Abstract
Introduction
Pregnancy in women on dialysis is associated with a higher risk of adverse events, and the best care for this population remains to be established.
Methods
In this series, we aimed to identify factors associated with the risk of adverse fetal outcomes among 93 pregnancies in women on hemodialysis. Dialysis dose was initially assigned according to the presence of residual diuresis, body weight, and years on dialysis. Subsequent adjustments on dialysis dose were performed according to several parameters.
Results
The overall successful delivery rate was 89.2%, with a dialysis regimen of 2.6 ± 0.7 h/d, 15.4 ± 4.0 h/wk, and mean weekly standard urea Kt/V of 3.3 ± 0.6. In the logistic models, preeclampsia, lupus, primigravida, and average midweek blood urea nitrogen (BUN) level were positively related to the risk of a composite outcome of perinatal death or extreme prematurity, whereas polyhydramnios was inversely related to it. In multivariable linear regression, preeclampsia, polyhydramnios, primigravida, average midweek BUN, and residual diuresis remained significantly and independently related to fetal weight, which is a surrogate marker of fetal outcome. An average midweek BUN of 35 mg/dl was the best value for discriminating the composite outcome, and BUN ≥35 mg/dl was associated with a significant difference in a Kaplan-Meier curve (P = 0.01).
Conclusion
Our results showed that a good fetal outcome could be reached and that preeclampsia, lupus, primigravida, residual diuresis, polyhydramnios, and hemodialysis dose were important variables associated with this outcome. In addition, we suggested that a midweek BUN <35 mg/dl might be used as a target for adjusting dialysis dose until hard data were generated.
Keywords: chronic kidney disease, fetal outcomes, hemodialysis, hemodialysis adequacy, preeclampsia, pregnancy
Although still uncommon, dialysis during gestation is becoming more frequent.1, 2, 3, 4 In the only study that evaluated trends along time, the pregnancy rate in women on dialysis has risen from 0.54 to 3.3 pregnancies per 1000 patients-years in the last 3 decades.5 Although a substantial improvement in pregnancy outcome has occurred,6 pregnancy in patients with chronic kidney disease (CKD) who are on dialysis still carries a significant risk of adverse events.7, 8
In this sense, nephrologists are faced with the difficult task of dealing with high-risk pregnancy, which carries increased odds of adverse events for both the mother and fetus. Several questions arise in the attempt to establish the best care for pregnant women on dialysis, and issues such as the best moment to start renal replacement therapy, dialysis dose, and schemes are a matter of intense debate. Comparisons are hard to make due to differences in patient profiles, dialysis modality, dialysis schemes, obstetric definitions, and obstetric protocols, as previously discussed.6 Protocols vary widely, and there is clearly a need for standardization and establishment of guidelines that particularly focus on improvement of fetal outcomes. Although there is some evidence that suggests that a more intensive dialysis dose is related to a better fetal outcome, the optimal dialysis regimen and dose remain to be established.9, 10, 11, 12, 13
In the present study, we reported our experience with 93 pregnancies in women who underwent hemodialysis (HD) from 2000 to 2017, which is currently the largest single-center series. In the analysis, we aimed to identify baseline risk factors for pregnancy outcomes and to evaluate the association between several dialysis parameters and the risk of adverse events.
Methods
Study Design and Population
This retrospective cohort study consisted of 93 pregnancies in women who underwent HD at Hospital das Clínicas, Faculty of Medicine, University of Sao Paulo, Brazil, from January 2000 to January 2017. During that period, 100 pregnant women were referred to our dialysis center. Seven patients were not included in this case series: 1 patient with acute renal failure, 2 patients who had HD for <15 days before delivery, 1 patient with multiple fetal losses before developing renal failure, 2 patients with a severe lupus flare that required intensive immunosuppression when the referral to the nephrological team was made, and 1 patient with osteogenesis imperfecta, a disease associated with poor fetal prognosis,14 which left 93 pregnancies for the analysis. For patients with >1 pregnancy (n = 4), all pregnancies were included. The study was approved by the Ethics Committee and was conducted in accordance with the Declaration of Helsinki. In a previous publication,13 we reported outcomes in 52 pregnancies that occurred from 1988 to 2008. In the present analysis, we excluded 18 pregnancies that occurred from 1988 to 1999 that were included in the previous publication because by that time a different dialysis regimen was prescribed (time-fixed, 3-hour sessions, 4−6 times weekly). Erythropoietin was not provided for 9 patients, and many advances in obstetric surveillance and neonatal care were not available. Thus, the present report included 34 pregnancies that occurred from 2000 to 2008 reported in the 2010 publication and 59 new pregnancies that occurred after 2008.
Dialysis Protocol
Pregnant women who underwent HD received a high-flux, high-efficiency, 6 times/week HD scheme (dialyzer 1.8 m2, high-flux polysulfone, Kuf 55 ml/h per mm Hg, blood flow 350 ml/min, and dialysate flow 800 ml/min). The dialysis regimen was individualized. Patients with diuresis of >1000 ml/d, <1 year on HD therapy, or with a body weight <70 kg were initially assigned to 1.5- to 2 hour sessions, whereas patients with diuresis of <1000 ml/d, >1 year on HD therapy, or body weight >70 kg were assigned to a 2- to 3-hour session. Throughout pregnancy, adjustment of dialysis dose followed 2 different protocols. In protocol 1, from January 2000 to December 2008, the dialysis regimen was adjusted according to the laboratory, ultrasonographic, and clinical parameters. Severe hypertension, anorexia, frequent nausea, excessive weight gain, and persistent polyhydramnios, were all treated with a 30-minute increase in HD time. In protocol 2, from January 2009 to January 2017, in accordance with our findings that an average midweek blood urea nitrogen (BUN) <35 mg/dl was associated with a better fetal outcome,13 in addition to the parameters from protocol 1, the dialysis dose was also increased as needed to keep the midweek BUN at <35 mg/dl. For those who were not on dialysis, this treatment was started when an ascending creatinine reached 3.5 to 4.0 mg/dl. However, most of the patients arrived as late referrals, with creatinine values far above this value (initial creatinine range: 3.3−9.7 mg/dl, and a median creatinine clearance of 11.7 ml/min with an interquartile range [IQR] of 7.6−15 ml/min). Recombinant human erythropoietin dose (median dose: 24,000 IU/wk; range: 4000−48,000 IU/wk) was adjusted to maintain maternal hematocrit at 30% in both protocols.
Several dialysis parameters were measured, including average BUN (mean values for midweek predialysis BUN were collected Wednesdays or Thursdays), peripartum BUN, and creatinine. Single-pool Kt/V was determined using a 2-point urea model based on the intradialytic decrease in the blood urea level, intradialytic weight loss, and session length.15 Kt/V reported here was the mean of several values collected throughout the pregnancy; number of measurements 3.7 ± 2.3). Weekly standard urea Kt/V (stdKt/V)16 was estimated for all patients using the Leypoldt proposed formula.17 Hours on dialysis per week were defined as the longer scheme prescribed during treatment. Diuresis in milliliters per day and residual creatinine clearance were measured in a 24-hour urine collection (at the initiation of renal replacement therapy for those starting dialysis after conception or soon after pregnancy diagnosis for patients already on dialysis), and renal Kt/V was ascertained. Creatinine clearance and renal Kt/V were not ascertained in 20 patients because these measurements were not regularly performed in the beginning of our series. In 3 patients with diuresis of <200 ml/d, renal Kt/V was considered zero.
