content and ABA content were measured according to Szekely et al.48 and Wang et al.49. H2O2 content and stained with DAB, superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities were measured in leaves using a method described previously19,50–52.
Seed germination and root length of transgenic and WT plants
Approximately 100 seeds of WT plants and BoNAC019-OE plants were germinated on MS medium containing 1 μM ABA for one week. The germination rate was calculated based on radicle protrusion. Each experiment was performed in triplicate. Seedlings grown on MS medium for five days were transferred to MS medium containing 1 μM ABA for five days. Each experiment was performed in triplicate. The mock control (CK) were the seedlings without ABA treatment.
Stomatal closure and aperture
We used epidermal strips of Arabidopsis for measuring the stomatal aperture. We merged the epidermal strips into the 30 mM KCl and 10 mM MES-KOH (pH 6.15) solution for 2.5 h at 22 °C and put them under light for fully opening the stomata53–55. Then, added ABA to the same solution for 2.5 h more. We measured more than 130 stomata of each lines using IMAGEJ 1.36b software (Broken Symmetry Software). Each experiment was performed in triplicates.
Anthocyanin content
WT plants and transgenic plants were germinated on MS medium for five days and were then transferred to MS containing 100 mM mannitol for five days to assess dehydration-induced accumulation of anthocyanin.
The absorbance spectra of anthocyanin are strikingly different with a change in pH56. The anthocyanin content of WT plants and BoNAC019-OE plants leaves were measured according to Zhang et al.32.
Results
Sequence analysis and transcriptional activation of the BoNAC019 gene
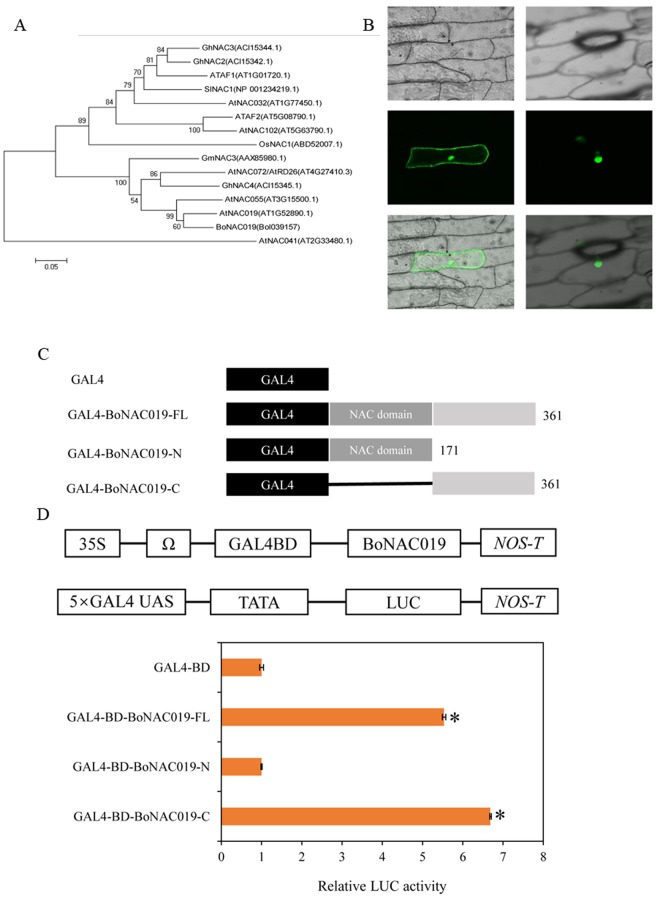
BoNAC019 (Bol039157) was cloned from cabbage and was the closest homologue to AtNAC019 gene (AT1G52890) in Arabidopsis. The multiple sequence alignment analysis showed that the N-terminal domain was highly conserved, while the C- terminal domain had low similarity with Arabidopsis proteins (Fig. S1). BoNAC019 clustered in the same clade as AtNAC019 and AtNAC055 by phylogenetic tree analysis (Fig. 1A). BoNAC019 is a putative transcription factor, and transient expression assays showed that BoNAC019 was located in nuclei (Fig. 1B).
Figure 1.
Sequence analysis of BoNAC019, nuclear localization and transcriptional activation of BoNAC019. (A) Phylogenetic tree of BoNAC019 and NAC members from other plant species. (B) Subcellular localization of the BoNAC019 protein in onion epidermal cells. (C) Transcription activation activity of BoNAC019. The full-length proteins (BoNAC019-FL), N-terminal fragment (BoNAC019-N) and C-terminal fragment (BoNAC019-C) were fused with the vector GAL4BD. (D) The plasmids containing the fusion genes and the empty control plasmid pGBKT7 were introduced into protoplasts. The GAL4BD vector was used as a negative control.
A GAL4 transient expression assay was used to investigate the transcriptional activity of BoNAC019 in Arabidopsis protoplasts. The relative LUC activities of Arabidopsis protoplasts transformed with GAL4BD-BoNAC019, GAL4BD-BoNAC019-C were significantly higher than negative control and GAL4BD-BoNAC019-N (Fig. 1C,D). These results showed that BoNAC019 functioned as a transcriptional activator.
Expression pattern of BoNAC019
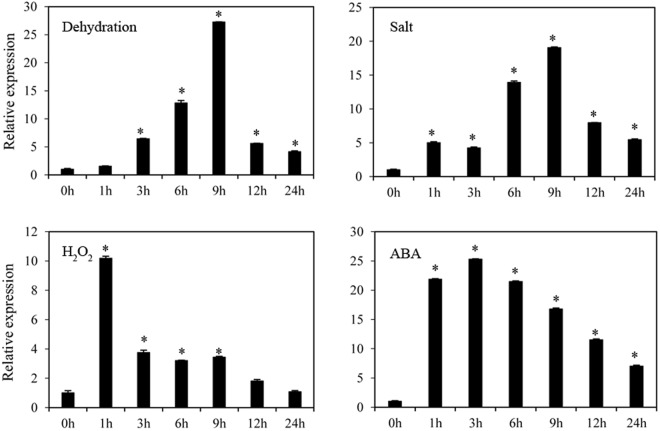
A variety of NAC transcription factors have been reported to respond to abiotic stress. QPCR experiments were used to detect the expression of BoNAC019 to further test whether BoNAC019 responds to abiotic stress. Under the dehydration treatment, BoNAC019 expression increased more than 25-fold after 12 h. Under the salt treatment, BoNAC019 expression increased more than 20-fold after 9 h. BoNAC019 expression was increased more than 12- and 20-fold under the ABA and H2O2 treatments, respectively (Fig. 2).
Figure 2.
Expression pattern of BoNAC019 in cabbage after stress treatments. Expression pattern of BoNAC019 in cabbage leaves after 150 mmol/L NaCl, 10% PEG6000, 10% H2O2, and 100 μM ABA treatments was performed via qPCR analysis. BoActin was used as the endogenous control. Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences from controls (*P < 0.05).
