Abstract
Geologically abrupt carbon perturbations such as the Palaeocene–Eocene Thermal Maximum (PETM, approx. 56 Ma) are the closest geological points of comparison to current anthropogenic carbon emissions. Associated with the rapid carbon release during this event are profound environmental changes in the oceans including warming, deoxygenation and acidification. To evaluate the global extent of surface ocean acidification during the PETM, we present a compilation of new and published surface ocean carbonate chemistry and pH reconstructions from various palaeoceanographic settings. We use boron to calcium ratios (B/Ca) and boron isotopes (δ11B) in surface- and thermocline-dwelling planktonic foraminifera to reconstruct ocean carbonate chemistry and pH. Our records exhibit a B/Ca reduction of 30–40% and a δ11B decline of 1.0–1.2‰ coeval with the carbon isotope excursion. The tight coupling between boron proxies and carbon isotope records is consistent with the interpretation that oceanic absorption of the carbon released at the onset of the PETM resulted in widespread surface ocean acidification. The remarkable similarity among records from different ocean regions suggests that the degree of ocean carbonate change was globally near uniform. We attribute the global extent of surface ocean acidification to elevated atmospheric carbon dioxide levels during the main phase of the PETM.
This article is part of a discussion meeting issue ‘Hyperthermals: rapid and extreme global warming in our geological past’.
Keywords: Palaeocene–Eocene Thermal Maximum, ocean acidification, boron isotope, boron/calcium, planktonic foraminifera
1. Introduction
A hallmark of the Palaeocene–Eocene Thermal Maximum (PETM, approx. 56 Ma) is a negative carbon isotope excursion (CIE) of 3–5‰, signifying a large injection of isotopically light carbon into the atmosphere–ocean reservoirs [1–4]. Although the exact source of carbon is a matter of debate, the event provides a test for our understanding of the ocean's response to the rapid invasion of carbon and heat. The uptake of carbon dioxide (CO2) in seawater should result in a decrease in ocean pH and calcium carbonate (CaCO3) saturation state (Ω), commonly termed ocean acidification [5]. In the modern ocean, these environmental changes (i.e. warming, deoxygenation and ocean acidification) are connected by a single driver: an increase in atmospheric pCO2 [6]. Current and predicted anthropogenic changes in ocean biogeochemistry inform us that the coastal regions and high latitudes are particularly vulnerable. Changes in continental run-off and nutrient fluxes along with seasonal stratification could amplify pH changes in coastal waters. Southern Ocean waters are inherently low in Ω, making them more susceptible to undersaturation with respect to CaCO3 during ocean acidification events. The geological record can potentially provide insight into the extent to which such environments and their biotas can be impacted by ocean acidification.
The PETM represents a natural experiment of the global ocean response to multiple, covarying environmental stressors. The degree of ocean warming is now well constrained to be 5–8°C [2,7], whereas deep-ocean acidification is largely inferred from widespread dissolution of seafloor carbonate sediments [4,8,9]. Evidence of surface ocean acidification, however, has only recently emerged from planktonic foraminiferal boron isotope (δ11B) and boron/calcium (B/Ca) proxy records [10–12]. Model studies provide ancillary support for whole ocean acidification [13–15]. Marine ecosystems experienced dynamic changes during the PETM but the relative impact of warming, deoxygenation and acidification on organisms remains unclear [16,17]. With improved spatial coverage of boron proxy records, we aim to better constrain the global magnitude of surface ocean acidification.
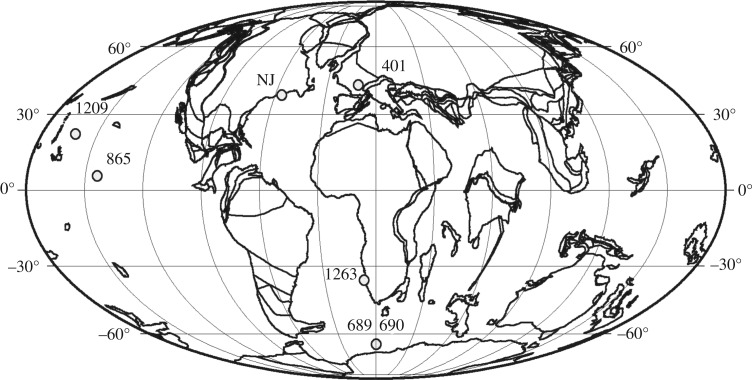
Here we quantify ocean acidification and its spatial extent within the upper ocean (surface to thermocline) during the PETM, synthesizing new and published records of planktonic foraminifera δ11B and B/Ca proxies. The study sites span pelagic and coastal environments, and low and high latitudes in the Atlantic and Pacific Ocean basins (figure 1). We generated planktonic foraminifera B/Ca records at high-latitude Southern Ocean (Atlantic Sector, Ocean Drilling Program (ODP) sites 689 and 690) and continental shelf site ODP Ancora, as well as δ11B data from thermocline-dwelling species at continental shelf sites (ODP sites Bass River and Millville). These new records complement published records from the pelagic, low-latitude Pacific ODP site 1209 [10], continental shelf ODP site Bass River [11] located along the eastern coast of North America and Deep Sea Drilling Project (DSDP) site 401 in the Northeast Atlantic [12]. Seawater properties (temperature, salinity, nutrients and oxygen concentration) and foraminiferal preservation state vary between the sites, and we exploit these differences to determine the global extent of surface ocean acidification across a diverse range of palaeoceanographic and depositional settings.
Figure 1.
Palaeocene–Eocene (approx. 56 Ma) palaeogeographic reconstruction and core study sites. Pelagic ODP sites include 1209, 1263, 865, 689, 690 and DSDP 401. The map is modified from an Ocean Drilling Stratigraphic Network (ODSN)-generated map using www.odsn.de. New Jersey coastal plain ODP sites include Bass River (BR), Ancora (AN) and Millville (MV).
2. Methods and material
(a). Site locations and depositional environments
High-latitude pelagic sites 689 (64°31.009′ S, 03°05.996′ E) and 690 (65°9.629′ S, 1°12.296′ E) are located on Maud Rise in the Weddell Sea (figure 1). The PETM palaeodepths of site 689 and site 690, composed of calcareous ooze, are estimated to be 1100 m and 1900 m, respectively [18]. A detailed study has shown that the CIE and its ensuing recovery are preserved within the site 690 PETM section, whereas a coring gap appears to have omitted the upper half of the CIE and the recovery at site 689 [18]. Acarinina soldadoensis (250–355 µm size fraction), A. praepentacamerata (180–250 µm size fraction) and Subbotina spp. (250–355 µm size fraction) were picked from washed sediment samples taken at 1–5 cm resolution at these sites. Because of foraminiferal species assemblage changes during the PETM [19] at sites 689 and 690, A. praepentacamerata was measured in the Palaeocene and A. soldadoensis was analysed within the CIE interval.
Coastal sites Bass River (BR; 39°36.70′ N, 74°26.20′ W), Millville (39°24.2778′ N, 75°05.3332′ W) and Ancora (39°41.5329′ N, 74°50.9410′ W) are located in New Jersey in the mid-Atlantic coastal plain [20–22] (figure 1). Ancora was closest to the palaeo-coastline, followed by Millville, and the most distal and down-dip site is Bass River, with PETM palaeodepths estimated at 50–150 m, indicative of middle to outer shelf environments [23,24]. These sections are composed of uppermost Palaeocene age micaceous silts and glauconitic sands (Vincentown Formation) transitioning to lower Eocene kaolinitic clay (Marlboro Formation) which yields well-preserved foraminifera tests with glassy textures. This is in contrast to the pelagic sites used in this study, which display a ‘frosty’ texture indicative of some degree of post-depositional recrystallization.
At Ancora, trace element to calcium ratios were analysed on multi-specimen monogeneric samples of surface-dwelling photosymbiont-bearing Acarinina and Morozovella spp., and thermocline-dwelling Subbotina spp. from the 250–355 µm size fraction to obtain approximately 200–400 µg of CaCO3 material. The gap in trace element data at the CIE onset (BR: 357.42–357.09 m, AN: 171.59–170.98 m) is due to an absence of foraminifera attributed to carbonate dissolution. At Ancora, resolution is low for the upper Palaeocene, due to low foraminifera abundances. Closely spaced samples were often combined to achieve sufficient sample material for boron isotopes (δ11B) at sites Bass River and Millville. δ11B measurements were carried out on the few species with adequate abundance, Subbotina triangularis (Palaeocene) and S. roesnaesensis (Eocene), using the 180–300 µm size fraction to obtain 1–4 mg of CaCO3 material.
(b). Age models
All the sites used in this study have published planktonic foraminifera and bulk carbonate δ13C records. However, for the purpose of refining age models between sites, we correlate the bulk carbonate records, which benefit from a higher temporal resolution (figure 2d). Although the bulk carbonate records differ in magnitude between sites, their temporal δ13C trends parallel the planktonic foraminiferal records. We provide more details on age constraints for New Jersey and Southern Ocean sites generated in this study in the electronic supplementary material.
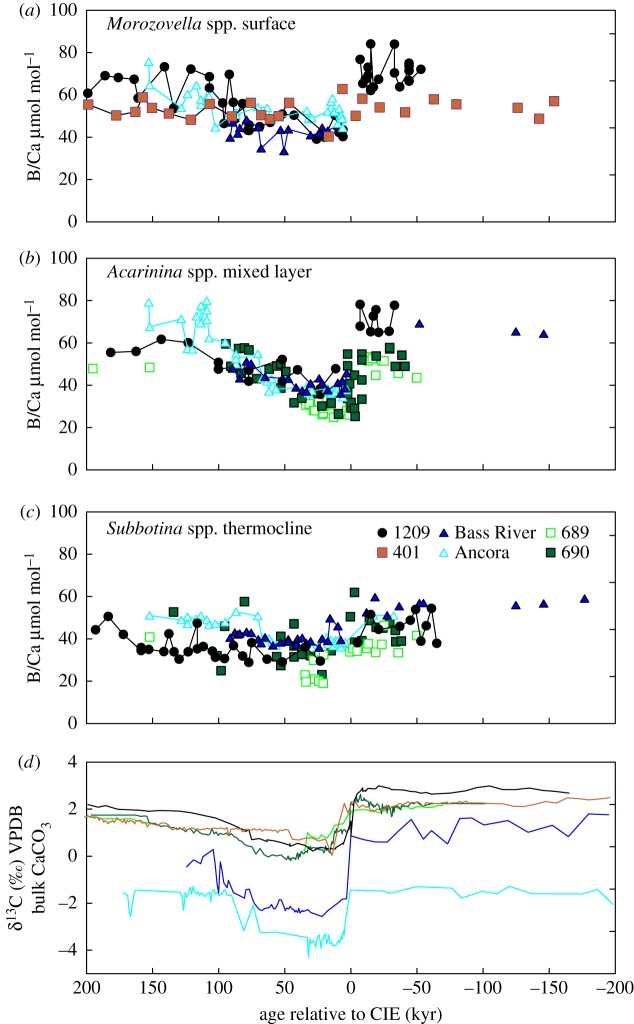
Figure 2.
