Abstract
Introduction
Early detection of diabetes mellitus (DM) and diabetic kidney disease (DKD) is important for preventing end-stage renal failure and reducing cardiovascular complications. Availability of a validated point-of-care (PoC) device that can measure various DKD markers would be useful in this respect, especially in resource-poor parts of the world.
Methods
We validated a novel nanotechnology-based multianalyte PoC device (minimally invasive and does not require trained medical personnel) against laboratory gold standard tests for the detection of 5 biomarkers related to management of DM and DKD. The prospective study was funded by an International Society of Nephrology American Nephrologists of Indian Origin grant in 2 phases: (i) proof of concept: random samples were tested for the analytes with the PoC device and correlated with the laboratory gold standard; and (ii) clinical validation in a well-characterized cohort of patients. A nonenzymatic- and nonantibody-based electrochemical PoC device for quantitative measurement of markers—glycosylated hemoglobin (HbA1c), hemoglobin, serum albumin, microalbuminuria, urine creatinine, and albumin-to-creatinine ratio−was developed and used in this study. The disposable strips were interfaced with a multipotentiostat hand-held PoC device (3.7-V rechargeable lithium battery, 5-inch touch screen, Bluetooth enabled) working in amperometry mode, which provided the results in <1 minute. Data were analyzed using linearity plots and Bland-Altman difference plot analysis.
Results
A total of 4717 individuals were screened during the study (phase 1: 2576 and phase 2: 2141.) In phase 2, samples were tested in 529 subjects (346 females)—120 subjects with type 1 DM, 255 subjects with type 2 DM, 54 subjects without DM, 400 subjects with stage 2 chronic kidney disease, and 30 subjects with stage 3 chronic kidney disease.
Conclusion
A nanotechnology-based PoC device for quantitative measurement of HbA1c, hemoglobin, serum albumin, microalbuminuria, and the urine albumin-to-creatinine ratio was developed for detection of early DKD and showed excellent correlation between the device and laboratory results. This device has the potential for early detection of DM and/or DKD, especially in remote communities in underserved areas of the world where prevalence of diabetes is rapidly increasing.
Keywords: albuminuria, anemia, chronic kidney disease, diabetes, diabetic nephropathy, obesity
Chronic kidney disease (CKD) is increasingly being recognized as a public health problem in developing countries like India and rest of the world.1, 2 Approximately 10% of the world population is affected by CKD, and millions die each year because of the lack of diagnostic tools and timely treatment.3 Diabetes mellitus (DM) is the major risk factor for CKD worldwide. Both experimental and cohort studies support the pathogenetic role of hyperglycemia and CKD.4 This problem is particularly of major importance in India where there is an epidemic of patients with new-onset type 2 DM, and where diabetic nephropathy has been shown to be the most important cause of end-stage renal disease.5, 6 The delivery of care to these patients, especially in the rural areas of India, is woefully inadequate.7 There is an acute need for comprehensive, continuous, and cost-effective health care delivery for these underserved people.8 Early detection and strategies for prevention of progression to diabetic kidney disease (DKD) would make a major difference for these patients and would also be economically beneficial for a resource-constrained country.9 Early diagnostics in remote and resource-challenged settings is difficult without access to costly well-equipped clinical laboratories and trained medical personnel. Consequently, developing cost-effective and easy-to-implement diagnostic tools remains an important goal in global health. One promising approach to achieve this goal is to detect disease biomarkers from accessible body fluids with point-of-care (PoC) biosensors. PoC biosensors can potentially improve patient care through real-time and remote health monitoring. We report the use of a multianalyte PoC device based on novel electrochemical sensing technology. This device quantitatively tests for glycosylated hemoglobin (HbA1c), hemoglobin, serum, and urine albumin and urine creatinine. Realizing the importance of anemia in diabetes, especially in DKD, we believe that this device will be extremely useful in detection of early DKD in most of the rural population of not only India, but the rest of the world.
Materials and Methods
The PoC device technology was developed at the Indian Institute of Science, Bangalore, over the last 5 years, and it is based on nonenzymatic- and nonantibody-based electrochemical biosensing technology.10, 11, 12, 13, 14 The PoC measurement is done on electrochemical disposable test strips that contain a membrane impregnated with patented sensing chemistries. This device, unlike many other devices, performs quantitative measurement of the analytes in question, at any remote area, with absolutely no infrastructure requirements.