Overexpression of BoNAC019 reduces tolerance to drought in Arabidopsis
To investigate whether BoNAC019 functions in the response to drought stress, transgenic Arabidopsis plants were generated. After kanamycin resistance and PCR analyses, four transgenic lines were selected for further study. Among these, the expression levels of BoNAC019 in the OE1 and OE2 lines were much higher than those in the other lines by qPCR analysis (Fig. S2).
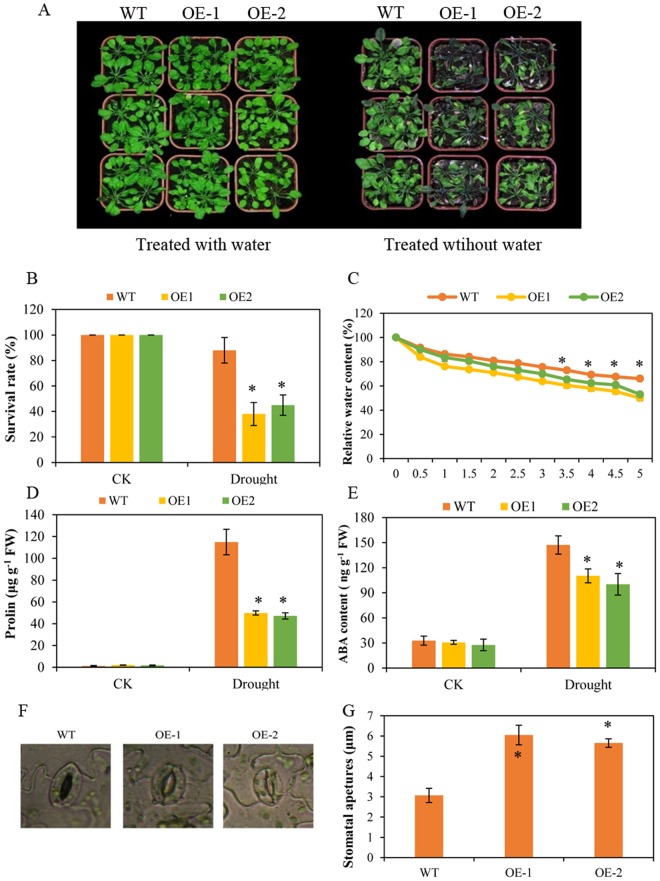
The growth performances of WT plants and transgenic plants were basically similar with well-watering condition. Without watering for three weeks, only 38% of the BoNAC019-OE plants survived, and was significantly lower than that of WT plants. Leaf wilting of BoNAC019-OE plants was much more serious than that observed in WT plants (Fig. 3A,B). Relative water content (RWC) in the OE1 and OE2 lines decreased to 50% and 53%, respectively after a 5 h incubation, while WT plants retained almost 65% of their fresh weight (Fig. 3C), and the stomatal apertures of overexpressing plants were larger than those of WT plants (Fig. 3F,G). These results indicating that the water retaining capacity of overexpressing plants was weaker. The free proline content and ABA content increased under abiotic stress conditions to cope with these stresses, the results showed that after drought treatment, the proline and ABA contents of WT plants and OE plants increased and proline and ABA contents were much lower in OE plants (Fig. 3D,E). BoNAC019 as the closest homologue gene to AtNAC019, the growth performances of the overexpression plants in response to drought stress were quite different. Overexpression of AtNAC019 showed higher survival rate and more tolerant to drought (Fig. S3).
Figure 3.
Overexpression of BoNAC019 reduced tolerance to drought stress. (A) Phenotypes of WT and OE plants after water withholding for three weeks. (B) Survival rate of WT and OE plants after drought treatment. (C) Water loss rate of the detached leaves. (D) Proline content. (E) MDA content. (F,G) Representative images (F) and stomatal aperture (G) of WT and OE plants after drought treatment. Seedlings treated with water were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
Overexpression of BoNAC019 decreases sensitivity to ABA
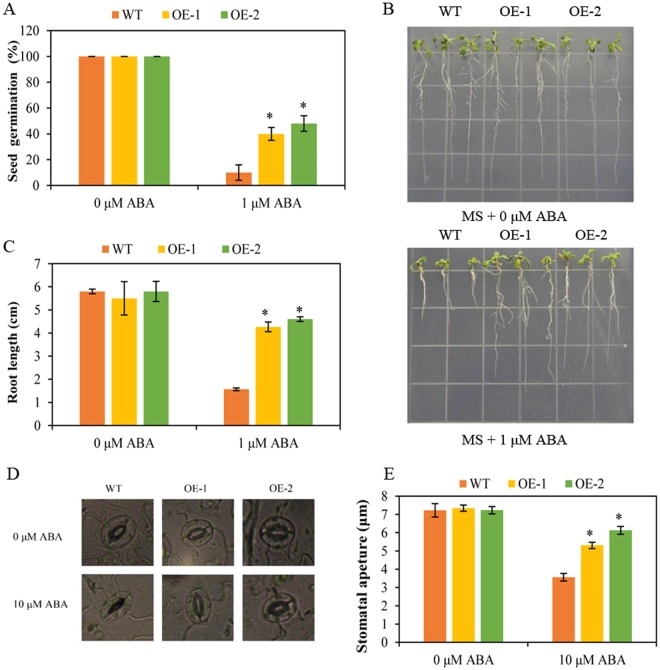
ABA is an important hormone involved in drought stress resistance in plants57,58. The germination rate and root elongation of BoNAC019-OE plants were analyzed to understand the role of BoNAC019 in the ABA signaling pathway. No obvious difference was observed between the germination rate of WT and BoNAC019 OE plants after 5 days of germination on MS medium without ABA. However, the germination rate of BoNAC019 OE plants was much higher than that in WT plants when the MS medium contained 1 μM ABA (Fig. 4A). The root elongation analysis showed no obvious difference in root lengths between WT and BoNAC019-OE plants, but root lengths of BoNAC019-OE plants were longer than those of WT plants on MS medium containing 1 μM ABA (Fig. 4B,C). ABA also mediates stomatal closure57,59. The stomatal apertures of BoNAC019-OE plants decreased by 20% in the 2.5 h 10 μM ABA treatment, while WT plants decreased by 20% (Fig. 4D,E). These results show that overexpressing BoNAC019 decreases sensitivity to ABA.
Figure 4.
