Abstract
Mutations in BRAF are common to many cancers, including CRC. The MEK inhibitors are being investigated in BRAF-mutant CRC. In this study, we aimed to investigate how MEK inhibitor suppresses growth of BRAF-mutated CRC cells as well as its potential mechanisms. Our findings indicated that MEK inhibitor promote PUMA expression via ERK/FoxO3a signaling pathway. In addition, PUMA induction is essential for MEK inhibitor-induced apoptosis. Moreover, PUMA induction is required for MEK inhibitors to induced apoptosis in combination with cisplatin, dabrafenib, or Gefitinib. Knockdown of PUMA suppressed the anticancer effect of the MEK inhibitor in vivo. Our findings indicate a novel role for PUMA as a regulator of the antitumor effects of MEK inhibitor, suggesting that PUMA induction may modulate MEK inhibitor sensitivity.
Subject terms: Chemotherapy, Target identification
Introduction
MEK1/2 are kinases that are linked with cell survival and regulation of proliferation1–3. Currently, MEK inhibitors are in clinical trials for several cancers, including colorectal cancer (CRC), specifically those with mutations in RAS or its downstream signaling component BRAF, which occur in several types of cancers4,5. The RAS/RAF/MEK/ERK signaling pathway is always activated in many types of cancers, with its activation often occurring through gain-of-function RAS and RAF mutations2. Previous studies have indicated that tumor cell lines with KRAS or BRAF mutations are more sensitive to MEK inhibitors in vitro3. Recently, trametinib, a MEK inhibitor, was approved for treatment of melanomas in which BRAF was mutated6,7. Selumetinib (AZD6244; ARRY-142886) is a MEK inhibitor for oral use, which is being investigated for the treatment of several cancer types8,9. According to preclinical evidence, we examined the effects of the MEK inhibitors in CRC.
p53 up-regulated modulator of apoptosis (PUMA; also known as BBC3) is a BH3-only pro-apoptotic protein that belongs to the Bcl-2 family10,11. PUMA is both a direct p53 target, and can be induced via apoptosis that is independent of p53 in response to a variety of stimulations12,13. Normally, the localization of PUMA is mainly in the mitochondria in cells14. In the apoptotic process, PUMA-mediated mitochondrial dysfunction leads to apoptosis15. PUMA antagonizes the functions of Bcl-2/Mcl-1 via Bax/Bak15. In previous studies, PUMA has been demonstrated to be a potential target of CRC cells16,17.
In this study, we found that MEK inhibitors induce PUMA induction via the ERK/FoxO3a signaling pathway, and we further determined that PUMA may modulate responses to MEK inhibitors in CRC cells. These results ultimately suggest that the induction of PUMA may be a key indicator of the therapeutic efficacy of the MEK inhibitors.
Results
MEK inhibitors induce apoptosis in CRC
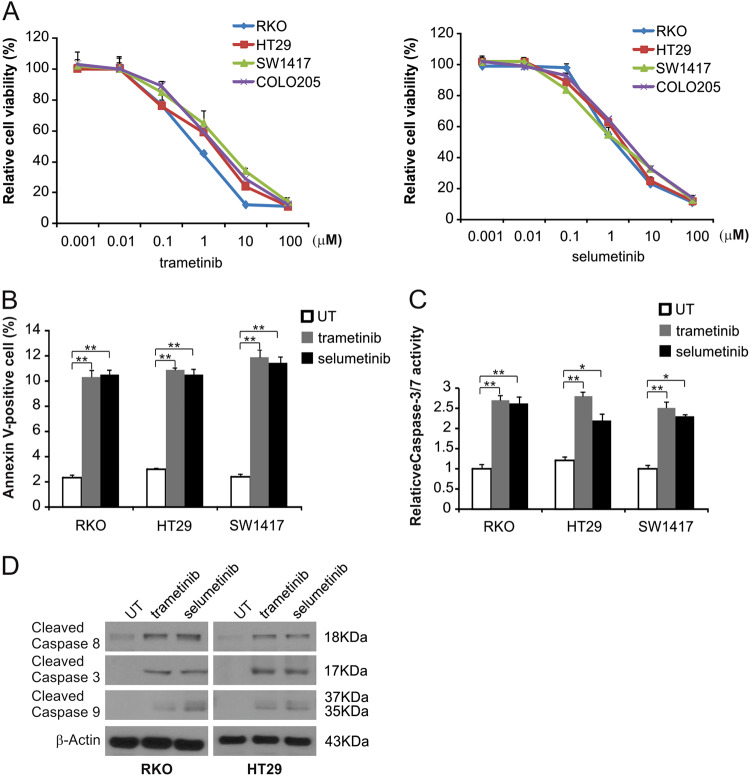
To examine the effects of the MEK inhibitor on BRAF mutation-induced CRC, 4 BRAF-mutated CRC cell lines were treated with trametinib or selumtinib for 72 h, and then the MTS assay was used to analyze cell growth. As shown in Fig. 1a, treatment of CRC cells with trametinib or selumetinib led to a reduction in cell growth. Next, apoptosis was detected by flow cytometry in trametinib-treated RKO, HT29, and SW1417 cells. MEK inhibitors enhanced the Annexin V-positive cell rate in these cells (Fig. 1b and S1A). MEK inhibitors also induced the activation of caspases 3/7 (Fig. 1c and S1B). In RKO and HT29 cells treated with the MEK inhibitors, Western blotting was used to assess caspase 3, 8 and 9 activation and both trametinib and selumetinib enhanced the activation of these caspases in these cells (Fig. 1d and S1C). These results suggest that MEK inhibitor inhibit cell growth and promote caspase-dependent apoptosis in CRC cells with BRAF mutations.