Obstetric Protocol
All participants followed a high-risk antenatal care protocol, defined as frequent prenatal and fetal monitoring, a low threshold for hospitalization, and a well-timed delivery. Low-dose aspirin and calcium supplementation were prescribed before gestational week 12, if not otherwise contraindicated, for preeclampsia prevention. Prenatal office visits were made every month up to 20 weeks and twice a month or weekly thereafter. Patients were actively monitored for signs and symptoms of preeclampsia. Both clinical (severe headache, visual change, and epigastric and right hypochondrium pain) and laboratory parameters (low platelet counts, increased liver enzymes, hemolysis, and increased proteinuria) were checked. Fetal ultrasonography, and uterine and umbilical artery Doppler studies were performed every 15 to 30 days in ambulatory patients and every week after hospitalization. Delivery was done in accordance with the recommendation of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy.18 Importantly, in our center, due to the high risk of preeclampsia in women on dialysis and difficulty with its diagnosis, we applied expectant management up to 37 weeks of gestation.18, 19 We allowed a pregnancy to go beyond 37 weeks only with special exceptions (n = 4). In fact, only 4 pregnancies reached beyond 37 weeks of gestation to avoid delivery on the non-dialysis day.
A multidisciplinary team with long-term experience in pregnancy among CKD patients was involved in the care of these patients. The team consisted of the nephrology staff, 4 obstetricians experts in CKD and high-risk pregnancies, 1 ultrasonographer specialist in fetal medicine, nurses, a nutritionist, and the neonatal team. Before every HD session, patients were questioned about contractions, vaginal discharges, and fetal movements. The neonatal intensive care unit was completely available on demand, and admission was also appointed to all babies born at <2200 g and/or at <36 weeks.
Hypertension was considered present if there were 2 blood pressure measurements >140 mm Hg for systolic blood pressure and/or >90 mm Hg for diastolic blood pressure, or if the patient was receiving antihypertensive therapy. Preeclampsia was defined as: (i) the abrupt onset of hypertension after 20 weeks of gestation associated with the appearance of proteinuria ≥300 mg/24 hour for women without baseline hypertension and proteinuria; (ii) doubling of protein excretion associated with exacerbation of hypertension after week 20 for those with baseline hypertension and proteinuria; and (iii) the presence of systemic manifestations of preeclampsia (thrombocytopenia, increased hepatic transaminase levels, hemolysis, persistent headache, blurred vision, epigastric pain) and/or the presence of abnormal placental Doppler waveform velocimetry combined with fetal growth restriction in patients with hypertension exacerbation and who could not have proteinuria measured due to anuria.20, 21, 22, 23, 24 Polyhydramnios and excess amniotic fluid were defined as an amniotic fluid index ≥25 cm and ≥18 cm on sonography, respectively.25
Stillbirth was defined as the occurrence of intrauterine fetal death after 24 weeks of gestation. Neonatal death was defined as a live infant dying within 28 days after delivery. Perinatal death included stillbirth and neonatal death. Preterm delivery was defined as a live birth before the 37th week of gestation (with early preterm defined as a live birth between the 30th and 34th weeks and late preterm between the 34th and 37th weeks of gestation). Extreme preterm birth was defined as a live birth before the 30th week of gestation. Pregnancy was considered successful if it resulted in a live infant who was discharged from the hospital. Emergency deliveries were defined as deliveries in the presence of acute fetal distress, premature placenta detachment, bleeding, and acute maternal decompensation.
Statistical Analysis
The primary adverse fetal outcome was defined as a composite outcome of perinatal death or extreme preterm birth. Categorical variables were compared using χ2 or Fisher exact tests, and continuous variables were compared using the Mann-Whitney test. Univariate logistic regression models were performed on the risk of the composite fetal outcome. Models were repeated after adjustments for preeclampsia, average BUN, and for residual diuresis. A receiver-operating characteristic curve on the composite fetal outcome was generated for average midweek BUN, and Kaplan-Meier curves were built using the best cutoff value defined by Youden’s index and compared by log-rank test. We also performed unadjusted and adjusted linear regression models using fetal weight and the predicted values of gestational age-adjusted fetal weight as the dependent variables. For the 2 twin pregnancies, fetal weight was entered as the mean value of the twins. All tests were 2-tailed, and a P value <0.05 was required for significance. SPSS (version 20; IBM, Armonk, NY) was used for analysis.
Results
In Table 1, we show the baseline variables, dialysis and biochemical parameters, and pregnancy outcomes in all 93 pregnancies in 89 women on HD. Mean maternal age was 30 ± 5 years, 31% self-reported race as black, and 78% were hypertensive before pregnancy. Median diuresis volume was 1.0 L (range: 0–4.0 L), and median residual creatinine clearance was 5.8 ml/min per 1.73 m2 (range: 0–20 ml/min per 1.73 m2). There were many CKD etiologies, with a predominance of chronic glomerulonephritis (32.3%). The average delivered regimen of dialysis was 2.6 ± 0.7 h/d, for a total of 15.4 ± 4.0 h/wk, with a mean weekly stdKt/V of 3.3 ± 0.6, a mean total stdKt/V of 4.3 ± 0.8 (residual renal function plus dialysis dose), and average BUN of 36.9 ± 9.4 mg/dl. In our series, 50.5% of the women were already on dialysis treatment before conception, with a mean previous time on dialysis of 24 months (range: 1−192 months).
Table 1.