Comparison of planktonic foraminiferal B/Ca (a–c) and bulk carbonate δ13C records (d). ODP 1209 (solid black circles) [10], 689 (open light green squares), 690 (solid green squares) and DSDP 401 (solid orange squares) [12] are pelagic sites (a–c). ODP sites Bass River (solid blue triangles) [11] and Ancora (light blue triangles) are continental shelf sites (a–c). Bulk carbonate δ13C records (d) are labelled in the same colour scheme as B/Ca records (a–c). Sites 1209 [9], 401 [12], 690 [25], 689 [18], Bass River [26] and Ancora [27] are plotted versus age in kiloyears relative to the onset of the CIE. A detailed explanation of the age model construction for each site can be found in the Methods section. Analytical reproducibility of B/Ca based on repeated analysis of in-house laboratory consistency standards is 6% and 7% (2 s.d.) at Rutgers University and University of California Santa Cruz, respectively [10,11]. Vienna Pee Dee Belemnite (VPDB) carbon isotope standard. (Online version in colour.)
(c). Analytical methodology
Trace element analyses were conducted on a Thermo Element XR inductively coupled plasma mass spectrometer at Rutgers University (sites Bass River and Ancora) and the University of California Santa Cruz (ODP 1209, 689 and 690) (figure 2a–c). Boron isotopes were measured on a Thermo Triton Thermal Ionization multi-collector mass spectrometer at Lamont-Doherty Earth Observatory (ODP 1209, Bass River and Millville) (figure 3a). Foraminiferal cleaning and instrument protocol carried out for records generated in this study (trace elements at sites Ancora, 689 and 690; boron isotopes at sites Millville and Bass River) follow previous work and detailed methodologies are provided therein [10,11].
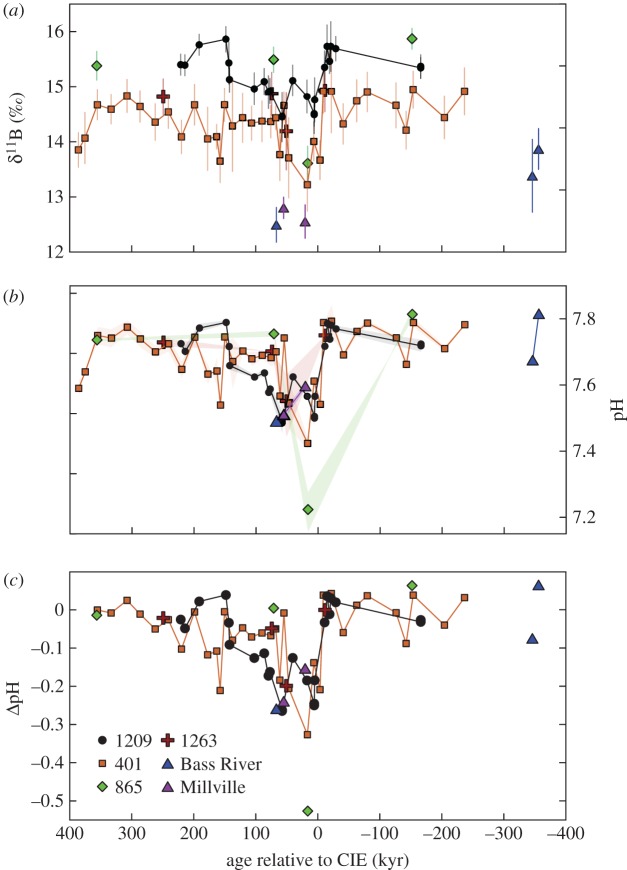
Figure 3.
Relative changes in ocean pH derived from planktonic foraminiferal boron isotope (δ11B) measurements (a). Surface dweller M. velascoensis was used to generate the 1209 (black circles), 1263 (red crosses), 865 (green triangles) [10] and M. subbotinae 401 (orange squares) [12] δ11B records (a). Deep dweller Subbotina spp. were used in δ11B reconstructions at Bass River (blue diamonds) and Millville (purple diamonds) (a). All records were normalized to a pre-event ocean pH = 7.75 by adjusting the intercept of the assumed δ11Bforam to 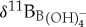 calibration following a similar approach to previous studies [10,12] (b,c). Ocean pH anomalies (ΔpH) were estimated by computing a relative δ11B change compared with an average of pre-CIE values (c). A constant seawater δ11B value of 38.9 ± 0.4‰ for the duration of the PETM was assumed [12] and no vital effect
calibration following a similar approach to previous studies [10,12] (b,c). Ocean pH anomalies (ΔpH) were estimated by computing a relative δ11B change compared with an average of pre-CIE values (c). A constant seawater δ11B value of 38.9 ± 0.4‰ for the duration of the PETM was assumed [12] and no vital effect 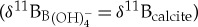 was considered to estimate ΔpH (c). Age is denoted in kiloyears relative to the CIE. Error envelopes on ocean pH estimates include the analytical uncertainty of 2 s.e. of repeat sample analyses (n = 2–3), and conservative uncertainty estimates for temperature (±2°C) and salinity (±2 units) (b). (Online version in colour.)
was considered to estimate ΔpH (c). Age is denoted in kiloyears relative to the CIE. Error envelopes on ocean pH estimates include the analytical uncertainty of 2 s.e. of repeat sample analyses (n = 2–3), and conservative uncertainty estimates for temperature (±2°C) and salinity (±2 units) (b). (Online version in colour.)
3. Results
New B/Ca data from sites 689, 690 and Ancora (electronic supplementary material, tables S1 and S2) are presented with published B/Ca records from sites 1209, Bass River and 401 (figure 2a–c) [10–12]. At site 690, using A. praepentacamerata and A. soldadoensis to represent the mixed layer, B/Ca declines by 30% (from 53 to 37 µmol mol−1) within the CIE, then recovers to pre-CIE values (figure 2b). Subbotina B/Ca, representing the thermocline, is slightly lower throughout but shows a similar 25% decline (from 45 to 34 µmol mol−1) and recovery (figure 2c). At site 689, the B/Ca patterns recorded by A. praepentacamerata, A. soldadoensis and Subbotina are similar to those of 690, though slightly offset (figure 2b,c). At Ancora, average B/Ca ratios for Subbotina spp. decline by 25% (from 51 to 38 µmol mol−1) within the CIE (figure 2c). Although no data were generated for the Palaeocene, within the CIE Morozovella spp. and Acarinina spp. values average 50 µmol mol−1 and increase to 70 and 60 µmol mol−1, respectively, in the recovery interval (figure 2a,b).
δ11B data from the Bass River and Millville sites are plotted along with published δ11B data from sites 1209, 1263, 865 [10] and 401 [12] (figure 3a; electronic supplementary material, table S3). Below the CIE onset at site Bass River, average δ11B values for Subbotina spp. are 13.6‰ and decrease to average minimum δ11B values within the CIE core of 12.5‰ and 12.7‰ at sites Bass River and Millville, respectively (figure 3a). To test a potential size bias, δ11B data were measured in multiple size fractions but no significant offset was found over the size range covered in this study (electronic supplementary material, table S3). This is consistent with data from modern deep dwellers, the ecological parallel of Subbotina spp., which show only a negligible δ11B-test size relationship [28].
4. Discussion
(a). Global trends in boron proxy records during the Palaeocene–Eocene Thermal Maximum
At all locations, and in each of the three foraminiferal taxa measured, the records follow a common pattern (table 1). Coincident with the onset of the CIE, B/Ca and δ11B decrease, indicating acidification of upper ocean waters. In all the boron proxy records that span the duration of the event, this sharp decline is followed by a plateau of consistently depressed B/Ca and δ11B values that persist for approximately as long as the CIE itself (approx. 70 kyr in our age models). Coeval planktonic foraminiferal δ13C values closely track B/Ca records through the CIE onset but δ13C appears to recover faster than B/Ca and δ11B (figures 2 and 3). This could be indicative of decoupling between the key ocean carbonate system parameters (i.e. pH, dissolved inorganic carbon (DIC) and 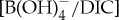 ) driving each individual proxy [10]. This divergence pattern can be explained by shifting the relative contributions of organic carbon burial versus silicate weathering to carbon sequestration during the CIE recovery. While both processes elevate pH, organic carbon burial is more efficient in raising δ13C because silicate weathering only accelerates dilution of this excess light carbon while organic carbon burial preferentially removes light 12C from exogenic reservoirs. Indeed, geochemical model studies invoke an intensified organic carbon burial feedback to facilitate carbon sequestration during the CIE recovery [12,29]. Finally, near the start of the CIE recovery, B/Ca and δ11B begin to increase coincident with a recovery and temporary overshoot of %CaCO3 in sediments globally [30], including an overshoot in the calcite compensation depth (CCD) [31] and/or a build-up of alkalinity in deep waters [32], suggesting a restoration of carbonate chemistry. Taken together, our foraminiferal boron proxy records support rapid PETM surface ocean acidification from pelagic to coastal, and tropical to polar settings, lasting tens of thousands of years.
) driving each individual proxy [10]. This divergence pattern can be explained by shifting the relative contributions of organic carbon burial versus silicate weathering to carbon sequestration during the CIE recovery. While both processes elevate pH, organic carbon burial is more efficient in raising δ13C because silicate weathering only accelerates dilution of this excess light carbon while organic carbon burial preferentially removes light 12C from exogenic reservoirs. Indeed, geochemical model studies invoke an intensified organic carbon burial feedback to facilitate carbon sequestration during the CIE recovery [12,29]. Finally, near the start of the CIE recovery, B/Ca and δ11B begin to increase coincident with a recovery and temporary overshoot of %CaCO3 in sediments globally [30], including an overshoot in the calcite compensation depth (CCD) [31] and/or a build-up of alkalinity in deep waters [32], suggesting a restoration of carbonate chemistry. Taken together, our foraminiferal boron proxy records support rapid PETM surface ocean acidification from pelagic to coastal, and tropical to polar settings, lasting tens of thousands of years.
Table 1.