Overexpression of BoNAC019 increased sensitivity to ABA. (A) Seed germination rate of WT and OE plants under ABA treatment. (B) Comparison of primary root length of WT and OE plants with ABA treatment. Five-day old seedlings grown on MS medium containing 1 μM ABA for 5 days. (C) Quantification of primary root length. (D,E) Representative images (D) and stomatal aperture (E) of WT and OE plants before and after ABA treatments. Seedlings treated without ABA were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
Overexpression of BoNAC019 increases H2O2 and reduces antioxidant enzyme activities
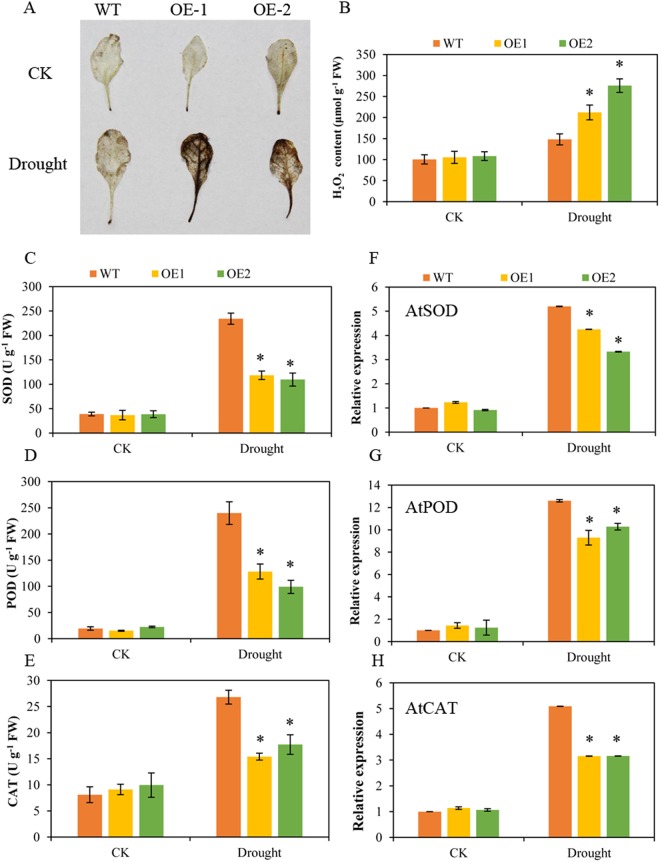
Adverse environmental conditions cause ROS accumulation in plants. Histochemical staining with diaminobenzidine (DAB) was used to detect the accumulation of ROS in WT plants and BoNAC019-OE plants. There was no obvious differences in leaf staining were observed between WT plants and BoNAC019-OE plants under normal condition, they stained negligibly with DAB. Under drought condition, the leaves of BoNAC019-OE plants were stained deeper than those of WT plants (Fig. 5A). The H2O2 content of BoNAC019-OE plants was much higher, which was consistent with the leaf staining result (Fig. 5B).
Figure 5.
Overexpression of BoNAC019 accumulated H2O2 and decreased antioxidant enzyme activity under drought condition. (A) Histochemical staining of WT and OE plants by diaminobenzidine (DAB) under normal and drought conditions. (B) H2O2 content of WT and OE plants under normal and drought conditions. (C–E) SOD, POD and CAT activity of WT and OE plants under normal and drought conditions. (F–H) The relative expressions of AtSOD, AtPOD and AtCAT. Seedlings treated without ABA were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
Enhanced antioxidant enzyme activities are an important way to scavenge ROS in plants. To further detect the ability of plants to scavenge ROS, the activities of the antioxidant enzymes SOD, POD, and CAT were evaluated. Under normal condition, no obvious differences in the activities of SOD, POD and CAT were detected in WT plants or BoNAC019-OE plant. Under drought treatment, the SOD, POD, and CAT activities were increased in both BoNAC019-OE plants and WT plants, but the enzyme activities were much lower in BoNAC019-OE plants than those in WT plants (Fig. 5C–E). These genes were reported encoding these antioxidant enzymes (AtSOD, AtPOD, and AtCAT) were selected for further study. As results, the expressions of these genes were much lower in BoNAC019-OE plants than that in WT plants under drought conditions (Fig. 5F–H). These results were consistent with the antioxidant enzyme activities, indicating that overexpressing BoNAC019 accumulated more ROS by reducing antioxidant enzyme activities to scavenge ROS.
Overexpression of BoNAC019 reduces anthocyanin accumulation by decreasing expressions of anthocyanin genes
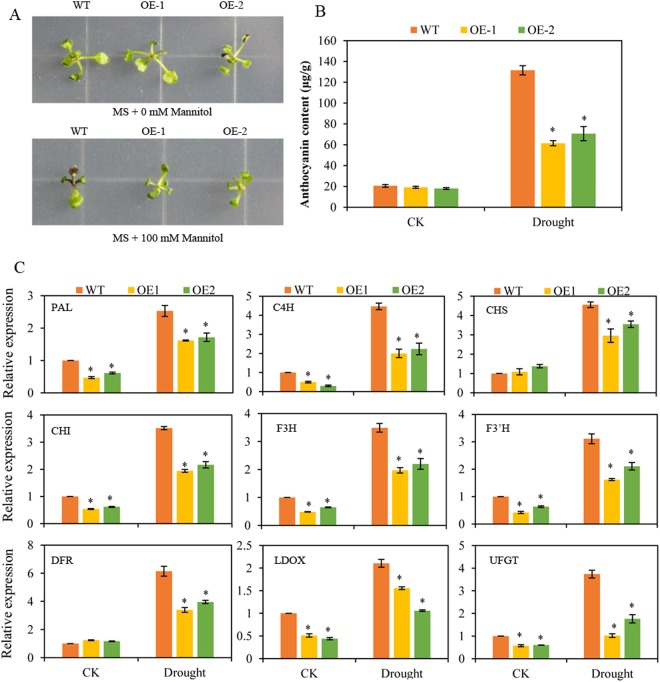
Anthocyanin is a flavonoid that plays important roles in plants. Previous studies have shown that accumulating flavonoids enhances abiotic stress tolerance by improving ROS scavenging ability35,60,61. As shown in Fig. 6A, with 100 μM mannitol treatment, the leaves of WT plants exhibited a deeper purple than that of OE plants, and anthocyanin content increased six-fold in WT plants and two-fold in BoNAC019-OE plants (Fig. 6B). Under normal conditions, there is almost no anthocyanin accumulation.
Figure 6.
Regulation of dehydration-induced anthocyanin in WT and OE plants. (A) Phenotype of 5 days old WT and OE seedlings on MS medium containing 0 and 100 mM mannitol for 5 days. (B) Anthocyanin content of WT and OE plants under normal and drought condition. (C) The expression of anthocyanin biosynthesis genes in WT and OE plants under normal and drought conditions were analyzed by qPCR. Seedlings on MS medium containing 0 mannitol were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
In addition, to test whether BoNAC019 participated in anthocyanin biosynthesis, we detected the expressions of the anthocyanin biosynthetic genes (PAL, C4H, CHS, CHI, F3H, F3′H, DFR, LDOX, and UFGT) in WT plants and BoNAC019-OE plants under normal and drought conditions. Expressions of these anthocyanin biosynthetic genes (PAL, C4H, CHS, F3H, ANS, and UFGT) were lower in BoNAC019-OE plants than that in WT plants under normal and drought conditions, which was consistent with the anthocyanin content results (Fig. 6C).