Descriptive data among all 93 pregnancies and according to the composite fetal outcome (death or birth ≤30 weeks of gestation)
| Total (n = 93) | Composite fetal outcome |
P valuea | ||
|---|---|---|---|---|
| (death or birth ≤30 wks of gestation) | ||||
| Adverse (n = 17) | Favorable (n = 76) | |||
| Baseline variables | ||||
| Maternal age (yr) | 30 ± 5 | 29 ± 6 | 31 ± 5 | 0.26 |
| Race (black) | 29 (31.2) | 2 (11.8) | 27 (35.5) | 0.08 |
| Primigravida (yes) | 24 (25.8) | 8 (47.1) | 16 (21.1) | 0.03 |
| Parity | 2 (1−8) | 2 (1−5) | 2 (1−8) | 0.13 |
| Dialysis before gestation (yes) | 47 (50.5) | 9 (52.9) | 38 (50) | 0.83 |
| Time on dialysisb (mos) | 24 (1−192) | 18 (5−96) | 28.5 (1−192) | 0.55 |
| Diuresis volume (ml) | 1000 (0−4000) | 500 (0−2450) | 1000 (0−4000) | 0.11 |
| Creatinine clearancec (ml/min per 1.73 m2) | 5.8 (0−20) | 2.45 (0−12) | 7.5 (0−20) | 0.06 |
| Renal stdKt/Vc | 0.7 (0−3.4) | 0.4 (0−1.7) | 0.85 (0−3.4) | 0.08 |
| Hypertension at baseline (yes) | 73 (78.5) | 16 (94.1) | 57 (75) | 0.11 |
| Hematocrit at baseline (%) | 29 ± 5 | 28 ± 5 | 29 ± 52 | 0.41 |
| Etiology of renal failure (classification 1) | 0.07 | |||
| Chronic glomerulonephritis | 30 (32.3) | 5 (29.4) | 25 (32.9) | |
| Polycystic kidney disease | 4 (4.3) | 0 | 4 (5.3) | |
| Lupus nephritis | 9 (9.7) | 5 (29.4) | 4 (5.3) | |
| Focal and segmental glomerulosclerosis | 6 (6.5) | 0 | 6 (7.9) | |
| Diabetes nephropathy | 6 (6.5) | 0 | 6 (7.9) | |
| Urologic diseases | 11 (11.8) | 3 (17.6) | 8 (10.5) | |
| Hypertension | 10 (10.8) | 1 (5.9) | 9 (11.8) | |
| Kidney transplantation | 5 (5.4) | 0 | 5 (6.6) | |
| Other | 12 (12.9) | 3 (17.6) | 9 (11.8) | |
| Etiology of renal failure (classification 2) | ||||
| Lupus nephritis vs. all other etiologies | 9 (9.7) | 5 (29.4) | 4 (5.3) | 0.009 |
| Dialysis parameters | ||||
| Hours/day | 2.6 ± 0.7 | 2.3 ± 0.5 | 2.6 ± 0.7 | 0.046 |
| Hours/week | 15.4 ± 4.0 | 13.7 ± 3.2 | 15.7 ± 4.0 | 0.046 |
| Peripartum BUN (mg/dl) | 35.3 ± 12.1 | 40.8 ± 12.6 | 34.1 ± 11.8 | 0.035 |
| Average BUN (mg/dl) | 36.9 ± 9.4 | 42.5 ± 9.7 | 35.7 ± 8.9 | 0.015 |
| Peripartum creatinine (mg/dl) | 5.2 ± 1.6 | 6.0 ± 1.4 | 5.0 ± 1.6 | 0.01 |
| HD stdKt/V | 3.3 ± 0.6 | 3.3 ± 0.7 | 3.3 ± 0.6 | 0.85 |
| Total stdKt/Vc | 4.3 ± 0.8 | 3.9 ± 0.7 | 4.4 ± 0.8 | 0.06 |
| Peripartum hematocrit (%) | 34 ± 4 | 33 ± 4 | 34 ± 4 | 0.23 |
| Pregnancy outcome | ||||
| Successful delivery (yes) | 83 (89.2) | — | — | — |
| Gestational age at delivery (wk)d | 35 (25−39) | — | — | — |
| Preeclampsia (yes) | 13 (14) | 9 (52.9) | 4 (5.3) | <0.0001 |
| Fetal weight (kg) | 1698 ± 719 | 627 ± 248 | 1938 ± 550 | <0.001 |
| Small-for-gestational age (yes) | 45 (48.4) | 11 (69) | 34 (45) | 0.10 |
| Polyhydramnios (yes) | 49 (52.7) | 2 (11.8) | 47 (61.8) | 0.0002 |
| Cesarean section (yes) | 69 (74.2) | 9 (52.9) | 60 (79) | 0.01 |
BUN, blood urea nitrogen; HD, hemodialysis; std, standard; Total stdKt/V, renal stdKt/V + HD stdKt/V.
Data are presented as no. (%), mean ± SD, or median (range).
P = χ2 or Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables.
For 47 patients already on dialysis before conception.
Variables for 73 of the 93 patients (78.5%).
Excluding 5 stillbirths.
The overall successful delivery rate was 89.2% (83 of 93). There were 2 successful twin pregnancies, and therefore, 85 infants were sent home. Of the 10 perinatal deaths, 5 were neonatal deaths, and 5 were stillbirths. There were 25.8% emergency deliveries, 70% neonatal intensive care unit admissions, and median neonatal intensive care unit stays of 30 days (range: 1−150 days). Mean fetal weight in all 93 pregnancies was 1698 ± 719 g and 1775 ± 660 g among live births only. Our median gestational age at delivery (excluding 5 stillbirths) was 35 weeks (range: 25–39). As expected from our obstetric protocol of labor induction, our prematurity rate was high (74%). However, nearly one-half of these were late preterm birth (n = 33).
Table 1 also shows the results of baseline, dialysis, and outcome parameters according to the composite fetal outcome. When looking at maternal variables, there was a higher percentage of primigravidas and lupus nephritis in those with adverse events. Among dialysis parameters, participants with favorable outcomes showed a significantly higher delivered dose of dialysis (reflected by hours per week, average BUN, and peripartum creatinine, although HD weekly stdKt/V was similar) compared with those with adverse events. In addition, adverse fetal outcome was associated with a higher frequency of preeclampsia, strikingly lower mean fetal weight, and was inversely related to the occurrence of polyhydramnios.
Despite differences in residual renal function and the dialysis scheme, there was no difference in fetal outcomes in women already on dialysis compared with those who started dialysis after conception and in women assigned to different dialysis protocols (Supplementary Table S1). In Supplementary Table S1, we also depicted data comparing women according to renal Kt/V availability, showing that those 20 women who did not have Kt/V ascertained were less likely to be already on dialysis before pregnancy and were prescribed less hours of dialysis per week.
The variables associated with preeclampsia are shown in Table 2. Preeclampsia was associated with a negative impact on the successful delivery rate and accounted for 40% of perinatal deaths and 53% of the adverse composite outcomes. As expected, preeclampsia was also associated with a significantly lower gestational age and lower birth weight. All delivered infants from patients with preeclampsia were premature, and 9 were extremely premature.
Table 2.
Descriptive data of 93 pregnancies according to the occurrence of preeclampsia
| Preeclampsia |
Pa value | ||
|---|---|---|---|
| Yes (n = 13) | No (n = 80) | ||
| Baseline variables | |||
| Maternal age (yr) | 29 ± 5 | 30 ± 6 | 0.73 |
| Race (black) | 2 (15.4) | 27 (33.8) | 0.33 |
| Primigravida (yes) | 3 (23.1) | 21 (26.3) | 1.00 |
| Dialysis before gestation (yes) | 5 (38.5) | 42 (52.5) | 0.39 |
| Diuresis (ml) | 400 (0−2500) | 1000 (0−4000) | 0.22 |
| Hypertension at baseline (yes) | 12 (92.3) | 61 (76.3) | 0.28 |
| Initial hematocrit (%) | 28 ± 5 | 29 ± 5 | 0.85 |
| Lupus nephritis vs. all other etiologies | 2 (15.4) | 7 (8.8) | 0.61 |
| Dialysis parameters | |||
| Hours/week | 14.8 ± 4.0 | 15.5 ± 4.0 | 0.55 |
| Average BUN (mg/dl) | 41.9 ± 9.7 | 36.1 ± 9.2 | 0.07 |
| Peripartum creatinine (mg/dl) | 5.1 ± 1.5 | 5.2 ± 1.6 | 0.93 |
| Residual creatinine clearanceb | 3.4 (4.9) | 7.4 (5.8) | 0.04 |
| Renal stdKt/Vb | 0 (0−1.5) | 0.8 (0−3.4) | 0.04 |
| HD stdKt/V | 3.3 ± 0.8 | 3.3 ± 0.6 | 0.96 |
| Total stdKt/Vb | 3.9 ± 0.7 | 4.3 ± 0.8 | 0.05 |
| Pregnancy outcome | |||
| Death or premature birth ≤30 wks (yes) | 9 (69.2) | 8 (10) | <0.0001 |
| Polyhydramnios (yes) | 3 (23.1) | 46 (57.5) | 0.03 |
| Fetal weight (kg) | 850 ± 324 | 1836 ± 670 | <0.001 |
| Gestational age at delivery (wks)c | 28.9 ± 2.7 | 34.0 ± 3.8 | <0.001 |
BUN, blood urea nitrogen; HD, hemodialysis; std, standard; total stdKt/V, renal stdKt/V + HD stdKt/V.
Data are presented as no. (%), mean ± SD, or median (range).
P = χ2 or Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables.