Average planktonic foraminiferal B/Ca and δ11B data during the PETM.
| average B/Ca µmol mol−1a |
|||||
|---|---|---|---|---|---|
| core site | species | below CIE onset | CIE core | post-CIE | ΔB/Cab |
| 1209 | M. velascoensis | 70.4 | 44.5 | 62.7 | 25.9 |
| 1209 | A. soldadoensis | 71.0 | 43.9 | 53.5 | 27.1 |
| 401 | M. subbotinae | 51.9 | 51.4 | 52.6 | 0.5 |
| 689 | Acarinina spp. | 49.3 | 29.8 | 49.2 | 19.5 |
| 690 | Acarinina spp. | 53.4 | 37.0 | 52.7 | 16.4 |
| Bass River | Acarinina spp. | 65.0 | 40.0 | 48.0 | 25.0 |
| Bass River | Morozovella spp. | 42.0 | 45.0 | ||
| Ancora | Acarinina spp. | 38.0 | 65.0 | ||
| Ancora | Morozovella spp. | 51.0 | 58.0 | ||
| 1209 | Subbotina spp. | 46.0 | 31.3 | 37.5 | 14.8 |
| 689 | Subbotina spp. | 37.3 | 24.9 | 39.2 | 12.4 |
| 690 | Subbotina spp. | 45.3 | 33.8 | 42.6 | 11.5 |
| Bass River | Subbotina spp. | 57.0 | 40.0 | 42.0 | 17.0 |
| Ancora | Subbotina spp. | 51.0 | 38.0 | 49.0 | 13.0 |
| average δ11B (‰)a | Δδ11B (‰)b | ||||
| 1209 | M. velascoensis | 15.5 | 14.7 | 15.3 | 0.9 |
| 401 | M. subbotinae | 14.7 | 14.0 | 14.3 | 0.7 |
| Bass River | Subbotina spp. | 13.5 | 12.5 | 1.1 | |
| Millville | Subbotina spp. | 12.7 | |||
aThe onset of the CIE is identified as the initial decrease in δ13C, core CIE is an interval of sustained low δ13C values (less than 70 kyr) and the post-CIE interval represents the recovery of δ13C to values below the CIE onset (70–200 kyr) (figure 2d).
bDifference between average values prior to the CIE onset and the CIE core interval.
A partial exception to the above trends occurs with the geochemical proxy records based on the surface dweller M. subbotinae at DSDP site 401 [12]. At site 401, one extreme minimum δ13C point and one extreme minimum δ11B point occur approximately 25 kyr after the CIE onset and are directly followed by the CIE recovery phase (figure 3a). Unlike all the other sites, B/Ca at site 401 shows only a very slight decrease at the CIE onset (figure 2a and table 1). Furthermore, the foraminifera δ13C records at site 401 exhibit a relatively small CIE magnitude compared with regional and global records [33]. Incomplete recovery by rotary drilling (RCB in International Ocean Discovery Program (IODP) terminology) at site 401 probably resulted in a truncated or disturbed PETM interval [34], which helps explain the discrepancies with other sites [35]. During the recovery of core 401–14R, which contains the PETM, drilling advanced 9.5 m but only 7.2 m of sediment was recovered, indicating the loss of over 2 m of sediment within this interval [34]. Given the abrupt lithological change at the Palaeocene–Eocene boundary, it is likely that at least some of this sediment loss occurred at the base of the PETM, truncating the CIE and associated geochemical records. A similar problem occurred during attempts to recover the clay layer at ODP 1263 by rotary coring [36]. We include the site 401 boron proxy records in the global compilation while recognizing that the CIE is only partially represented (figures 2a and 3).
(b). Quantifying changes in seawater carbonate chemistry
Following the approach of Penman et al. [10], we use the boron isotope data from mixed-layer and thermocline-dwelling foraminifera to quantify the decrease in surface seawater pH during the onset of the PETM. We focus on the relative magnitude of acidification rather than estimating absolute pH, and use the more established δ11B proxy to corroborate B/Ca trends. Knowledge of past seawater δ11B (δ11Bsw) as well as a species-specific calibration are required to derive absolute ocean pH using foraminiferal δ11B data. An estimation of temperature, salinity and major seawater ion concentration is also required to calculate the dissociation constant of boric acid (K*B). The experimentally determined boron isotopic fractionation factor of 1.0272 was used [37]. We applied an estimated δ11Bsw value of 38.9 ± 0.4‰ [12], which is consistent with δ11Bsw estimates over the Eocene [38]. To account for potential laboratory offsets, pre-event δ11B values were normalized to pH = 7.75, following the approach of previous PETM boron proxy model studies [10,12]. K*B was calculated with the MyAMI model assuming Eocene seawater (approx. 50 Ma) [Mg] = 30 mM and [Ca] = 20 mM [39]. Surface and thermocline temperatures are estimated using Mg/Ca palaeothermometry with a modern multi-species calibration [40]. In addition to temperature, variation in past seawater Mg/Ca composition affects the Mg distribution coefficient and possibly Mg/Ca proxy sensitivity to temperature. A nonlinear correction scheme to account for the influence of variable past Mg/Casw developed for the modern planktonic foraminifer Trilobatus sacculifer was applied to compute ocean temperatures [41]. We used previously generated Mg/Ca records in M. velascoensis at site 1209 [10,42], M. subbotinae at site 401 [12] and Subbotina spp. at site Bass River [11]. Pre- and post-event salinity values of 37 (ODP 1209) and 35 (sites Bass River, Millville and ODP 401) were used and are similar to climate model regional estimates [43]. A salinity increase of 1.5 units was previously determined at site 1209 [42], whereas a 2 unit decrease is estimated for coastal New Jersey sites and a 1.5 unit decrease at site 401 (further discussion on estimation of salinity anomalies in §4c Contribution of regional salinity shifts on B/Ca).
Culture calibration studies with modern planktonic foraminifera document species-specific δ11Bforam to 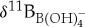 relationships that are variably offset from seawater borate
relationships that are variably offset from seawater borate 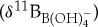 values [44]. These isotopic offsets are thought to be caused by the physiological processes of symbiont photosynthesis, respiration and calcification that act to modify pH within the calcifying microenvironment around foraminifera and, subsequently, alter the δ11B recorded in foraminiferal calcite [44]. To estimate ocean pH from the extinct species used in this study, we need to approximate their
values [44]. These isotopic offsets are thought to be caused by the physiological processes of symbiont photosynthesis, respiration and calcification that act to modify pH within the calcifying microenvironment around foraminifera and, subsequently, alter the δ11B recorded in foraminiferal calcite [44]. To estimate ocean pH from the extinct species used in this study, we need to approximate their 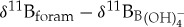 relationships. The first option is to assume equilibrium between δ11B of foraminiferal calcite (δ11Bc) and δ11B of seawater borate
relationships. The first option is to assume equilibrium between δ11B of foraminiferal calcite (δ11Bc) and δ11B of seawater borate 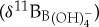 , which is equivalent to assuming the absence of any vital effects
, which is equivalent to assuming the absence of any vital effects 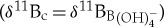 . Alternatively, habitat ecology (i.e. surface dwelling and photosymbiont bearing) determined by previous studies on the basis of carbon and oxygen isotope offsets can be used as a means to assign a modern calibration relationship to an analogous extinct species. However, ascribing such a modern analogue to an extinct species assumes modern vital effects were similar during the Palaeogene, which is currently poorly understood.
. Alternatively, habitat ecology (i.e. surface dwelling and photosymbiont bearing) determined by previous studies on the basis of carbon and oxygen isotope offsets can be used as a means to assign a modern calibration relationship to an analogous extinct species. However, ascribing such a modern analogue to an extinct species assumes modern vital effects were similar during the Palaeogene, which is currently poorly understood.
Several lines of evidence argue for diminished vital effects of Eocene age planktonic foraminifera relative to modern species. Reduced δ13C test size relationships in muricate PETM species (acarininids and morozovellids) compared with modern species (Globigerinoides) suggest overall lower photosymbiont activity rates [45–47]. If we assume that lower photosynthetic rates translate to reduced vital effects, we would expect foraminiferal δ11B to be closer to seawater 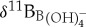 values. Indeed, δ11B values analysed for a suite of planktonic foraminifera occupying the upper water column show a narrower isotopic range in the Eocene relative to modern day, implying reduced vital effects [38,48]. This is further corroborated by similar δ11B values obtained in time-equivalent Eocene samples of S. roesnaesensis and benthic foraminifera Cibicidoides [38], genera which are interpreted to have minimal δ11B-related vital effects in modern ocean sediments [44]. When modern ecologically equivalent
values. Indeed, δ11B values analysed for a suite of planktonic foraminifera occupying the upper water column show a narrower isotopic range in the Eocene relative to modern day, implying reduced vital effects [38,48]. This is further corroborated by similar δ11B values obtained in time-equivalent Eocene samples of S. roesnaesensis and benthic foraminifera Cibicidoides [38], genera which are interpreted to have minimal δ11B-related vital effects in modern ocean sediments [44]. When modern ecologically equivalent 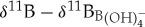 calibration equations are applied to Eocene surface (morozovellids) and thermocline (subbotinids) species, an unrealistic reverse ocean pH depth profile is obtained [38]. This suggests that modern δ11B to
calibration equations are applied to Eocene surface (morozovellids) and thermocline (subbotinids) species, an unrealistic reverse ocean pH depth profile is obtained [38]. This suggests that modern δ11B to 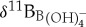 calibrations overestimate Eocene planktonic foraminifer vital effects. For these reasons, we consider no vital effect
calibrations overestimate Eocene planktonic foraminifer vital effects. For these reasons, we consider no vital effect 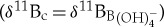 with a slope of 1.0 to be a closer approximation of surface and thermocline ocean pH change (ΔpH) at the CIE relative to pre-event values (figure 3c). We calculate an average pH decrease for M. velascoensis of 0.22 units (extrema 0.12–0.30) at site 1209, 0.15 units (0.04–0.34) for M. subbotinae at site 401 and for Subbotina spp. 0.22 units (0.23–0.52) in New Jersey (figure 3c). By contrast, if we apply the pH sensitivity of the modern photosymbiont-bearing T. sacculifer (slope = 0.82) (refitted in [28,49]) at site 1209, a larger average pH decrease for M. velascoensis of 0.26 units (extrema 0.14–0.37) is estimated (electronic supplementary material, figure S1c). Whether we include or exclude any vital effects, our multi-site pH estimates produce a uniform degree of acidification in the coastal ocean compared with pelagic sites (figure 3c; electronic supplementary material, figure S1c) in agreement with model predictions, lending support to our observations [12]. This spatial similarity is perhaps unsurprising, as an increase in atmospheric pCO2 should be absorbed by the surface ocean everywhere, leading to a relatively uniform pH decrease globally. A decline in the degree of surface ocean acidification from the mixed layer to the thermocline is also consistent with model predictions of attenuated acidification with depth [14]. Regardless of the calibration uncertainties, both δ11B records are consistent in providing evidence for upper ocean acidification in support of our interpretation of complementary B/Ca records.
with a slope of 1.0 to be a closer approximation of surface and thermocline ocean pH change (ΔpH) at the CIE relative to pre-event values (figure 3c). We calculate an average pH decrease for M. velascoensis of 0.22 units (extrema 0.12–0.30) at site 1209, 0.15 units (0.04–0.34) for M. subbotinae at site 401 and for Subbotina spp. 0.22 units (0.23–0.52) in New Jersey (figure 3c). By contrast, if we apply the pH sensitivity of the modern photosymbiont-bearing T. sacculifer (slope = 0.82) (refitted in [28,49]) at site 1209, a larger average pH decrease for M. velascoensis of 0.26 units (extrema 0.14–0.37) is estimated (electronic supplementary material, figure S1c). Whether we include or exclude any vital effects, our multi-site pH estimates produce a uniform degree of acidification in the coastal ocean compared with pelagic sites (figure 3c; electronic supplementary material, figure S1c) in agreement with model predictions, lending support to our observations [12]. This spatial similarity is perhaps unsurprising, as an increase in atmospheric pCO2 should be absorbed by the surface ocean everywhere, leading to a relatively uniform pH decrease globally. A decline in the degree of surface ocean acidification from the mixed layer to the thermocline is also consistent with model predictions of attenuated acidification with depth [14]. Regardless of the calibration uncertainties, both δ11B records are consistent in providing evidence for upper ocean acidification in support of our interpretation of complementary B/Ca records.