Fig. 1. MEK inhibitor induces apoptosis in CRC cells.
a The indicated cell lines were treated with increasing doses of trametinib or selumetinib for 72 h. Cell proliferation was determined by MTS assay. b The indicated cell lines were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h at the indicated concentrations. Apoptosis was analyzed by Annexin V/PI staining followed by flow cytometry. c The indicated cell lines were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h at the indicated concentrations. Caspase 3/7 activity was determined by fluorogenic analysis. d The indicated cell lines were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib at the indicated time points. Cleaved caspase 3, 8, and 9 were analyzed by Western blotting. The results in a, b, and c are expressed as the means ± SD of three independent experiments. **P < 0.01, *P < 0.05
MEK inhibitors promote p53-independent PUMA induction
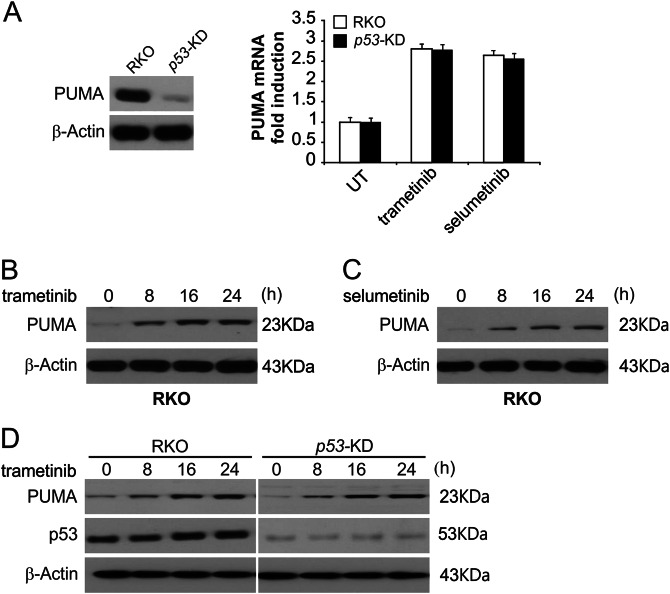
We then analyzed how the MEK inhibitors induce apoptosis in CRC cells. We treated RKO cells with trametinib or selumetinib, and the PUMA mRNA and protein levels were increased significantly (Fig. 2b, c and S2A–S2D). In addition, the same effects of MEK inhibitors were observed in HT29 cells (Figure S2E–S2G). Moreover, following trametinib treatment, PUMA protein and mRNA levels were increased in RKO cell with stable p53-knockdown (p53-KD) (Fig. 2a–d). Therefore, expression of PUMA was selectively induced in response to MEK inhibition without regard for p53 activity and may drive apoptosis in these cells.
Fig. 2. MEK inhibitors induce p53-independent PUMA induction.