Variables for 73 of the 93 patients (78.5%).
Excluding 5 stillbirths.
Table 3 shows the unadjusted and adjusted logistic regression models on the risk of the composite fetal outcome. In the unadjusted models, preeclampsia, primigravida, lupus nephritis, BUN, and peripartum creatinine were all positively associated with adverse composite fetal outcomes, whereas polyhydramnios and dialysis session length were inversely related to this risk. Next, models were repeated for the significant variables, adjusting for the effect of preeclampsia only, average BUN only, and diuresis volume only. All variables related to adverse events in the unadjusted models were still significantly related to the composite fetal outcomes after adjustment for preeclampsia. When the models were adjusted for average midweek BUN or diuresis volume, preeclampsia, polyhydramnios, lupus nephritis, and primigravida were still related to the composite fetal outcomes, but not to the HD session length. Unfortunately, because we had only 17 events in the adverse group, we could not run a full multivariable model, a strategy that would have probably caused overfitting of estimates.
Table 3.
Logistic regression models on the risk of adverse composite fetal outcome (17 events among 93 pregnancies)
| OR | 95% CI | P value | |
|---|---|---|---|
| Unadjusted | |||
| Preeclampsia (yes) | 20.2 | 5.1−81 | <0.0001 |
| Polyhydramnios (yes) | 0.08 | 0.02−0.39 | 0.002 |
| Primigravida (yes) | 3.3 | 1.11−10.0 | 0.03 |
| Lupus nephritis | 7.5 | 1.76−32.0 | 0.01 |
| Hours/week | 0.87 | 0.76−1.00 | 0.06 |
| Peripartum BUN (mg/dl) | 1.04 | 1.00−1.09 | 0.04 |
| Average BUN (mg/dl) | 1.08 | 1.02−1.15 | 0.01 |
| Peripartum creatinine (mg/dl) | 1.45 | 1.04−2.04 | 0.03 |
| Diuresis volume (per 100 ml) | 0.95 | 0.87−1.02 | 0.16 |
| Residual creatinine clearance (ml/min per 1.73 m2)a | 0.89 | 0.78−1.01 | 0.07 |
| Renal stdKt/Va | 0.44 | 0.17−1.16 | 0.10 |
| HD stdKt/V | 0.84 | 0.35−1.98 | 0.68 |
| Total stdKt/Va | 0.49 | 0.22−1.10 | 0.08 |
| Adjusted for preeclampsia | |||
| Polyhydramnios (yes) | 0.10 | 0.02−0.53 | 0.01 |
| Primigravida (yes) | 6.8 | 1.6−29.5 | 0.01 |
| Lupus nephritis | 11.0 | 2.0−59.8 | 0.01 |
| Hours/week | 0.85 | 0.72−1.01 | 0.06 |
| Average BUN (mg/dl) | 1.07 | 1.00−1.15 | 0.05 |
| Peripartum creatinine (mg/dl) | 1.78 | 1.16−2.73 | 0.01 |
| Adjusted for average BUN | |||
| Preeclampsia (yes) | 17.4 | 4.2−72.6 | <0.0001 |
| Polyhydramnios (yes) | 0.10 | 0.02−0.51 | 0.01 |
| Lupus nephritis | 3.27 | 1.03−10.4 | 0.04 |
| Primigravida (yes) | 7.66 | 1.61−36.5 | 0.01 |
| Hours/week | 0.88 | 0.76−1.01 | 0.07 |
| Peripartum creatinine (mg/dl) | 1.41 | 0.99−2.00 | 0.06 |
| Adjusted for diuresis volume | |||
| Preeclampsia (yes) | 19.8 | 4.8−81.2 | <0.0001 |
| Polyhydramnios (yes) | 0.06 | 0.01−0.30 | 0.0006 |
| Primigravida (yes) | 3.61 | 1.17−11.15 | 0.03 |
| Lupus nephritis | 6.61 | 1.53−28.7 | 0.01 |
| Average BUN (mg/dl; mean/SD) | 1.09 | 1.02−1.16 | 0.01 |
| Peripartum creatinine (mg/dl) | 1.39 | 0.97−1.99 | 0.08 |
BUN, blood urea nitrogen; CI, confidence interval; HD, hemodialysis; OR, odds ratio; std, standard; Total stdKt/V, renal stdKt/V + HD stdKt/V.
Variables for 73 of the 93 patients (78.5%).
We next performed unadjusted and adjusted linear regression models on fetal weight (Table 4), which is a surrogate marker of gestational age and fetal outcome. Data on fetal weight was available for all the 93 pregnancies. The univariable models showed that preeclampsia, average midweek BUN, primigravida, lupus nephritis, peripartum creatinine, and previous hypertension were all negatively associated with fetal weight, and polyhydramnios and dialysis session length were positively related to it. Three multivariable models are also shown in Table 4. The first model considered only maternal variables and HD dialysis dose. In this model, HD stdKt/V was not associated with weight, whereas the other variables had significant or near-significant associations. The second model substituted HD stdKt/V for average BUN, showing that this variable was importantly associated with fetal weight, together with lupus and primigravida. The third model included all variables associated with fetal weight and showed that preeclampsia, polyhydramnios, primigravida, average midweek BUN, session length, and diuresis volume were all independently related to fetal weight. We also repeated the models using the predicted values of gestational age-adjusted fetal weight, with similar results. Lastly, we worked with the residual effect of that regression, and most variables were not related to the residual, except for preeclampsia (β: −180; 95% confidence interval [CI]: −374 to 14; P = 0.07), polyhydramnios (β: 138; 95% CI: 4−273; P = 0.04), and BUN (β: −13; 95% CI: −20 to −7; P = 0.0002). When these 3 variables were entered into a multivariable model, only BUN remained significantly associated with the residual of gestational age-adjusted fetal weight (β: −12; 95% CI: −19 to −5; P = 0.002).
Table 4.