Culturing experiments established a carbonate system control on B/Ca in planktonic foraminiferal calcite [50–53]. A first-order approximation is to simply relate modern B/Ca proxy systematics to interpret PETM records. However, when modern B/Ca calibrations are applied to B/Ca data from extinct PETM taxa, unrealistic negative 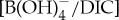 values are estimated [10]. In general, the PETM B/Ca values are significantly lower, extending beyond the modern calibration range. We instead consider new empirical culture calibrations that relate B/Ca to
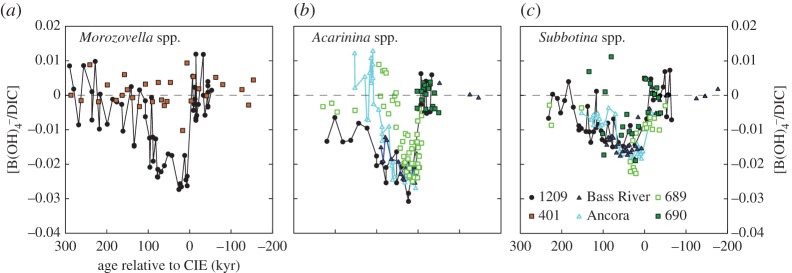
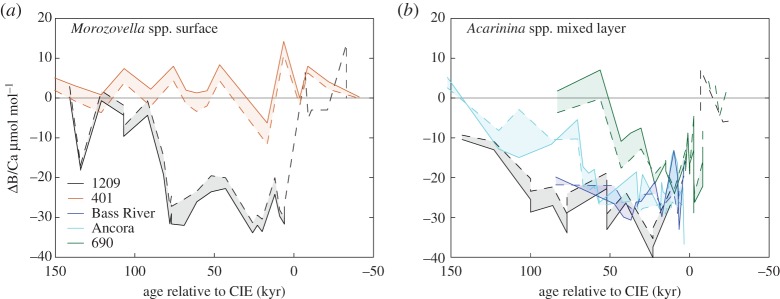
values are estimated [10]. In general, the PETM B/Ca values are significantly lower, extending beyond the modern calibration range. We instead consider new empirical culture calibrations that relate B/Ca to 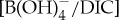 of modern photosymbiont-bearing species Orbulina universa under simulated Palaeocene seawater chemistry (high [Ca], low [Mg] and low total boron concentration [BT]) [53] (figure 4). We translate B/Ca excursion anomalies (average pre-CIE baseline compared with core CIE values) to estimate
of modern photosymbiont-bearing species Orbulina universa under simulated Palaeocene seawater chemistry (high [Ca], low [Mg] and low total boron concentration [BT]) [53] (figure 4). We translate B/Ca excursion anomalies (average pre-CIE baseline compared with core CIE values) to estimate 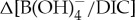 as in previous studies [10,11] and apply ‘Palaeocene’-derived calibration sensitivity [53] (figure 4). We only consider B/Ca excursion anomalies of mixed-layer (acarininids) and thermocline (subbotinids) dwellers, for which there are pre-CIE values recorded at all locations used in this study. We estimate a
as in previous studies [10,11] and apply ‘Palaeocene’-derived calibration sensitivity [53] (figure 4). We only consider B/Ca excursion anomalies of mixed-layer (acarininids) and thermocline (subbotinids) dwellers, for which there are pre-CIE values recorded at all locations used in this study. We estimate a 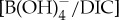 decrease for M. velascoensis of 0.023 at site 1209 and essentially no change for M. subbotinae at site 401 (figure 4a). For Acarinina spp., all sites (NJ, 1209, 689 and 690) show an average decrease of 0.022 (extrema 0.021–0.024) (figure 4b) and Subbotina spp. show an average decrease of 0.015 (extrema 0.013–0.018) (figure 4c). Using the new Palaeocene calibration yields
decrease for M. velascoensis of 0.023 at site 1209 and essentially no change for M. subbotinae at site 401 (figure 4a). For Acarinina spp., all sites (NJ, 1209, 689 and 690) show an average decrease of 0.022 (extrema 0.021–0.024) (figure 4b) and Subbotina spp. show an average decrease of 0.015 (extrema 0.013–0.018) (figure 4c). Using the new Palaeocene calibration yields 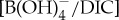 anomalies are closer to previous estimates based on calibrations conducted in modern seawater [10] but still larger than predicted carbonate and borate chemistry estimated by model outputs [53]. This model–data difference could be a result of the Palaeocene calibration being too insensitive to fully resolve the B/Ca anomalies across the PETM [53]. Another explanation is that a larger carbon release (greater than 3000 Pg C) is required to better approximate the B/Ca-based estimates of carbonate chemistry change. Both B/Ca and δ11B proxies estimate a shift in surface ocean carbonate chemistry towards lower pH and saturation state. Within the calibration uncertainties, both boron-based proxies support a uniform pattern of ocean acidification during the PETM. Coincident biogeochemical changes, such as salinity, dissolution and symbiont loss, could influence B records and we evaluate these factors in addition to seawater carbonate chemistry.
anomalies are closer to previous estimates based on calibrations conducted in modern seawater [10] but still larger than predicted carbonate and borate chemistry estimated by model outputs [53]. This model–data difference could be a result of the Palaeocene calibration being too insensitive to fully resolve the B/Ca anomalies across the PETM [53]. Another explanation is that a larger carbon release (greater than 3000 Pg C) is required to better approximate the B/Ca-based estimates of carbonate chemistry change. Both B/Ca and δ11B proxies estimate a shift in surface ocean carbonate chemistry towards lower pH and saturation state. Within the calibration uncertainties, both boron-based proxies support a uniform pattern of ocean acidification during the PETM. Coincident biogeochemical changes, such as salinity, dissolution and symbiont loss, could influence B records and we evaluate these factors in addition to seawater carbonate chemistry.
Figure 4.
Ocean carbonate chemistry reconstructions derived from planktonic foraminiferal B/Ca records (a–c). B/Ca records are presented for ODP sites 1209 (black circles) [10], 689 (open light green squares), 690 (solid green squares), Bass River (solid blue triangles) [11], Ancora (light blue triangles) and DSDP 401 (orange squares) [12]. Seawater 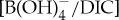 anomalies are relative to average pre-CIE values and are based on
anomalies are relative to average pre-CIE values and are based on 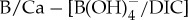 sensitivity determined in modern photosymbiont-bearing planktonic foraminifera species O. universa under simulated Palaeocene seawater chemistry [53]. (Online version in colour.)
sensitivity determined in modern photosymbiont-bearing planktonic foraminifera species O. universa under simulated Palaeocene seawater chemistry [53]. (Online version in colour.)
(c). Contribution of regional salinity shifts on B/Ca
Culture experiments reveal a small but significant influence of salinity on boron incorporation and, consequently, B/Ca ratios in planktonic foraminifera [50]. Regional shifts in salinity during the PETM could augment or dampen B/Ca anomalies and, if unaccounted for, bias our interpretation of the B/Ca component related to carbonate chemistry. We can disentangle the influences of salinity and carbonate chemistry by normalizing B/Ca records to a common salinity value. However, accurate estimates of salinity using the paired Mg/Ca-δ18O method [42] require accurate estimates of temperature and an appropriate δ18Osw–salinity regional relationship, which are difficult to constrain. As an alternative, we chose to reconstruct salinity anomalies relative to pre-CIE baseline values [42]. Morozovellid and acarininid Mg/Ca and δ18O datasets at sites Bass River [11,26], Ancora [27], 401 [12], 1209 [10,42] and 690 [2,54] were used to estimate relative salinity anomalies. For each site, an average pre-CIE δ18O and Mg/Ca value was subtracted from each data point to estimate the relative changes across the event, Δδ18O and ΔMg/Ca. Ocean temperature anomalies were estimated using a multi-species calibration, as inter-species variability in Mg/Ca temperature sensitivity is quite small in modern subtropical planktonic foraminifera [40]. In this way, we can quantify uncertainty of non-thermal controls on Mg/Ca that are necessary to consider for absolute temperature reconstructions. The temperature component of Δδ18O was calculated with a Δδ18O/ΔT sensitivity of 0.21‰/°C, derived for modern symbiont-bearing O. universa [55]. Orbulina universa was selected as its temperature sensitivity is a mid-range value for modern planktonic foraminifer species and B/Ca salinity sensitivity is also known [50]. The salinity anomaly estimate assumes the discrepancy between δ18O and Mg/Ca temperature anomalies is solely from regional surface water δ18Osw. The residual non-temperature δ18O component corresponds to Δδ18Osw and was converted to ΔSSS (surface seawater salinity) by using the same slope as the modern regional Δδ18Osw to ΔSSS relationship. The residual Δδ18O was converted to ΔSSS using a slope of 0.15‰/SSS at sites Bass River and Ancora [56], 0.38‰/SSS for site 1209 [42], 0.24‰/SSS for site 690 [57,58] and 0.55‰/SSS for site 401 [59].