a Parental and p53-KD RKO cells were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h. PUMA mRNA induction by trametinib was analyzed by real-time reverse transcriptase (RT) PCR, with β-actin as the control. b RKO cells were treated with 0.1 μmol/L trametinib at the indicated time points. PUMA expression was analyzed by Western blotting. c RKO cells were treated with 0.1 μmol/L selumetinib at the indicated time points. PUMA expression was analyzed by Western blotting. d Parental and p53-KD RKO cells were treated with 1 μmol/L trametinib at the indicated time points. PUMA expression was analyzed by Western blotting
PUMA mediates MEK inhibitor-induced apoptosis
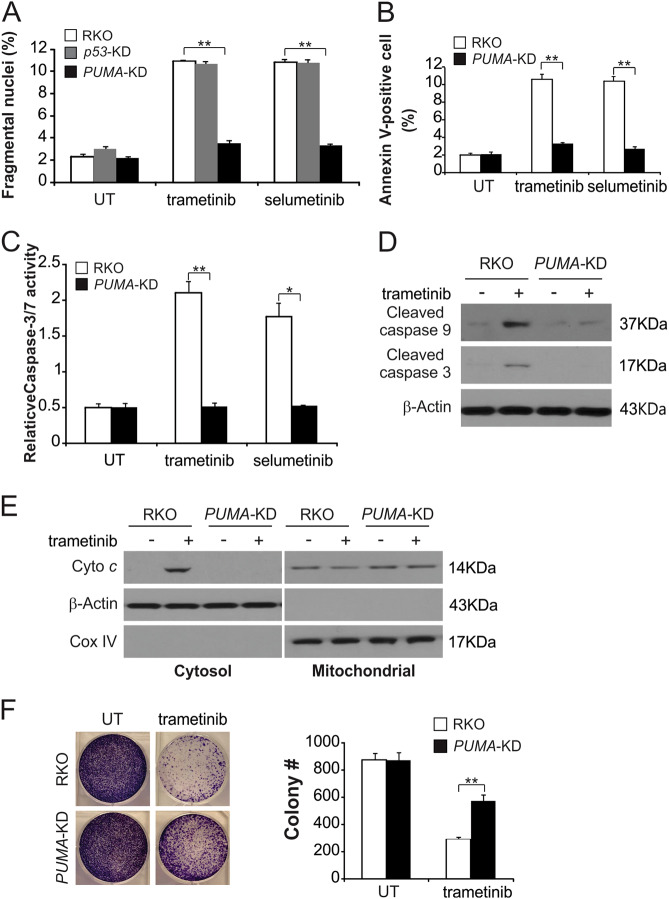
To investigate the role of PUMA induction in MEK inhibitor response, we examined the role of PUMA in the context of MEK inhibitor-induced apoptosis through the use of PUMA cells bearing a stable knockdown of PUMA (PUMA-KD). Compared with parental cells, PUMA-KD RKO cells had significantly lower rates of apoptosis, which was induced by the MEK inhibitors (Fig. 3a and S3A). Deficiency of p53 could not abolish MEK inhibitor-induced apoptosis (Fig. 3a and S3A). Annexin V/PI staining was used to confirm this apoptotic reduction in MEK inhibitors-treated PUMA-KD RKO cells (Fig. 3b and S3B). Consistently, activation of caspases 3 and 7 was reduced in response to MEK inhibitors in PUMA-KD RKO cells (Fig. 3c and S3C). PUMA deficiency abrogated trametinib-induced intrinsic apoptotic events, including activation of caspases 3 and 9 (Fig. 3d and S3D) and the cytochrome c release (Fig. 3e and S3E). We also found PUMA-KD HT29 cells blocked MEK inhibitors induced apoptosis in comparison with parental cells (Figure S3F and S3G). It is noteworthy that in long-term colony formation assays, survival was enhanced in PUMA-KD cells after trametinib treatment compared to parental cells (Fig. 3f). Together, these results show that apoptosis in CRC cells treated with trametinib is dependent on PUMA.
Fig. 3. PUMA is required for the apoptotic activity of MEK inhibitors.
a Parental, p53-KD and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h. Apoptosis was analyzed by a nuclear fragmentation assay. b Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h. Apoptosis was analyzed by Annexin V/PI staining followed by flow cytometry. c Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h. Caspase 3/7 activity was determined by fluorogenic analysis. d Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib for 24 h. Cleaved caspase 3 and 9 were analyzed by Western blotting. e The cytoplasm and mitochondria were fractionated from parental and PUMA-KD RKO cells treated with 0.1 μmol/L trametinib for 24 h. The distribution of cytochrome c was analyzed by Western blotting. β-Actin and cytochrome oxidase subunit IV (Cox IV) were analyzed as the control for loading and fractionation. f Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib for 24 h. Colony formation assay was performed by seeding an equal number of treated cells in 12-well plates and then staining attached cells with crystal violet 10 days later. Left, representative pictures of colonies; Right, quantification of colony numbers. The results in a, b, c and f are expressed as the means ± SD of three independent experiments. **P < 0.01, *P < 0.05
MEK inhibitors induced FoxO3a-dependent PUMA induction
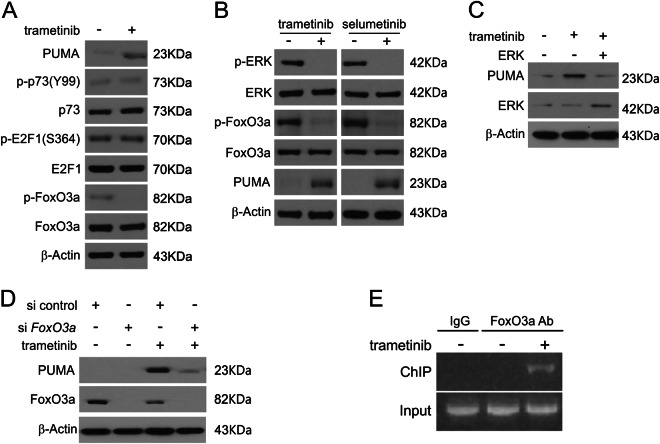
We then investigated the transcription factor involved in MEK inhibitor-induced PUMA upregulation in CRC cells. E2F1 and p73 were excluded due to the lack of total and phosphorylation changes following trametinib treatment (Fig. 4a and S4A). Moreover, as shown in Fig. 4a, b, S4A and S4B, trametinib was found to decrease ERK phosphorylation as well as dephosphorylate FoxO3a, promoting FoxO3a nuclear translocation. Moreover, treatment of cells with selumetinib was sufficient to induce up-regulation of PUMA following ERK and FoxO3a dephosphorylation in RKO and HT29 cells (Fig. 4b, S4B and S4C). Exogenous expression of ERK1 inhibited trametinib-mediated PUMA induction (Fig. 4c). In addition, as shown in Fig. 4d, knockdown of FoxO3a eliminated trametinib-induced PUMA upregulation in RKO cells. Next, chromatin immunoprecipitation (ChIP) was performed to establish if FoxO3a directly activates transcription of PUMA. Trametinib promoted FoxO3a binding to the PUMA promoter (Fig. 4e). The above data show that FoxO3a binds directly to the PUMA promoter driving its transcription upon MEK inhibitors treatment.
