Abstract
Introduction
The soluble receptor of urokinase plasminogen activator (suPAR) is an innate immunity/inflammation biomarker predicting cardiovascular (CV) and non-CV events in various conditions, including type 2 diabetic patients on dialysis. However, the relationship between suPAR and clinical outcomes in the hemodialysis population at large has not been tested.
Methods
We measured plasma suPAR levels (R&D enzyme-linked immunosorbent assay [ELISA]) in 1038 hemodialysis patients with a follow-up of 2.9 years (interquartile range = 1.7−4.2) who were enrolled in the PROGREDIRE study, a cohort study involving 35 dialysis units in 2 regions in Southern Italy.
Results
suPAR was strongly (P < 0.001) and independently related to female gender (β = −0.160), age (β = 0.216), dialysis vintage (β = 0.264), CV comorbidities (β = 0.105), alkaline phosphatase (β = 0.136), albumin (β = −0.147), and body mass index (BMI; β = 0.174) (all P < 0.006). In fully adjusted analyses, suPAR tertiles predicted the risk of all-cause mortality (third tertile vs. first tertile hazard ratio (HR) = 1.91, 95% confidence interval (CI) = 1.47 – 2.48, P < 0.001), CV mortality (HR = 1.47, 95% CI = 1.03–2.09, P = 0.03), and non-CV mortality (HR = 1.94, 95% CI = 1.28–2.93, P = 0.002); these relationships were not modified by diabetes or other risk factors. suPAR added only modest prognostic risk discrimination and reclassification power for these outcomes to parsimonious models based on simple clinical variables.
Conclusion
In conclusion, suPAR robustly predicted all-cause and both CV and non-CV mortality in a large unselected hemodialysis population. Intervention studies are needed to definitively test the hypothesis that suPAR is causally implicated in clinical outcomes in this population.
Keywords: cardiovascular mortality, hemodialysis, mortality, noncardiovascular mortality, soluble urokinase plasminogen activator receptor (suPAR)
In 2010, more than 2.5 million people with end-stage kidney disease (ESKD) were on chronic dialysis treatment worldwide, and the number of ESKD on dialysis is projected to more than double by 2030.1 The prognosis of these patients is notoriously dismal, with a death rate of about 20% per year among patients on stable hemodialysis treatment, both in the United States2 and in Europe.3 Most clinical trials aimed at testing interventions targeting the excess death risk of ESKD have been disappointing,4 and reducing the high mortality rate in the dialysis population—an inherently multifactorial issue5—remains a largely unmet clinical need.
Among the risk factors implicated in the high risk of death from ESKD, altered innate immunity/inflammation has emerged as a major risk factor for the systemic complications of this condition.6, 7 In general, studies looking at the link between innate immunity/inflammation and mortality and/or cardiovascular (CV) disease have been based mainly on C-reactive protein (CRP),8 whereas only a minority of studies applied interleukin-6 (IL-6) and other innate immunity biomarkers. Soluble urokinase plasminogen activator receptor (suPAR) is 1 of the strongest biomarkers of innate immunity activation. and several studies have documented that high suPAR levels consistently associates with mortality in septic patients in intensive care units9, 10 as well as with death and CV events in various populations,11, 12, 13 including patients with mild to moderate CKD.14 This biomarker directly orchestrates fundamental processes in the pathogenesis of atherosclerosis, including monocyte adhesion to the endothelium, migration, and proliferation,9 and it is more strongly associated with vascular damage and adverse clinical outcomes than classical innate immunity/inflammation markers such as CRP. Indeed, suPAR is superior to CRP for predicting CV disease events in high-risk patients.15 suPAR appears to be of particular relevance in CKD because it is not only a likely causal risk factor for renal damage16 but also a powerful prognostic factor predicting faster renal function loss in patients with suspected CV disease and normal glomerular filtration rate (GFR).17 suPAR levels are, on average, markedly raised in ESKD patients maintained on chronic hemodialysis treatment.18 For the foregoing, a thorough assessment of the relationship between suPAR and all-cause and CV mortality appears to be of obvious relevance in ESKD. The sole study in ESKD patients performed so far was based on the 4D Study, a landmark clinical trial testing the effect of atorvastatin in type 2 diabetic patients maintained on chronic dialysis.18 Observational analyses in the framework of clinical trials as in the 4D Study are an unquestionable source of information for generating new hypotheses about the etiology and prognosis; however, findings in these studies are restricted to the specific population of these trials, for example, type 2 diabetic patients in the 4D Study.
Until now, there has been no cohort study in the dialysis population investigating the relationship between suPAR and mortality in unselected patients on chronic dialysis. With this background in mind, we investigated the relationship between suPAR and all-cause and CV mortality in a large cohort of dialysis patients, including the vast majority of the dialysis population in a region of Italy with approximately 2 million inhabitants.
Methods
The study protocol was approved by the ethical committee of our institution. All participants gave their informed consent before enrollment.
