Abstract
Introduction
Stroke rate and mortality are greater in individuals with end-stage renal disease (ESRD) than in those without ESRD. We examined discrepancies in stroke care in ESRD patients and their influence on mortality.
Methods
This is a national record linkage cohort study of hospitalized stroke individuals from 2005 to 2013. Presentation, measures of care quality (admission to stroke unit, swallow assessment, antithrombotics, or thrombolysis use), and outcomes were compared in those with and without ESRD after propensity score matching (PSM). We examined the effect of being admitted to a stroke unit on survival using Kaplan-Meier and Cox survival analyses.
Results
A total of 8757 individuals with ESRD and 61,367 individuals with stroke were identified. ESRD patients (n =486) experienced stroke over 34,551.9 patient-years of follow-up; incidence rates were 25.3 (dialysis) and 4.5 (kidney transplant)/1000 patient-years. After PSM, dialysis patients were less likely to be functionally independent (61.4% vs. 77.7%; P < 0.0001) before stroke, less frequently admitted to stroke units (64.6% vs. 79.6%; P < 0.001), or to receive aspirin (75.3% vs. 83.2%; P = 0.01) than non-ESRD stroke patients. There were no significant differences in management of kidney transplantation patients. Stroke with ESRD was associated with a higher death rate during admission (dialysis 22.9% vs.14.4%, P = 0.002; transplantation: 19.6% vs. 9.3%; P = 0.034). Managing ESRD patients in a stroke unit was associated with a lower risk of death at follow-up (hazard ratio: 0.68; 95% confidence interval: 0.55−0.84).
Conclusion
Stroke incidence is high in ESRD. Individuals on dialysis are functionally more dependent before stroke and less frequently receive optimal stroke care. After a stroke, death is more likely in ESRD patients. Acute stroke unit care may be associated with lower mortality.
Keywords: ESRD, dialysis, stroke, transplantation outcomes
Declining kidney function is associated with increased cardiovascular risk,1 and the presence of chronic kidney disease (CKD) is incorporated into risk prediction algorithms used to guide preventative treatments for cardiovascular disease.2, 3 Risk is greatest in end-stage renal disease (ESRD), which is approximately 5 to 20 times that of the age-matched controlled general population,4, 5 and treatments such as dialysis or immunosuppression for kidney transplantation may increase risk further.6, 7 Risk of stroke is reported to be 10 time more common in ESRD,8, 9 yet case mix presentation, management, and outcomes of stroke in individuals with ESRD remains poorly understood.
In ESRD, provision of evidence-based management of cardiovascular disease and stroke prevention is hindered10, 11, 12 by limited evidence of benefit with interventions such as statins13 or anticoagulation.14 Advanced CKD is a common exclusion criterion in trials that target cardiovascular risk.15 Individuals with ESRD are typically admitted to renal units when hospitalized for intercurrent illness.10, 16 This could affect access to stroke unit care and treatments shown to improve stroke outcomes.17
We assessed the incidence of stroke in ESRD and if the presence of ESRD influenced presentation, management, and outcome of stroke by merging data from the Scottish Renal Registry and Scottish Stroke Care Audit. We hypothesized that key care quality indicators in stroke would be less commonly achieved in those with ESRD compared with those without ESRD.
Methods
We performed a national data linkage study using data within the Scottish Renal Registry (SRR), Scottish Stroke Care Audit (SSCA), Scottish Morbidity Records (SMR), and the National Records of Scotland. We used propensity score matching (PSM) and assessed case mix presentation, measures of care quality, and outcomes after stroke in individuals with and without ESRD.
Data Sets
SRR is a nation-wide data set, contributed to by all renal units in Scotland.18, 19 All patients who receive renal replacement therapy (RRT) for ESRD are included. The SRR records patient demographics, renal replacement modality, clinical measurements, laboratory results, and cause of death.
SSCA monitors performance of stroke care against guideline-derived standards throughout Scotland. Data held by the SSCA includes all cases of stroke and transient ischemic attack admitted to hospital. Ascertainment of stroke cases in the SSCA is carried out by trained audit coordinators supported by stroke clinicians. Data are collected on demographics, case mix at presentation, stroke classification, use of brain imaging, antithrombotic medication, stroke unit care, and discharge outcomes. This data set has provided coverage of all hospitals managing acute stroke since 2005.
SMR01 has been collecting data on all surgical and medical hospital admissions since 1968. Since 1989, SMR01 has been used to plan hospital finances to ensure a high completion rate.
The National Records of Scotland is a department of Scottish Government, responsible for registration of life events, including deaths.
All 4 datasets were linked, from January 1, 2005 to December 31, 2013. We included only adults (16 years or older at stroke) in our analyses. Supplementary Figures S1 and S2 demonstrate the construction of the linked data sets for analysis.
Definitions
Stroke
Only cases of confirmed stroke as a final diagnosis were included. The SMR01 was interrogated for International Classification of Diseases (ICD-10) codes for stroke (I60, I61, I62.9, I63, and I64). All stroke diagnoses were ordered by date of stroke from January 1, 2005 and the first recorded stroke date, with the source of diagnosis recorded. In cases in which SMR01 and SSCA diagnoses overlapped, the SSCA was used as the source.
End-Stage Renal Disease
All patients commencing RRT for ESRD in Scotland are recorded within the SRR. ESRD is a diagnosis determined by the clinician initiating RRT or by continued use of RRT for >90 days for acute kidney injury without renal recovery.
Modality of RRT
We considered 3 groups of ESRD patients; (i) all patients with ESRD regardless of RRT modality, (ii) patients receiving dialysis (either hemodialysis [HD] or peritoneal dialysis [PD] at the start of study, or who began HD or PD as their first RRT modality during the study), and (iii) individuals with a kidney transplant (either at commencement of the study or who were transplanted during the study de novo or after a period of dialysis).
Baseline Characteristics
The SMR01 was interrogated from January 1, 1981 to December 31, 2013 for presence of ICD-10 codes related to atrial fibrillation and/or flutter (I48), ischemic heart disease (I21, 24, 24.8, I24.9, I25, I25.1, I25.2, I25.5, and I25.8), diabetes mellitus (E10 and E11), and hypertension (I10 and I15). To prevent overlap of diagnoses, ICD codes for stroke (as previously described) were pulled from 1981 until date of the first stroke in individuals who experienced stroke or until December 31, 2013 in individuals who did not have a stroke.
Using Scottish Government urban-rural and socioeconomic classifications based on patient postcode, we subdivided patients into the most or least deprived and urban or rural as described elsewhere.20 We used the postcode at commencing RRT in patients on RRT or with stroke for the non-ESRD stroke population.