For the first time, the single hand-held device tests for 5 different biomarkers (hemoglobin, HbA1c, serum albumin, urine microalbumin, and urine creatinine) and will be extendable to other markers (glycated albumin, serum creatinine, serum bilirubin, and so on) in the future. The range of analytes measured in this device are HbA1c (5.0%−15%), hemoglobin (2−25 g/dl), serum albumin (1−6.0 g/dl), microalbuminuria (2 mg/l−1 g/l), and urine creatinine (50 mg/l−2 g/l). The device is further being modified to extend the upper limit of detection, especially for urine analytes. The device is shown in Figure 1.
Figure 1.
anuPath multianalyte diagnostic device (Pathshodh Healthcare Pvt Ltd., Entrepreneurship Centre, Indian Institute of Science, Bangalore).
This study was done in 2 phases. The first phase was the clinical proof-of-concept phase, in which samples were tested with the PoC device and compared with laboratory gold standard methodologies. During the clinical validation phase, the technology was verified in the clinical setting. (Samatvam Endocrinology Diabetes Centre – Jnana Sanjeevini Diabetes Hospital and Medical Centre, Bangalore, India). All the samples (blood and urine) that were tested by the PoC device were simultaneously tested at a reference laboratory. Laboratory methods used for urine albumin, urine creatinine, hemoglobin, serum albumin, and HbA1c were immunoturbidimetry, the Jaffe method, the sodium lauryl sulphate colorimetric method, the bromocresol green method, high-performance liquid chromatography, and the Bio-Rad method, respectively. The albumin-to-creatinine ratio (ACR) values were calculated from the measured urinary albumin and creatinine. The PoC device can store the test data of 60,000 patients, which can be transferred to a computer and/or mobile device via Bluetooth. All the samples were tested on the same day for microalbuminuria, urine creatinine, ACR, HbA1c, hemoglobin, and serum albumin using the multianalyte PoC device. The same samples were tested at a central pathology laboratory for validation. The technology used for each of the analytes is briefly described in the following.
PoC Technology: Hemoglobin
Hemoglobin sensing chemistry relies on Aza heterocyclic receptors (pyridine and imidazole) that convert the hemoglobin molecule into electrochemical active hemichrome.10, 14 The disposable test strips were laminated with a membrane on top of the active area of strips. A “Micro Droplet” of Aza heterocyclic sensing chemistry was then dispensed on top of this membrane. The strips impregnated with the sensing chemistry were allowed to dry in a controlled ambient temperature. After application of the sample blood drop, the sensing chemistry reacted with the hemoglobin molecule and converted it into electrochemical active hemichrome. The hemichrome formation prevented dimer formation, thus enabling an unhindered diffusion-controlled reaction at the electrode surface of the test strip. This resulted in iron redox current from the heme center, which was directly proportional to the concentration of hemoglobin present in the sample.
PoC Technology: HbA1c
Disposable carbon-printed electrode test strips with 2 working electrodes were used for HbA1c measurements. Both the working electrodes were laminated with a membrane impregnated with sensing chemistry. One of the working electrodes was laminated with a second membrane impregnated with boron acid. The HbA1c component was filtered with boronate affinity principle by using a phenyl boronic acid membrane. The PoC hand-held device could measure currents from both of the working electrodes: the current due to total hemoglobin and the current due to nonglycated hemoglobin after filtering the glycated component. The difference in redox currents from the 2 working electrodes was used to calculate HbA1c.
PoC Technology: Serum Albumin
Serum albumin is a molecular taxi in human body and can bind a wide variety of metal ions and organic molecules (e.g., copper, fatty acids, and so on). In the present innovation, we exploited the metal binding property of the molecule. Serum albumin was detected electrochemically by using the metal ion sensing chemistry by exploiting the concept of inherent affinity of the albumin molecule toward metal ions such as copper (II).11
PoC Technology: Urine Albumin, Urine Creatinine, and Urine ACR
Copper with oxidation state +2 is 1 of the most highly binding metal ions with albumin, and at same time, the copper (II) metal ion is an electrochemical active ion. The micro droplet of copper (II) with a controlled pH value was dispensed on a membrane laminated on electrochemical active disposable test strips. In contrast, creatinine forms chelates with iron molecules. The iron-sending chemistry in which iron oxidation states were +2 were used as a sensing chemistry for urine creatinine detection.