Study Population
The study population is part of a cohort of 1189 dialysis patients enrolled from February 2009 to October 2010 in the Prospective Registry of The Working Group of Epidemiology of Dialysis Region Calabria (PROGREDIRE), a cohort study involving 35 dialysis units in 2 regions in southern Italy (Calabria and Sicily) and covering a geographical area with approximately 2 million residents. We included in this analysis 1038 hemodialysis (HD) patients in whom suPAR measurements could be performed. Patients had been on regular HD for a median time of 3.8 years (interquartile range [IQR] = 1.8−7.4 years) and were being treated with standard bicarbonate dialysis with noncellulosic membrane filters of various type. Arteriovenous fistula was the vascular access most commonly used (in 86.3% of patients). Patients were on dialysis 3 ± 0.4 times a week, with a mean dialysis time of 233 ± 18 minutes and a blood flux of 288 ± 33 ml. A total of 559 patients were treated with various antihypertensive drugs (240 on mono-therapy with angiotensin-converting enzyme inhibitors, calcium channel blockers, α- and β-blockers, vasodilators, diuretics, or other drugs, with 188 on double therapy, 73 on triple therapy, and 58 patients on quadruple or quintuple therapy with various combinations of these drugs). The main demographic, somatometric, clinical, and biochemical characteristics of the study population are detailed in Table 1. A total of 88 patients were censored at the time of renal transplantation and 50 patients because they were lost to follow-up.
Table 1.
Main demographic, somatometric, and clinical characteristics in the whole study population and in patients divided according to suPAR tertiles
| Characteristics | Whole group (N = 1038) | suPAR tertiles |
Among- groups comparison P |
r (P) |
||
|---|---|---|---|---|---|---|
| First tertile (n = 351) | Second tertile (n = 336) | Third tertile (n = 351) | ln suPAR versus | |||
| Age, yr | 65 ± 14 | 61 ± 15 | 66 ± 12 | 68 ± 12 | <0.001 | r = 0.222, P < 0.001 |
| Body mass index, kg/m2 | 25 ± 5 | 24 ± 4 | 25 ± 4 | 26 ± 5 | <0.001 | r = 0.121, P < 0.001 |
| Waist circumference, cm | 98 ± 14 | 95 ± 13 | 98 ± 14 | 100 ± 14 | <0.001 | r = 0.129, P < 0.001 |
| Male sex, n (%) | 662 (64) | 258 (74) | 217 (65) | 187 (53) | <0.001 | r = –0.172, P < 0.001 |
| Smoker, n (%) | 141 (14) | 58 (17) | 46 (14) | 37 (11) | 0.08 | r = –0.038, P=0.226 |
| Diabetic, n (%) | 281 (27) | 71 (22) | 98 (31) | 112 (34) | 0.001 | r = 0.088, P = 0.006 |
| Dialysis vintage, mo | 46 (21–88) | 35 (16–67) | 45 (21–89) | 62 (28–114) | <0.001 | r = 0.220, P < 0.001 |
| With central catheter or arterial graft, n (%) | 129 (14) | 33 (10) | 37 (12) | 59 (20) | 0.004 | r = 0.082, P = 0.01 |
| Kt/V | 1.29 ± 0.42 | 1.29 ± 0.39 | 1.29 ± 0.49 | 1.28 ± 0.39 | 0.99 | r = 0.026, P = 0.45 |
| With cardiovascular comorbidities, n (%) | 525 (51) | 142 (41) | 179 (53) | 204 (59) | <0.001 | r = 0.156, P < 0.001 |
| Coronary heart disease,a n (%) | 179 (17) | 44 (13) | 62 (19) | 73 (21) | 0.012 | r = 0.089, P = 0.004 |
| Heart failure | 113 (15) | 20 (8) | 46 (18) | 47 (18) | 0.001 | r = 0.119, P < 0.001 |
| Cerebrovascular disease,b n (%) | 117 (11) | 38 (11) | 37 (11) | 42 (12) | 0.88 | r = 0.046, P = 0.14 |
| Peripheral vascular disease, n (%) | 197 (19) | 44 (13) | 62 (19) | 91 (26) | <0.001 | r = 0.117, P < 0.001 |
| On antihypertensive treatment, n (%) | 559 (54) | 200 (57) | 186 (56) | 173 (50) | 0.014 | r = –0.106, P = 0.001 |
| Systolic blood pressure, mm Hg | 135 ± 22 | 136 ± 20 | 136 ± 23 | 133 ± 23 | 0.18 | r = –0.076, P = 0.02 |
| Diastolic blood pressure, mm Hg | 74 ± 12 | 76 ± 11 | 73 ± 12 | 72 ± 12 | <0.001 | r = –0.160, P < 0.001 |
| Cholesterol, mg/dl | 155 ± 39 | 153 ± 37 | 156 ± 40 | 155 ± 42 | 0.55 | r = 0.041, P = 0.21 |
| Hemoglobin, g/dl | 11.3 ± 1.5 | 11.3 ± 1.4 | 11.3 ± 1.5 | 11.2 ± 1.5 | 0.83 | r = –0.014, P = 0.67 |
| Leukocyte count, 103/ml | 6.7 ± 2.0 | 6.5 ± 1.8 | 6.8 ± 2.0 | 6.9 ± 2.2 | 0.04 | r = 0.066, P = 0.04 |
| Albumin, g/dl | 3.9 ± 0.5 | 4.0 ± 0.5 | 3.9 ± 0.5 | 3.8 ± 0.6 | 0.002 | r = –0.138, P < 0.001 |
| C-reactive protein, mg/l | 5.0 (3.0–12.0) | 4.0 (3.0–9.0) | 5.0 (3.0–13.8) | 6.0 (3.0–15.0) | 0.005 | r = 0.073, P = 0.003 |
| Fibrinogen, mg/dl | 385 ± 110 | 363 ± 97 | 401 ± 112 | 392 ± 116 | <0.001 | r = 0.115, P = 0.001 |
| Calcium, mg/dl | 9.1 ± 0.9 | 9.2 ± 0.9 | 9.2 ± 0.9 | 9.1 ± 1.0 | 0.17 | r = –0.056, P = 0.09 |
| Phosphate, mg/dl | 5.0 ± 1.6 | 5.2 ± 1.6 | 4.9 ± 1.6 | 5.0 ± 1.8 | 0.13 | r = -0.043, P = 0.19 |
| Parathyroid hormone, pg/ml | 239 (116–460) | 239 (122–494) | 252 (119–463) | 226 (109–440) | 0.38 | r = –0.021, P = 0.53 |
| Alkaline phosphatase, UI/l | 85 (65–116) | 75 (59–97) | 85 (64–123) | 98 (77–145) | <0.001 | r = 0.161, P < 0.001 |
Data are expressed as mean ± SD, as median and interquartile range, or as percent frequency, as appropriate. Comparisons among groups were made by one-way analysis, the Kruskal−Wallis test, or the χ2 test, as appropriate. Linear correlation for continuous variables and point biserial correlation were applied to correlate continuous and binary variables, respectively. Bold values indicate significant correlations.