SSCA Auditable Standards
SSCA annually reports standards of stroke care compared with audited targets, specifically, the percentage with access to a stroke unit within 1 day of admission, brain imaging within 24 hours of admission, swallow screen on day of admission, aspirin within 1 day of admission (if the index stroke was ischemic), and thrombolysis within 1 hour of arrival to hospital. We present 2 forms of data regarding these standards. First, the percentage of cases that received the following at any point during their admission included stroke unit care, brain imaging, swallow screen, aspirin, or thrombolysis. Second, we reanalyzed, using date and time of admission to hospital and date and time of each care standard, to determine time-specific outcomes as per SSCA standards.
Mortality
Using the national death records, we determined vitality status until September 25, 2015 for all persons. Death during stroke admission and vitality status at end of follow-up are presented. When exploring the impact of stroke care on mortality, we omitted cases of stroke diagnosed only at death and analyzed both ESRD and non-ESRD populations based on the source of the initial diagnosis: SSCA or SMR01. Causes of death for all patients are presented as per ICD-10 coding rules in Supplementary Tables S1 to S3.
Statistical Analyses
Data cleaning and analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC). Graphs were produced using Stata version 14.1 (Statacorp, College Station, TX).
Incidence rates are expressed per 1000 patient-years and calculated using the number of stroke events divided by the follow-up time. Follow-up time is calculated using date of study entry (January 1, 2005) or start of RRT for ESRD until the date of stroke, death, change of renal replacement modality, or end of study.
We compared patient demographics split by the source of stroke diagnosis: SSCA versus SMR01 and stroke versus no stroke. Demographic values are presented as mean ± SD or median (interquartile range [IQR]), and comparison between groups was assessed using the t-test, Mann-Whitney U test, or the χ2 test as appropriate. To account for differences in cohort size, we compared pre- and postmatching demographics using standardized differences.
Propensity Score Matching
Individuals with ESRD and stroke are more likely to have comorbidities than individuals without. To reduce the potential for confounding, we performed propensity score matching (PSM) for all ESRD patients, then individually for each of those on dialysis and with a renal transplant. Matching was based on factors predictive of stroke or outcome. Specifically, we matched on age, sex, deprivation and rurality status, and medical history of stroke, ischemic heart disease, hypertension, diabetes, and atrial fibrillation. We matched using nearest neighbour matching, at a ratio of 5:1. Data were tabulated for all ESRD, dialysis, and kidney transplantation cohorts. Data were presented before and after PSM in each case, with comparison between groups using the Mann-Whitney U or the χ2 tests as appropriate. Because our primary aim was to directly compare cohorts, missing data were not imputed, but rather were presented within each table. PSM was performed using the computing environment R, MatchIt package (R Foundation, Vienna, Austria).21
Survival analyses were performed using the Kaplan-Meier estimator and Cox proportional hazard model. We assessed differences between survival probabilities after stroke in dialysis, kidney transplantation, and non-ESRD stroke populations. Finally, to explore effects of care on outcome, we used admission to a stroke unit as a surrogate for optimal stroke care in ESRD (adjusted for age, sex, use of dialysis, previous diabetes, ischemic heart disease, hypertension, and atrial fibrillation) to examine its impact on death at follow-up. The strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed in this observational study (Supplementary Figure 4).
Ethical Approval
The data sets used work within the National Health Service Code of Practice on Protecting Patient Confidentiality, incorporating requirements of statutes and common law including the Data Protection, Human Rights, and the Adults with Incapacity (Scotland) Acts. Access and use of data were approved following National Services Scotland proportionate governance review by the Privacy Advisory Committee of Information Services Division, National Health Service Scotland Reference 55/14.
Results
ESRD Stroke Incidence Rate and Stroke Record Source
There were 8757 individuals aged 16 or older in the SRR from January 1, 2005 to December 31, 2013. A total of 6749 were receiving dialysis at study entry, and 3699 had a kidney transplant at entry or were transplanted during follow-up. A total of 486 (5.5%) individuals experienced stroke during 34,551.9 patient-years of follow-up. The stroke incidence rate per 1000 patient-years for all individuals with ESRD was 14.1; for individuals on dialysis, it was 25.3 (26.7 for HD and 17.3 for PD), and for individuals with a kidney transplant, it was 4.5.
Of the first recorded stroke during the study period, 230 (47.3%) were recorded in the SSCA, 219 (45.1%) in the SMR01, and 37 (7.6%) as a primary cause of death (having not been diagnosed prior to death). No significant differences existed in baseline demographics between individuals with ESRD and stroke in the SMR01 database compared with the those in the SSCA database (Table 1).
Table 1.
Baseline demographics of all end-stage renal disease patients with stroke, split by source of diagnosis
| Variable | SSCA stroke (n = 230) | SMR01 stroke (n = 219) | P value |
|---|---|---|---|
| Age at stroke, yr, median (IQR) | 71 (13) | 71 (17) | 0.406 |
| Sex | |||
| Female (%) | 111 (48.3) | 93 (42.5) | |
| Male (%) | 119 (51.7) | 126 (57.5) | 0.220 |
| Primary renal diagnosis group | |||
| Glomerulonephritis | 36 (15.7) | 31 (14.2) | 0.692 |
| Interstitial disease | 48 (20.9) | 46 (21.0) | 1.000 |
| Multisystem | 53 (23.0) | 46 (21.0) | 0.649 |
| Diabetic nephropathy | 57 (24.8) | 60 (27.4) | 0.591 |
| Other | 36 (15.7) | 36 (16.4) | 0.898 |
| Urban-rural | |||
| Rural | 34 (14.8) | 35 (16.0) | |
| Urban | 196 (85.2) | 184 (84.0) | 0.794 |
| SIMD | |||
| Least deprived | 171 (74.4) | 154 (70.3) | |
| Most deprived | 59 (25.7) | 65 (29.7) | 0.344 |
| Era of stroke | |||
| 2005−2006 | 54 (23.5) | 88 (40.2) | |
| 2007−2008 | 50 (21.7) | 44 (20.1) | |
| 2009−2010 | 43 (18.7) | 48 (21.9) | |
| 2011−2013 | 83 (36.1) | 39 (17.8) | <0.001 |
| Medical history | |||
| Atrial fibrillation | 21 (9.1) | 30 (13.7) | 0.139 |
| Ischemic heart disease | 81 (35.2) | 86 (39.3) | 0.381 |
| Previous stroke | 20 (8.7) | 24 (11.0) | 0.432 |
| Diabetes | 90 (39.1) | 90 (41.1) | 0.701 |
| Hypercholesterolemia | 41 (17.8) | 34 (15.5) | 0.530 |
| Obesity | 15 (6.5) | 14 (6.4) | 1.000 |
| HBP | 171 (74.5) | 164 (74.9) | 0.914 |
| Clinical variables, median (IQR) | |||
| Systolic BP, mm Hg | 146.0 (30.0) | 141.0 (28.8) | 0.475 |
| Diastolic BP, mm Hg | 69.8 (22.0) | 68.5 (17.5) | 0.894 |
| Weight, kg | 68.4 (28.0) | 71.8 (23.1) | 0.213 |
| Laboratory variables, median (IQR) | |||
| Hemoglobin, g/l | 11.5 (1.8) | 11.4 (1.7) | 0.825 |
| Albumin, mmol/l | 36.0 (7.0) | 37.8 (6.0) | 0.171 |
| Serum phosphate, mmol/l | 1.45 (0.61) | 1.48 (0.62) | 0.449 |
| Adjusted calcium, mmol/l | 2.33 (0.2) | 2.35 (0.2) | 0.220 |
| ESA use | |||
| Death at follow-up | 191 (83.0) | 205 (93.6) | <0.001 |
ESA, erythropoietin-stimulating agent; BP, blood pressure; HBP, high blood pressure; IQR, interquartile range; SIMD, Scottish Index of Multiple Deprivation; SMR01, Scottish Morbidity Records 01; SSCA, Scottish Stroke Care Audit.