Fig. 4. Induction of PUMA by MEK inhibitors is mediated through ERK inhibition.
a RKO cells were treated with 0.1 μmol/L trametinib for 24 h. PUMA, p73, E2F1, p-FoxO3a and FoxO3a expression were analyzed by Western blotting. b RKO cells were treated with 0.1 μmol/L trametinib or 0.1 μmol/L selumetinib for 24 h. Indicated protein levels were analyzed by Western blotting. c RKO cells were transfected with ERK plasmid for 6 h and then treated with 1 μmol/L trametinib for 24 h. PUMA and ERK expression was analyzed by Western blotting. d RKO cells were transfected with either a control scrambled siRNA or FoxO3a siRNA for 24 h and then treated with 1 μmol/L trametinib for 24 h. FoxO3a and PUMA expression was analyzed by Western blotting. e Chromatin immunoprecipitation (ChIP) was performed using an anti-FoxO3a antibody on RKO cells following trametinib treatment for 12 h. ChIP with the control IgG was used as a control. PCR was carried out using primers surrounding the FoxO3a binding sites in the PUMA promoter
PUMA mediates chemosensitization in response to MEK inhibitors
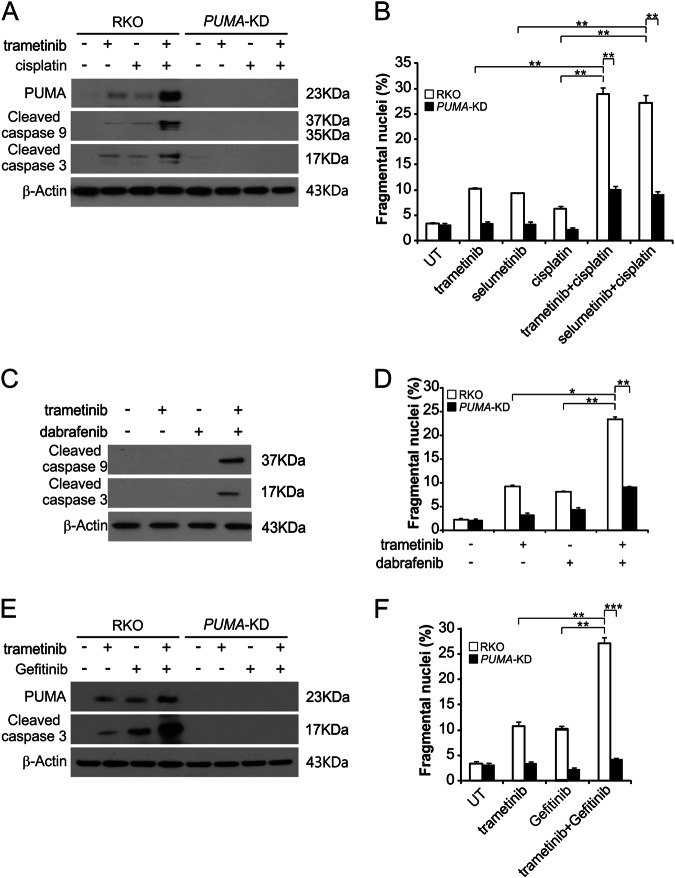
The combination of a MEK inhibitors and chemotherapy drugs has been described previously18,19. However, the mechanism behind the influence of chemosensitization of MEK inhibitors is unclear. We hypothesized that induction of PUMA could facilitate such chemosensitization. PUMA induction occurs simultaneously with MEK inhibitors and other drug treatment by different routes. As shown in Fig. 5a, our results suggested, in comparison to single drug treatment, that the combination of trametinib and cisplatin induced much higher levels of PUMA. Our results are in agreement with concurrent PUMA upregulation by MEK inhibitors and DNA damage agents via p53-independent and p53-dependent mechanisms, respectively17,20. Apoptosis and cleaved-caspase 3 and 9 were also significantly improved in RKO and HT29 cells after combination treatment; however, knockdown of PUMA blocked these effects (Fig. 5a, b and S5A). Furthermore, RKO cells were stimulated by trametinib and dabrafenib, and the effect of PUMA was analyzed. The level of apoptosis and caspase 3 and 9 activity were significantly enhanced in parental, but not PUMA-KD, RKO and HT29 cells following the combination treatment (Fig. 5c, d and S5B). We also found trametinib induced EGFR phosphorylation in both parental and PUMA-KD RKO and HT29 cells (Figure S5C and S5D). Trametinib and Gefitinib synergistically induced PUMA upregulation and apoptosis in these cells (Fig. 5e, f, S5E and S5F). PUMA knockdown attenuated apoptosis and activation of caspase 3 in response to combination treatment (Fig. 5e, f, S5E and S5F). The above findings demonstrate that the anticancer effects of trametinib can be promoted by PUMA-dependent chemosensitization.