Past myocardial infarction or angina, coronary angioplasty, or surgery.
Stroke or transient ischemic attack.
Laboratory Measurements
Blood sampling was performed at baseline after an overnight fast. Blood was drawn with ethylenediamine tetraacetic acid as an anticoagulant during a mid-week day (brief dialysis interval). suPAR was assayed by enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN) from banked (at –80°), never-defrosted specimens. At baseline, we separated collected serum in several aliquots to prevent defreezing/refreezing cycles and sample deterioration. The coefficient of variation of this assay was 4.6% (intra-assay) and 5.5% (interassay). The upper limit of the normal range of plasma suPAR by this method in the healthy population is 3829 pg/ml (Quantikine ELISA human uPAR immunoassay; R&D Systems, Minneaplis, MN) Cholesterol, albumin, calcium, phosphate, alkaline phosphatase, parathyroid hormone (intact molecule), CRP, fibrinogen, and hemoglobin measurements were made using standard methods in the routine clinical laboratory. Residual renal function was estimated according to Stel et al., that is, by calculating the residual estimated glomerular filtration rate (eGFR).19
Study Endpoints
Mortality and CV mortality, fatal CV events, and nonfatal CV events were the main study endpoints. Cardiovascular events were centrally adjudicated and classified as follows: stroke (ischemic or hemorrhagic), documented by computed tomography, magnetic resonance imaging, and/or clinical and neurological evaluation; transient ischemic attacks; myocardial infarction confirmed by serial changes of electrocardiographic and cardiac biomarkers; electrocardiographically documented angina episodes; electrocardiographically documented arrhythmia; and unexpected, sudden death highly suspected as of cardiac origin. De novo chronic heart failure was defined as chronic heart failure in a patient without this condition at baseline. To be classified as having chronic heart failure, patients had to show mild or more severe dyspnea during ordinary activities (New York Heart Association class II or higher) plus evidence of anatomical/functional left ventricular disease on echocardiography. Each cause of death was assessed by 3 independent physicians. In doubtful cases, diagnosis was attributed by consensus. During the review process, the involved physicians used all available medical information, including hospitalization forms and medical records. In case of death occurring at home, family members and/or general practitioners were interviewed to better understand the circumstances that led to death.
Statistical Analysis
Data were expressed as mean ± SD (normally distributed data), median and IQR (non−normally distributed data), or as percent frequency (categorical data). Comparisons among groups were made by 1-way analysis of variance, the Kruskal−Wallis test, or the χ2 test, as appropriate. Pearson correlation analyses were performed to investigate the correlates of suPAR. Independent correlates of suPAR were investigated by including, in a linear regression model, all variables associated with this biomarker in correlation analyses. Because of the nonnormal distribution of suPAR, this variable was log transformed before analysis. The final functional form of suPAR for survival analyses was investigated by using Martingale residuals.20 This analysis showed 2 steps at 5625 and 6999 pg/ml, corresponding approximately to the upper limits of the first and second tertiles. We therefore adopted suPAR categorization by tertiles for subsequent analyses. Survival analyses were performed by using both univariate and multivariate Cox regression analyses, including suPAR as a categorical (tertiles) variable, traditional (age, gender, current smoking, diabetes, cholesterol, arterial pressure, antihypertensive treatment, and CV comorbidities), inflammation and nutritional status (CRP, albumin, fibrinogen, body mass index [BMI]), and ESKD-related risk factors (dialysis vintage, Kt/V, fistula/catheters or grafts, hemoglobin, parathyroid hormone, alkaline phosphatase). The number of missing values was less than 10% for each variable. In multiple models, we adopted the most conservative approach to replace missing data, that is, we replaced missing data by the mean or median values (according to the data distribution). The date of renal transplantation or last observation for patients lost to follow-up was carefully recorded. As these events were not the endpoint of the study, these patients were considered as censored in both models. Survival curves were made to compare the risk of the considered outcome. The association between suPAR and CV or non-CV mortality was assessed by using competitive risk regression models.21, 22 Nonlinear associations were assessed by estimating hazard ratios of mortality and CV mortality according to suPAR with its linear spline terms23 with knots at 5625 and 6999 pg/ml. The potential effect modification by diabetes, somatometric measurements (BMI), dialysis vintage, blood pressure and antihypertensive drug use, biomarkers of protein-energy wasting and inflammation (albumin, CRP, fibrinogen) and Kt/V on the relationship between suPAR and death/CV death was investigated by standard analyses by creating appropriate multiplicative terms in Cox regression analyses or in competitive risk regression models, as appropriate. The prognostic power of suPAR was assessed by using the Harrell C Index, the Net Reclassification Index, and the Integrated Discrimination Index. In each test, we compared a parsimonious model based on backwardly selected variables (backward selection concluded after 13 steps for all-cause mortality, 15 steps for CV mortality) with a full model, including the same variables as in the base model and suPAR as categorical variable. For the Net Reclassification Index, the cut-offs for defining risk categories (all-cause death: <34%, 34%−51%, >51%; CV death: <17%, 17%−30%, >30%) were chosen according to the distribution of the predicted probability of death/CV death. Statistical analysis was performed by using standard statistical packages (SPSS for Windows, Version 24; SPSS Inc., Chicago, Illinois, USA; STATA for Windows, Version 13; Stata Corp., College Station, Texas, USA).