At each site, the pre-CIE B/Ca average of each species was used as the baseline, and ΔB/Ca was calculated for each sample that also has a paired Mg/Ca and δ18O-based salinity anomaly estimate (figure 5). For Acarinina spp., the pre-CIE B/Ca average from site Bass River was applied to proximal site Ancora to compute ΔB/Ca because there are no Palaeocene B/Ca values at Ancora (figure 5b). B/Ca records were corrected for ΔSSS using the O. universa sensitivity of ΔB/Ca/ΔSSS (2.6 µmol mol−1 per salinity unit) as determined in laboratory culture [50]. Both the uncorrected and salinity-corrected ΔB/Ca are shown in figure 5 with the exception of Morozovella spp. at sites Bass River and Ancora, as the B/Ca records do not extend into the Palaeocene (figure 2a). At the shelf NJ sites Bass River and Ancora, North Atlantic site 401 and Southern Ocean sites 689 and 690 regional salinity decreases amplify the total B/Ca anomaly, while the estimated salinity increase at North Pacific site 1209 dampens the total anomaly. These estimated salinity changes can account for a 13% increase in the total B/Ca anomaly at site 1209, and an 11% and 22% decrease in NJ and Southern Ocean sites, respectively. In our salinity correction on B/Ca, we assume that modern B/Ca salinity sensitivities apply to extinct PETM taxa. This should be considered an approximation because modern studies document species-specific B/Ca salinity responses [50], and it is unclear whether these modern salinity sensitivities might have been different under Palaeocene seawater composition. In light of these assumptions, we consider our salinity-corrected reconstructions as a first-order sensitivity test to evaluate the potential bias in B/Ca records. While regional salinity variations can account for some minor differences between B/Ca anomalies among studied sites, ultimately the trends remain robust and most consistent with ocean carbonate chemistry as the main driver of PETM B/Ca records.
Figure 5.
Estimation of the potential contribution of regional changes in salinity to B/Ca records. B/Ca anomalies (ΔB/Ca, µmol mol−1) were computed by subtracting a pre-CIE average value from each point in the record (a,b). Paired planktonic foraminiferal Mg/Ca and δ18O were used to estimate δ18Osw changes; regional δ18Osw–salinity relationships were used to translate δ18Osw to ΔSSS. Plotted here are ΔB/Ca records uncorrected (dashed line) and corrected (bold line) for regional changes in salinity using B/Ca salinity sensitivity of modern O. universa derived in laboratory culture experiments [50]. The shaded region represents the potential contribution of local salinity changes to ΔB/Ca records. Age is denoted in kiloyears relative to the CIE. (Online version in colour.)
(d). Impact of preservation
With multiple sites from a diverse range of palaeoceanographic settings, palaeodepths, sediment burial depths, lithology and diagenetic pathways, we can assess the potential impact of preservation on individual boron proxy records. Differential dissolution tends to lower B/Ca of surface planktonic foraminifera [60], but shows little impact on thermocline dwellers [61]. It was speculated that variable dissolution response between species is the result of heterogeneous B distribution within the test and relatively greater dissolution susceptibility of certain test textures [61]. δ11B of T. sacculifer was found to lower with increasing water depth, again as a result of enhanced dissolution [62–64]. However, δ11B in Globigerinoides ruber does not follow a similar dissolution pattern [63,64]. This variable dissolution susceptibility between different species is argued to be a result of differential δ11B heterogeneity within the test or variable dissolution susceptibility of ontogenetic versus gametogenic calcite [65]. The proximity of sites 689 (1100 m) and 690 (1900 m) but with different palaeo water depths provides a scenario to test a depth-related dissolution influence on B/Ca. The close similarity of the absolute values and trends in sites 689 and 690 B/Ca records despite disparate palaeodepths suggests a minimal and consistent influence of dissolution (figure 2b,c).
An additional opportunity to assess the foraminiferal preservation state comes with the strontium/calcium ratio (Sr/Ca) of biogenic CaCO3, which is released into pore fluids during calcite dissolution and excluded during inorganic calcite precipitation. Indeed, detailed Sr/Ca measurements of diagenetically altered foraminiferal calcite show lower values, clearly demonstrating the effect of dissolution and recrystallization [66]. Sr/Ca values at Bass River and Ancora are generally higher than the value typical of diagenetic calcite (1.0 mmol mol−1) [66], indicating that diagenesis did not compromise the geochemical records, with the exception of samples within two discrete depth intervals not included in the reconstructions [11]. Foraminiferal Sr/Ca at site 1209 is lower (near 1 mmol mol−1 [42]), consistent with the ‘frosty’ texture of foraminifers at that site. However, Sr/Ca is near constant throughout the PETM interval, suggesting that if recrystallization has altered foraminiferal trace element compositions it has done so uniformly over this short time interval and, therefore, relative changes in B/Ca should be preserved.
Another strategy to evaluate the effects of preservation on geochemical signals is to compare sites with identical environmental conditions but demonstrably different diagenetic sediment histories. Edgar et al. [65] compared time-equivalent (Middle Eocene) planktonic foraminiferal B/Ca and δ11B records from glassy foraminifers (Tanzanian Drilling Project, Indian Ocean) with a site that contains recrystallized foraminifera (ODP 865, central Pacific Ocean). Planktonic foraminiferal B/Ca are lower in the recrystallized (frosty) foraminifers from site 865, yet, by contrast, δ11B values were similar, suggesting that exchange between pore fluids and recrystallized carbonate can result in a lowering of boron concentration without isotopic fractionation. This predicts that B/Ca in diagenetically precipitated inorganic calcite is lower than biogenic calcite, which is consistent with the idea of preferential B exclusion during recrystallization. While this raises some concern for the pelagic records, site 865 represents an extreme example of recrystallization as the entire sediment column consists of a high-porosity ooze dominated by foraminifera sand so that recrystallization is occurring in a completely or partially open system at high seawater to CaCO3 ratios [67]. This contrasts with the lower porosity pelagic sites in this study, where pore water chemistry is mainly controlled by diffusion and diagenesis occurs in a relatively closed system at lower seawater to CaCO3 ratios. Furthermore, the continental shelf sites (sites Bass River and Ancora) exhibit exceptionally well-preserved foraminifera with classic glassy shell textures similar to the preservation seen in Eocene Tanzania sections [68]. The relatively impermeable nature of clay-rich sediments limits or even prevents interaction of foraminiferal calcite with pore fluids, thus preserving primary chemical signatures. Visual evaluation of the site Bass River foraminifera by scanning electron microscopy supports this claim [11]. Remarkably, despite these different diagenetic histories and preservation states between the shelf and pelagic sites, a similar pattern of B/Ca is recorded, suggesting that any diagenetic bias on relative trends of individual records is minimal (figure 2a–c).
(e). Symbiont loss
A potential biological consequence of warming is loss of photosymbionts (bleaching) in planktonic foraminifera. Evidence of symbiont bleaching in planktonic foraminifera was previously suggested for the Middle Eocene [69,70]. Since the early stages of δ11B proxy development, it was apparent that biogenic CaCO3 is likely not to be simply a recorder of ambient seawater pH but also carries the signal of local pH variation within the calcifying microenvironment. Physiological processes including symbiont photosynthesis, respiration and calcification can modify local pH [71,72] and, in turn, B/Ca and δ11B of foraminiferal calcite [73,74]. Detailed intra-test measurements in symbiont-bearing benthic foraminifera [75], light intensity in planktonic foraminifera culture experiments [53,73] and sediment-trap work [76] collectively substantiate the potential impact of symbiont activity on B/Ca and δ11B.
Test size δ13C gradients in PETM planktonic foraminifera taxa are used as an indicator of photosymbiont activity [45] and loss of gradients as a proxy for bleaching in symbiont-bearing species [70]. Preferential use of light carbon (12C) during photosynthesis in combination with either increased photosymbiont abundance or activity in larger individuals can explain the positive relationship between test size and δ13C. The δ13C test size gradients in symbiont-bearing species (A. soldadoensis and Morozovella spp.) in shelf sites are maintained within the CIE, suggesting that, at temperate latitudes, bleaching did not occur [11]. Deeper dwelling Subbotina spp. also record a B/Ca and δ11B change, corroborating the trends shown in symbiotic surface dwellers within the same cores, signifying that even if bleaching did occur it cannot explain the entire signal (figures 2a–c and 3). In addition, Southern Ocean sites (689, 690) were located in comparatively colder polar waters where foraminifera would presumably be less vulnerable to warming-induced bleaching. The close similarity of the B/Ca trends between the cold high latitudes (sites 689 and 690), the warm low latitudes (site 1209), and the temperate continental shelf (NJ margin) argues against symbiont bleaching as a major driver of the B/Ca trends at all sites.
(f). Biological implications
Our global compilation of boron proxy (δ11B and B/Ca) reconstructions reveals near-uniform upper ocean acidification (figures 3c and 4). The similarity in B/Ca values between records implies minimal spatial variability in absolute ocean carbonate chemistry (e.g. 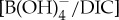 ) (figure 4). Since the degree of acidification was uniformly expressed in the surface ocean then there appears to be no refuge. The carbonate saturation state, on the other hand, could be spatially variable, which is suggested during the PETM by model output [77]. However, if we follow this logic, the high latitudes would be closer to undersaturated values with respect to CaCO3 and thus are more vulnerable to acidification. There could be short-term responses to ocean acidification during the CIE onset that are not captured in our B proxy records, as dissolution gaps at the CIE onset are common in most sections, even in the shallow marine shelf sections. Another possibility is that the combined effects of ocean acidification and warming on marine biota could be more significant than ocean carbonate chemistry as an independent stressor.
) (figure 4). Since the degree of acidification was uniformly expressed in the surface ocean then there appears to be no refuge. The carbonate saturation state, on the other hand, could be spatially variable, which is suggested during the PETM by model output [77]. However, if we follow this logic, the high latitudes would be closer to undersaturated values with respect to CaCO3 and thus are more vulnerable to acidification. There could be short-term responses to ocean acidification during the CIE onset that are not captured in our B proxy records, as dissolution gaps at the CIE onset are common in most sections, even in the shallow marine shelf sections. Another possibility is that the combined effects of ocean acidification and warming on marine biota could be more significant than ocean carbonate chemistry as an independent stressor.
Several major plankton groups experienced dynamic changes during the PETM, including evolutionary turnover and shifts in latitudinal distribution, probably in response to warming. Moreover, geochemical evidence indicates that tropical sea-surface temperatures probably exceeded 30°C, thereby pushing thermal thresholds observed in the modern ocean [78,79]. In the tropics, mixed-layer dwelling planktonic foraminifera (morozovellids and acarininids) exhibit a transient increase in morphological variation that included the appearance of distinctive (malformed) ecophenotypes, whereas populations of thermocline-dwelling taxa (subbotinids) temporarily collapsed at some locations [80]. A similar pattern of migration and elevated levels of taxonomic turnover is also observed in calcareous nannoplankton [81], Apectodinium spp. dinocysts [82] and radiolarians [83]. These lines of evidence are consistent with the heterogeneous latitudinal pattern of ocean warming [7].