Fig. 5. MEK inhibitors synergize with cisplatin, dabrafenib or Gefitinib to induce apoptosis via PUMA in CRC cells.
a Parental and PUMA-KD RKO cells were treated with 0.5 μmol/L trametinib, 0.5 μmol/L selumetinib, 20 mg/L cisplatin, or their combination for 24 h. PUMA, cleaved caspase 3 and 9 were analyzed by Western blotting. b Parental and PUMA-KD RKO cells were treated with 0.5 μmol/L trametinib, 0.5 μmol/L selumetinib, 20 mg/L cisplatin, or their combination for 24 h. Apoptosis was analyzed by a nuclear fragmentation assay. c RKO cells were treated with 0.5 μmol/L trametinib, 1 μmol/L dabrafenib, or their combination for 24 h. Cleaved caspase 3 and 9 were analyzed by Western blotting. d Parental and PUMA-KD RKO cells were treated with 0.5 μmol/L trametinib, 1 μmol/L dabrafenib, or their combination for 24 h. Apoptosis was analyzed by a nuclear fragmentation assay. e Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib, 0.2 μmol/L gefitinib, or their combination for 24 h. PUMA and cleaved caspase 3 were analyzed by Western blotting. f Parental and PUMA-KD RKO cells were treated with 0.1 μmol/L trametinib, 0.2 μmol/L gefitinib, or their combination for 24 h. Apoptosis was analyzed by a nuclear fragmentation assay. The results in b, d and f are expressed as the means ± SD of three independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05
PUMA mediates the anticancer activity of MEK inhibitors in vivo
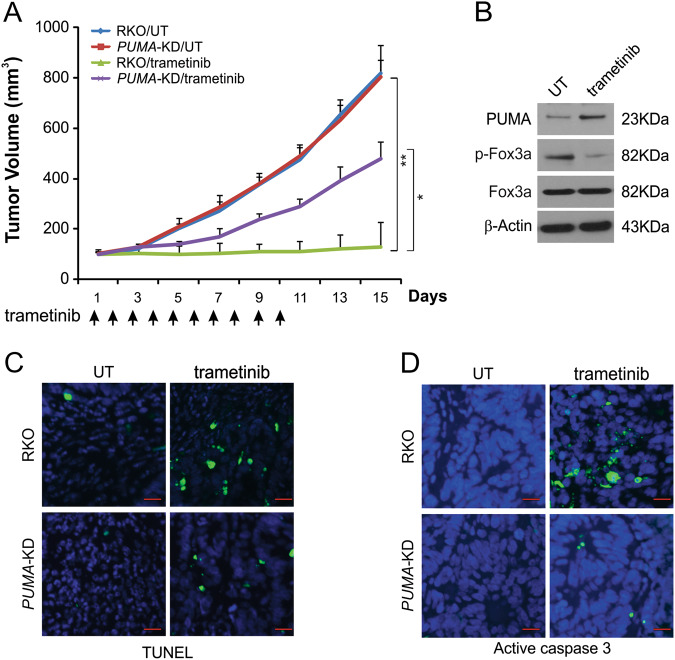
To assess how PUMA affects the anticancer efficacy of MEK inhibitors in vivo, nude mice were injected with parental or PUMA-KD RKO. After 7 days, mice were treated daily for 10 days with vehicle control or 1 mg/kg trametinib by oral gavage. There was no significant difference in tumor growth characteristics between parental and PUMA-KD tumors after control treatment (Fig. 6a). Trametinib treatment inhibited tumors driven by parental RKO cells by more than 70% (Fig. 6a). Tumors from PUMA-KD cells were significantly resistant to trametinib treatment (Fig. 6a). The above data indicate that PUMA expression is required for the anticancer functions of trametinib in vivo. The Western blotting results demonstrated that FoxO3a phosphorylation was suppressed and PUMA expression was increased in trametinib-treated parental tumors (Fig. 6b). In trametinib-treated mice, but not in control mice, apoptosis was significantly induced. In PUMA-KD tumors, trametinib-induced TUNEL-positive cells were abolished (Fig. 6c). Furthermore, the active caspase 3 staining results demonstrated a similar trend (Fig. 6d). The above data indicate that PUMA is a key mediator of the anticancer efficacy of MEK inhibitors in mice.