Our published references can be further referred for any of the fine details of the technology, method, and sensing chemistries for hemoglobin, HbA1c, serum albumin, urine albumin, and ACR.10, 11, 12, 13, 14
Statistical Analysis
All continuous variables were measured as mean ± SD. The PoC results for urine albumin, urine creatinine, hemoglobin, serum albumin, and HbA1c were compared with pathology laboratory gold standards. The linear correlation curves were plotted for all these biomarkers to establish the correlation between PoC results and laboratory gold standards. Bland-Altman difference plot analysis was performed for all these tested samples. A Bland-Altman plot (difference plot) was used in analyzing the agreement between 2 different assays. All statistical analyses were done with statistical software Prism GraphPad Ver. 7 (GraphPad Software, La Jolla, CA).
Results
Phase 1: Clinical Proof-of-Concept Phase
During the initial proof-of-concept phase, samples from the patients were collected from the laboratory and/or clinic and tested with the PoC device, and then were compared with laboratory gold standard. In this phase, between January 2015 and December 2016, the PoC device was used for 2576 samples for the various analytes. Table 1 shows the samples details.
Table 1.
Details of samples during phase 1
| Investigation | Number of samples analyzed by the PoC (n = 2576) |
|---|---|
| Hemoglobin | 546 |
| Serum albumin | 523 |
| HbA1c | 503 |
| Urine creatinine | 501 |
| Urine albumin | 503 |
HbA1c, glycosylated hemoglobin; PoC, point of care.
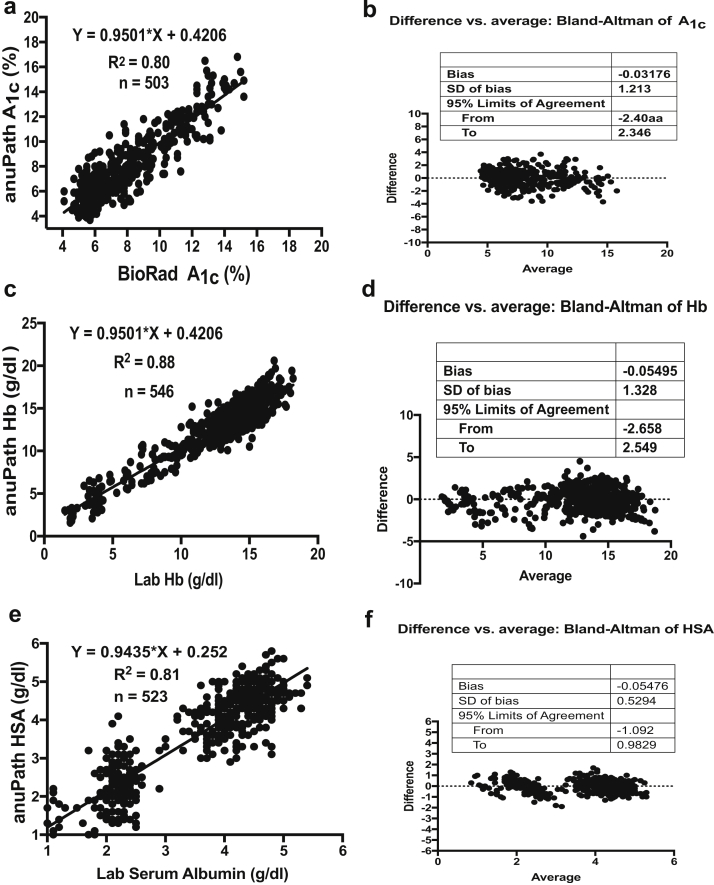
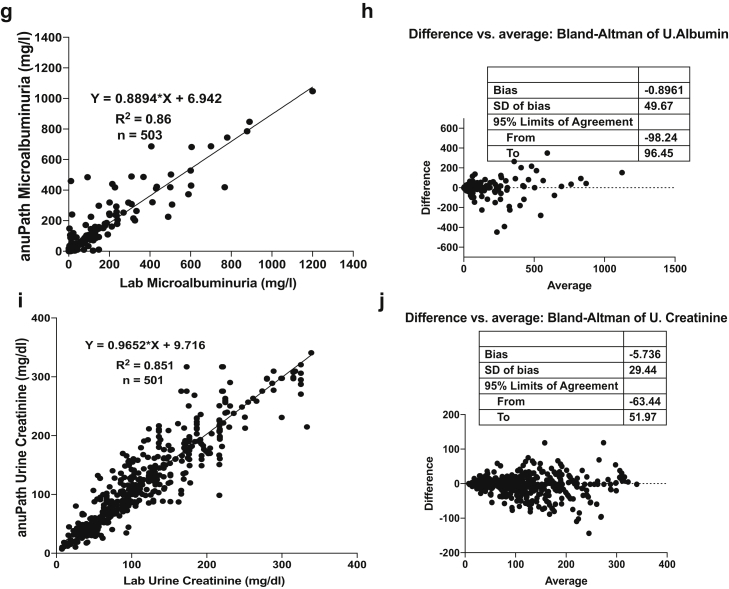
The statistical analysis showed excellent linear correlation in the proof-of-concept in phase 1. Table 2 shows the summary of analytics in phase 1. Figure 2 shows the linearity plots and Bland-Altman plots for HbA1c, hemoglobin, serum albumin, urine albumin, and urine creatinine during the proof-of-concept phase (phase 1).