There is a wealth of evidence implicating warming as an agent of change amongst plankton communities, yet the high incidence of malformed nannoplankton and thinning of coccolithophores found at numerous sites during the peak of the CIE suggests that ocean acidification also played a role [84–87]. Still, the impact of undersaturation on nannoplankton was exceeded by that of temperature; extracellular coccolithophore groups thought to be sensitive to ocean chemistry took refuge in cool waters at high latitudes [77]. Moreover, increased rates of coccoliths and foraminifera malformation in coastal sites suggest that the pH decline in combination with warming and eutrophication increased stress on planktonic calcifiers [88]. A similar combination of stressors was previously suggested for the collapse of framework-building corals during the PETM [89]. Disentangling the biotic effects of multiple abiotic stressors is proving to be a challenging task and should be a focus of future research. Nevertheless, the relative impacts of future ocean acidification are likely to be more severe as anthropogenic emission rates are considerably faster than estimated for the PETM [12,90,91].
5. Conclusion
The similarities between foraminiferal boron proxy records over the PETM, from a variety of palaeoceanographic settings and diagenetic histories, is consistent with globally uniform ocean acidification as the primary driver for the first-order feature common to all records: the large, rapid decrease in B/Ca and δ11B at the onset of the CIE. Subtle differences in absolute values between sites can be explained by differences in local oceanographic conditions, including salinity and dissolution. The boron isotope records at several of our sites allow us to quantify the degree of ocean acidification and suggest approximately a 0.15–0.3 pH unit decrease in the upper ocean. These estimates overlap with the higher end of carbon emissions scenarios considered in carbon cycle model simulations of the event [14,15]. This degree of acidification is less severe than the 0.4 pH unit decrease predicted for the end of the twenty-first century due to anthropogenic carbon emissions [92] and probably occurred over several thousand years [90,91], an order of magnitude slower than current ocean acidification. This might explain the general lack of extinctions among mixed-layer dwelling calcifiers during the PETM.
Finally, all boron records indicate a prolonged state of moderate acidification lasting as long as the CIE, adding to the mounting evidence (including the ‘body’ of the CIE itself [15] and a delayed CCD overshoot [31]) that carbon emissions continued for several tens of thousands of years following the onset of the PETM.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Gavin Foster for helpful discussions, Rob Franks for analytical support, Dick Olsson for assistance with planktonic foraminifera identification, Jim Browning and Ken Miller for sampling assistance of New Jersey IODP core material. We thank two anonymous reviewers for their constructive comments that improved the manuscript.
Data accessibility
The geochemical datasets supporting this article have been uploaded as part of the electronic supplementary material, tables S1–S3.
Authors' contributions
T.L.B. collected data, constructed age models, performed pH and carbonate chemistry estimates and drafted the manuscript and figures. D.E.P. collected data, constructed age models and assisted in drafting the manuscript. B.H. collected New Jersey boron isotope data and assisted in drafting the manuscript. D.C.K. assisted in planktonic foraminifera taxonomy. D.C.K. and T.J.B. contributed ODP 689 and 690 sample materials. D.C.K., T.J.B. and Y.R. assisted in drafting the manuscript. J.C.Z. directed the study and assisted in drafting the manuscript. T.L.B., D.E.P., T.J.B., Y.R. and J.C.Z. conceived of and designed the project. All authors read and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This research was supported by NSF grant no. OISE-1107787, Schlanger Ocean Drilling Fellowship and Joanna Resig Foraminiferal Research Fellowship awarded to T.L.B., a Flint Postdoctoral Fellowship granted to D.E.P., NSF OCE 12-20554 to B.H. and NSF grant no. OCE-1415958 awarded to T.J.B. and J.C.Z.
References
- 1.McInerney FA, Wing SL. 2011. The Paleocene-Eocene thermal maximum: a perturbation of carbon cycle, climate, and biosphere with implications for the future. Annu. Rev. Earth Planet. Sci. 39, 489–516. ( 10.1146/annurev-earth-040610-133431) [DOI] [Google Scholar]
- 2.Kennett JP, Stott LD. 1991. Abrupt deep-sea warming, paleoceanographic changes and benthic extinctions at the end of the Paleocene. Nature 353, 225–229. ( 10.1038/353225a0) [DOI] [Google Scholar]
- 3.Koch PL, Zachos JC, Gingerich PD. 1992. Correlation between isotope records in marine and continental carbon reservoirs near the Palaeocene/Eocene boundary. Nature 358, 319–322. ( 10.1038/358319a0) [DOI] [Google Scholar]
- 4.Dickens GR, Castillo MM, Walker JCG. 1997. A blast of gas in the latest Paleocene: simulating first-order effects of massive dissociation of oceanic methane hydrate. Geology 25, 259–262. ( 10.1130/0091-7613(1997)025%3C0259:abogit%3E2.3.co;2) [DOI] [PubMed] [Google Scholar]
- 5.Caldeira K, Wickett ME. 2003. Anthropogenic carbon and ocean pH. Nature 425, 365 ( 10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 6.Gruber N. 2011. Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Phil. Trans. R. Soc. A 369, 1980–1996. ( 10.1098/rsta.2011.0003) [DOI] [PubMed] [Google Scholar]
- 7.Dunkley Jones T, Lunt DJ, Schmidt DN, Ridgwell A, Sluijs A, Valdes PJ, Maslin M. 2013. Climate model and proxy data constraints on ocean warming across the Paleocene–Eocene Thermal Maximum. Earth Sci. Rev. 125, 123–145. ( 10.1016/j.earscirev.2013.07.004) [DOI] [Google Scholar]
- 8.Zachos JC, et al. 2005. Rapid acidification of the ocean during the Paleocene-Eocene Thermal Maximum. Science 308, 1611–1615. ( 10.1126/science.1109004) [DOI] [PubMed] [Google Scholar]
- 9.Colosimo AB, Bralower TJ, Zachos JC. 2006. Evidence for lysocline shoaling and methane hydrate dissociation at the Paleocene-Eocene Thermal Maximum on Shatsky Rise, ODP Leg 198. In Proc. of the Ocean Drilling Program, scientific results (eds Bralower TJ, Premoli Silva I, Malone M), pp. 1–36. College Station, TX: Ocean Drilling Program. [Google Scholar]
- 10.Penman DE, Hönisch B, Zeebe RE, Thomas E, Zachos JC. 2014. Rapid and sustained surface ocean acidification during the Paleocene-Eocene Thermal Maximum. Paleoceanography 29, 2014PA002621 ( 10.1002/2014PA002621) [DOI] [Google Scholar]
- 11.Babila TL, Rosenthal Y, Wright JD, Miller KG. 2016. A continental shelf perspective of ocean acidification and temperature evolution during the Paleocene-Eocene Thermal Maximum. Geology 44, 275–278. ( 10.1130/g37522.1) [DOI] [Google Scholar]
- 12.Gutjahr M, Ridgwell A, Sexton PF, Anagnostou E, Pearson PN, Pälike H, Norris RD, Thomas E, Foster GL. 2017. Very large release of mostly volcanic carbon during the Palaeocene–Eocene Thermal Maximum. Nature 548, 573 ( 10.1038/nature23646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panchuk K, Ridgwell A, Kump LR. 2008. Sedimentary response to Paleocene-Eocene Thermal Maximum carbon release: a model-data comparison. Geology 36, 315–318. ( 10.1130/g24474a.1) [DOI] [Google Scholar]
- 14.Ridgwell A, Schmidt DN. 2010. Past constraints on the vulnerability of marine calcifiers to massive carbon dioxide release. Nat. Geosci. 3, 196–200. ( 10.1038/ngeo755) [DOI] [Google Scholar]
- 15.Zeebe RE, Zachos JC, Dickens GR. 2009. Carbon dioxide forcing alone insufficient to explain Palaeocene-Eocene Thermal Maximum warming. Nat. Geosci. 2, 576–580. ( 10.1038/ngeo578) [DOI] [Google Scholar]
- 16.Thomas E. 2007. Cenozoic mass extinctions in the deep sea: what perturbs the largest habitat on Earth? Geol. Soc. Am. Spec. Pap. 424, 1–23. ( 10.1130/2007.2424(01)) [DOI] [Google Scholar]
- 17.Thomas E. 1998. Biogeography of the Late Paleocene Benthic foraminiferal extinction. In Late Paleocene-Early Eocene climatic and biotic events in the marine and terrestrial records (eds Aubry M-P, Lucas SG, Berggren WA), pp. 214–234. New York, NY: Columbia University Press. [Google Scholar]
- 18.Kelly DC, Nielsen TMJ, Schellenberg SA. 2012. Carbonate saturation dynamics during the Paleocene–Eocene Thermal Maximum: bathyal constraints from ODP sites 689 and 690 in the Weddell Sea (South Atlantic). Mar. Geol. 303–306, 75–86. ( 10.1016/j.margeo.2012.02.003) [DOI] [Google Scholar]
- 19.Kelly DC. 2002. Response of Antarctic (ODP Site 690) planktonic foraminifera to the Paleocene–Eocene Thermal Maximum: faunal evidence for ocean/climate change. Paleoceanography 17, 23-1–23-13. ( 10.1029/2002pa000761) [DOI] [Google Scholar]
- 20.Miller KG, et al. 1999. Ancora site. In Proc. of the Ocean Drilling Program, Initial Reports 174AX (eds Miller KG, Sugarman PJ, Browning JV et al.), pp. 1–65. College Station, TX: Ocean Drilling Program. [Google Scholar]