Data are presented as no. (%).
Demographics in ESRD: Stroke Versus No Stroke
In all individuals with ESRD, there were differences between those who experienced stroke and those who did not (Table 2). Individuals with stroke were older at commencing RRT, more likely to have atrial fibrillation, a history of stroke, and diabetes. In the dialysis group, previous ischemic heart disease was not associated with stroke, whereas higher serum phosphate was associated with stroke. In the transplantation group, the inverse was true; previous ischemic heart disease and lower serum phosphate were associated with stroke. Further details are outlined in Supplementary Tables S4 and S5.
Table 2.
Baseline demographics of all end-stage renal disease patients, no stroke versus stroke
| Variable | No stroke (n = 8271) | Stroke (n = 486) | P value |
|---|---|---|---|
| Age at first RRT, yr, median (IQR) | 56.4 (30.6) | 66.3 (19.3) | <0.0001 |
| Sex (%) | |||
| Female | 3494 (41.7) | 221 (45.5) | |
| Male | 4893 (58.3) | 265 (54.5) | 0.098 |
| Primary renal diagnosis group | |||
| Glomuleronephritis | 1532 (18.5) | 70 (14.4) | 0.022 |
| Interstitial disease | 2205 (26.7) | 102 (21.0) | 0.006 |
| Multisystem | 1680 (20.3) | 114 (23.5) | 0.105 |
| Diabetic nephropathy | 1463 (17.7) | 122 (25.1) | <0.0001 |
| Other | 1379 (16.7) | 78 (16.1) | 0.754 |
| Missing | 12 (0.1) | 0 | |
| Urban-rural | |||
| Rural | 1380 (16.7) | 75 (15.4) | |
| Urban | 6891 (83.3) | 411 (84.6) | 0.491 |
| SIMD | |||
| Least deprived | 6129 (74.1) | 356 (73.3) | |
| Most deprived | 2142 (25.9) | 130 (26.8) | 0.670 |
| Medical history | |||
| Atrial fibrillation | 502 (6.1) | 52 (10.7) | <0.001 |
| Ischemic heart disease | 2519 (30.5) | 176 (36.2) | 0.012 |
| Stroke | 272 (3.3) | 48 (9.9) | <0.001 |
| Diabetes | 2468 (29.8) | 193 (39.7) | <0.001 |
| Hypercholesterolemia | 1053 (12.7) | 80 (16.5) | 0.022 |
| Obesity | 464 (5.6) | 31 (6.4) | 0.480 |
| HBP | 5894 (71.3) | 361 (74.3) | 0.233 |
| Clinical variables, median (IQR) | |||
| Systolic BP, mm Hg | 138 (29.0) | 142 (30.0) | <0.001 |
| Diastolic BP, mm Hg | 72 (18.0) | 69 (20.0) | 0.077 |
| Weight, kg | 72.7 (23.0) | 70.3 (25.6) | 0.008 |
| Laboratory variables | |||
| Hemoglobin, g/l | 11.7 (1.9) | 11.45 (1.5) | <0.001 |
| Albumin, mmol/l | 38.0 (7.0) | 37.0 (7.0) | <0.001 |
| Serum phosphate, mmol/l | 1.35 (0.6) | 1.46 (0.6) | <0.001 |
| Adjusted calcium, mmol/l | 2.37 (0.2) | 2.35 (0.2) | 0.004 |
| ESA use | 4873 (58.9) | 268 (55.1) | 0.107 |
| Death at follow-up | 4188 (50.6) | 433 (89.1) | <0.001 |
ESA, erythropoietin-stimulating agent; BP, blood pressure; HBP, high blood pressure; IQR, interquartile range; RRT, renal replacement therapy; SIMD, Scottish Index of Multiple Deprivation.
Data are presented as no. (%).
Demographics: SSCA Stroke in the ESRD Population Versus SSCA Stroke in the Non-ESRD Population
We identified 61,367 individuals registered in the SSCA with stroke during our study period. A total of 280 (0.5%) individuals in the SSCA data set were on RRT for ESRD. Compared with the background population, individuals with ESRD developed stroke at a younger age, and had a greater prevalence of previous ischemic heart disease, hypertension, and diabetes (Table 3). Similar results were found in individuals on dialysis (Table 4). In the renal transplantation population, there were differences in age, sex, hypertension, and diabetes status (Table 5). PSM removed all demographic differences (Table 3, Table 4, Table 5).
Table 3.