Univariable and multivariable linear regression models on fetal weight and the predicted values of gestational age-adjusted fetal weight
| Fetal weight |
Predicted fetal weight |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI β | p | (gestational age-adjusted) |
|||
| β | 95% CI β | P value | ||||
| Univariable | ||||||
| Age (yr) | 16 | −11 to 43 | 0.25 | 18 | −6 to 42 | 0.14 |
| Primigravida | −504 | −828 to −180 | 0.003 | −379 | −670 to −87 | 0.01 |
| Lupus nephritis | −760 | −1238 to −281 | 0.002 | −619 | −1047 to −191 | 0.01 |
| Dialysis before gestation | 60 | −238 to 357 | 0.69 | 67 | −197 to 331 | 0.62 |
| Diuresis volume (per 100 ml increase) | 12 | −6 to 30 | 0.19 | 10 | −6 to 26 | 0.23 |
| Hypertension at baseline | −481 | −829 to −132 | 0.01 | −277 | −593 to 40 | 0.09 |
| Residual creatinine clearance (ml/min per 1.73 m2)a | 8 | −21 to 37 | 0.59 | 4 | −22 to 30 | 0.78 |
| Renal stdKt/Va | 71 | −133 to 275 | 0.49 | 36 | −149 to 221 | 0.70 |
| Hours/week | 47 | 11 to 84 | 0.01 | 44 | 11 to 76 | 0.01 |
| Peripartum creatinine (mg/dl) | −88 | −181 to 4 | 0.06 | −74 | −157 to 8 | 0.08 |
| Average BUN (mg/dl) | −36 | −50 to −22 | <0.0001 | −22 | −36 to −9 | 0.001 |
| Average BUN ≥35 mg/dl | −671 | −941 to −400 | <0.0001 | −425 | −681 to −170 | 0.001 |
| HD stdKt/V | 154 | −93 to 401 | 0.22 | 175 | −43 to 393 | 0.11 |
| Total stdKt/Va | 171 | −38 to 380 | 0.11 | 129 | −61 to 320 | 0.18 |
| Death or birth <30 wks of gestation | −1311 | −1583 to −1039 | <0.0001 | −1323 | −1526 to −1120 | <0.0001 |
| Preeclampsia | −987 | −1364 to −609 | <0.0001 | −807 | −1149 to −465 | <0.0001 |
| Polyhydramnios | 711 | 453 to 970 | <0.0001 | 573 | 337 to 809 | <0.0001 |
| Cesarean section | 248 | −88 to 584 | 0.15 | 396 | 106 to 687 | 0.008 |
| Multivariable model 1 | ||||||
| Lupus nephritis | −447 | −933 to 40 | 0.07 | −368 | −814 to 78 | 0.10 |
| Primigravida | −394 | −717 to −72 | 0.02 | −294 | −589 to 2 | 0.05 |
| Hypertension at baseline | −365 | −699 to −30 | 0.03 | −156 | −463 to 150 | 0.31 |
| Diuresis volume (per 100 ml increase) | 17 | −2 to 37 | 0.08 | 17 | −0.4 to 35 | 0.06 |
| HD stdKt/V | 183 | −79 to 446 | 0.17 | 241 | −0.2 to 482 | 0.05 |
| Multivariable model 2 | ||||||
| Lupus nephritis | −437 | −877 to 3 | 0.05 | −394 | −827 to 38 | 0.07 |
| Primigravida | −351 | −646 to −56 | 0.02 | −263 | −552 to 27 | 0.07 |
| Hypertension at baseline | −222 | −531 to 88 | 0.16 | −104 | −408 to 201 | 0.50 |
| Diuresis volume (per 100 ml increase) | 13 | −3 to 28 | 0.10 | 10 | −5 to 25 | 0.19 |
| Average BUN (mg/dl) | −31 | −44 to −17 | <0.0001 | −19 | −33 to −6 | 0.01 |
| Multivariable model 3 | ||||||
| Preeclampsia | −654 | −961 to −346 | <0.0001 | −570 | −884 to −256 | 0.001 |
| Polyhydramnios | 291 | 45 to 536 | 0.02 | 249 | −2 to 499 | 0.05 |
| Primigravida | −255 | −508 to −2 | 0.05 | −169 | −428 to 89 | 0.20 |
| Lupus nephritis | −280 | −654 to 94 | 0.14 | −259 | −641 to 124 | 0.18 |
| Hypertension at baseline | −126 | −384 to 133 | 0.34 | −13 | −278 to 251 | 0.92 |
| Average BUN (mg/dl) | −23 | −35 to −11 | 0.0002 | −13 | −25 to 0 | 0.04 |
| Hours/week | 37 | 5 to 68 | 0.02 | 38 | 5 to 70 | 0.02 |
| Diuresis volume (per 100 ml increase) | 21 | 7 to 35 | 0.005 | 18 | 3 to 33 | 0.02 |
BUN, blood urea nitrogen; CI, confidence interval; HD, hemodialysis; std, standard; Total stdKt/V, renal stdKt/V + HD stdKt/V.
Variables for 73 of the 93 patients (78.5%).
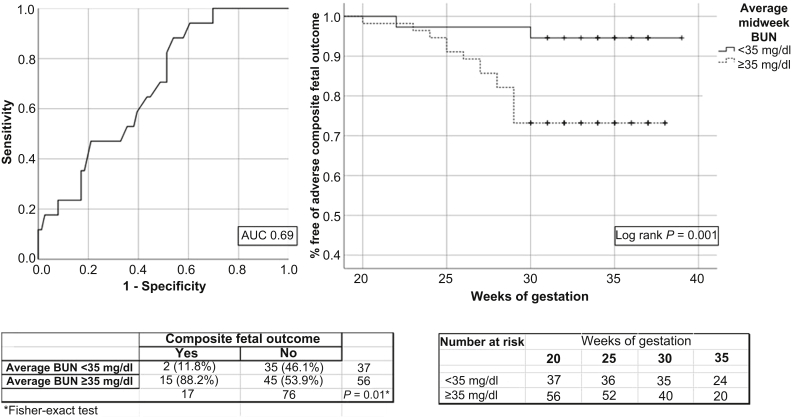
To assess the ability of average midweek BUN to discriminate the composite adverse fetal outcome, we built a receiver-operating characteristic curve (Figure 1). The area under the curve was 0.69 (95% CI: 0.57–0.81), and an average BUN value of 35 mg/dl was the best cutoff value for that curve. Among the 17 adverse events, 15 (88.2%) presented with an average BUN value of ≥35 mg/dl. An average midweek BUN ≥35 mg/dl was associated with an odds ratio (OR) of 6.4 (95% CI: 1.4–30.0; P = 0.02), increased risk of adverse fetal outcome, and a significant difference in the Kaplan-Meier survival curve (Figure 1) (log-rank P = 0.01).
Figure 1.
Receiver-operating characteristic curve for midweek blood urea nitrogen (BUN) (milligrams per deciliter) in relation to adverse composite fetal outcome and a Kaplan-Meier curve on the composite fetal outcome according to a cutoff of midweek BUN of 35 mg/dl. AUC, area under the curve.
Discussion
In the present report of 93 pregnancies in women on HD, the live birth rate was 89.2%, and the composite fetal outcome (perinatal death or extremely premature) occurred in 18.3%. No maternal deaths occurred. Preeclampsia was diagnosed in 14% of pregnancies, a percentage that might be underestimated because of the limitations on the diagnosis of this condition in dialysis patients, and that the expected incidence of preeclampsia in women with preexisting renal disease is as high as 70%.26, 27, 28, 29, 30
The average dialysis dose was 2.6 ± 0.7 h/d (15.4 ± 4.0 h/wk), with mean weekly urea stdKt/V of 3.3 ± 0.6. Throughout pregnancy, clinical, sonographic, and laboratory parameters were used to adjust for dialysis dose. Importantly, residual diuresis and years on dialysis were important factors that determined the initial dialysis dose in our protocol. Our obstetric protocol included labor induction for pregnancies reaching 37 weeks of gestation. The decision to adopt this practice, similar to the recommended guidelines for pregnant women with superimposed preeclampsia without severe features,18, 19, 31 was based on 3 main facts: the high rate of hypertension in our population, the high risk of preeclampsia, and the challenges of diagnosing preeclampsia. Prolonging gestation for patients with mild preeclampsia beyond 37 weeks was associated with a poor maternal outcome in a large multicenter randomized trial.32
When we analyzed the composite fetal outcome of perinatal death and extreme prematurity, several variables emerged as being related to the occurrence of adverse events (preeclampsia, polyhydramnios, primigravida, lupus, hours per week, and BUN). Although we could not run a full multivariable model on the composite outcome due to the low number of events, the multivariable model, which used fetal weight as a surrogate marker of fetal outcome, essentially confirmed the findings seen in the logistic regression models. The reason why we chose to work with fetal weight was because this variable is a sum of effects related to fetal growth, most importantly gestational age (R2 = 0.79 in our sample), as well as other factors (e.g., genetics, smoking, systemic diseases, nutrition, and placental function). We repeated the analysis on the predicted values of the gestational age-adjusted fetal weight, thus separating the variability in weight that was solely due to gestational age. Because reaching term in CKD is a challenge, this variable can be seen as a proxy for gestational age and used as an outcome.