The MYB (TT2, PAP1, PAP2, MYB113, and MYB114) and bHLH (TT8, GL3, and EGL3) transcription factors interacted with WD40 protein (TTG1) to regulate the anthocyanin biosynthetic genes. Therefore, we detected the expression levels of these transcription factors. Under normal and drought conditions, only the expressions of TT2, MYB113, and TT8 were much lower in BoNAC019-OE plants than that in WT plants. No obvious differences were observed between the expressions of other genes in BoNAC019-OE plants and WT plants (Fig. S4). These results indicated that overexpressing of BoNAC019 reduced anthocyanin accumulation by decreasing the expressions of anthocyanin biosynthetic genes.
Stress-responsive genes are regulated in BoNAC019 overexpressing plants under drought stress
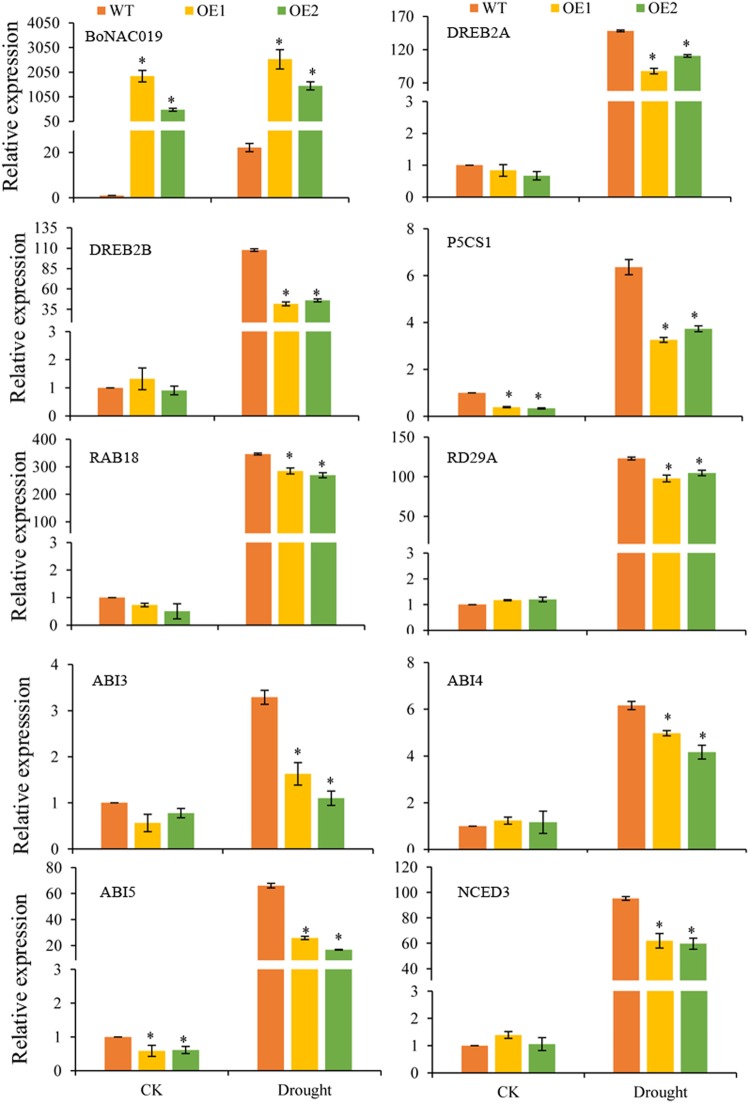
We selected several stress-responsive genes and compared their expressions in BoNAC019-OE plants and WT plants to further investigate the molecular mechanism of BoNAC019. Overexpression of BoNAC019 reduced drought tolerance, therefore, eight stress-related genes (AtDREB2A, AtDREB2B, AtRD29A, AtRAB18, and AtP5CS1) were selected for further study. Under normal conditions, the expressions of AtP5CS1 and AtRAB18 were significantly lower in BoNAC019-OE plants. All the stress responsive genes were induced, and the expressions were much lower in BoNAC019-OE plants (Fig. 7).
Figure 7.
Expression levels of stress responsive genes of WT and OE plants. Cabbage seedlings treated without water were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
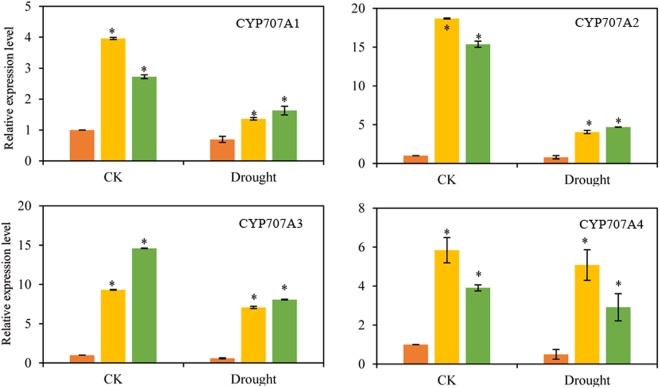
ABA synthetic gene (AtNCED3), ABA catabolism genes, ABA signaling genes (AtPYL1, AtPYL3, AtPP2CA, AtSnRK2.2, AtSnRK2.4, and AtSnRK2.6) and ABA response genes (AtABI3, AtABI4, and AtABI5) were selected for further study. Under normal condition, the expressions of ABA catabolism genes (AtCYP707A1- AtCYP707A4) were significantly higher and the expressions of ABA response genes AtABI3 and AtABI5 were much lower in BoNAC019-OE plants. No obvious difference of the ABA synthetic gene (AtNCED3) and ABA signaling genes expressions were detected between BoNAC019 OE plants and WT plants. In BoNAC019-OE plants, these stress responsive genes expressions were much lower compared to WT plants under drought condition (Fig. 8 and S5).
Figure 8.
Expression levels of ABA catabolism genes of WT and OE plants. Cabbage seedlings treated without water were used as a mock control (CK). Error bars show the standard deviations for three independent replicates. Asterisks indicate statistically significant differences compared to WT at the same time point (*P < 0.05).