Results
The main baseline characteristics of the study population are reported in Table 1. The median value of suPAR was 6250 pg/ml (IQR = 5319−7633). In all, 64% of patients were male, and the mean age was 65 years. A total of 281 patients were diabetic (27%). The cause of ESKD was hypertension-related nephropathy in 321 patients (31%), glomerulonephritis in 129 (12%), pyelonephritis or interstitial nephritis in 80 (8%), autosomal dominant polycystic kidney disease or other genetic diseases in 120 (12%), and unknown in 192 (19%). suPAR levels were higher in female patients (median = 6605 pg/ml, IQR = 5666−8303 pg/ml) than in male patients (median = 6043 pg/ml, IQR = 5120−7144 pg/ml) and exceeded the upper limit of the normal range (3829 pg/ml) in the vast majority of patients (1007 of 1038, 97%). In Table 1, patients are presented by suPAR tertiles. Patients with higher levels of suPAR (third tertile) were older, with a higher BMI and waist circumference, were more frequently diabetic, and had a higher burden of CV comorbidities and a longer dialysis vintage as compared to patients in the other tertiles. They were also more frequently dialyzed by a central catheter or an arterial graft (Table 1). Furthermore, the same patients exhibited deranged levels of protein-energy wasting and inflammation biomarkers (lower serum albumin and higher CRP, leucocyte count, and fibrinogen), and had higher alkaline phosphatase and lower systolic and diastolic blood pressure. The use of antihypertensive agents was less frequent in the third than in the other two suPAR tertles. These associations were fully confirmed in correlation analyses (Table 1, last column).
Independent Correlates of suPAR
On multiple regression analysis, including all significant correlates of suPAR (Table 1), only gender (β = −0.160), age (β = 0.216), dialysis vintage (β = 0.264), CV comorbidities (β = 0.105), alkaline phosphatase (β = 0.136), albumin (β = −0.147), and BMI (β = 0.174, all P ≤ 0.006) maintained an independent association with suPAR.
Survival Analysis
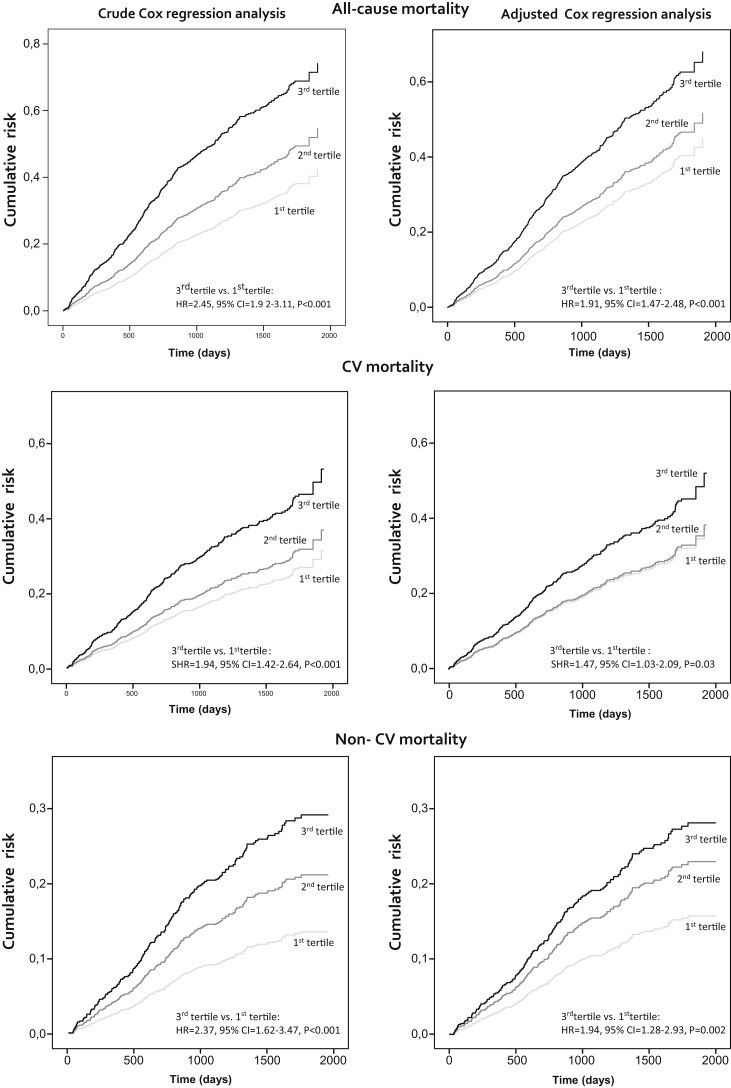
During a median follow-up of 2.9 years (IQR = 1.7≤4.2 years), 436 patients died, 247 of them of CV causes. The causes of death are shown in Supplementary Table S1. A categorical analysis by suPAR tertiles (see Methods) showed a dose−response relationship between suPAR tertiles and risk of death (Figure 1), and this relationship held true after adjustment for traditional, inflammation, nutritional status, and ESKD-related risk factors (third tertile vs. first tertile HR = 1.84, 95% CI = 1.42–2.39, P < 0.001). Notably, the HR remained the same after exclusion of albumin (1.85, 95% CI = 1.43−2.40, P < 0.001) or CRP (1.83, 95% CI = 1.41−2.37, P < 0.001) or both of these factors (1.84, 95% CI = 1.42−2.39, P < 0.001) from the Cox model (Supplementary Table S2), suggesting that the relationship between suPAR and all-cause death is independent of these biomarkers of inflammation.