Table 2.
Analytics summary during phase 1
| Analyte | Number tested | Correlation (R2) | Bland-Altman bias | 95% limits of agreement |
|---|---|---|---|---|
| Hemoglobin | 546 | 0.88 | 0.05495 | −2.658 to 2.549 |
| Serum albumin | 523 | 0.81 | −0.05476 | −1.092 to 0.9829 |
| HbA1c | 503 | 0.80 | −0.03176 | −2.409 to 2.346 |
| Urine creatinine | 501 | 0.85 | −5.736 | −63.44 to 51.97 |
| Urine albumin | 503 | 0.86 | −0.8961 | −98.24 to 96.45 |
HbA1c, glycosylated hemoglobin.
Figure 2.
Linearity plots and Bland-Altman plots for glycosylated hemoglobin (HbA1c), hemoglobin (Hb), serum albumin (HSA), urine albumin, and urine creatinine during clinical proof-of-concept phase (phase 1). (a) Linearity plot of HbA1c lab values versus anuPath POC device values. (b) Difference versus average plot (Bland-Altman plot) for HbA1c. (c) Linearity plot of Hb lab values versus anuPath POC device values. (d) Difference versus average plot (Bland-Altman plot) for Hb. (e) Linearity plot of serum albumin lab values versus anuPath POC device values. (f) Difference versus average plot (Bland-Altman plot) for serum albumin.
Phase 2: Clinical Validation Phase
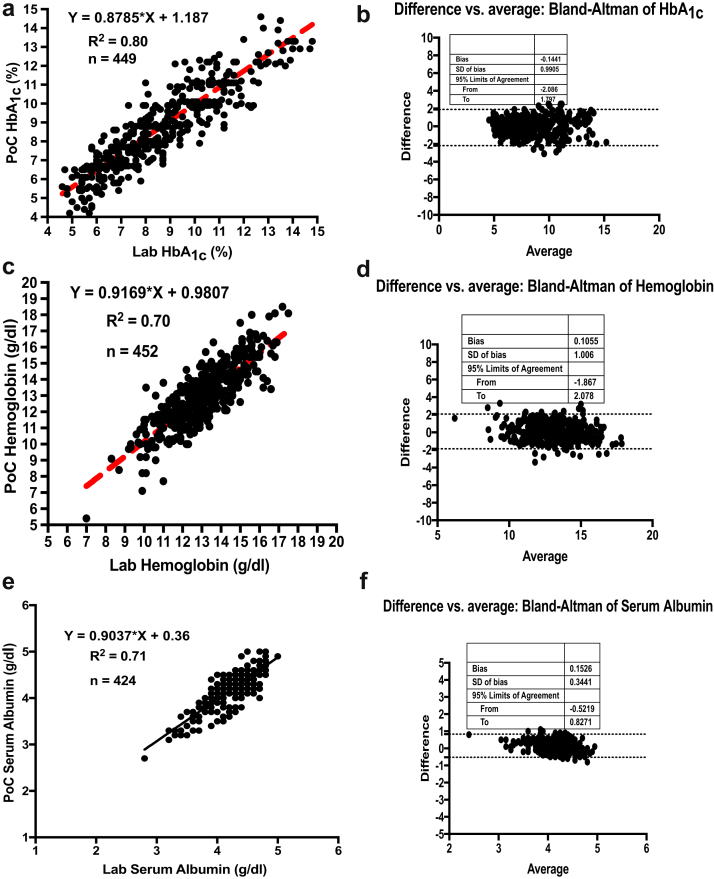
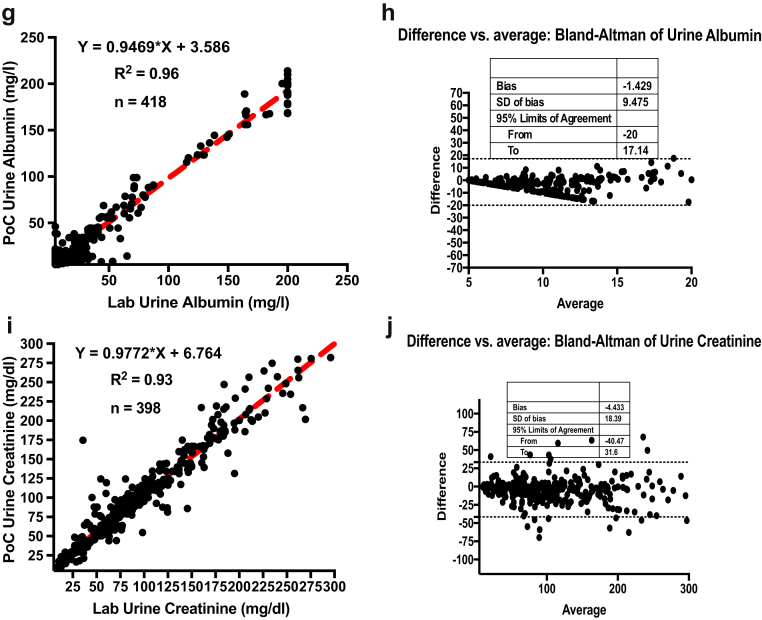
A total of 529 subjects from Samatvam-Jnana Sanjeevini Medical Centre and Diabetes Hospital were studied for the clinical validation phase (phase 2). There were 183 (35%) men and 346 (65%) women who consented to participate. Of these 529 subjects, 299 had hypertension (56%). This cohort consisted of 54 subjects without DM, 120 subjects with type 1 DM, and 355 subjects with type 2 DM. The mean ± SD age of the entire