Fig. 6. PUMA mediates the anticancer effects of trametinib in a xenograft model.
a Nude mice were injected s.c. with 4 × 106 parental and PUMA-KD RKO cells. After 1 week, mice were treated with 1 mg/kg trametinib or buffer for ten consecutive days. The tumor volume at the indicated time points after treatment was calculated and plotted (n = 6 in each group). Arrows indicate trametinib injection. The results are expressed as the means ± SD of three independent experiments. **P < 0.01; *P < 0.05. b Parental RKO xenograft tumors were treated with 1 mg/kg trametinib or the control buffer as in a for four consecutive days. The indicated protein in representative tumors was analyzed by Western blotting. c Paraffin-embedded sections of tumor tissues from mice treated as in b were analyzed by TUNEL staining. d Tissue sections from c were analyzed by active caspase 3 staining. Scale bars 25 μm
Discussion
As RAS/MEK/ERK signaling is activated in several cancer types, many MEK inhibitors that target this signaling pathway are being developed21,22. The V600E or V600E/K mutation of BRAF causes its constitutive kinase activity, activating MEK1/2 as downstream effectors, which in turn mediates ERK1/2 activation22,23. In non-cellular systems, the BRAF V600 mutation has been shown to activate MEK1/2 kinase activity23. Previous studies demonstrated that trametinib is involved in antiproliferative, proapoptotic, and anticancer effects through inhibition of aberrant ERK pathway activation in cell lines and animal models24,25. In this study, we assessed how trametinib affects CRC cells. The findings indicate that trametinib’s suppression of tumor growth is modulated by apoptosis and FoxO3a activation, which drives the induction of PUMA and subsequent apoptosis that is dependent on mitochondrial signaling pathways.
PUMA is an important mediator of apoptosis in response to a range of chemotherapeutic compounds, and it may therefore be a good predictor of chemosensitivity26,27. PUMA, a Bcl-2 family protein, can be induced in p53-dependent or -independent manners; once induced it interacts with the mitochondria to induce apoptosis26,28,29. PUMA involves the important process of tumorigenesis30. In cancer cells, PUMA could be a potential target of chemotherapy because activating PUMA expression suppresses cancer growth by restoring apoptosis31. Non-genetically stimulatory conditions include the deprivation of growth factors, inflammatory cytokines, and kinase inhibitors. PUMA upregulation can also be p53-independent, mediated by factors such as FoxO3a, E2F1, NF-κB, and p7316,32–34. PUMA induction promotes apoptosis induction through activation of Bax and other BH3-only proteins, leading to active caspase cascades in cancer cells35. A recent study has further shown that the response of isolated tumor cell mitochondria to the Bcl-2 homology 3 (BH3) peptide of PUMA was predictive of patients’ therapeutic responses to chemotherapy36. In the current research, our findings demonstrated trametinib-mediated PUMA induction in parental as well as p53-KD RKO cells. This suggests that trametinib may mediate cytotoxicity via induction of PUMA through a p53-independent mechanism.
Our findings indicate that PUMA is induced following ERK inhibition after MEK inhibitors treatment and initiates apoptosis in CRC cells via the intrinsic apoptosis pathway. Previous studies have also shown that PUMA induction is vital for induction of apoptosis in response to many chemotherapeutic agents, making it a key marker of chemosensitivity. Our results confirm that in CRC cells treated with MEK inhibitors, PUMA upregulation is a good predictor of responses to treatment. Approaches that allow for assessment of PUMA induction via non-invasive techniques may be possible, and would be powerful predictive tools.
In conclusion, our findings revealed a new anticancer mechanism by PUMA-mediated apoptosis after MEK inhibitors treatment. The ERK/FoxO3a signaling pathway participates in MEK inhibitor-mediated PUMA overexpression. The results of our research suggest that PUMA expression could be applied as a modulator in clinical trials of MEK inhibitors, potentially having significant relevance for future drug development efforts.
Materials and methods
Cell culture
The RKO, HT29, SW1417, and COLO205 CRC cell lines were bought from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in DMEM supplemented with 10% newborn calf serum, 100 µg/mL streptomycin, and 100 units/mL penicillin (Invitrogen, Carlsbad, CA, USA). Trametinib, selumetinib, dabrafenib, Gefitinib (Selleckchem, Houston, TX, USA), and cisplatin (Sigma, St Louis, MO, USA) were resuspended in DMSO. The construct of ERK1 was obtained from Addgene.