Discussion
Unlike positive regulation of AtNAC019, overexpression of BoNAC019 reduces drought tolerance and decreases expressions of stress responsive genes
Overexpression of BoNAC019 reduced drought tolerance in Arabidopsis, as BoNAC019-OE plants showed a lower survival rate and lower RWC, indicating that overexpression of BoNAC019 reduced drought tolerance by lowering the plants water retaining abilities. Proline accumulation acts in stress-related signaling and cross tolerance62–64. The P5CS gene encodes elta-1-pyrroline-5-carboxylate synthase (P5CS) and is an important stress responsive gene in plants65–67. Overexpressing AtP5CS1 increased drought tolerance in transgenic plants48,68–71. In this study, the expression of AtP5CS1 was lower in BoNAC019-OE plants than that in WT plants under normal and drought conditions. These results were consistent with the proline content of BoNAC019-OE plants. AtP5CS1 might be the target gene of BoNAC019. Further study is needed to verify the target gene of BoNAC019.
DREB belongs to the AP2 (APETALA2)/ERF superfamily and participates in the abiotic stress response. DREB1 and DREB2 are two members of DREB transcription factors. DREB1 respond to low temperatures, and DREB2 respond to drought and salt treatments72–75. AtRD29A and AtRAB18 were stress and ABA responsive marker genes, and their overexpression enhanced drought tolerance and reduced water loss rate under drought conditions46,76,77. AtDREB2A, AtDREB2B, AtRD29A, and AtRAB18 were selected for further study, as expressions of these genes were induced by dehydration, while the expressions of these stress genes were down-regulated in BoNAC019 OE plants (Fig. 7), indicating that overexpression of BoNAC019 reduced drought tolerance and increased water loss by downregulating stress responsive genes. BoNAC019 may regulate other stress responsive genes or interact with other factors to participate in a complex drought signaling pathway; however, further study is needed to confirm this hypothesis.
AtNAC019 was a well-known stress-responsive NAC transcription factor, and overexpression of AtNAC019 enhanced drought tolerance (Fig. S3) and upregulated stress gene AtERD116. Although BoNAC019 is highly homologous with AtNAC019, the function of BoNAC019 in drought stress was completely different. The N-terminal regions which were the DNA binding domains (1–150aa) of BoNAC019 and AtNAC019 were basically the same. The function of the NAC transcription factors was determined by the C-terminal region, and the alignment analysis showed that the C-terminal region of BoNAC019 had a low similarity to AtNAC019 (Fig. S6), which might result in different regulation of downstream genes and different functions in drought stress responses.
BoNAC019 mediates drought tolerance via an ABA-dependent pathway
ABA is involved in abiotic stress resistance in plants57,58 and meditates a series of developmental processes, including seed germination, root elongation, and stomatal movement78,79. Abscisic acid is a key endogenous messenger in plants, and it has a crucial role in various plant stresses80–82. After drought treatment, the ABA content was induced and much lower in OE plants (Fig. 3E). 9-cis-epoxy carotenoid dioxygenase (NCED) the key enzyme in ABA biosynthesis. AtNCED3 was induced strongly by dehydration, and overexpression of AtNCED3 promoted synthesis of ABA. Endogenous ABA content increases to participate in a series of physiological and cellular processes to resist water deficit stress83,84. The CYP707A family contains four members in Arabidopsis. ABA 80-hydroxylase was coded by CYP707A1- CYP707A4. This enzyme plays a key role in ABA catabolism and in mediating plant response to adverse stress conditions. Suppressing the expressions of CYP707As in cherry fruit by RNAi enhanced drought tolerance in cherry fruits, the water loss rates of transgenic plants were much lower than WT plants85. Our results showed that under normal condition, no obvious difference of AtNCED3 expression was detected in BoNAC019-OE and WT plants. Under normal and drought conditions, the expressions of CYP707A1-CYP707A4 were induced in BoNAC019-OE plants (Fig. 7 and 8).
The signaling mechanism of ABA is critical for improving plant tolerance to stress environments. ABA signaling contains three core components: pyrabactin resistance (PYR)/pyrabactin resistance-like (PYL)/regulatory component of ABA receptors (RCAR), protein phosphatase 2C and SNF1(Sucrose non-fermenting)- related protein kinase 2. In the presence of ABA, PP2C activity which functioned as a negative regulator was inhibited by PYR/PYL/RCAR-PP2C complex formation. Inhibition of PP2C activity activated SnRK2 and then phosphorylates downstream substrate proteins such as transcription factors, and thus facilitating transcription of ABA-responsive genes81. The ABI3, ABI4, and ABI5 proteins have been reported to participate in seed germination and early seedling growth and development86–88. ABI5 was induced by dehydration treatment, and overexpression of ABI5 enhanced stress tolerance86,89. In addition, the expression levels of AtNCED3, ABA signaling genes, ABA response genes were lower in BoNAC019-OE plants than those in WT plants, while no obvious difference of these genes expressions were detected under normal conditions (Fig. 7 and S5). BoNAC019 might activate the ABA catabolism genes to decrease the tolerance to drought stress. These genes might be the target genes of BoNAC019. The other genes involved in ABA biosynthesis, ABA signaling pathway and ABA response genes were not directly regulated by BoNAC019. BoNAC019 might interact with the other factors to regulate these genes expressions. BoNAC019 might negatively regulate dehydration response by inducing ABA catabolism genes, resulting in decreasing ABA content and drought tolerance.
Overexpression of BoNAC019 reduces ROS scavenging ability by decreasing antioxidant enzyme activities and anthocyanin accumulation
Enzymatic antioxidants include SOD, POD, and CAT. Overexpression of these genes encoding relevant enzymes improved ROS scavenging ability to protect plants against abiotic stress90–92. Under drought conditions, overexpressing plants accumulated more H2O2, and the activities of the antioxidant enzymes were lower than those in WT plants (Fig. 5A–E). Expressions of AtSOD, AtPOD, and AtCAT encoding antioxidant enzymes were detected in BoNAC019-OE plants and WT plants, and their expression levels all decreased in BoNAC019-OE plants under the drought condition (Fig. 5F–H). Therefore, BoNAC019 probably participates in ROS scavenging by reducing antioxidant enzyme activities.
Non-enzymatic antioxidants include ascorbate, GSH, carotenoids, tocopherols, and flavonoids31. Anthocyanin is flavonoid that plays important roles in plants. Previous studies have shown that flavonoid accumulation could enhance abiotic stress tolerance by improving ROS scavenging ability35,60,61,93. In this study, the anthocyanin content of BoNAC019-OE plants was significantly lower than that of WT plants under drought conditions (Fig. 6A).
Synthesis of anthocyanins in Arabidopsis is well understood40,94,95. PAL, C4H, CHS, CHI, F3H, F3′H, DFR, LDOX, and UFGT are important enzymes in the anthocyanin biosynthetic pathway96,97. In this study, the expression levels of these genes were lower in BoNAC019-OE plants than those in WT plants under normal and drought conditions, indicating that BoNAC019 participates in anthocyanin biosynthesis by regulating the anthocyanin biosynthetic genes (Fig. 6B).