Generally, all the analysis showed the same results. First, the most important factor related to either adverse fetal outcome or decreasing fetal weight was preeclampsia. The presence of preeclampsia was a strong predictor of adverse outcome (OR: 20.2; 95% CI: 5.1–81; P < 0.0001). In the absence of preeclampsia, the probability of a successful delivery was 93.5%, whereas with preeclampsia, this rate was reduced to 69%. CKD is largely known to be an important risk factor for superimposed preeclampsia,28, 33, 34 and patients on dialysis seem to have an additional risk. Several factors might underlie that relationship, such as endothelial dysfunction, increased oxidative stress, imbalance in angiogenic factors, chronic inflammation, and severity of hypertension, among others, but our current knowledge is still limited. Interestingly, the only variable related to preeclampsia diagnosis in our series was residual renal function (renal KtV and creatinine clearance). Women with no preeclampsia presented with higher residual renal function. This relation was unlikely to be confounded by dialysis dose because those with more residual renal function were assigned to shorten the initial dialysis dose. This finding suggests that residual renal function may be related to the risk of preeclampsia, and that triggers the question of whether measures to protect residual renal function may also protect women from the risk of preeclampsia. In addition, a better understanding of the mechanism underlying the strong association between CKD and preeclampsia is essential for development of therapeutic measures.
In our series, polyhydramnios was associated with a favorable fetal outcome (OR: 0.08; 95% CI: 0.02−0.39; P = 0.002). In the presence of excess amniotic fluid or polyhydramnios, the probability of a successful pregnancy was 98% (1 fetal loss of 49 pregnancies). Because women who developed polyhydramnios were changed to a more intense regimen of dialysis for polyhydramnios treatment in our protocol, this association could be mediated by the increased dialysis dose. However, even after adjustments for parameters of dialysis doses (average BUN in the logistic model and average BUN, hours of dialysis per week and residual diuresis in the linear regression on fetal weight), polyhydramnios remained significantly and independently related to a better fetal outcome. Polyhydramnios is a common complication in pregnant women on dialysis, with an incidence ranging from 30% to 70%.3, 13, 35, 36, 37 Although the mechanisms are not fully understood, increased fetal urine production secondary to urea osmotic diuresis is the most acceptable cause. Osmotic diuresis is also the established mechanism to the increased risk of polyhydramnios in diabetic pregnant women.38, 39 Different reports have suggested that polyhydramnios may be treated by increasing the HD dose and decreasing maternal and fetal blood urea levels, and therefore, osmotic diuresis.3, 40, 41, 42, 43 One hypothesis to explain the association of polyhydramnios with favorable outcome in the setting of CKD is that polyhydramnios may serve as an indirect marker of adequate placental blood flow.13 It is plausible that the occurrence of osmotic diuresis in the fetus of dialytic women is dependent on a normal fetal renal blood flow. Fetuses with uteroplacental insufficiency habitually shunt blood flow away from nonessential organs (e.g., the kidney), thus decreasing urine output and amniotic fluid volume. The incidence of oligohydramnios secondary to placental insufficiency in severe preeclampsia ranges from 20% to 50%.44, 45, 46 Once osmotic diuresis, secondary to a high fetal blood urea level, is often present in pregnant women on dialysis, we speculate that only patients with inadequate placental blood flow will not develop polyhydramnios.47
In our data, several parameters of increasing dialysis dose were related to a better fetal outcome and fetal weight, particularly average midweek BUN and hours on dialysis. This result was in accordance with other studies that showed that increased dialysis dose might be the optimal approach to pregnancy in dialysis.1, 10, 11, 12, 13 However, despite this common agreement, much debate exists regarding the establishment of an optimal target for dialysis dose. One group, from Canada performed nocturnal HD in 17 pregnant women and reported 86.4% live births, one of the best results in the literature. Simply by comparing their results with the American Registry for Pregnancy in Dialysis Patients, the authors showed a positive association of the live birth rate with the tertiles of hours of dialysis.12 However, the American Registry for Pregnancy in Dialysis Patients presented a poor fetal outcome (61.4%) compared with many other published studies, including 1 from the United States48 and our own,13, 49 a fact that might have influenced the results. In addition, analysis might have been biased considering that Canadian and US patients were different in many other aspects, especially for the presence of an experienced multidisciplinary team.6, 37, 50, 51
When we compared our study with the Canadian study,12 it was striking that similar survival rates were reached with the different dialysis schemes provided. It might be argued that because our maximum dialysis dose offered was not higher than the HD stdKt/V of 4.4 (4 h/d), we could not really evaluate the effect of an even higher dialysis dose. Although this is true, our survival rate of 89.2% was slightly superior to the 86.4% survival rate reported in the Canada study, although we used less than one-third of the HD dose. We did present a higher frequency of prematurity, possibly due to the fact that we induced labor by 37 weeks of gestation, but had similar percentages of prematurity at <34 weeks of gestation.
Whether performing long intensive dialysis is better than short daily dialysis is an important question that we believe can only be truly answered with a randomized clinical trial. Long intensive dialysis might cause detrimental effects. It might reduce residual diuresis and accelerate loss of residual kidney function. In the Frequent Hemodialysis Network Trial, it was demonstrated that long nocturnal treatment was associated with more rapid loss of residual kidney function than conventional HD.52 Residual kidney function has many reported benefits.53, 54, 55, 56 Recently, a large retrospective cohort study found that the decline in residual kidney function during the first year of dialysis was associated with a 2-fold higher risk of death among incident HD patients.57 Other adverse effects reported in pregnant women submitted to long intensive dialysis included a potential increase in the rate of cervical shortening and incompetence, which could lead to an enhanced risk of prematurity.11, 12, 49 In the 2 studies that performed the most intense HD regimens, the rate of cervical incompetence was 60%11 and 18%,12 percentages that were far larger than those reported for the general pregnant population (1.7%).58 In our study, we had only 2 cases (2.2%) of cervical incompetence. Mechanisms related to the association between dialysis dose and cervical incompetence are still unclear, but might be related to decreased levels of progesterone.59, 60
The best measure for assessing dialysis dose is also an important and unresolved issue. Defining dose in terms of hours of dialysis might be limited, although this variable was related to fetal outcomes in our sample. Total and HD stdKt/V showed a trend that was associated with better outcomes in all analyses but did not have the best performance in discriminating events. In contrast, an average midweek BUN <35mg/dl had a fairly good performance in discriminating adverse composite fetal outcomes. Only 2 among 17 events occurred in women with an average midweek BUN of <35 mg/dl, with a sensibility of 88.2% for the composite fetal outcome. However, BUN has other determinants, and whether an average BUN primarily reflects small molecule clearance throughout pregnancy or if it also reflects other factors (protein consumption, catabolism, severity of maternal disease, vascular disease, corticosteroids use) is an important question that we could not address in this study.
We also observed that patients who started dialysis therapy after conception did not have a better fetal outcome compared with those who were already on dialysis, as has been frequently reported.61, 62 Keeping midweek BUN to <35 mg/dl and adjusting initial HD dose according to the presence of residual renal function are factors that could eventually be related to this finding, triggering the question of whether women already on dialysis should be targeted with a higher initial dialysis dose compared with those who start dialysis during pregnancy.