Figure 1.
Crude and adjusted Cox regression analysis showing the effect of soluble urokinase plasminogen activator receptor (suPAR) on all-cause, cardiovascular, and noncardiovascular mortality, respectively. Variables included are suPAR and all variables listed in Table 1. Survival curves were made to compare the risk of the considered outcome. The association between suPAR and cardiovascular (CV) or non-CV mortality was assessed by using competitive risk regression models (see Materials and Methods for further details). CI, confidence interval; HR, hazard ratio; SHR, subdistribution hazard ratio.
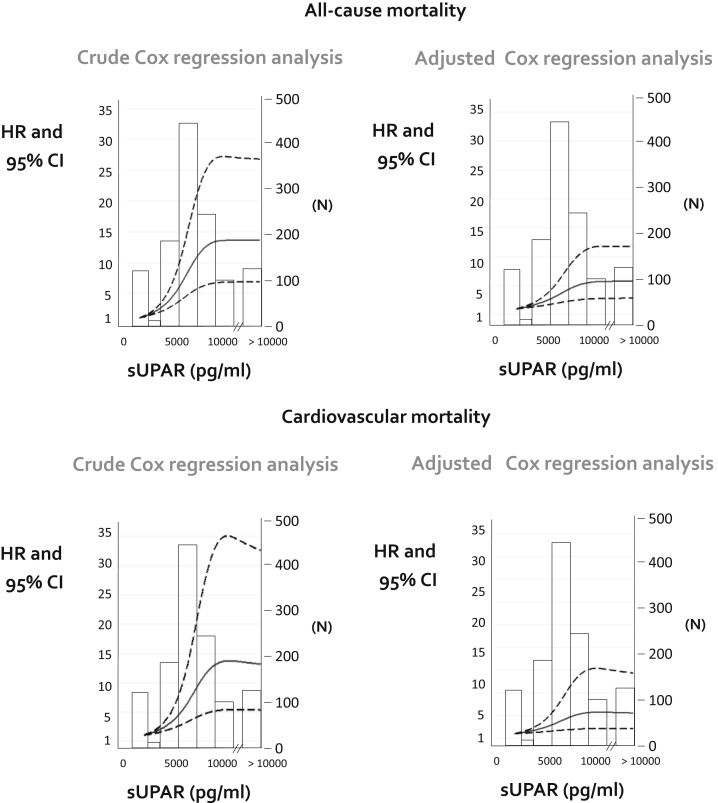
The effect of suPAR was also tested for CV mortality and non-CV mortality separately, by using competing risk models. As shown in Figure 1, the risk of CV mortality was highest in the third suPAR tertile, and this relationship was confirmed in fully adjusted analyses (third tertile vs. first tertile subdistribution: HR = 1.48, 95% CI = 1.04–2.10, P = 0.03), and an even stronger association was observed for non-CV mortality (third tertile vs. first tertile subdistribution hazard ratio = 1.89, 95% CI = 1.24–2.87, P = 0.003). Comparison of HR of suPAR (third tertile vs. first tertile) for non-cardiovascular and cardiovascular mortality obtained by standard Cox regression analysis and competing risk regression analysis are shown in Supplementary Table S3. Similarly, analysis of the association of suPAR with all-cause and CV mortality by nonlinear models (see Methods) again confirmed robust links between suPAR and these outcomes (Figure 2).
Figure 2.
Analysis of the association of soluble urokinase plasminogen activator receptor (suPAR) with all-cause and cardiovascular (CV) mortality by nonlinear models. Models were adjusted for the full list of variables presented in Table 1. Nonlinear associations were assessed by estimating hazard ratios of mortality and CV mortality according to suPAR with its linear spline terms 36 with knots at 5625 and 6999 pg/ml. (See Methods for further details). n = Number of patients corresponding to specific values of suPAR. CI, confidence interval; HR, hazard ratio.
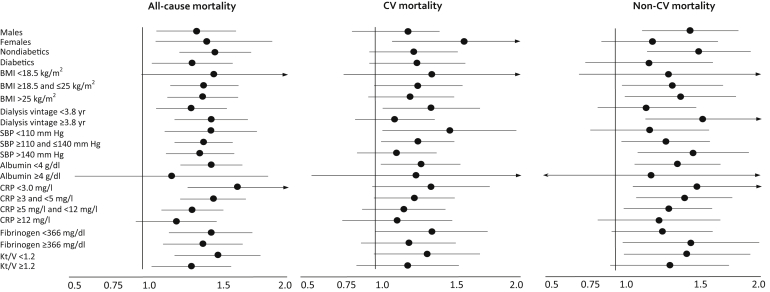
To investigate whether the link between suPAR and clinical outcomes differed in diabetic and nondiabetic patients, we performed an effect modification analysis by this disease. As shown in Figure 3, the hazard rates for all-cause (nondiabetic: 1.48; 95% CI = 1.25−1.75; diabetic: 1.31, 95% CI = 1.06−1.62, P = 0.36), CV (nondiabetic 1.23, 95% CI = 0.96−1.57); diabetic: 1.25, 95% CI = 0.96−1.62, P = 0.92), and non-CV (nondiabetic: 1.54, 95% CI = 1.22−1.94; diabetic: 1.15, 95% CI = 0.79−1.68, P = 0.19) death were almost identical in diabetic and nondiabetic patients with no effect modification. Similarly, no effect modification by gender, BMI, dialysis vintage, systolic blood pressure, albumin, CRP, fibrinogen, and Kt/V was registered (Figure 3 and Supplementary Table S4).