Demographics, presentation, management, and outcomes after stroke from the Scottish Stroke Care Audit showing data before and after propensity score matching for all patients with end-stage renal disease
| Variable | Non-ESRD population (n = 61,087) | ESRD population (n = 280) | Standardized differencea/P value | Propensity-matched non-ESRD population (n = 1,395) | Propensity-matched renal population (n = 279) | Standardized differencea/P value |
|---|---|---|---|---|---|---|
| Patient demographics | ||||||
| Age at stroke, yr, median (IQR) | 76 (17.0) | 71 (16.0) | −0.48 | 70 (17.0) | 70 (17.0) | −0.01 |
| Female | 31,368 (51.4) | 140 (50.0) | −0.03 | 713 (51.1) | 139 (49.8) | −0.03 |
| Rural | 10,015 (16.4) | 41 (14.6) | −0.05 | 187 (13.4) | 41 (14.7) | 0.04 |
| Deprived | 29,709 (48.6) | 138 (49.3) | 0.01 | 713 (51.1) | 138 (49.5) | −0.03 |
| Previous AF | 5019 (8.2) | 26 (9.3) | 0.03 | 102 (7.3) | 26 (9.3) | 0.07 |
| Previous CeVD | 3555 (5.8) | 27 (9.6) | 0.14 | 111 (8.0) | 26 (9.3) | 0.05 |
| Previous IHD | 12,025 (19.7) | 97 (34.6) | 0.33 | 486 (34.8) | 96 (34.4) | −0.01 |
| Previous hypertension | 25,818 (42.3) | 208 (74.3) | 0.66 | 1073 (76.9) | 207 (74.2) | −0.06 |
| Previous diabetes | 8536 (14.0) | 113 (40.4) | 0.61 | 531 (38.1) | 112 (40.1) | 0.04 |
| Case mix | ||||||
| Independent in ADLs before admission | 47,184 (77.2) | 179 (63.9) | <0.0001 | 1096 (78.6) | 178 (63.8) | <0.0001 |
| Missing | 5109 (8.4) | 37 (13.2) | 134 (9.6) | 37 (13.3) | ||
| Living alone before admission | 22,065 (36.1) | 64 (22.9) | <0.0001 | 430 (30.8) | 64 (22.9) | 0.025 |
| Missing | 4453 (7.3) | 36 (12.9) | 124 (8.9) | 36 (12.9) | ||
| Talking at first assessment | 42,795 (70.1) | 196 (70.0) | 0.386 | 1020 (73.1) | 195 (69.9) | 0.678 |
| Missing | 3707 (6.1) | 26 (9.3) | 93 (6.7) | 26 (9.3) | ||
| Orientated at first assessment | 35,846 (58.7) | 160 (57.1) | 0.789 | 895 (64.2) | 159 (57.0) | 0.094 |
| Missing | 5325 (8.7) | 35 (12.5) | 128 (9.2) | 35 (12.5) | ||
| Able to lift arms at first assessment | 35,622 (58.3) | 178 (63.6) | 0.013 | 833 (59.7) | 178 (63.8) | 0.092 |
| Missing | 4817 (7.9) | 29 (10.4) | 123 (8.8) | 29 (10.4) | ||
| Able to walk on first assessment | 23,732 (38.9) | 95 (33.9) | 0.197 | 561 (40.2) | 95 (34.1) | 0.091 |
| Missing | 5529 (9.1) | 34 (12.1) | 141 (10.1) | 34 (12.2) | ||
| Index stroke | ||||||
| Ischemic | 51,606 (84.5) | 255 (91.1) | 1186 (85.0) | 245 (91.0) | ||
| Hemorrhagic | 6954 (11.4) | 23 (8.2) | 0.063 | 140 (10.0) | 23 (8.2) | 0.277 |
| Missing | 2527 (4.1) | 2 (0.7) | 69 (5.0) | 2 (0.7) | ||
| Admission source | ||||||
| Acute hospital | 1984 (3.3) | 22 (7.9) | <0.001 | 31 (2.2) | 21 (7.5) | <0.0001 |
| Sheltered/care home | 2758 (4.5) | 2 (0.7) | <0.001 | 41 (2.9) | 2 (0.7) | 0.035 |
| Home | 54,613 (89.4) | 240 (85.7) | 0.250 | 1277 (91.5) | 240 (86.0) | 0.050 |
| Rehab/other | 904 (1.5) | 7 (2.5) | 0.133 | 31 (2.2) | 7 (2.5) | 0.661 |
| Missing | 828 (1.4) | 9 (3.2) | 15 (1.1) | 9 (3.2) | ||
| Management | ||||||
| Admitted to stroke unit | 47,619 (78.0) | 189 (67.5) | 0.002 | 1110 (79.6) | 188 (67.4) | <0.001 |
| Missing | 1415 (2.3) | 16 (5.7) | 24 (1.7) | 16 (5.7) | ||
| Thrombolysis: IS only | 2487 (4.8) | 10 (3.9) | 0.649 | 54 (4.6) | 10 (3.9) | 0.863 |
| Missing | 25,009 (40.9) | 126 (45) | 560 (40.1) | 125 (44.8) | ||
| Aspirin: IS only | 43,194 (83.7) | 190 (74.5) | <0.001 | 991 (83.6) | 190 (74.8) | 0.002 |
| Missing | 601 (1.0) | 4 (1.4) | 13 (0.9) | 4 (1.4) | ||
| Swallow screen | 48,692 (79.7) | 214 (76.4) | 0.191 | 1098 (78.7) | 213 (76.3) | 0.433 |
| Missing | 2519 (4.1) | 13 (4.6) | 59 (4.2) | 13 (4.7) | ||
| Brain imaging | 59,680 (97.7) | 278 (99.3) | 0.257 | 1365 (97.9) | 277 (99.3) | 0.754 |
| Missing | 284 (0.5) | 0 | 14 (1.0) | 0 | ||
| Duration of admission (IQR) | 13 (37.0) | 16 (38.0) | 0.211 | 12 (33.0) | 16 (38.0) | 0.079 |
| Discharge destination | ||||||
| NHS bed | 3969 (6.5) | 42 (15.0) | <0.0001 | 66 (4.7) | 42 (15.1) | <0.0001 |
| Care home | 6390 (10.5) | 15 (5.4) | 0.003 | 100 (7.2) | 15 (5.4) | 0.302 |
| Home/sheltered | 39,125 (64.1) | 153 (54.6) | <0.001 | 979 (70.2) | 152 (54.5) | <0.0001 |
| Other | 782 (1.3) | 4 (1.4) | 0.786 | 25 (1.8) | 4 (1.4) | 0.806 |
| Died | 9694 (15.9) | 62 (22.1) | 0.007 | 188 (13.5) | 62 (22.2) | 0.001 |
| Missing | 1127 (1.8) | 4 (1.4) | 37 (2.7) | 4 (1.4) | ||
| Death at follow-up | 33,169 (54.3) | 229 (81.8) | <0.0001 | 676 (48.5) | 228 (81.7) | <0.0001 |
ADL, activities of daily living; AF, atrial fibrillation; CeVD, Cerebrovascular Disease; IHD, ischemic heart disease; IQR, interquartile range; IS, ischemic stroke; NHS, National Health Service.
Data are presented as no. (%).
Standardized difference: difference in means or proportions divided by SE; imbalance defined as absolute values >0.20.
Table 4.