MYB, bHLH, and WD40 are three important transcription factors involved in anthocyanin biosynthesis and directly regulate the anthocyanin biosynthetic genes40,98,99. MYB12 regulates the expressions of CHS, CHI, and F3H100. Expressions of DFR and ANS were dramatically induced by overexpressing FtWD40101. PtrMYB75 interacted with bHLH113 and TTG1 to repress the expressions of anthocyanin biosynthetic genes by directly binding to the promoters of these genes in poplar102. In this study, we also detected the expressions of these above transcription factors. Under normal and drought conditions, only TT2 and MYB113 expression levels were significantly lower in BoNAC019-OE plants than those in WT plants. No obvious difference was observed between the expressions of other genes in BoNAC019-OE plants and WT plants (Fig. S4). Therefore, we hypothesized that WD40 and bHLH might be the upstream transcription factors of BoNAC019 or that WD40 and bHLH might interact with BoNAC019 to regulate the anthocyanin biosynthetic genes. Li et al. reported that suppressing CPY707A2 by RNAi in cherry fruit induced anthocyanin accumulation and upregulating the anthocyanin biosynthesis genes85. In this study, we found that the expressions of CPY707A genes were activated by BoNAC019 (Fig. 8) and the ABA content (Fig. 3E) was significantly lower in OE plants. Therefore, we speculated that BoNAC019 might inhibit the synthesis of anthocyanin by activating the expression of CPY707A genes. Further study is needed to confirm this hypothesis.
In summary, overexpressing BoNAC019 showed decreased drought tolerance and accumulated more ROS by decreasing antioxidant enzyme activities and anthocyanin accumulation to scavenge ROS, the ability to scavenge ROS was weaker in BoNAC019-OE plants than that in WT plants. Relevant studies of NAC transcription factors participating in anthocyanin biosynthesis in response to drought stress are limited. Our study provides some new insight into the molecular mechanism of drought resistance in the Brassica family.
Electronic supplementary material
Acknowledgements
This work was partly supported by the grants to Guo (2016YFD0101007, BLVT-03) and Li (tszy20140808).
Author Contributions
J.-F.W., W.-R.L. and Y.-D.G. designed research; J.-F.W., W.-R.L., Y.-Y.C., G.-L.W., X.-Y.W., C.-D.Q., L.-L. and S.-J.Q. performed the experiments; J.-F.W., W.-R.L., Y.-Y.C., and G.-L.W. analyzed the data; S.-X.R., X.-W.Y., X.-S.L. and Y.-D.G. revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinfang Wang, Weiran Lian and Yunyun Cao contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31690-1.
References
- 1.Zhu J-K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedoroff NV, et al. Radically Rethinking Agriculture for the 21st Century. Science. 2010;327:833–834. doi: 10.1126/science.1186834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, X., Cai, X., Xu, C., Wang, Q. & Dai, S. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. International journal of molecular sciences17 (2016). [DOI] [PMC free article] [PubMed]
- 4.Zhang Q. Strategies for developing green super rice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16402–16409. doi: 10.1073/pnas.0708013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu, S., Ramegowda, V., Kumar, A. & Pereira, A. Plant adaptation to drought stress. F1000Research5, 10.12688/f1000research.7678.1 (2016). [DOI] [PMC free article] [PubMed]
- 6.Lata C, Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. Journal of Experimental Botany. 2011;62:4731–4748. doi: 10.1093/jxb/err210. [DOI] [PubMed] [Google Scholar]
- 7.Nuruzzaman, M., Sharoni, A. M. & Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Frontiers in Microbiology4, 10.3389/fmicb.2013.00248 (2013). [DOI] [PMC free article] [PubMed]
- 8.Joshi, R. et al. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Frontiers in Plant Science7, 10.3389/fpls.2016.01029 (2016). [DOI] [PMC free article] [PubMed]
- 9.Singh, D. & Laxmi, A. Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Frontiers in Plant Science6, 10.3389/fpls.2015.00895 (2015). [DOI] [PMC free article] [PubMed]
- 10.Hu H, et al. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Molecular Biology. 2008;67:169–181. doi: 10.1007/s11103-008-9309-5. [DOI] [PubMed] [Google Scholar]
- 11.Rushton PJ, et al. Tobacco transcription factors: Novel insights into transcriptional regulation in the Solanaceae. Plant Physiology. 2008;147:280–295. doi: 10.1104/pp.107.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y, You J, Xie K, Xie W, Xiong L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Molecular Genetics and Genomics. 2008;280:547–563. doi: 10.1007/s00438-008-0386-6. [DOI] [PubMed] [Google Scholar]
- 13.Seo PJ, Kim S-G, Park C-M. Membrane-bound transcription factors in plants. Trends in Plant Science. 2008;13:550–556. doi: 10.1016/j.tplants.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Seo PJ, et al. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant Journal. 2010;61:661–671. doi: 10.1111/j.1365-313X.2009.04091.x. [DOI] [PubMed] [Google Scholar]
- 15.Lam Son Phan T, Nishiyama R, Yamaguchi-Shinozaki K, Shinozaki K. Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach. GM Crops. 2010;1:32–39. doi: 10.4161/gmcr.1.1.10569. [DOI] [PubMed] [Google Scholar]
- 16.Tran LSP, et al. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16:2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H, et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita M, et al. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant Journal. 2004;39:863–876. doi: 10.1111/j.1365-313X.2004.02171.x. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, et al. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. Journal of Experimental Botany. 2015;66:6803–6817. doi: 10.1093/jxb/erv386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X, et al. RhNAC3, a stress- associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress- related genes in rose petals. Plant Biotechnology Journal. 2014;12:38–48. doi: 10.1111/pbi.12114. [DOI] [PubMed] [Google Scholar]
- 21.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 22.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic Acid: Emergence of a Core Signaling Network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 23.Aswath CR, Kim SH, Mo SY, Kim DH. Transgenic plants of creeping bent grass harboring the stress inducible gene, 9-cis-epoxycarotenoid dioxygenase, are highly tolerant to drought and NaCl stress. Plant Growth Regulation. 2005;47:129–139. doi: 10.1007/s10725-005-3380-6. [DOI] [Google Scholar]
- 24.Bao G, et al. Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnology Journal. 2016;14:206–214. doi: 10.1111/pbi.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S-Y, et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, Y. Regulators of PP2C phosphatase activity function as abscisic acid sensors (vol 324, pg 1064, 2009). Science 324, 1266–1266 (2009). [DOI] [PubMed]
- 27.Wang J, et al. CsATAF1 positively regulates drought stress tolerance by ABA-dependent pathway and promoting ROS scavenging in cucumber. Plant & Cell Physiology. 2018;59:930–945. doi: 10.1093/pcp/pcy030. [DOI] [PubMed] [Google Scholar]