Figure 3.
Absence of effect modification attributable to diabetes, body mass index (BMI), dialysis vintage, systolic blood pressure (SBP), albumin, C-reactive protein (CRP), fibrinogen, and Kt/V on the link between soluble urokinase plasminogen activator receptor (suPAR)−considered outcome (all-cause mortality/cardiovascular mortality/noncardiovascular mortality).
Prognostic Power of suPAR
The addition of suPAR to parsimonious models of all-cause and CV mortality in dialysis patients (including age, diabetes, cholesterol, CV comorbidities, dialysis vintage, albumin, vascular access, and alkaline phosphatase for all-cause death; age, diabetes, cholesterol, CV comorbidities, vascular access and alkaline phosphatase for CV death) added only 3% (all-cause death) or 2% (CV death) of explanatory power. The gain in discriminating power, as assessed by the Harrell C Index, was less than 1% for both outcomes. suPAR was also scarcely useful to correctly reclassify the risk categories (all-cause death: Net Reclassification Index = 0.6%, P = 0.77; Integrated Discrimination Index = 1%, P = 0.0007; CV death: Net Reclassification Index = −0.2%, P = 0.94; Integrated Discrimination Index = 0.4%, P = 0.09).
Discussion
In a cohort of patients formed by the 80% the dialysis population in a geographical area in Italy with approximately 2 million inhabitants, suPAR was independently associated with female gender, age, dialysis vintage, and background CV comorbidities, as well as with protein energy wasting and inflammation biomarkers. Independently of other factors, suPAR levels were related to the risk of all-cause and both CV and non-CV death in diabetic and nondiabetic patients across various risk factors strata, including diabetes and other risk factors, suggesting a possible causal implication of this molecule in adverse clinical outcomes in ESKD. On the other hand, suPAR added quite modest predictive power to parsimonious risk models based on standard risk factors in the dialysis population.
The suPAR molecule is one of the most interesting innate immunity signaling molecules and biomarkers in chronic kidney disease.24 It belongs to the lysine 6−neurotoxin family of signaling proteins known to bind integrins to regulate cell adhesion.25 Well beyond being causally implicated in focal glomerulosclerosis,26 suPAR, a 50-kDa protein, associates strongly with the GFR17 and predicts the incidence of CKD in patients with suspected coronary heart disease16 and individuals in the general population,21 as well as the rate of GFR loss in children.27 We found that in ESKD patients, circulating suPAR, a compound that is not removed by dialysis, exceeded the upper limit of the normal range in the vast majority (97%) of patients in our cohort.
Interest in suPAR rests mainly on the strong relationship between circulating levels of this compound and all-cause and CV mortality and other adverse clinical outcomes in patients with severe multiorgan diseases admitted into intensive care units,9, 10 in the general population,11 as well as in patients with CV disease,12, 13 cancer,11 sepsis,28 and HIV.29 In the present study, suPAR associated with all-cause, CV, and non-CV mortality in a dose-dependent fashion, and these associations held true both in diabetic and in nondiabetic patients. Thus, the risk implications of high suPAR levels in hemodialysis patients, a population with virtually no renal function, are not confined to diabetic patients18 but apply to the hemodialysis population at large also irrespective of the presence of background CV comorbidities and other risk factors. We found that suPAR was independently related to biomarkers of protein-energy wasting and inflammation, a syndromic complex consistently associated with all-cause and CV mortality in ESKD.30 However, the predictive power of suPAR was completely independent of protein-energy wasting and inflammation as quantified by serum albumin, CRP, and BMI, indicating that inflammatory pathways other than protein-energy wasting and CRP are involved in the high risk of elevated suPAR levels. Urokinase plasminogen activator receptor (uPAR) is a receptor for urokinase and binding partner for integrins produced in various cell species including the endothelium, macrophages, T cells, fibroblasts, and epithelial cells, and circulating suPAR reflects cellular shedding of uPAR.
Recently, the production of suPAR from immature myeloid cells has been shown to be a constant source in focal and segmental glomerulosclerosis.31 On binding its receptor, uPAR triggers the production plasmin as well as cell adhesion, migration, and proliferation, which are wide-ranging phenomena that participate in atherosclerosis,32 infectious diseases,28 and cancer.11, 33
While suggesting an etiologic role of suPAR in adverse health outcomes in ESKD, our analyses show that this biomarker per se adds quite modest predictive power to parsimonious models based on standard risk factors in the ESKD population. Indeed, both discrimination analyses and risk reclassification analyses showed just small gains in the predictive power for all-cause and CV death by adding suPAR to equations based on backwardly selected, simple clinical variables such as age, diabetes, cholesterol, CV comorbidities, vascular access, dialysis vintage, and albumin. This is not surprising, given the plethora of pathophysiological changes that occur in patients with end-stage renal disease. However, our findings that suPAR remains independently predictive of outcomes in this population suggest that the CV manifestations caused by or associated with suPAR do not require functioning kidneys.