- 21.Miller KG, et al. 1998. Bass River site. In Proc. of the Ocean Drilling Program, Initial Reports 174AX (eds Miller KG, Sugarman PJ, Browning JV et al.), pp. 5–43. College Station, TX: Ocean Drilling Program. [Google Scholar]
- 22.Sugarman PJ, et al. 2005. Millville site. In Proc. of the Ocean Drilling Program, Initial Reports 174AX (eds Miller KG, Sugarman PJ, Browning JV et al.), pp. 1–94. College Station, TX: Ocean Drilling Program. [Google Scholar]
- 23.Harris AD, Miller KG, Browning JV, Sugarman PJ, Olsson RK, Cramer BS, Wright JD. 2010. Integrated stratigraphic studies of Paleocene-lowermost Eocene sequences, New Jersey Coastal Plain: evidence for glacioeustatic control. Paleoceanography 25, PA3211 ( 10.1029/2009pa001800) [DOI] [Google Scholar]
- 24.Stassen P, Thomas E, Speijer RP. 2012. Integrated stratigraphy of the Paleocene-Eocene Thermal Maximum in the New Jersey Coastal Plain: toward understanding the effects of global warming in a shelf environment. Paleoceanography 27, PA4210 ( 10.1029/2012PA002323) [DOI] [Google Scholar]
- 25.Bains S, Corfield RM, Norris RD. 1999. Mechanisms of climate warming at the end of the Paleocene. Science 285, 724–727. ( 10.1126/science.285.5428.724) [DOI] [PubMed] [Google Scholar]
- 26.John CM, Bohaty SM, Zachos JC, Sluijs A, Gibbs S, Brinkhuis H, Bralower TJ. 2008. North American continental margin records of the Paleocene-Eocene Thermal Maximum: implications for global carbon and hydrological cycling. Paleoceanography 23, A2217 ( 10.1029/2007pa001465) [DOI] [Google Scholar]
- 27.Makarova M, Wright JD, Miller KG, Babila TL, Rosenthal Y, Park JI. 2017. Hydrographic and ecologic implications of foraminiferal stable isotopic response across the U.S. mid-Atlantic continental shelf during the Paleocene-Eocene Thermal Maximum. Paleoceanography 32, 56–73. ( 10.1002/2016PA002985) [DOI] [Google Scholar]
- 28.Henehan MJ, Foster GL, Bostock HC, Greenop R, Marshall BJ, Wilson PA. 2016. A new boron isotope-pH calibration for Orbulina universa, with implications for understanding and accounting for ‘vital effects’. Earth Planet. Sci. Lett. 454, 282–292. ( 10.1016/j.epsl.2016.09.024) [DOI] [Google Scholar]
- 29.Bowen GJ, Zachos JC. 2010. Rapid carbon sequestration at the termination of the Palaeocene–Eocene Thermal Maximum. Nat. Geosci. 3, 866 ( 10.1038/ngeo1014) [DOI] [Google Scholar]
- 30.Kelly DC, Nielsen TMJ, McCarren HK, Zachos JC, Röhl U. 2010. Spatiotemporal patterns of carbonate sedimentation in the South Atlantic: implications for carbon cycling during the Paleocene–Eocene Thermal Maximum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 293, 30–40. ( 10.1016/j.palaeo.2010.04.027) [DOI] [Google Scholar]
- 31.Penman DE, et al. 2016. An abyssal carbonate compensation depth overshoot in the aftermath of the Palaeocene-Eocene Thermal Maximum. Nat. Geosci. 9, 575–580. ( 10.1038/ngeo2757) [DOI] [Google Scholar]
- 32.Luo Y, Boudreau BP, Dickens GR, Sluijs A, Middelburg JJ. 2016. An alternative model for CaCO3 over-shooting during the PETM: biological carbonate compensation. Earth Planet. Sci. Lett. 453, 223–233. ( 10.1016/j.epsl.2016.08.012) [DOI] [Google Scholar]
- 33.Bornemann A, Norris RD, Lyman JA, D'Haenens S, Groeneveld J, Röhl U, Farley KA, Speijer RP. 2014. Persistent environmental change after the Paleocene–Eocene Thermal Maximum in the eastern North Atlantic. Earth Planet. Sci. Lett. 394, 70–81. ( 10.1016/j.epsl.2014.03.017) [DOI] [Google Scholar]
- 34.Party SS. 1979. Site 401. In Initial reports of the deep sea drilling project (eds Montadert L, Roberts DG), pp. 77–123. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- 35.Zeebe RE, Zachos JC. 2007. Reversed deep-sea carbonate ion basin gradient during Paleocene-Eocene Thermal Maximum. Paleoceanography 22, PA3201 ( 10.1029/2006pa001395) [DOI] [Google Scholar]
- 36.Zachos JC, et al. 2004. Leg 208. In Proc. of the Ocean Drilling Program, Initial Reports. College Station, TX: Ocean Drilling Program.
- 37.Klochko K, Kaufman AJ, Yao W, Byrne RH, Tossell JA. 2006. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285. ( 10.1016/j.epsl.2006.05.034) [DOI] [Google Scholar]
- 38.Anagnostou E, John EH, Edgar KM, Foster GL, Ridgwell A, Inglis GN, Pancost RD, Lunt DJ, Pearson PN. 2016. Changing atmospheric CO2 concentration was the primary driver of early Cenozoic climate. Nature 533, 380–384. ( 10.1038/nature17423) [DOI] [PubMed] [Google Scholar]
- 39.Hain MP, Sigman DM, Higgins JA, Haug GH. 2015. The effects of secular calcium and magnesium concentration changes on the thermodynamics of seawater acid/base chemistry: implications for Eocene and Cretaceous ocean carbon chemistry and buffering. Glob. Biogeochem. Cycles 29, 517–533. ( 10.1002/2014GB004986) [DOI] [Google Scholar]
- 40.Anand P, Elderfield H, Conte MH. 2003. Calibration of Mg/Ca thermometry in planktonic foraminifera from a sediment trap time series. Paleoceanography 18, 15 PA1050 ( 10.1029/2002pa000846) [DOI] [Google Scholar]
- 41.Evans D, Müller W. 2012. Deep time foraminifera Mg/Ca paleothermometry: nonlinear correction for secular change in seawater Mg/Ca. Paleoceanography 27, PA4205 ( 10.1029/2012PA002315) [DOI] [Google Scholar]
- 42.Zachos JC, Wara MW, Bohaty S, Delaney ML, Petrizzo MR, Brill A, Bralower TJ, Premoli-Silva I. 2003. A transient rise in tropical sea surface temperature during the Paleocene-Eocene Thermal Maximum. Science 302, 1551–1554. ( 10.1126/science.1090110) [DOI] [PubMed] [Google Scholar]
- 43.Kiehl JT, Shields CA. 2013. Sensitivity of the Palaeocene–Eocene Thermal Maximum climate to cloud properties. Phil. Trans. R. Soc. A 371, 20130093 ( 10.1098/rsta.2013.0093) [DOI] [PubMed] [Google Scholar]
- 44.Foster GL, Rae JWB. 2016. Reconstructing ocean pH with boron isotopes in foraminifera. Annu. Rev. Earth Planet. Sci. 44, 207–237. ( 10.1146/annurev-earth-060115-012226) [DOI] [Google Scholar]
- 45.D'Hondt S, Zachos JC, Schultz G. 1994. Stable isotopic signals and photosymbiosis in Late Paleocene planktic foraminifera. Paleobiology 20, 391–406. ( 10.1017/S0094837300012847) [DOI] [Google Scholar]
- 46.Birch HS, Coxall HK, Pearson PN. 2012. Evolutionary ecology of Early Paleocene planktonic foraminifera: size, depth habitat and symbiosis. Paleobiology 38, 374–390. ( 10.1666/11027.1) [DOI] [Google Scholar]
- 47.Quillévéré F, Norris RD, Moussa I, Berggren WA. 2001. Role of photosymbiosis and biogeography in the diversification of Early Paleogene Acarininids (Planktonic Foraminifera). Paleobiology 27, 311–326. ( 10.1666/0094-8373(2001)027%3C0311:ROPABI%3E2.0.CO;2) [DOI] [Google Scholar]
- 48.Pearson PN, Palmer MR. 1999. Middle Eocene seawater pH and atmospheric carbon dioxide concentrations. Science 284, 1824–1826. ( 10.1126/science.284.5421.1824) [DOI] [PubMed] [Google Scholar]
- 49.Sanyal A, Bijma J, Spero H, Lea DW. 2001. Empirical relationship between pH and the boron isotopic composition of Globigerinoides sacculifer: implications for the boron isotope paleo-pH proxy. Paleoceanography 16, 515–519. ( 10.1029/2000PA000547) [DOI] [Google Scholar]
- 50.Allen KA, Hönisch B, Eggins SM, Rosenthal Y. 2012. Environmental controls on B/Ca in calcite tests of the tropical planktic foraminifer species Globigerinoides ruber and Globigerinoides sacculifer. Earth Planet. Sci. Lett. 351–352, 270–280. ( 10.1016/j.epsl.2012.07.004) [DOI] [Google Scholar]
- 51.Henehan MJ, Foster GL, Rae JWB, Prentice KC, Erez J, Bostock HC, Marshall BJ, Wilson PA. 2015. Evaluating the utility of B/Ca ratios in planktic foraminifera as a proxy for the carbonate system: a case study of Globigerinoides ruber. Geochem. Geophys. Geosyst. 16, 1052–1069. ( 10.1002/2014GC005514) [DOI] [Google Scholar]
- 52.Howes EL, Kaczmarek K, Raitzsch M, Mewes A, Bijma N, Horn I, Misra S, Gattuso JP, Bijma J. 2017. Decoupled carbonate chemistry controls on the incorporation of boron into Orbulina universa. Biogeosciences 14, 415–430. ( 10.5194/bg-14-415-2017) [DOI] [Google Scholar]
- 53.Haynes LL, Hönisch B, Dyez KA, Holland K, Rosenthal Y, Fish CR, Subhas AV, Rae JWB. 2017. Calibration of the B/Ca proxy in the planktic foraminifer Orbulina universa to Paleocene seawater conditions. Paleoceanography 32, 580–599. ( 10.1002/2016PA003069) [DOI] [Google Scholar]
- 54.Thomas DJ, Zachos JC, Bralower TJ, Thomas E, Bohaty S. 2002. Warming the fuel for the fire: evidence for the thermal dissociation of methane hydrate during the Paleocene-Eocene Thermal Maximum. Geology 30, 1067–1070. ( 10.1130/0091-7613(2002)030%3C1067:WTFFTF%3E2.0.CO;2) [DOI] [Google Scholar]
- 55.Bemis BE, Spero HJ, Bijma J, Lea DW. 1998. Reevaluation of the oxygen isotopic composition of planktonic foraminifera: experimental results and revised paleotemperature equations. Paleoceanography 13, 150–160. ( 10.1029/98pa00070) [DOI] [Google Scholar]