Demographics, presentation, management, and outcomes after stroke form the Scottish Stroke Care Audit showing data before and after propensity score matching for individuals on dialysis for end-stage renal disease.
| Variable | Non-ESRD population (n = 61,087) | Dialysis population (n = 224) | Standardized differencea/P value | Propensity-matched non-ESRD population (n = 1115) | Propensity-matched dialysis population (n = 223) | Standardized differencea/P value |
|---|---|---|---|---|---|---|
| Patient demographics | ||||||
| Age at stroke, yr (IQR) | 76 (17.0) | 72 (16.0) | −0.37 | 73 (15.0) | 72 (16.0) | −0.09 |
| Female | 31,368 (51.4) | 119 (53.1) | 0.04 | 613 (55.0) | 118 (52.9) | −0.04 |
| Rural | 10,015 (16.4) | 35 (15.6) | −0.02 | 169 (15.2) | 35 (15.7) | 0.01 |
| Deprived | 29,709 (48.6) | 108 (48.2) | −0.01 | 542 (48.6) | 108 (48.4) | 0.00 |
| Previous AF | 5019 (8.2) | 22 (9.8) | 0.05 | 107 (9.6) | 22 (9.9) | 0.01 |
| Previous CeVD | 3555 (5.8) | 22 (9.8) | 0.14 | 88 (7.9) | 21 (9.4) | 0.05 |
| Previous IH | 12,025 (19.7) | 82 (36.6) | 0.37 | 435 (39.0) | 81 (36.3) | −0.06 |
| Previous hypertension | 25,818 (42.3) | 167 (74.6) | 0.66 | 872 (78.2) | 166 (74.4) | −0.09 |
| Previous diabetes | 8536 (14.0) | 93 (41.5) | 0.63 | 445 (39.9) | 92 (41.3) | 0.03 |
| Case mix | ||||||
| Independent in ADLs before admission | 47,184 (77.2) | 138 (61.6) | <0.0001 | 886 (77.7) | 137 (61.4) | <0.0001 |
| Missing | 5109 (8.4) | 28 (12.5) | 118 (10.6) | 28 (12.6) | ||
| Living alone before admission | 22,065 (36.1) | 52 (23.2) | <0.001 | 346 (31.0) | 52 (23.3) | 0.046 |
| Missing | 4453 (7.3) | 27 (12.1) | 95 (8.5) | 27 (12.1) | ||
| Talking at first assessment | 42,795 (70.1) | 159 (71.0) | 0.377 | 797 (71.5) | 158 (70.9) | 0.856 |
| Missing | 3707 (6.1) | 19 (8.5) | 74 (6.6) | 19 (8.5) | ||
| Orientated at first assessment | 35,846 (58.7) | 13 (58.0) | 0.824 | 686 (61.5) | 129 (57.9) | 0.508 |
| Missing | 5325 (8.7) | 25 (11.2) | 101 (9.1) | 25 (11.2) | ||
| Able to lift arms at first assessment | 35,622 (58.3) | 141 (63.0) | 0.080 | 650 (58.3) | 141 (63.2) | 0.125 |
| Missing | 4817 (7.9) | 21 (9.4) | 99 (8.9) | 21 (9.4) | ||
| Able to walk on first assessment | 23,732 (38.9) | 69 (30.8) | 0.026 | 420 (37.7) | 69 (30.9) | 0.068 |
| Missing | 5529 (9.1) | 26 (11.6) | 118 (10.6) | 26 (11.7) | ||
| Index stroke | ||||||
| Ischemic | 51,606 (84.5) | 203 (90.6) | 947 (84.9) | 202 (90.6) | ||
| Hemorrhagic | 6954 (11.4) | 19 (8.5) | 122 (10.9) | 19 (8.5) | ||
| Missing | 2527 (4.1) | 2 (0.9) | 0.145 | 46 (4.1) | 2 (0.9) | 0.239 |
| Admission source | ||||||
| Acute hospital | 1984 (3.3) | 22 (9.8) | <0.0001 | 27 (2.4) | 21 (9.4) | <0.0001 |
| Sheltered/care home | 2758 (4.5) | 2 (0.9) | 0.005 | 30 (2.7) | 2 (0.9) | 0.147 |
| Home | 54613 (89.4) | 186 (83.0) | 0.019 | 1020 (91.5) | 186 (83.4) | 0.001 |
| Rehab/Other | 904 (1.5) | 7 (3.1) | 0.047 | 18 (1.6) | 7 (3.1) | 0.166 |
| Missing | 828 (1.4) | 7 (3.1) | 20 (1.8) | 7 (3.1) | ||
| Management | ||||||
| Admitted to stroke unit | 49,619 (78.0) | 145 (64.7) | <0.001 | 888 (79.6) | 144 (64.6) | <0.001 |
| Missing | 1415 (2.3) | 16 (7.1) | 27 (2.4) | 16 (7.2) | ||
| Thrombolysis: IS only | 2487 (4.8) | 7 (3.5) | 0.496 | 47 (5.0) | 7 (3.5) | 0.568 |
| Missing | 25,009 (40.9) | 100 (44.6) | 439 (39.4) | 99 (44.4) | ||
| Aspirin: IS only | 43,194 (83.7) | 152 (74.9) | 0.002 | 788 (83.2) | 152 (75.3) | 0.010 |
| Missing | 601 (1.0) | 3 (1.3) | 11 (1.0) | 3 (1.3) | ||
| Swallow screen | 48,692 (79.7) | 173 (77.2) | 0.315 | 903 (81.0) | 172 (77.1) | 0.229 |
| Missing | 2519 (4.1) | 9 (4.0) | 38 (3.4) | 9 (4.0) | ||
| Brain imaging | 59,680 (97.7) | 222 (99.1) | 0.450 | 1095 (98.2) | 221 (99.1) | 1.000 |
| Missing | 284 (0.5) | 0 | 6 (0.5) | 0 | ||
| Duration of admission (IQR) | 13 (37.0) | 17 (39.0) | 0.101 | 12 (36.0) | 17 (39.0) | 0.027 |
| Discharge destination, n (%) | ||||||
| NHS bed | 3969 (6.5) | 38 (17.0) | <0.0001 | 53 (4.8) | 38 (17.0) | <0.0001 |
| Care home | 6390 (10.5) | 14 (6.3) | 0.037 | 97 (8.7) | 14 (6.3) | 0.287 |
| Home/sheltered | 39,125 (64.1) | 114 (50. 9) | <0.0001 | 765 (68.6) | 113 (50.7) | <0.0001 |
| Other | 782 (1.3) | 4 (1.8) | 0.541 | 19 (1.7) | 4 (1.8) | 1.000 |
| Died | 9694 (15.9) | 51 (22.7) | 0.008 | 160 (14.4) | 51 (22.9) | 0.002 |
| Missing | 1127 (1.8) | 3 (1.3) | 21 (1.9) | 3 (1.3) | ||
| Death at end follow-up | 33,169 (54.3) | 192 (85.7) | <0.0001 | 590 (52.9) | 191 (85.7) | <0.0001 |
ADL, activities of daily living; AF, atrial fibrillation; CeVD, Cerebrovascular Disease; IHD, ischemic heart disease; IQR, interquartile range; IS, ischemic stroke; NHS, National Health Service.
Data are presented as no. (%).
Standardized difference: difference in means or proportions divided by SE; imbalance defined as absolute values >0.20.
Table 5.