Our data remain hypothesis generating and can only suggest, but not prove, causality. Although large, our cohort included white individuals only. Further studies are therefore needed to determin whether the predictive power of suPAR in white hemodialysis patients also applies to other ethnicities, as it does in diseases different from ESKD.34, 35 The possibility that the strong link between suPAR and clinical outcomes in the present study is due to residual confounding by unmeasured variables cannot be excluded. Data in the present study indicate that the link between suPAR and mortality, initially described in type 2 diabetic patients,18 can be extended to patients with various etiologies of ESKD. In addition, the data imply that high suPAR levels reflect a specific innate-immunity/inflammation pathway in large part independent of the classical inflammatory pathway, including high CRP and hypoalbuminemia, which is eventually conducive to a high risk of death.
In conclusion, suPAR levels robustly predict all-cause and both CV and non-CV mortality in a reasonably large, unselected dialysis population. Interference with uPAR is a potential pathway for countering CV36 and non-CV37 events, and therefore our findings may be relevant for designing new studies aimed at curbing the high mortality burden of ESKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are very grateful to the PROGREDIRE Working Group COLLABORATORS for their invaluable support in data collection: Giovanni Alati, Eleonora Barreca, Rosalia Boito, Margherita Bovino, Vincenzo Bruzzese, Maria Capria, Simonetta Cassani, Salvatore Chiarella, Antonio Chippari, Teresa Cicchetti, Edoardo Crifò-Gasparro, Carlo Curti, Francesco D’Agostino, Emanuela D’Anello, Maria De Gaudio, Aldo Foscaldi, Cesare Fornaciari, Corrado Franco, Alfredo Gaglioti, Domenico Galati, Francesco Grandinetti, Maurizio Gullo, Maria Rosa La Gamba, Domenico Logozzo, Iginia Maimone, Maria Letizia Mannino, Elena Mazzuca, Agazio Mellace, Giuseppe Natale, Vincenzo Panuccio, Domenico Plutino, Antonio Pugliese, Anna Reina, Rita Roberti, Mariagrazia Santangelo, Arcangelo Sellaro, Rosalba Scicchitano, Carmela Vardè, Francesco Zingone.
Acknowledgments
Author Contributions
CZ designed the study and JR contributed with CZ in developing the idea of the same study. CT and MP were responsible for data collection and data analysis and performed statistical analyses with GT. PP and SC performed biochemical measurements and FM gave critical contribution to data analysis and interpretation. CZ has had full access to the data in the study and final responsibility for the decision to submit for publication. CT and CZ wrote the first draft and the final version of the paper that was read and approved by all authors.
Footnotes
Table S1. Main causes of death recorded during the follow-up.
Table S2. HR of all-cause mortality in the fully adjusted model (model 1), in a model without albumin (model 2) or without CRP (model 3) or without albumin and CRP (model 4).
Table S3. Comparison of HR of suPAR (third tertile vs. first tertile) for noncardiovascular and cardiovascular mortality obtained by standard Cox regression analysis and competing risk regression analysis. Both models are adjusted for traditional, inflammation and nutritional status, and ESKD-related risk factors.
Table S4. HR of suPAR for all-cause mortality/cardiovascular mortality/noncardiovascular mortality across gender, diabetes, BMI, dialysis vintage, systolic blood pressure, albumin, CRP, fibrinogen, and Kt/V categories. All P values for the effect modification are >0.05.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Contributor Information
Carmine Zoccali, Email: carmine.zoccali@tin.it.
PROGREDIRE Working Group:
Giovanni Alati, Eleonora Barreca, Rosalia Boito, Margherita Bovino, Vincenzo Bruzzese, Maria Capria, Simonetta Cassani, Salvatore Chiarella, Antonio Chippari, Teresa Cicchetti, Edoardo Crifò-Gasparro, Carlo Curti, Francesco D’Agostino, Emanuela D’Anello, Maria De Gaudio, Aldo Foscaldi, Cesare Fornaciari, Corrado Franco, Alfredo Gaglioti, Domenico Galati, Francesco Grandinetti, Maurizio Gullo, Maria Rosa La Gamba, Domenico Logozzo, Iginia Maimone, Maria Letizia Mannino, Elena Mazzuca, Agazio Mellace, Giuseppe Natale, Vincenzo Panuccio, Domenico Plutino, Antonio Pugliese, Anna Reina, Rita Roberti, Mariagrazia Santangelo, Arcangelo Sellaro, Rosalba Scicchitano, Carmela Vardè, and Francesco Zingone
Supplementary Material
Main causes of death recorded during the follow-up.
HR of all-cause mortality in the fully adjusted model (model 1), in a model without albumin (model 2) or without CRP (model 3) or without albumin and CRP (model 4).
Comparison of HR of suPAR (third tertile vs. first tertile) for noncardiovascular and cardiovascular mortality obtained by standard Cox regression analysis and competing risk regression analysis. Both models are adjusted for traditional, inflammation and nutritional status, and ESKD-related risk factors.
HR of suPAR for all-cause mortality/cardiovascular mortality/noncardiovascular mortality across gender, diabetes, BMI, dialysis vintage, systolic blood pressure, albumin, CRP, fibrinogen, and Kt/V categories. All P values for the effect modification are >0.05.
References
- 1.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 2.USRDS. 2015 USRDS annual data report volume 2: ESRD in the United States. 2016;2:1–274.