- 56.Zachos JC, Schouten S, Bohaty S, Quattlebaum T, Sluijs A, Brinkhuis H, Gibbs SJ, Bralower TJ. 2006. Extreme warming of mid-latitude coastal ocean during the Paleocene-Eocene Thermal Maximum: inferences from TEX86 and isotope data. Geology 34, 737–740. ( 10.1130/g22522.1) [DOI] [Google Scholar]
- 57.Ostland GH. 1987. GEOSECS Atlantic, Pacific and Indian Ocean expeditions. Shorebased data and graphica. GEOSECS Atlas Ser. 7, 200. [Google Scholar]
- 58.Mackensen A, Hubberten H-W, Scheele N, Schlitzer R. 1996. Decoupling of δ13C ∑CO2 and phosphate in recent Weddell Sea deep and bottom water: implications for glacial Southern Ocean paleoceanography. Paleoceanography 11, 203–215. ( 10.1029/95PA03840) [DOI] [Google Scholar]
- 59.LeGrande AN, Schmidt GA. 2006. Global gridded data set of the oxygen isotopic composition in seawater. Geophys. Res. Lett. 33, L12604 ( 10.1029/2006gl026011) [DOI] [Google Scholar]
- 60.Coadic R, Bassinot F, Dissard D, Douville E, Greaves M, Michel E. 2013. A core-top study of dissolution effect on B/Ca in Globigerinoides sacculifer from the tropical Atlantic: potential bias for paleo-reconstruction of seawater carbonate chemistry. Geochem. Geophys. Geosyst. 14, 1053–1068. ( 10.1029/2012GC004296) [DOI] [Google Scholar]
- 61.Dai Y, Yu J, Johnstone HJH. 2016. Distinct responses of planktonic foraminiferal B/Ca to dissolution on seafloor. Geochem. Geophys. Geosyst. 17, 1339–1348. ( 10.1002/2015GC006199) [DOI] [Google Scholar]
- 62.Hönisch B, Hemming NG. 2004. Ground-truthing the boron isotope-paleo-pH proxy in planktonic foraminifera shells: partial dissolution and shell size effects. Paleoceanography 19, 13 PA4010 ( 10.1029/2004PA001026) [DOI] [Google Scholar]
- 63.Ni Y, Foster GL, Bailey T, Elliott T, Schmidt DN, Pearson P, Haley B, Coath C. 2007. A core top assessment of proxies for the ocean carbonate system in surface-dwelling foraminifers. Paleoceanography 22, PA3212 ( 10.1029/2006PA001337) [DOI] [Google Scholar]
- 64.Seki O, Foster GL, Schmidt DN, Mackensen A, Kawamura K, Pancost RD. 2010. Alkenone and boron-based Pliocene pCO2 records. Earth Planet. Sci. Lett. 292, 201–211. ( 10.1016/j.epsl.2010.01.037) [DOI] [Google Scholar]
- 65.Edgar KM, Anagnostou E, Pearson PN, Foster GL. 2015. Assessing the impact of diagenesis on δ11B, δ13C, δ18O, Sr/Ca and B/Ca values in fossil planktic foraminiferal calcite. Geochim. Cosmochim. Acta 166, 189–209. ( 10.1016/j.gca.2015.06.018) [DOI] [Google Scholar]
- 66.Kozdon R, Kelly DC, Kitajima K, Strickland A, Fournelle JH, Valley JW. 2013. In situ δ18O and Mg/Ca analyses of diagenetic and planktic foraminiferal calcite preserved in a deep-sea record of the Paleocene-Eocene Thermal Maximum. Paleoceanography 28, 517–528. ( 10.1002/palo.20048) [DOI] [Google Scholar]
- 67.Party SS. 1993. Site 865. In Proceedings of the Ocean Drilling Program, Initial Reports 143 (eds Sager WW, Winterer EL, Firth JV, Party SS), pp. 111–180. College Station, TX: Ocean Drilling Program. [Google Scholar]
- 68.Pearson PN, Ditchfield PW, Singano J, Harcourt-Brown KG, Nicholas CJ, Olsson RK, Shackleton NJ, Hall MA. 2001. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature 413, 481–487. ( 10.1038/35097000) [DOI] [PubMed] [Google Scholar]
- 69.Wade B, Al-Sabouni N, Hemleben C, Kroon D. 2008. Symbiont bleaching in fossil planktonic foraminifera. Evol. Ecol. 22, 253–265. ( 10.1007/s10682-007-9176-6) [DOI] [Google Scholar]
- 70.Edgar KM, Bohaty SM, Gibbs SJ, Sexton PF, Norris RD, Wilson PA. 2012. Symbiont ‘bleaching’ in planktic foraminifera during the Middle Eocene Climatic Optimum. Geology 41, 15–18. ( 10.1130/g33388.1) [DOI] [Google Scholar]
- 71.Rink S, Kühl M, Bijma J, Spero HJ. 1998. Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa. Mar. Biol. 131, 583–595. ( 10.1007/s002270050350) [DOI] [Google Scholar]
- 72.Jørgensen BB, Erez J, Revsbech NP, Cohe Y. 1985. Symbiotic photosynthesis in a planktonic foraminiferan, Globeriginoides sacculifer (Brady), studied with microelectrodes. Limnol. Oceanogr. 30, 1253–1267. ( 10.4319/lo.1985.30.6.1253) [DOI] [Google Scholar]
- 73.Hönisch B, Bijma J, Russell AD, Spero HJ, Palmer MR, Zeebe RE, Eisenhauer A. 2003. The influence of symbiont photosynthesis on the boron isotopic composition of foraminifera shells. Mar. Micropaleontol. 49, 87–96. ( 10.1016/S0377-8398(03)00030-6) [DOI] [Google Scholar]
- 74.Zeebe RE, Wolf-Gladrow DA, Bijma J, Hönisch B. 2003. Vital effects in foraminifera do not compromise the use of δ11B as a paleo-pH indicator: evidence from modelling. Paleoceanography 18, 1043 ( 10.1029/2003PA000881) [DOI] [Google Scholar]
- 75.Rollion-Bard C, Erez J. 2010. Intra-shell boron isotope ratios in the symbiont-bearing benthic foraminiferan Amphistegina lobifera: implications for δ11B vital effects and paleo-pH reconstructions. Geochim. Cosmochim. Acta 74, 1530–1536. ( 10.1016/j.gca.2009.11.017) [DOI] [Google Scholar]
- 76.Babila TL, Rosenthal Y, Conte MH. 2014. Evaluation of the biogeochemical controls on B/Ca of Globigerinoides ruber white from the Oceanic Flux Program, Bermuda. Earth Planet. Sci. Lett. 404, 67–76. ( 10.1016/j.epsl.2014.05.053) [DOI] [Google Scholar]
- 77.Gibbs SJ, Bown PR, Ridgwell A, Young JR, Poulton AJ, O'Dea SA. 2016. Ocean warming, not acidification, controlled coccolithophore response during past greenhouse climate change. Geology 44, 59–62. ( 10.1130/g37273.1) [DOI] [Google Scholar]
- 78.Aze T, et al. 2014. Extreme warming of tropical waters during the Paleocene–Eocene Thermal Maximum. Geology 42, 739–742. ( 10.1130/g35637.1) [DOI] [Google Scholar]
- 79.Frieling J, Gebhardt H, Huber M, Adekeye OA, Akande SO, Reichart G-J, Middelburg JJ, Schouten S, Sluijs A. 2017. Extreme warmth and heat-stressed plankton in the tropics during the Paleocene-Eocene Thermal Maximum. Sci. Adv. 3, 1600891 ( 10.1126/sciadv.1600891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelly CD, Bralower TJ, Zachos JC, Silva IP, Thomas E. 1996. Rapid diversification of planktonic foraminifera in the tropical Pacific (ODP site 865) during the Late Paleocene Thermal Maximum. Geology 24, 423–426. ( 10.1130/0091-7613(1996)024%3C0423:rdopfi%3E2.3.co;2) [DOI] [Google Scholar]
- 81.Bralower TJ. 2002. Evidence of surface water oligotrophy during the Paleocene-Eocene Thermal Maximum: nannofossil assemblage data from Ocean Drilling Program Site 690, Maud Rise, Weddell Sea. Paleoceanography 17, 13-1–13-12 ( 10.1029/2001PA000662) [DOI] [Google Scholar]
- 82.Sluijs A, et al. 2006. Subtropical Arctic Ocean temperatures during the Palaeocene/Eocene Thermal Maximum. Nature 441, 610–613. ( 10.1038/nature04668) [DOI] [PubMed] [Google Scholar]
- 83.Hollis CJ. 2007. Radiolarian faunal turnover through the Paleocene-Eocene transition, Mead Stream, New Zealand. In Radiolaria: siliceous plankton through time (eds Baumgartner PO, Aitchison JC, De Wever P, Jackett S-J), pp. 79–99. Basel, Switzerland: Birkhäuser Basel. [Google Scholar]
- 84.Bralower TJ, Self-Trail JM. 2016. Nannoplankton malformation during the Paleocene-Eocene Thermal Maximum and its paleoecological and paleoceanographic significance. Paleoceanography 31, 1423–1439. ( 10.1002/2016PA002980) [DOI] [Google Scholar]
- 85.Raffi I, Backman J, Zachos JC, Sluijs A. 2009. The response of calcareous nannofossil assemblages to the Paleocene Eocene Thermal Maximum at the Walvis Ridge in the South Atlantic. Mar. Micropaleontol. 70, 201–212. ( 10.1016/j.marmicro.2008.12.005) [DOI] [Google Scholar]
- 86.Kahn A, Aubry M-P. 2004. Provincialism associated with the Paleocene/Eocene Thermal Maximum: temporal constraint. Mar. Micropaleontol. 52, 117–131. ( 10.1016/j.marmicro.2004.04.003) [DOI] [Google Scholar]
- 87.O'Dea SA, Gibbs SJ, Bown PR, Young JR, Poulton AJ, Newsam C, Wilson PA. 2014. Coccolithophore calcification response to past ocean acidification and climate change. Nat. Commun. 5, 5363 ( 10.1038/ncomms6363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Livsey CM. 2015. The planktonic foramininferal response to the Paleocene-Eocene Thermal Maximum on the Atlantic coastal plain. Masters Thesis, Pennsylvania State University, PA, USA. [Google Scholar]
- 89.Speijer R, Scheibner C, Stassen P, Morsi A-M. 2012. Response of marine ecosystems to deep-time global warming: a synthesis of biotic patterns across the Paleocene-Eocene Thermal Maximum (PETM). Aust J. Earth Sci.105, 6–16.
- 90.Zeebe RE, Ridgwell A, Zachos JC. 2016. Anthropogenic carbon release rate unprecedented during the past 66 million years. Nat. Geosci. 9, 325–329. ( 10.1038/ngeo2681) [DOI] [Google Scholar]
- 91.Kirtland Turner S, Ridgwell A. 2016. Development of a novel empirical framework for interpreting geological carbon isotope excursions, with implications for the rate of carbon injection across the PETM. Earth Planet. Sci. Lett. 435, 1–13. ( 10.1016/j.epsl.2015.11.027) [DOI] [Google Scholar]
- 92.Gattuso J-P, et al. 2015. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722 ( 10.1126/science.aac4722) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The geochemical datasets supporting this article have been uploaded as part of the electronic supplementary material, tables S1–S3.