group was 48 ± 20 years. The mean ± SD age of the type1 DM subjects was 18 ± 11 years, and type 2 DM subjects were aged 57 ± 20 years. The estimated glomerular filtration rate (eGFR) (CKD grades: nil, 1, 2, and 3/4) of the subjects were as follows: 378 (71%) subjects had an eGFR >90 ml/min per 1.73 m2 (CKD grade: nil or 1); 121 (23%) subjects had an eGFR range of 60 to 89 ml/min per 1.73 m2 (CKD grade: nil or 2); and 30 (6%) subjects had an eGFR range of 15 to 59 ml/min per 1.73 m2 (CKD grade: 3/4). Table 3 shows the summary of analytics in phase 2. The laboratory parameters of the clinical validation phase cohort as tested by standard laboratory techniques are given in Table 4. Table 5 shows the details of samples. The PoC results of these analytes were compared with laboratory gold standards. The data were analyzed the same way as phase 1, and the statistical analyses again showed significant correlation and agreement between the PoC device and laboratory gold standard assays. Figure 3 shows the linearity plots and Bland-Altman plots for HbA1c, hemoglobin, serum albumin, urine albumin, and urine creatinine during the clinical validation phase (phase 2).
Table 3.
Analytics summary during phase 2
| Analyte | Number tested | Correlation (R2) | Bland-Altman bias | 95% limits of agreement |
|---|---|---|---|---|
| Hemoglobin | 452 | 0.70 | 0.1055 | −1.867 to 2.078 |
| Serum albumin | 424 | 0.71 | −0.1526 | −0.5219 to 0.8271 |
| HbA1c | 449 | 0.80 | −0.1441 | −2.086 to 1.797 |
| Urine creatinine | 398 | 0.93 | −4.433 | −40.47 to 31.6 |
| Urine albumin | 418 | 0.96 | −1.429 | −20 to 17.14 |
HbA1c, glycosylated hemoglobin.
Table 4.