Concerning maternal variables, primiparity and lupus nephritis were related to a worse outcome. However, because the number of lupus patients was small, adjustments might be limited, and other studies should address the importance of lupus in pregnant women with CKD. Regarding primiparity, at first glance, the relationship to adverse fetal outcome could be mediated by the increased preeclampsia risk that these patients bear. However, the relationship between primigravida and fetal outcome was independent of preeclampsia in all analyses. In addition, when we compared patients with and without preeclampsia, there was no difference in the primigravida rate. Although the diagnosis of preeclampsia is challenging in this population, which could lead to an underadjustment of the preeclampsia effect, it is possible that multiparity is intrinsically related to a decreased risk of adverse events (natural selection bias).
Lastly, our favorable results were likely related to the experience gathered in our center by our multidisciplinary team in the last 30 years. This suggests that measures that foster experience and learning in the care of pregnant women in dialysis centers may affect outcomes. We believe the close contact with an experienced obstetric team is of utmost importance in the care of these patients.
Our study had several limitations. Most importantly, it was a retrospective case series, and this design limited our ability to truly adjust for confounding effects. Two different protocols were applied, and patients were assigned to different dialysis initial schemes and dialysis dose adjustments throughout pregnancy, factors that confounded the relationships observed, despite our efforts to perform adjustments. Second, due to a small number of events, some of our analyses were limited, and we could not run a multivariable model in the logistic regression analysis. This problem led us to use fetal weight as a surrogate marker of fetal outcome. By using this strategy, we were able to run a multivariable model and check if results were in the same direction than those indicated by the logistic regression models. In addition, renal clearance could not be ascertained for 20 patients, and we used diuresis output as a covariate in the multivariable models instead. Last, although we showed the dialysis dose associated with the best fetal outcome in our center, we could not test this dose against an even higher dialysis dose. Therefore, this finding should be interpreted considering its limitations. A randomized clinical trial would be the only way of addressing the important and unresolved question of the best dialysis dose that should be offered to pregnant women. Despite all these limitations, we believe that our data may contribute importantly to current literature, fostering discussion on the best care for these high-risk patients.
In conclusion, our results showed that a good fetal outcome could frequently be reached for pregnant women on HD with a specialized multidisciplinary team applying a multidimensional approach. In addition, we observed that preeclampsia, lupus nephritis, primigravida, residual diuresis, polyhydramnios, and HD dose were important variables associated with this outcome. We also suggested that a predialysis midweek serum BUN <35 mg/dl might be used as a target for adjusting dialysis dose until hard data from clinical trials are generated.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Descriptive data according to occurrence of pregnancy before or after starting hemodialysis, to 2 different protocols of dialysis dose tailoring, and to missing values on renal Ktv.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Descriptive data according to occurrence of pregnancy before or after starting hemodialysis, to 2 different protocols of dialysis dose tailoring, and to missing values on renal Ktv.
References
- 1.Bagon J.A., Vernaeve H., De Muylder X. Pregnancy and dialysis. Am J Kidney Dis. 1998;31:756–765. doi: 10.1016/s0272-6386(98)70060-5. [DOI] [PubMed] [Google Scholar]
- 2.Souqiyyeh M.Z., Huraib S.O., Saleh A.G. Pregnancy in chronic hemodialysis patients in the Kingdom of Saudi Arabia. Am J Kidney Dis. 1992;19:235–238. doi: 10.1016/s0272-6386(13)80003-0. [DOI] [PubMed] [Google Scholar]
- 3.Holley J.L., Reddy S.S. Pregnancy in dialysis patients: a review of outcomes, complications, and management. Semin Dial. 2003;16:384–388. doi: 10.1046/j.1525-139x.2003.16085.x. [DOI] [PubMed] [Google Scholar]
- 4.Hu S. Pregnancy in dialysis patients: where do we go from here? Semin Dial. 2003;16:376–378. doi: 10.1046/j.1525-139x.2003.16083.x. [DOI] [PubMed] [Google Scholar]
- 5.Shahir A.K., Briggs N., Katsoulis J. An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialyzed women using data from the ANZDATA Registry. Nephrology. 2013;18:276–284. doi: 10.1111/nep.12044. [DOI] [PubMed] [Google Scholar]
- 6.Piccoli B.P., Minelli F., Versino E. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant. 2016;31:1915–1934. doi: 10.1093/ndt/gfv395. [DOI] [PubMed] [Google Scholar]
- 7.Bramham K. Dialysis and pregnancy: no longer the impossible. Nephrol Dial Transplant. 2016;31:1763–1765. doi: 10.1093/ndt/gfw216. [DOI] [PubMed] [Google Scholar]
- 8.Groothoff J. Pregnancy during dialysis: still a challenge to get there, but worth the effort. Nephrol Dial Transplant. 2015;30:1053–1055. doi: 10.1093/ndt/gfv124. [DOI] [PubMed] [Google Scholar]
- 9.Shemin D. Dialysis in pregnant women with chronic kidney disease. Semin Dial. 2003;16:379–383. doi: 10.1046/j.1525-139x.2003.16084_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Asamiya Y., Otsubo S., Matsuda Y. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217–1222. doi: 10.1038/ki.2009.48. [DOI] [PubMed] [Google Scholar]
- 11.Haase M., Morgera S., Bamberg C. A systematic approach to managing pregnant dialysis patients – the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant. 2005;20:2537–2542. doi: 10.1093/ndt/gfi044. [DOI] [PubMed] [Google Scholar]
- 12.Hladunewich M.A., Hou S., Odutayo A. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol. 2014;25:1103–1109. doi: 10.1681/ASN.2013080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luders C., Castro M.C.M., Titan S.M. Obstetric outcome in pregnant women on long-term dialysis: a case series. Am J Kidney Dis. 2010;56:77–85. doi: 10.1053/j.ajkd.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Cubert R., Cheng E.Y., Mack S. Osteogenesis imperfecta: mode of delivery and neonatal outcome. Obstet Gynecol. 2001;97:66–69. doi: 10.1016/s0029-7844(00)01100-5. [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 16.Gotch F.A. The current therapy of urea kinetic modeling with respect to different dialysis modalities. Nephrol Dial Transplant. 1998;13(Suppl 6):S10–S14. doi: 10.1093/ndt/13.suppl_6.10. [DOI] [PubMed] [Google Scholar]
- 17.Leypoldt J.K., Jaber B.L., Zimmerman D.L. Predicting treatment dose for novel therapies using urea standard Kt/V. Semin Dial. 2004;17:142–145. doi: 10.1111/j.0894-0959.2004.17212.x. [DOI] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 19.Magee L.A., Pels A., Helewa M., on behalf of the Canadian Hypertensive Disorders of pregnancy (HDP) Working Group Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Piccoli G.B., Gaglioti P., Attini R. Pre-eclampsia or chronic kidney disease? The flow hypothesis. Neprhol Dial Transplant. 2013;28:1199–1206. doi: 10.1093/ndt/gfs573. [DOI] [PubMed] [Google Scholar]
- 21.Sibai B.M., Stella C.L. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200:481.e1–481.e7. doi: 10.1016/j.ajog.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Harrington K., Fayyad A., Thakur V. The value of uterine artery Doppler in prediction of uteroplacental complications in multiparous women. Ultrasound Obstet Gynecol. 2004;23:50–55. doi: 10.1002/uog.932. [DOI] [PubMed] [Google Scholar]