- 28.Huang, X.-S., Liu, J.-H. & Chen, X.-J. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. Bmc Plant Biology10, 10.1186/1471-2229-10-230 (2010). [DOI] [PMC free article] [PubMed]
- 29.You, J. & Chan, Z. ROS Regulation During Abiotic Stress Responses in CropPlants. Frontiers in Plant Science 6, 10.3389/fpls.2015.01092 (2015). [DOI] [PMC free article] [PubMed]
- 30.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, N. et al. Melatonin Improved Anthocyanin Accumulation by Regulating Gene Expressions and Resulted in High Reactive Oxygen Species Scavenging Capacity in Cabbage. Frontiers in Plant Science7, 10.3389/fpls.2016.00197 (2016). [DOI] [PMC free article] [PubMed]
- 33.Mahmood, K., Xu, Z., El-Kereamy, A., Casaretto, J. A. & Rothstein, S. J. The Arabidopsis Transcription Factor ANAC032 Represses Anthocyanin Biosynthesis in Response to High Sucrose and Oxidative and Abiotic Stresses. Frontiers in Plant Science7, 10.3389/fpls.2016.01548 (2016). [DOI] [PMC free article] [PubMed]
- 34.Castellarin SD, et al. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell and Environment. 2007;30:1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakabayashi R, et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant Journal. 2014;77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey CC, Strahle JT, Selinger DA, Chandler VL. Mutations in the pale aleurone color1 regulatory gene of the Zea mays anthocyanin pathway have distinct phenotypes relative to the functionally similar TRANSPARENT TESTA GLABRA1 gene in Arabidopsis thaliana. Plant Cell. 2004;16:450–464. doi: 10.1105/tpc.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiology. 2005;139:1840–1852. doi: 10.1104/pp.105.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quattrocchio F, et al. PH4 of petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell. 2006;18:1274–1291. doi: 10.1105/tpc.105.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baudry A, Caboche M, Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant Journal. 2006;46:768–779. doi: 10.1111/j.1365-313X.2006.02733.x. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant Journal. 2008;53:814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 41.Morishita T, et al. Arabidopsis NAC Transcription Factor, ANAC078, Regulates Flavonoid Biosynthesis under High-light. Plant and Cell Physiology. 2009;50:2210–2222. doi: 10.1093/pcp/pcp159. [DOI] [PubMed] [Google Scholar]
- 42.Wu A, et al. JUNGBRUNNEN1, a Reactive Oxygen Species-Responsive NAC Transcription Factor, Regulates Longevity in Arabidopsis. Plant Cell. 2012;24:482–506. doi: 10.1105/tpc.111.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayum, M. A. et al. Alfin-like transcription factor family: characterization and expression profiling against stresses in Brassica oleracea. Acta Physiologiae Plantarum3810.1007/s11738-016-2139-1 (2016).
- 44.Yu, J. et al. Bolbase: a comprehensive genomics database for Brassica oleracea. Bmc Genomics14, 10.1186/1471-2164-14-664 (2013). [DOI] [PMC free article] [PubMed]
- 45.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 46.Mao X, et al. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. Journal of Experimental Botany. 2012;63:2933–2946. doi: 10.1093/jxb/err462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, et al. Identification of an Apoplastic Protein Involved in the Initial Phase of Salt Stress Response in Rice Root by Two-Dimensional Electrophoresis. Plant Physiology. 2009;149:916–928. doi: 10.1104/pp.108.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szekely G, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant Journal. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, et al. The role of abscisic acid in regulating cucumber fruit development and ripening and its transcriptional regulation. Plant Physiology and Biochemistry. 2013;64:70–79. doi: 10.1016/j.plaphy.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell and Environment. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 51.Ranieri A, Petacco F, Castagna A, Soldatini GF. Redox state and peroxidase system in sunflower plants exposed to ozone. Plant Science. 2000;159:159–167. doi: 10.1016/S0168-9452(00)00352-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhang LA, et al. Cotton GhMPK2 is involved in multiple signaling pathways and mediates defense responses to pathogen infection and oxidative stress. Febs Journal. 2011;278:1367–1378. doi: 10.1111/j.1742-4658.2011.08056.x. [DOI] [PubMed] [Google Scholar]
- 53.Khokon MAR, et al. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell and Environment. 2011;34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 54.Mori IC, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. Plos Biology. 2006;4:1749–1762. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramirez V, et al. Drought tolerance in Arabidopsis is controlled by the OCP3 disease resistance regulator. Plant Journal. 2009;58:578–591. doi: 10.1111/j.1365-313X.2009.03804.x. [DOI] [PubMed] [Google Scholar]
- 56.Pazmino-Duran EA, Giusti MM, Wrolstad RE, Gloria MBA. Anthocyanins from Oxalis triangularis as potential food colorants. Food Chemistry. 2001;75:211–216. doi: 10.1016/S0308-8146(01)00201-1. [DOI] [Google Scholar]
- 57.Li T, Wu X-Y, Li H, Song J-H, Liu J-Y. A Dual-Function Transcription Factor, AtYY1, Is a Novel Negative Regulator of the Arabidopsis ABA ResponseNetwork. Molecular Plant. 2016;9:650–661. doi: 10.1016/j.molp.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Hong, Y., Zhang, H., Huang, L., Li, D. & Song, F. Overexpression of a Stress-Responsive NAC Transcription Factor Gene ONACO22 Improves Drought and Salt Tolerance inRice. Frontiers in Plant Science 7, 10.3389/fpls.2016.00004 (2016). [DOI] [PMC free article] [PubMed]
- 59.Chen H-Y, et al. ORA47 (octadecanoid-responsive AP2/ERF-domain transcription factor 47) regulates jasmonic acid and abscisic acid biosynthesis and signaling through binding to a novel cis-element. New Phytologist. 2016;211:599–613. doi: 10.1111/nph.13914. [DOI] [PubMed] [Google Scholar]
- 60.Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling & Behavior. 2011;6:709–711. doi: 10.4161/psb.6.5.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aung Htay, N. et al. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biology 17, (23 March 2017)-(2023 March 2017) (2017). [DOI] [PMC free article] [PubMed]
- 62.Aleksza D, Horvath GV, Sandor G, Szabados L. Proline Accumulation Is Regulated by Transcription Factors Associated with Phosphate Starvation. Plant Physiology. 2017;175:555–567. doi: 10.1104/pp.17.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin X, Cui Y, Wang M, Xia X. Overexpression of a novel MYB-related transcription factor, OsMYBR1, confers improved drought tolerance and decreased ABA sensitivity in rice. Biochemical and Biophysical Research Communications. 2017;490:1355–1361. doi: 10.1016/j.bbrc.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 64.Kaur G, Asthir B. Proline: a key player in plant abiotic stress tolerance. Biologia Plantarum. 2015;59:609–619. doi: 10.1007/s10535-015-0549-3. [DOI] [Google Scholar]