Details of samples during phase 2: clinical validation phase
| Investigation | Number of samples analyzed by the PoC (n = 2141) |
|---|---|
| Hemoglobin | 452 |
| Serum albumin | 424 |
| HbA1c | 449 |
| Urine creatinine | 398 |
| Urine albumin | 418 |
HbA1c, glycosylated hemoglobin; PoC, point of care.
Table 5.
Clinical validation phase (phase 2): laboratory parameters (N = 529) cohort
| Investigation | Value (mean ± SD) |
|---|---|
| Hemoglobin (g/dl) | 12.3 ± 3.3 |
| HbA1c (%) | 8.01 ± 3.1 |
| Serum Albumin (g/l) | 3.7 ± 1.4 |
| Serum creatinine (mg/dl) | 0.7 ± 0.7 |
| eGFR (ml/min per 1.73 m2) | 90 ± 38 |
| Total cholesterol (mg/dl) | 157 ± 70 |
| LDL cholesterol (mg/dl) | 83 ± 46 |
| Triglycerides (mg/dl) | 183 (149) |
eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein.
Figure 3.
Linearity plots and Bland-Altman plots for glycosylated hemoglobin (HbA1c), hemoglobin (Hb), serum albumin, urine albumin, and urine creatinine during the clinical validation phase (phase 2). (a) Linearity plot of HbA1c lab values versus anuPath point of care (POC) device values. (b) Difference versus average plot (Bland-Altman plot) for HbA1c. (c) Linearity plot of Hb lab values versus anuPath POC device values. (d) Difference versus average plot (Bland-Altman plot) for Hb. (e) Linearity plot of serum albumin lab values versus anuPath POC device values. (f) Difference versus average plot (Bland-Altman plot) for serum albumin.
Discussion
Clark and Lyons, through their seminal publication on glucose oxidase electrodes, laid the foundation for the modern PoC biosensor. The PoC glucometer is an excellent example of how a simple and elegant idea can impact millions of lives. The PoC biosensor market is a multibillion dollar market, but the harsh reality is that the glucose biosensor is the only major contributor to this growth, and we are still waiting for more biosensors for other tests that are as robust and cost effective as the glucometer. There are 2 major components of a chemical biosensor; one is the receptor or sensing chemistry, and the second is the transducer. The commercialization and accuracy mainly depend on these 2 factors. The sensing chemistry should be specific to the biomolecule of interest in the pool of biofluid. The choice of sensing chemistry is more critical because it can directly influence the cost and robustness of the PoC device.
Immunoassays have become the first choice as the receptor molecule in PoC biosensor publications. There is no doubt that antibody-based immunoassays are highly sensitive and can be used together with biochemistry analyzers in a pathology laboratory. However, by definition, the requirements of a PoC biosensor are different from those of a biochemistry analyzer. A biosensor is a self-contained device that can be used for PoC settings without any special storage and handling requirements. The stability of antibodies and enzymes, after functionalization on disposable strips and cartridges, are still a serious problem in PoC biosensors. Moreover, the accuracy is also affected by temperature, humidity, and pH variations. Before immunoassays, the clinical pathology mainly relied on the analytical techniques for various pathology tests without sophisticated instrumentation. The accuracy and reliability of those analytical techniques are still relevant in pathology laboratories. With advancements in engineering and materials, it has become possible to miniaturize the big biochemistry analyzer in a pathology laboratory into a table-top device or so-called PoC device, but in most of the cases, the sensing chemistry element has remained the same; it is either an antibody or an enzyme. In this scenario, where it appears that biosensor research is facing a bottleneck, it is worth analyzing the possibilities of novel sensing chemistry for PoC biosensors that are more robust, more cost effective, and accurate.
We demonstrated a PoC device that was not only an integration of different tests, but the heart of a technology that is “physiology inspired sensing chemistry,” which is novel and unique. This novel concept of sensing chemistries relies on the natural reactions of biomolecules in the human body. For example, for the detection of hemoglobin, we converted the hemoglobin into hemichrome. Reversible and nonreversible hemichrome formation is a natural reaction in the human body. The device uses a rechargeable lithium ion battery and can be continuously used for 5 hours in the field after the battery is fully charged. The device is Bluetooth-enabled and can store 60,000 tests reports. Reports can easily be transferred from a remote location via mobile phone. All these features make this device best suited for remote locations and resource-challenged settings. The operation of the device is similar to that of the glucometer, and in developing country like India, where >1 billion users carry mobile phones, any person with basic knowledge of a mobile phone can easily operate this device in the field. The only consumables with this device are the disposable test strips; there is no need for any sample preparation and mixing. The device has another unique feature that has allowed the technical team to remote access the device in the field from the research and development center to diagnose any problem. After manufacturing of the test strips, we tested each lot. We supplied the control solutions for calibration of the device, but it was only required in cases of ambiguity, and there was no need of daily calibration.