- 23.Hladunewich M.A., Melamad N., Bramhan K. Pregnancy across the spectrum of chronic kidney disease. Kidney Int. 2016;89:995–1007. doi: 10.1016/j.kint.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Rolfo A., Attini R., Tavassoli E. Is it possible to differentiate chronic kidney disease and preeclampsia by means of new and old biomarkers? A prospective study. Dis Markers. 2015;2015:127083. doi: 10.1155/2015/127083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moise K.J. Polyhydramnios. Clin Obstet Gynecol. 1997;40:266–279. doi: 10.1097/00003081-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Davison J.M., Lindheimer M.D. Pregnancy and chronic kidney disease. Semin Nephrol. 2011;31:86–99. doi: 10.1016/j.semnephrol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Fisher M.J. Chronic kidney disease and pregnancy. Maternal and fetal outcomes. Adv Chronic Kidney Dis. 2007;14:1321–1345. doi: 10.1053/j.ackd.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Williams D., Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;336:211–215. doi: 10.1136/bmj.39406.652986.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevis I.F., Reitsma A., Dominic A. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J.J., Xin-Xin M., Hao L. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol. 2015;10:1964–1978. doi: 10.2215/CJN.09250914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(suppl 1):S1–S22. [PubMed] [Google Scholar]
- 32.Koopma C.M., Bijlenga D., Groen H. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicenter, open-label randomised controlled trial. Lancet. 2009;374:979–988. doi: 10.1016/S0140-6736(09)60736-4. [DOI] [PubMed] [Google Scholar]
- 33.Fink J.C., Schwartz S.M., Benedetti T.J. Increased risk of adverse maternal and infant outcomes among women with renal disease. Paediatr Perinat Epidemiol. 1998;12:277–287. doi: 10.1046/j.1365-3016.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- 34.Hall M. Pregnancy in women with CKD: a success story. Am J Kidney Dis. 2016;68:633–639. doi: 10.1053/j.ajkd.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Reddy S.S., Holley J.L. Management of the pregnant chronic dialysis patient. Adv Chronic Kidney Dis. 2007;14:146–155. doi: 10.1053/j.ackd.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Chou C.Y., Ting I.W., Lin T.H. Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eu J Obstet Gynecol. 2008;136:165–170. doi: 10.1016/j.ejogrb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Furaz-Czerpak K.R., Fernadez-Juarez G., Moreno-de la Higuera A.M. Pregnancy in women on chronic dialysis: a review. Nefrologia. 2012;32:287–294. doi: 10.3265/Nefrologia.pre2012.Jan.11319. [DOI] [PubMed] [Google Scholar]
- 38.Bramhan K., Rajasingham D. Pregnancy in diabetes and kidney disease. J Renal Care. 2012;38:78–89. doi: 10.1111/j.1755-6686.2012.00270.x. [DOI] [PubMed] [Google Scholar]
- 39.Forgas J.S., Romero R., Wildman D.E. Relationship between maternal and fetal plasma glucose and insulin concentrations during graded maternal hyperglycemic states in primates. Am J Perinatol. 2006;23:369–375. doi: 10.1055/s-2006-947725. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Menchero R., Albero M.D., Cabeza B. Gestación con éxito em una paciente con lúpus eritematoso sistémico en hemodiálises. Nefrologia. 2004;24:70–74. [PubMed] [Google Scholar]
- 41.Reister F., Reister B., Heyl W. Dialysis and pregnancy – a case report and review of the literature. Ren Fail. 1999;21:533–539. doi: 10.3109/08860229909045193. [DOI] [PubMed] [Google Scholar]
- 42.Hou S. Modification of dialysis regimens for pregnancy. Int J Artif Organs. 2002;25:823–826. doi: 10.1177/039139880202500902. [DOI] [PubMed] [Google Scholar]
- 43.Manisco G., Poti M., Maggiulli G. Pregnancy in end-stage renal disease patients on dialysis: how to achieve a successful delivery. Clin Kidney J. 2015;8:293–299. doi: 10.1093/ckj/sfv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinillo A., Cesari S., Bariselli S. Placental lesions associated with oligohydramnios in fetal growth restricted (FGR) pregnancies. Placenta. 2015;36:538–544. doi: 10.1016/j.placenta.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Schucker J.L., Mercer B.M., Audibert F. Serial amniotic fluid index in severe preeclampsia: a poor predictor of adverse outcome. Am J Obstet Gynecol. 1996;175:1018–1023. doi: 10.1016/s0002-9378(96)80045-7. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien J.M., Mercer B.M., Friedman S.A. Amniotic fluid index in hospitalized hypertensive patients managed expectantly. Obstet Gynecol. 1993;82:247–250. [PubMed] [Google Scholar]
- 47.Luders C., Titan S., Carmo L.P.F. Pregnancy related polyhydramnios in chronic dialysis patients: a treatable pathology, a hemodialysis adequacy tool and an outcome predictor (abstract) J Am Soc Nephrol. 2010;21:465A. [Google Scholar]
- 48.Sachdeva M., Barta V., Thakkar J. Pregnancy outcomes in women on hemodialysis: a national survey. Clin Kidney J. 2017;10:276–281. doi: 10.1093/ckj/sfw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romão J.E., Jr., Luders C., Kahhale S. Pregnancy in women on chronic dialysis: a single-center experience with 17 cases. Nephron. 1998;78:416–422. doi: 10.1159/000044970. [DOI] [PubMed] [Google Scholar]
- 50.Hladunewich M., Schatell D. Intensive dialysis and pregnancy. Hemodialysis Int. 2016;20:339–348. doi: 10.1111/hdi.12420. [DOI] [PubMed] [Google Scholar]
- 51.Yang L.Y., Thia E.W.H., Tan L.K. Obstetric outcomes in women with end-stage renal disease on chronic dialysis: a review. Obstet Med. 2010;3:48–53. doi: 10.1258/om.2010.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daugirdas J.T., Greene T., Rocco M.V. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraus M.A., Kansal S., Copland M. Intensive hemodialysis and potential risks with increasing treatment. Am J Kidney Dis. 2016;68(suppl 1):S51–S58. doi: 10.1053/j.ajkd.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Shafi T., Jaar B.G., Plantinga L.C. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the choice for healthy outcomes in caring for end-stage renal disease (CHOICE) study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vilar E., Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 56.Shemin D., Boston A.G., Laliberty P. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 57.Obi Y., Rhee C.M., Mathew A.T. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27:3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werner E.F., Han C.S., Pettker C.M. Universal cervical-length screening to prevent preterm birth: a cost effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38:32–37. doi: 10.1002/uog.8911. [DOI] [PubMed] [Google Scholar]
- 59.Hubinont C.J., Goldman M., Vanherwedhem J.L. Effects of chronic renal failure and hemodialysis on hormonal evaluation of pregnancy. Am J Nephrol. 1988;8:57–61. doi: 10.1159/000167554. [DOI] [PubMed] [Google Scholar]
- 60.Brost B.C., Newman R.B., Hendricks S.K. Effect of hemodialysis in serum progesterone level in pregnant women. Am J Kidney Dis. 1999;33:917–919. doi: 10.1016/s0272-6386(99)70426-9. [DOI] [PubMed] [Google Scholar]
- 61.Okundaye I., Abrinko P., Hou S. Registry of pregnancy in dialysis patients. Am J Kidney Dis. 1998;31:766–773. doi: 10.1016/s0272-6386(98)70044-7. [DOI] [PubMed] [Google Scholar]
- 62.Jesudason S., Grace B.S., McDonald S.P. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol. 2014;9:143–149. doi: 10.2215/CJN.03560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive data according to occurrence of pregnancy before or after starting hemodialysis, to 2 different protocols of dialysis dose tailoring, and to missing values on renal Ktv.