Demographics, presentation, management, and outcomes after stroke from the Scottish Stroke Care Audit showing data before and after propensity score matching for individuals with a renal transplant
| Variable | Non-ESRD population (n = 61,087) | Kidney transplant (RT) population (n = 56) | Standardized differencea/P value | Propensity-matched non-ESRD population (n = 280) | Propensity-matched RT population (n = 56) | Standardized differencea/P value |
|---|---|---|---|---|---|---|
| Patient demographics | ||||||
| Age, yr, median (IQR) | 76 (17.0) | 65.5 (15.0) | −0.99 | 66 (15) | 65.5 (15.0) | −0.12 |
| Female | 31,368 (51.4) | 21 (37.5) | −0.28 | 101 (36.1) | 21 (37.5) | 0.03 |
| Rural | 10,015 (16.4) | 6 (10.7) | −0.17 | 22 (7.9) | 6 (10.7) | 0.10 |
| Deprived | 29,709 (48.6) | 30 (53.6) | 0.10 | 156 (55.7) | 30 (53.6) | −0.04 |
| Previous AF | 5019 (8.2) | 4 (7.1) | −0.05 | 12 (4.3) | 4 (7.1) | 0.12 |
| Previous CeVD | 3555 (5.8) | 5 (8.9) | 0.11 | 27 (9.6) | 5 (8.9) | −0.02 |
| Previous IHD | 12,025 (19.7) | 15 (26.8) | 0.15 | 79 (28.2) | 15 (26.8) | −0.03 |
| Previous hypertension | 25,818 (42.3) | 41 (73.2) | 0.63 | 207 (73.9) | 41 (73.2) | −0.02 |
| Prior diabetes | 8536 (14.0) | 20 (35.7) | 0.51 | 101 (36.1) | 20 (35.7) | −0.01 |
| Case mix | ||||||
| Independent in ADLs before admission | 47,184 (77.2) | 41 (73.2) | 0.692 | 236 (84.3) | 41 (73.2) | 0.597 |
| Missing | 5109 (8.4) | 9 (16.1) | 19 (6.8) | 9 (16.1) | ||
| Living alone before admission | 22,065 (36.1) | 12 (21.4) | 0.072 | 85 (30.4) | 12 (21.4) | 0.397 |
| Missing | 4453 (7.3) | 9 (16.1) | 18 (6.4) | 9 (16.1) | ||
| Talking at first assessment | 42795 (70.1) | 37 (66.1) | 1.000 | 218 (77.9) | 37 (66.1) | 0.322 |
| Missing | 3707 (6.1) | 7 (12.5) | 14 (5.0) | 7 (12.5) | ||
| Orientated at first assessment | 35,846 (58.7) | 30 (53.6) | 1.000 | 194 (69.3) | 30 (53.6) | 0.214 |
| Missing | 5325 (8.7) | 10 (17.9) | 18 (6.4) | 10 (17.9) | ||
| Able to lift arms at first assessment | 35,622 (58.3) | 37 (66.1) | 0.052 | 187 (66.8) | 37 (66.1) | 0.486 |
| Missing | 4817 (7.9) | 8 (14.3) | 18 (6.4) | 8 (14.3) | ||
| Able to walk on first assessment | 23,732 (38.9) | 26 (46.4) | 0.111 | 128 (45.7) | 26 (46.4) | 0.638 |
| Missing | 5529 (9.1) | 8 (14.3) | 21 (7.5) | 8 (14.3) | ||
| Index stroke | ||||||
| Ischemic | 51,606 (84.5) | 52 (92.9) | 242 (86.4) | 52 (92.9) | ||
| Hemorrhagic | 6954 (11.4) | 4 (7.1) | 30 (10.7) | 4 (7.1) | ||
| Missing | 2527 (4.1) | 0 | 0.405 | 8 (2.9) | 0 | 0.477 |
| Admission source | ||||||
| Acute hospital | 1984 (3.3) | 0 | 0.426 | 11 (3.9) | 0 | 0.223 |
| Sheltered/care home | 2758 (4.5) | 0 | 0.180 | 8 (2.9) | 0 | 0.362 |
| Home | 54,613 (89.4) | 54 (96.4) | 0.009 | 255 (91.1) | 54 (96.4) | 0.032 |
| Rehab/other | 904 (1.5) | 0 | 1.000 | 2 (0.7) | 0 | 1.000 |
| Missing | 828 (1.4) | 2 (3.6) | 4 (1.4) | 2 (3.6) | ||
| Management | ||||||
| Admitted to stroke unit | 47,619 (78.0) | 44 (78.6) | 0.868 | 221 (78.9) | 44 (78.6) | 0.712 |
| Missing | 1415 (2.3) | 0 | 7 (2.5) | 0 | ||
| Thrombolysis: IS only | 2487 (4.8) | 3 (5.8) | 0.731 | 8 (3.3) | 3 (5.8) | 0.407 |
| Missing | 25,009 (40.9) | 26 (46.4) | 119 (42.5) | 26 (46.4) | ||
| Aspirin: IS only | 43,194 (83.7) | 38 (73.1) | 0.051 | 200 (82.6) | 38 (73.1) | 0.157 |
| Missing | 601 (1.0) | 1 (1.8) | 3 (1.1) | 1 (1.8) | ||
| Swallow screen | 48,692 (79.7) | 41 (73.2) | 0.457 | 222 (79.3) | 41 (73.2) | 0.565 |
| Missing | 2519 (4.1) | 4 (7.1) | 9 (3.2) | 4 (7.1) | ||
| Brain imaging | 59,680 (97.7) | 56 (100.0) | 0.629 | 276 (98.6) | 56 (100) | 1.000 |
| Missing | 284 (0.5) | 0 | 4 (1.4) | 0 | ||
| Duration of admission (IQR) | 13 (37) | 11 (32) | 0.683 | 11 (22) | 11 (32) | 0.541 |
| Discharge destination | ||||||
| NHS bed | 3969 (6.5) | 4 (7.1) | 0.784 | 16 (5.7) | 4 (7.1) | 0.756 |
| Care home | 6390 (10.5) | 1 (1.8) | 0.027 | 15 (5.4) | 1 (1.8) | 0.488 |
| Home/sheltered | 39,125 (64.1) | 39 (69.6) | 0.479 | 214 (76.4) | 39 (69.6) | 0.292 |
| Other | 782 (1.3) | 0 | 1.000 | 3 (1.1) | 0 | 1.000 |
| Died | 9694 (15.9) | 11 (19.6) | 0.462 | 26 (9.3) | 11 (19.6) | 0.034 |
| Missing | 1127 (1.8) | 1 (1.8) | 6 (2.1) | 1 (1.8) | ||
| Death at follow−up | 33,169 (54.3) | 37 (66.1) | 0.082 | 117 (41.8) | 37 (66.1) | 0.001 |
ADL, activities of daily living; AF, atrial fibrillation; CeVD, Cerebrovascular Disease; IHD, ischemic heart disease; IQR, interquartile range; IS, ischemic stroke; NHS, National Health Service.
Standardized difference: difference in means or proportions divided by SE; imbalance defined as absolute values >0.20.