One of the stated goals of the International Society of Nephrology is to develop a cohesive and comprehensive plan for the management of CKD. One of the priorities of the stakeholders is to have continuous CKD surveillance, and to attain this objective, it is important to develop better diagnostic modalities for early detection and prevention of CKD. It is also acknowledged that DKD is the most important contributor to the global CKD burden. One of the ways to address this issue of early detection and prevention of DKD is to use novel diagnostic technology (PoC device) to reach most of the population in remote areas of the developing world. The volume of laboratory testing with PoC devices is steadily increasing all over the world.15, 16 There is progressive improvement in hand-held PoC technology, and the present PoC device tries to incorporate most of these advances.
One of the analytes chosen in our device for detection and management of DM was HbA1c. HbA1c is considered to be a better a marker for detection of DM as per medical guidelines.17, 18 A few PoC devices for HbA1c have been developed recently, but few of them have satisfied international standards, and most of them require reagents and sample preparation in the field.19 Similarly, various PoC devices have been developed for hemoglobin, but most of them use photochemical sensing, and some of them are often semiquantitative. Few have stood the test of time. Their use has been mostly restricted to blood donors.20 Our reasoning for testing hemoglobin along with other analytes is that it picks up one of the most common problems of anemia and malnutrition in our part of the world, besides the fact that patients with DM often present with anemia in early stages of their kidney involvement.21 We could not find any such device that tests for serum albumin in the literature. Patients with DKD who have nephrotic range proteinuria often have low-serum albumin. A low-serum albumin level interferes with the urinary excretion of albumin, and occasionally, the urinary albumin excretion rate is fallaciously low. Therefore, we endeavoured to test both the urine ACR and serum albumin. The device also compared favorably with semiquantitative testing of microalbuminuria.
Conclusions
PoC testing offers the inherent advantages of rapid availability of data, decreased turnaround time, immediate decision making, and easy implementation in resource-challenged settings. However, development of PoC devices is facing a bottleneck because of the limited knowledge of robust sensing chemistries. There are few working options in PoC testing for clinically relevant investigations other than blood glucose. A PoC glucometer is still the main driving force of PoC testing. We demonstrated the potential of a novel, patented PoC technology for early DKD evaluation. This study represents a major step forward in realizing a robust and scalable PoC multianalyte biosensor that uses novel dry sensing chemistry without any sample preparation steps. The assay was validated against pathology laboratory techniques for the quantitative measurements of HbA1c, hemoglobin, serum albumin, microalbuminuria, urine creatinine, and eventually, ACR, using clinical samples. An excellent linearity was demonstrated. The present technology could potentially improve patient care through real-time and remote health monitoring. This PoC device is currently being introduced for Community Diabetes Care for the rural and urban poor using the “Peers for Health” (Project: Madhura Sanjeevini) Community Health Workers.22
In conclusion, early, accurate, and effective detection of DKD in remote areas of the developing world is needed. This simple, easy to use PoC device, which simultaneously tests 5 analytes when used in remote underserved areas of the world, will go a long way in alleviating the burden of DKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We acknowledge the funding support of the International Society of Nephrology American Nephrologists of Indian Origin for this study. We also thank MHRD and the Ministry of Health, Government of India for funding support through IMPRINT project. We also thank BIRAC for supporting PathShodh through the BIPP program. We acknowledge the contribution of technical staff of PathShodh Healthcare Pvt. Ltd. and Clinical and Research Staff of Samatvam Jnana Sanjeevini Diabetes Hospital and Medical Centre, Bangalore, India during this study. The following is the list of Clinical Investigators who participated in the study as Members of Diabetes Collaborative Study Group of PathShodh Healthcare Pvt Ltd Bangalore, India, Samatvam Endocrinology Diabetes Center and Jnana Sanjeevini Medical Center and Diabetes Hospital, Bangalore, India: F. Syed, G. Naik, A. Kumar, A. Prasad, N. Kumar, G. Sharma, V. Nath, B.V. Reshma, T.D. Babitha, M. Kavitha, S. Chandraprabha, C.S. Muralidharakrishna, K. Rajiv, A. Govind, M.D. Chitra, S. Jayalakshmi, L. Reddy, T. Kamala, P. Ravikumar, K.M. Chandrika, K. Sumathi, and Vasudha Srikanth, along with Diabetes Educators/Physician Assistants–Research Coordinators: S.G. Rao, A. Hegde, R. Ashok, U. Dayashankar, P. Srinivasa, S. Chandrashekhar, H.K. Vasanthalakshmi, U. Rangaraj, N. Jayaram, V. Shivaraj, M. Marimuthu, M. Priya, and M. Suma.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modi G.K., Jha V. The incidence of end-stage renal disease in India: a population based study. Kidney Int. 2006;70:2131–2133. doi: 10.1038/sj.ki.5001958. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation Global facts: about kidney disease. https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease Available at: Accessed June 20, 2018.