- 65.Abu-Romman SM, et al. Cloning and expression patterns of the HvP5CS gene from barley (Hordeum vulgare) Journal of Food Agriculture & Environment. 2011;9:279–284. [Google Scholar]
- 66.Shaar-Moshe, L., Huebner, S. & Peleg, Z. Identification of conserved drought-adaptive genes using a cross-species meta-analysis approach. Bmc Plant Biology15, 10.1186/s12870-015-0493-6 (2015). [DOI] [PMC free article] [PubMed]
- 67.Wehner, G., Balko, C., Humbeck, K., Zyprian, E. & Ordon, F. Expression profiling of genes involved in drought stress and leaf senescence in juvenile barley. Bmc Plant Biology16, 10.1186/s12870-015-0701-4 (2016). [DOI] [PMC free article] [PubMed]
- 68.Chen J, et al. Cloning and genetic diversity analysis of a new P5CS gene from common bean (Phaseolus vulgaris L.) Theoretical and Applied Genetics. 2010;120:1393–1404. doi: 10.1007/s00122-010-1263-3. [DOI] [PubMed] [Google Scholar]
- 69.Kubala S, et al. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. Journal of Plant Physiology. 2015;183:1–12. doi: 10.1016/j.jplph.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Su M, et al. Cloning two P5CS genes from bioenergy sorghum and their expression profiles under abiotic stresses and MeJA treatment. Plant Science. 2011;181:652–659. doi: 10.1016/j.plantsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Silva Guerzoni JT, et al. Stress-induced Delta 1-pyrroline-5-carboxylate synthetase (P5CS) gene confers tolerance to salt stress in transgenic sugarcane. Acta Physiologiae Plantarum. 2014;36:2309–2319. doi: 10.1007/s11738-014-1579-8. [DOI] [Google Scholar]
- 72.Hichri I, et al. SIDREB2, a tomato dehydration-responsive element-binding 2 transcription factor, mediates salt stress tolerance in tomato and Arabidopsis. Plant Cell and Environment. 2016;39:62–79. doi: 10.1111/pce.12591. [DOI] [PubMed] [Google Scholar]
- 73.Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Egawa C, et al. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes & Genetic Systems. 2006;81:77–91. doi: 10.1266/ggs.81.77. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Xia X, Yin W. A poplar DRE-binding protein gene, PeDREB2L, is involved in regulation of defense response against abiotic stress. Gene. 2011;483:36–42. doi: 10.1016/j.gene.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Lang V, Palva ET. The Expression of A Rab-Related Gene, Rab18, is Induced By Abscisic-Acid During The Cold-Acclimation Process of Arabidopsis-Thaliana (L) Heynh. Plant Molecular Biology. 1992;20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- 77.Yamaguchi-Shinozaki K, Shinozaki K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Molecular and General Genetics. 1993;238:331–340. doi: 10.1007/BF00277130. [DOI] [PubMed] [Google Scholar]
- 78.Yang X, Bai Y, Shang J, Xin R, Tang W. The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of Abscisic Acid Insensitive 5 expression by Brassinazole Resistant 1. Plant Cell and Environment. 2016;39:1994–2003. doi: 10.1111/pce.12763. [DOI] [PubMed] [Google Scholar]
- 79.Huang, Y., Feng, C. -Z., Ye, Q., Wu, W. -H. & Chen, Y. -F. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. Plos Genetics12, 10.1371/journal.pgen.1005833 (2016). [DOI] [PMC free article] [PubMed]
- 80.Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and Challenges in Understanding Plant Abiotic Stress Responses and Tolerance. Plant and Cell Physiology. 2011;52:1569–1582. doi: 10.1093/pcp/pcr106. [DOI] [PubMed] [Google Scholar]
- 81.Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends in Plant Science. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research. 2011;124:509–525. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 83.Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant Journal. 2001;28:123–133. doi: 10.1046/j.1365-313X.2001.01131.x. [DOI] [PubMed] [Google Scholar]
- 84.Babak B, et al. Characterization of the Promoter Region of an Arabidopsis Gene for 9-cis-Epoxycarotenoid Dioxygenase Involved in Dehydration-Inducible Transcription. DNA Research. 2013;20:315–324. doi: 10.1093/dnares/dst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Q, et al. PacCYP707A2 negatively regulates cherry fruit ripening while PacCYP707A1 mediates drought tolerance. Journal of Experimental Botany. 2015;66:3765–3774. doi: 10.1093/jxb/erv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giraudat J, et al. Isolation Of The Arabidopsis-Abi3 Gene By Positional Cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skubacz, A., Daszkowska-Golec, A. & Szarejko, L. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Frontiers in Plant Science7, 10.3389/fpls.2016.01884 (2016). [DOI] [PMC free article] [PubMed]
- 90.Shafi A, et al. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Molecular Biology. 2015;87:615–631. doi: 10.1007/s11103-015-0301-6. [DOI] [PubMed] [Google Scholar]
- 91.Badawi GH, et al. Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiologia Plantarum. 2004;121:231–238. doi: 10.1111/j.0031-9317.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 92.Sun L, et al. NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Cat(2+) and nitric oxide in Arabidopsis guard cells. Plant Science. 2017;262:81–90. doi: 10.1016/j.plantsci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Li P, et al. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant Journal. 2017;89:85–103. doi: 10.1111/tpj.13324. [DOI] [PubMed] [Google Scholar]
- 94.Borevitz JO, Xia YJ, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–2393. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pesch M, et al. TRANSPARENT TESTA GLABRA1 and GLABRA1 Compete for Binding to GLABRA3 in Arabidopsis. Plant Physiology. 2015;168:584-+. doi: 10.1104/pp.15.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grotewold E. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 97.Allan AC, Hellens RP, Laing WA. MYB transcription factors that colour our fruit. Trends in Plant Science. 2008;13:99–102. doi: 10.1016/j.tplants.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 98.Zhang F, Gonzalez A, Zhao MZ, Payne CT, Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 2003;130:4859–4869. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]
- 99.Xu W, et al. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytologist. 2014;202:132–144. doi: 10.1111/nph.12620. [DOI] [PubMed] [Google Scholar]
- 100.Mehrtens F, Kranz H, Bednarek P, Weisshaar B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiology. 2005;138:1083–1096. doi: 10.1104/pp.104.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao P, et al. Fagopyrum tataricum FtWD40 Functions as a Positive Regulator of Anthocyanin Biosynthesis in Transgenic Tobacco. Journal of Plant Growth Regulation. 2017;36:755–765. doi: 10.1007/s00344-017-9678-6. [DOI] [Google Scholar]
- 102.Wan S, Li C, Ma X, Luo K. PtrMYB57 contributes to the negative regulation of anthocyanin and proanthocyanidin biosynthesis in poplar. Plant Cell Reports. 2017;36:1263–1276. doi: 10.1007/s00299-017-2151-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.