MTS
The MTS assay was conducted with the use of a CellTiter 96® Aqueous No-Radioactive Cell Proliferation Assay Kit (Promega, Fitchburgh, WI, USA). Briefly, cells were added to 96-well plates and grown overnight prior to treatment. After 72 h, viability was determined. Each experiment was repeated three times.
Western blotting
Western blotting was conducted as described previously37 using antibodies against PUMA (Abcam, Cambridge, MA, USA), ERK, phospho-ERK, cleaved-caspase 8, cleaved-caspase 9, cytochrome oxidase subunit IV (Cox IV), phospho-FoxO3a, EGFR, p-EGFR, cleaved-caspase 3, E2F1, p73, p-p73, FoxO3a, p-FoxO3a (Cell Signaling Technology, Danvers, MA, USA), cytochrome c, and β-actin (Santa Cruz Biotechnology, Dallas, TX, USA).
qRT-PCR
TRIzol was used for DNA extraction (Invitrogen, Carlsbad, CA, USA) as manufacturer’s instructions. One μg of RNA was used for reverse transcription with the Quantscript reverse transcription Kit (Applied Biosystems, Foster City, CA, USA). Bestar®SybrGreen qPCR mastermix (Invitrogen, Carlsbad, CA, USA) and a LightCycler 480® II Real-Time PCR System (Roche, Branchburg, NJ, USA) were used for the qPCR reaction. Primers are as list: PUMA, Forward: 5′-TGGGGTCTGCCCAGGCAT-3′, Reverse: 5′-GAGCTGCCCTCCTGGCGTG-3′; β-actin, Forward: 5′-GAGACAACCTACAACAGCATCAT-3′, Reverse: 5′-GAAGCCAAAATGGGACCACCG-3′.
Apoptosis
Nuclear staining was used to analyze apoptosis with Hoechst 33258 (Invitrogen, Carlsbad, CA, USA). A FITC Annexin V Apoptosis Detection Kit I (BD PharmingenTM, Franklin Lakes, NJ, USA) was used for further detection of apoptosis. A Cell Death Detection ELISAPlus Kit (Roche Molecular Biochemicals, Branchburg, NJ, USA) was applied to detect caspase 3/7 activity. For the colony formation assay, cells treated with trametinib for 24 h were plated in 6-well plates. After allowing cell growth for 10 days, cell colonies were stained by crystal violet38. Cytochrome c in cytosolic fractions was analyzed by Western blotting to detect cytochrome c release.
Transfection
Knockdown was performed using 200 pmol of siRNA with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 24 h, cells were treated with MEK inhibitors. FoxO3a siRNA and scrambled siRNA were obtained (Santa Cruz Biotechnology, Dallas, TX, USA). For stable transfection, plasmids containing a p53-targeting (CACCATCCACTACAACTACAT) or PUMA-targeting shRNA (CCTGGAGGGTCATGTACAATCTCTT) were transfected into RKO and HT29 cells. After transfection the cells were added into 96-well plates with 5 µg/mL puromycin. Western blot was used to confirm PUMA expression.
Chromatin immunoprecipitation (ChIP)
A Chromatin Immunoprecipitation Assay Kit (Millipore, Burlington, MA, USA) with a FoxO3a antibody (Cell Signaling Technology, Danvers, MA, USA) was used for all ChIP protocols. Primers used were: forward: 5′-GCGCACAGGTGCCTCGGC-3′ and reverse: 5′-TGGGTGTGGCCGCCCT-3′.
Xenografts
All animal experimental were performed in accordance with the guidelines of the First Affiliate Hospital of Dalian Medical University and in compliance with the institutional ethical guidelines. Mice were injected into either flank with both 4 × 106 parental and PUMA-KD RKO cells in 200 μl PBS (n = 6). After a week, mice with tumors were treated for ten consecutive days with 1 mg/kg trametinib by oral gavage. The formula for tumor volume calculation was: 1/2 × length × width2. When tumors were ~1.0 cm3 in size, mice were sacrificed. Tumors were collected from euthanized mice and were formalin fixed and paraffin embedded. Active caspase 3 and TUNEL staining were conducted on these embedded tumor sections with an Alexa Fluor 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
All data are means ± standard deviation (SD). One-way ANOVA or Student’s t test were conducted as appropriate with the GraphPad Prism V software. P < 0.05 was the threshold for statistical significance.
Electronic supplementary material
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41389-024-00543-0"
Change history
11/21/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41389-024-00543-0
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41389-018-0078-y).