- 4.Deferrari G., Repetto M., Calvi C. Diabetic nephropathy: from micro- to macroalbuminuria. Nephrol Dial Transplant. 1998;13 Suppl 8:11–15. doi: 10.1093/ndt/13.suppl_8.11. [DOI] [PubMed] [Google Scholar]
- 5.Kaveeshwar S.A., Cornwall J. The current state of diabetes mellitus in India. Australasian Med J. 2014;7:45–48. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S.K., Dash S.C., Irshad M. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–1642. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 7.Unnikrishnan R., Anjana R.M., Vishwanathan M. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12:357–370. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]
- 8.Jha V., Garcia-Garcia, Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 9.DeVries E.F., Rabelink T.J., vanden Hout W.B. Modelling the cost-effectiveness of delaying end stage renal disease. Nephron. 2016;133:89–97. doi: 10.1159/000446548. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Bhat N, inventors. Device and method for detection of haemoglobin and its complexes. PCT International Application PCT/IB2015/056832.
- 11.Kumar V, Bhat N, inventors. Electrochemical biosensor and a method of sensing albumin and its complexes. PCT International Application PCT/IB2015/056619.
- 12.Kumar V, Kashyap N, Bhat N, inventors. Device and method for detecting creatinine and albumin to creatinine ratio. PCT International Application PCT/IB2016/050311.
- 13.Kumar V., Hebbar S., Kalam R. Creatinine-iron complex and its use in electrochemical measurement of urine creatinine. IEEE Sensors J. 2017;18:1. [Google Scholar]
- 14.Kumar V., Kashyap N.D.M., Hebbar S. Aza-heterocyclic receptors for direct electron transfer haemoglobin biosensor. Nature Sci Rep. 2017;7:42031. doi: 10.1038/srep42031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jani I.V., Peter T.F. How point-of-care testing could drive innovation in global health. N Engl J Med. 2013;368:2319–2324. doi: 10.1056/NEJMsb1214197. [DOI] [PubMed] [Google Scholar]
- 16.Olansky L., Kennedy L. Fingerstick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33:948–949. doi: 10.2337/dc10-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Expert Committee International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks D.B. The diagnosis of diabetes is changing: how implementation of haemoglobin A1c will impact clinical laboratories. Clin Chem. 2009;55:1612–1614. doi: 10.1373/clinchem.2009.132704. [DOI] [PubMed] [Google Scholar]
- 19.Lenters-Westra E., Slingerland R.J. Six out of eight haemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 20.Singh A., Dubey A., Sonker A., Chaudhary R. Evaluation of various methods of point-of-care testing of haemoglobin concentration in blood donors. Blood Transfus. 2015;13:233–239. doi: 10.2450/2014.0085-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Achkar T.M., Ohmit S.E., McCullough P.A. Higher prevalence of anaemia with diabetes mellitus in moderate kidney insufficiency: the Kidney Early Evaluation Program. Kidney Int. 2005;67:1483–1488. doi: 10.1111/j.1523-1755.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 22.Manjunath M, Srikanta S, Ashwini KJ, et al., and the Diabetes Collaborative Study Group. Peers for Health: Community health worker led diabetes hypertension screening awareness program for rural and urban poor. Oral Presentation: OP 0085, World Diabetes Congress, International Diabetes Federation, Abu Dhabi, United Arab Emirates, December 5, 2017.