Output After PSM: Case Mix
Individuals with ESRD were less likely to be independent in activities of daily living (63.8% vs. 78.6%; P < 0.0001) and less likely to be living alone (22.9% vs. 30.8%; P = 0.025) before admission. There were similar results found in individuals on dialysis, but there were no significant differences between individuals with a kidney transplant and the non-ESRD stroke population (Table 3, Table 4, Table 5).
Output After PSM: Admission Source
Individuals with ESRD and individuals treated with dialysis were more likely to present with stroke from an acute hospital rather than from home (7.5% vs. 2.2%; P < 0.0001 and 9.4% vs. 2.4%; P < 0.0001, respectively). Transplant recipients were more likely to be admitted from home (96.4% vs. 91.1%; P = 0.032) compared with the non-ESRD stroke population (Table 3, Table 4, Table 5).
Output After PSM: Stroke Care and Audit Standards
Individuals with ESRD and individuals treated with dialysis were less likely to be managed on an acute stroke unit during their admission (67.4% vs. 79.6%; P < 0.001 and 64.6% vs. 79.6%; P < 0.001, respectively) and less likely to receive aspirin (74.8% vs. 83.6%; P = 0.002 and 75.3% vs. 83.2%; P = 0.01, respectively). There were no differences in management of transplantation recipients compared with the non-ESRD stroke population (Table 3, Table 4, Table 5).
Individuals on dialysis were less likely to be admitted to a stroke unit on day 0 or 1 (43.5% vs. 62.6%; P = 0.005), less likely to have a swallow screen within the first 24 hours (66.4% vs. 74.1%; P = 0.017), and less likely to receive thrombolysis within 60 minutes (1.5% vs. 3.9%; P = 0.016). These measures did not differ in kidney transplantation recipients (Supplementary Tables S6–S8).
PSM: Discharge Destination
Individuals with ESRD and individuals on dialysis were more likely to remain in an National Health Service inpatient bed (15.1% vs. 4.7%; P < 0.0001 and 17.0% vs. 4.8%; P < 0.0001, respectively) and were less likely to be discharged to home or to a sheltered accommodation (54.5% vs. 70.2%; P < 0.0001 and 50.7% vs. 68.6%; P < 0.0001). This association was not present in individuals with a kidney transplant. In both dialysis and kidney transplant patients, in-hospital mortality was almost double the rate demonstrated in individuals without ESRD (dialysis: 22.9% vs. 14.4%; P = 0.002; transplant: 19.6% vs. 9.3%; P = 0.034).
ESRD Mortality After Stroke
At end of follow-up, 4621 (52.8%) individuals with ESRD had died. Death was more likely in individuals who were older, who had high cardiovascular comorbidity burden, and who experienced stroke during follow-up. Further demographics are presented in Table 6.
Table 6.
Baseline demographics of all end-stage renal disease patients, alive versus dead at end of follow-up
| Variable | Alive (n = 4136) | Dead (n = 4621) | P value |
|---|---|---|---|
| Age at first RRT, yr, median (IQR) | 44.6 (27.0) | 67 (20.8) | <0.0001 |
| Sex | |||
| Female | 1743 (42.1) | 1932 (41.8) | |
| Male | 2393 (57.9) | 2689 (58.2) | 0.761 |
| Primary renal diagnosis group | |||
| Missing | 1 (0.02) | 11 (0.2) | |
| Glomuleronephritis | 989 (23.9) | 613 (13.3) | <0.0001 |
| Interstitial disease | 1403 (33.9) | 904 (19.6) | <0.0001 |
| Multisystem | 613 (14.8) | 1181 (25.6) | <0.0001 |
| Diabetic nephropathy | 540 (13.1) | 1045 (22.6) | <0.0001 |
| Other | 590 (14.3) | 867 (18.8) | <0.0001 |
| Urban-rural | |||
| Rural | 722 (17.5) | 733 (15.9) | |
| Urban | 3414 (82.5) | 3888 (84.1) | 0.047 |
| SIM | |||
| Least deprived | 3080 (74.5) | 3405 (73.7) | |
| Most deprived | 1056 (25.5) | 1216 (26.3) | 0.407 |
| Medical history | |||
| Atrial fibrillation | 76 (1.8) | 478 (10.3) | <0.0001 |
| Ischemic heart disease | 743 (18.0) | 1952 (42.2) | <0.0001 |
| Stroke | 60 (1.5) | 260 (5.6) | <0.0001 |
| Diabetes | 935 (22.6) | 1726 (37.4) | <0.0001 |
| Hypercholesterolemia | 423 (10.2) | 710 (15.4) | <0.0001 |
| Obesity | 201 (4.9) | 294 (6.4) | 0.004 |
| HBP | 2887 (69.8) | 3368 (72.9) | 0.017 |
| Clinical variables, median (IQR) | |||
| Systolic BP, mm Hg | 138.0 (27.0) | 138.5 (31.5) | 0.755 |
| Diastolic BP, mm Hg | 75.0 (17.0) | 69.0 (19.0) | <0.0001 |
| Weight, kg | 75.2 (23.0) | 70.3 (22.3) | <0.0001 |
| Laboratory variables | |||
| Hemoglobin, g/l | 12 (2.0) | 11.3 (1.8) | <0.0001 |
| Albumin, mmol/l | 39 (6.0) | 36 (7.0) | <0.0001 |
| Serum phosphate, mmol/l | 1.21 (0.6) | 1.45 (0.6) | <0.0001 |
| Adjusted calcium, mmol/l | 2.38 (0.2) | 2.36 (0.2) | <0.0001 |
| ESA use | |||
| Stroke during study period | 53 (1.3) | 433 (9.4) | <0.0001 |
ESA, erythropoietin-stimulating agent; BP, blood pressure; HBP, high blood pressure; IQR, interquartile range; RRT, renal replacement therapy; SIMD, Scottish Index of Multiple Deprivation.
Data are presented as no. (%).
A total of 229 (81.8%) of the 280 individuals with ESRD who experienced stroke died compared with 33,169 (54.3%) of 61,087 individuals in the general stroke population. The higher mortality rate remained after PSM (85.7% vs. 52.9% for dialysis; P < 0.0001 and 66.1% vs. 41.8% for transplant; P = 0.001) (Tables 4 and 5).