References
- 1.Solit, D. B. et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature439, 358–362 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao, Y. & Adjei, A. A. The clinical development of MEK inhibitors. Nat. Rev. Clin. Oncol.11, 385–400 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Luke, J. J., Ott, P. A. & Shapiro, G. I. The biology and clinical development of MEK inhibitors for cancer. Drugs74, 2111–2128 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Holderfield, M., Deuker, M. M., McCormick, F. & McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer14, 455–467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millington, G. W. Mutations of the BRAF gene in human cancer, by Davies et al. (Nature 2002; 417: 949–54). Clin. Exp. Dermatol.38, 222–223 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Corcoran, R. B. et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.33, 4023–4031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, D. B. et al. Combined BRAF (dabrafenib) and MEK inhibition (trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.32, 3697–3704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalanotti, F. et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res.19, 2257–2264 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, D. W. & Patel, S. P. Profile of selumetinib and its potential in the treatment of melanoma. OncoTargets Ther.7, 1631–1639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong, J. et al. FBW7-dependent Mcl-1 degradation mediates the anticancer effect of Hsp90 inhibitors. Mol. Cancer Ther.16, 1979–1988 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong, J., Tan, S., Zou, F., Yu, J. & Zhang, L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene36, 787–796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming, L., Wang, P., Bank, A., Yu, J. & Zhang, L. PUMA dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J. Biol. Chem.281, 16034–16042 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Wu, B. et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut56, 645–654 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambroise, G., Portier, A., Roders, N., Arnoult, D. & Vazquez, A. Subcellular localization of PUMA regulates its pro-apoptotic activity in Burkitt’s lymphoma B cells. Oncotarget6, 38181–38194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu, J., Wang, Z., Kinzler, K. W., Vogelstein, B. & Zhang, L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl Acad. Sci. USA100, 1931–1936 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun, Q. et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene28, 2348–2357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, D., Wei, L., Yu, J. & Zhang, L. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res.20, 3472–3484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata, A. N. et al. Synergistic activity and heterogeneous acquired resistance of combined MDM2 and MEK inhibition in KRAS mutant cancers. Oncogene36, 6581–6591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ElMokh, O. et al. Combined MEK and Pi3’-kinase inhibition reveals synergy in targeting thyroid cancer in vitro and in vivo. Oncotarget8, 24604–24620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, P., Yu, J. & Zhang, L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc. Natl Acad. Sci. USA104, 4054–4059 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caunt, C. J., Sale, M. J., Smith, P. D. & Cook, S. J. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat. Rev. Cancer15, 577–592 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Tran, K. A. et al. MEK inhibitors and their potential in the treatment of advanced melanoma: the advantages of combination therapy. Drug Des. Dev. Ther.10, 43–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzivassiliou, G. et al. ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol. Cancer Ther.11, 1143–1154 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Lugowska, I., Kosela-Paterczyk, H., Kozak, K. & Rutkowski, P. Trametinib: a MEK inhibitor for management of metastatic melanoma. OncoTargets Ther.8, 2251–2259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh, S. J. & Corrie, P. G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med. Oncol.7, 122–136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, H., Xie, Y., Yang, D. & Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget8, 25310–25322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roulston, A., Muller, W. J. & Shore, G. C. BIM, PUMA, and the achilles’ heel of oncogene addiction. Sci. Signal.6, pe12 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Tan, S.et al. PUMA mediates ER stress-induced apoptosis in portal hypertensive gastropathy. Cell Death Dis. 5, (2014).. [DOI] [PMC free article] [PubMed]
- 29.Qiu, W. et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J. Clin. Investig.121, 1722–1732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan, J. et al. MicroRNA-199a-3p inhibits tumorigenesis of hepatocellular carcinoma cells by targeting ZHX1/PUMA signal. Am. J. Transl. Res.9, 2457–2465 (2017). [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J. et al. Targeting the apoptotic Mcl-1-PUMA interface with a dual-acting compound. Oncotarget8, 54236–54242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng, X., He, K., Zhang, L. & Yu, J. Crizotinib induces PUMA-dependent apoptosis in colon cancer cells. Mol. Cancer Ther.12, 777–786 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu, W. et al. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology54, 1249–1258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudgeon, C. et al. PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Mol. Cancer Ther.9, 2893–2902 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, Q., Sakaida, T., Yue, W., Gollin, S. M. & Yu, J. Chemosensitization of head and neck cancer cells by PUMA. Mol. Cancer Ther.6(12 Pt 1), 3180–3188 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Heinicke, U., Kupka, J., Fichter, I. & Fulda, S. Critical role of mitochondria-mediated apoptosis for JNJ-26481585-induced antitumor activity in rhabdomyosarcoma. Oncogene35, 3729–3741 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Tong, J. et al. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res.77, 2512–2521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He, K. et al. BRAFV600E-dependent Mcl-1 stabilization leads to everolimus resistance in colon cancer cells. Oncotarget7, 47699–47710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.