- 3.Heaf J. Current trends in European renal epidemiology. Clin Kidney J. 2017;10:149–153. doi: 10.1093/ckj/sfw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramann R., Floege J., Ketteler M. Medical options to fight mortality in end-stage renal disease: a review of the literature. Nephrol Dial Transplant. 2012;27:4298–4307. doi: 10.1093/ndt/gfs400. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz A., Covic A., Fliser D. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C., Vanholder R., Massy Z.A. The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 7.Stenvinkel P., Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., He J., Zhang F. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol. 2013;26:243–253. doi: 10.5301/jn.5000169. [DOI] [PubMed] [Google Scholar]
- 9.Huttunen R., Syrjänen J., Vuento R. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med. 2011;270:32–40. doi: 10.1111/j.1365-2796.2011.02363.x. [DOI] [PubMed] [Google Scholar]
- 10.Giamarellos-Bourboulis E.J., Norrby-Teglund A., Mylona V. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16:R149. doi: 10.1186/cc11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eugen-Olsen J., Andersen O., Linneberg A. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 12.Eapen D.J., Manocha P., Ghasemzedah N. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3:e001118. doi: 10.1161/JAHA.114.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyngbæk S., Marott J.L., Sehestedt T. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904–2911. doi: 10.1016/j.ijcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Meijers B., Poesen R., Claes K. Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int. 2015;87:210–216. doi: 10.1038/ki.2014.197. [DOI] [PubMed] [Google Scholar]
- 15.Hodges G.W., Bang C.N., Wachtell K. suPAR: a new biomarker for cardiovascular disease? Can J Cardiol. 2015;31:1293–1302. doi: 10.1016/j.cjca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Hayek S.S., Koh K.H., Grams M.E. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;26:945–953. doi: 10.1038/nm.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayek S.S., Sever S., Ko Y.-A. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drechsler C., Hayek S.S., Wei C. Soluble urokinase plasminogen activator receptor and outcomes in patients with diabetes on hemodialysis. Clin J Am Soc Nephrol. 2017;12:1265–1273. doi: 10.2215/CJN.10881016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stel V.S., Dekker F.W., Ansell D. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24:3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 20.Therneau T.M., Grambsch P.M., Fleming T.R. Martingale-based residuals for survival models. Biometrika. 1990;77:147–160. [Google Scholar]
- 21.Hsu J.Y., Roy J.A., Xie D. Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol. 2017;12:1181–1189. doi: 10.2215/CJN.10301016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noordzij M., Leffondré K., van Stralen K.J. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 23.Wold S. Spline functions in data analysis. Technometrics. 1974;16:1–11. [Google Scholar]
- 24.Reiser J. Circulating permeability factor suPAR: from concept to discovery to clinic. Trans Am Clin Climatol Assoc. 2013;124:133–138. [PMC free article] [PubMed] [Google Scholar]
- 25.Wei C., El Hindi S., Li J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peev V., Hahm E., Reiser J. Unwinding focal segmental glomerulosclerosis. F1000Research. 2017;6:466. doi: 10.12688/f1000research.10510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer F., Trachtman H., Wühl E. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr. 2017;171:e172914. doi: 10.1001/jamapediatrics.2017.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni W., Han Y., Zhao J. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: a systematic review and meta-analysis. Sci Rep. 2016;6:39481. doi: 10.1038/srep39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrowski S.R., Katzenstein T.L., Pedersen M. Plasma levels of intact and cleaved urokinase receptor decrease in HIV-1-infected patients initiating highly active antiretroviral therapy. Scand J Immunol. 2006;63:478–486. doi: 10.1111/j.1365-3083.2006.001768.x. [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy C.P., Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahm E., Wei C., Fernandez I. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23:100–106. doi: 10.1038/nm.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tipping P.G., Davenport P., Gallicchio M. Atheromatous plaque macrophages produce plasminogen activator inhibitor type-1 and stimulate its production by endothelial cells and vascular smooth muscle cells. Am J Pathol. 1993;143:875–885. [PMC free article] [PubMed] [Google Scholar]
- 33.De Bock C.E., Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- 34.Botha S., Fourie C.M.T., Schutte R. Soluble urokinase plasminogen activator receptor as a prognostic marker of all-cause and cardiovascular mortality in a black population. Int J Cardiol. 2015;184:631–636. doi: 10.1016/j.ijcard.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y., Zhou H., Wu Y. Soluble urokinase plasminogen activator receptor and the risk of coronary artery disease in young Chinese patients. Dis Markers. 2017;2017:4719403. doi: 10.1155/2017/4719403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simone T.M., Higgins P.J. Low molecular weight antagonists of plasminogen activator inhibitor-1: therapeutic potential in cardiovascular disease. Mol Med Ther. 2012;1:101. doi: 10.4172/2324-8769.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gondi C.S., Lakka S.S., Dinh D.H. Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biol. 2004;1:165–176. doi: 10.1017/s1740925x04000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main causes of death recorded during the follow-up.
HR of all-cause mortality in the fully adjusted model (model 1), in a model without albumin (model 2) or without CRP (model 3) or without albumin and CRP (model 4).
Comparison of HR of suPAR (third tertile vs. first tertile) for noncardiovascular and cardiovascular mortality obtained by standard Cox regression analysis and competing risk regression analysis. Both models are adjusted for traditional, inflammation and nutritional status, and ESKD-related risk factors.
HR of suPAR for all-cause mortality/cardiovascular mortality/noncardiovascular mortality across gender, diabetes, BMI, dialysis vintage, systolic blood pressure, albumin, CRP, fibrinogen, and Kt/V categories. All P values for the effect modification are >0.05.