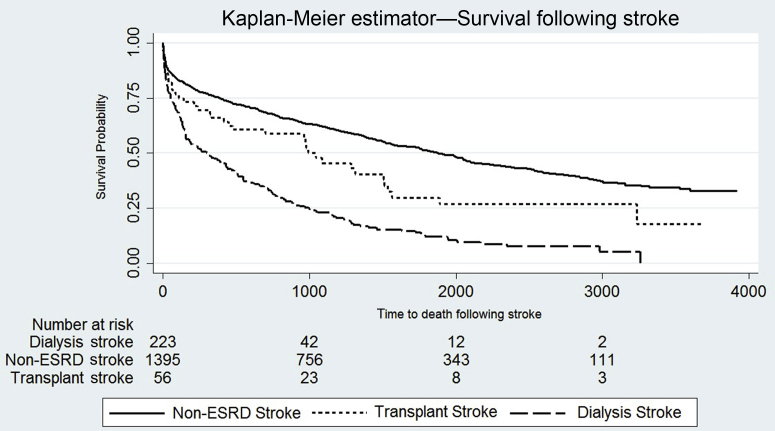
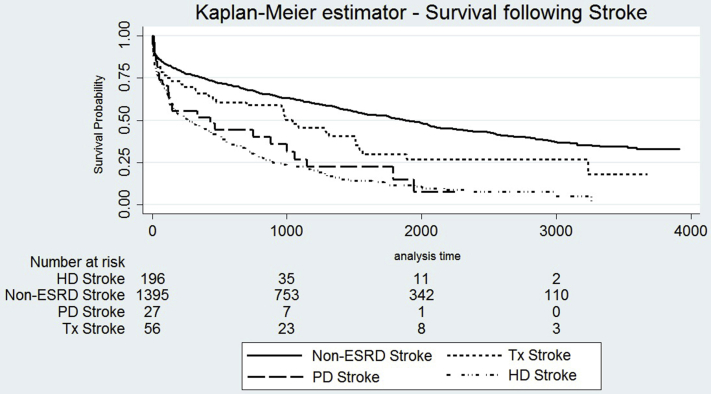
Median survival (IQR) after stroke was 0.8 (2.1) years in individuals on dialysis, 2.5 (3.8) years in individuals with a kidney transplant, and 3.1 (4.4) years in the non-ESRD stroke population (P < 0.0001) (Figure 1 and Supplementary Figure S3, demonstrating split by RRT modality).
Figure 1.
Kaplan-Meier estimator survival curves demonstrating time to death after stroke in the matched population without end-stage renal disease (ESRD), transplant recipients. and dialysis patients. Log-rank test; P < 0.0001.
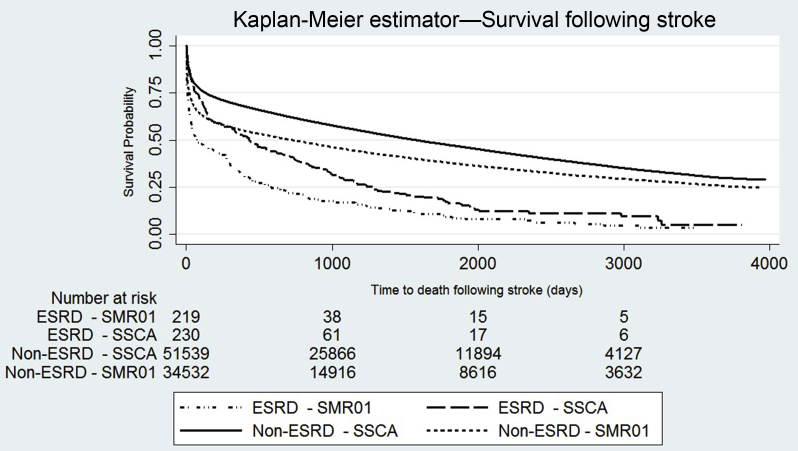
When we assessed all stroke episodes in both ESRD and non-ESRD populations, there was demonstrably lower survival in individuals with ESRD than those without ESRD. Furthermore, the presence of first recorded stroke episode during follow-up within the SMR01 was associated with lower survival than those recorded within the SSCA (Figure 2).
Figure 2.
Kaplan-Meier survival curves demonstrating time to death after following stroke in both end-stage renal disease (ESRD) and non-ESRD populations, split by source of stroke diagnosis: non-ESRD Scottish Stroke Care Audit (SSCA), non-ESRD Scottish Morbidity Records 01 (SMR01), ESRD population SSCA, and ESRD population SMR01. Log-rank test; P < 0.0001.
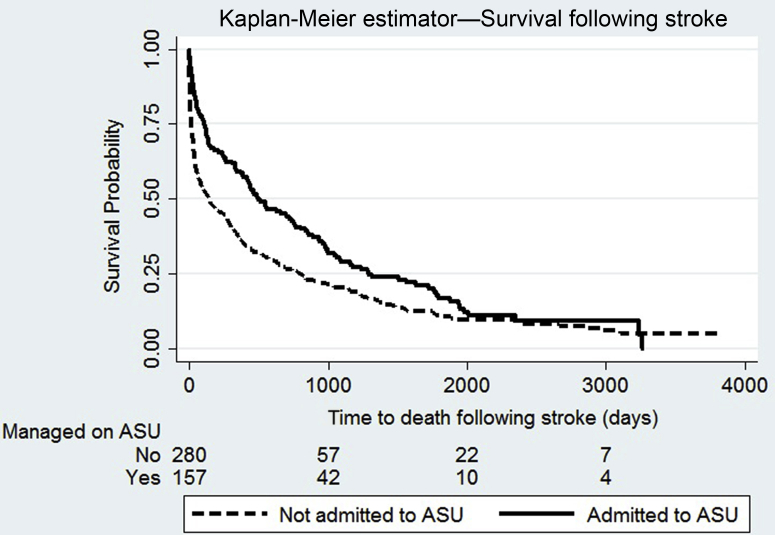
In individuals with ESRD alone, 157 (35.0%) patients with a first recorded stroke were admitted to a stroke unit. At follow-up, mortality was lower in those admitted to a stroke unit (82.8% vs. 91.1%; P = 0.014). On multivariable analysis, admission to a stroke unit was associated with lower risk of death (hazard ratio: 0.68; 95% confidence interval: 0.55−0.84; P < 0.001) (Figure 3 and Supplementary Table S9).
Figure 3.
Kaplan-Meier survival curves demonstrating time to death after stroke in patients with end-stage renal disease and the effect of admission to an acute stroke unit (ASU). Log-rank test P < 0.0001.
Discussion
In this population-based record linkage cohort study we demonstrated that incidence of stroke in ESRD is high and higher in individuals receiving dialysis compared with kidney transplant recipients. We found individuals with ESRD who experienced stroke were more functionally dependent at presentation, there were differences in delivery of stroke care to individuals with ESRD, and in-hospital death from stroke was higher. These findings suggest that provision of stroke care for individuals with ESRD needs urgent review.
The stroke rate of 25.3 and 4.5 per 1000 patient-years in individuals on dialysis or transplant recipients, respectively, is in keeping with previous published reports.8, 20, 22, 23 During the same period, the crude incidence rate for cerebrovascular disease in Scotland was 2.4 per 1000 patient-years,24 which demonstrated a 10-fold increase in stroke incidence in individuals who received dialysis. In real-world terms, in our renal unit, with prevalent population of approximately 650 dialysis recipients, this equated to 1 stroke every 22 days or, nationally, 1 stroke per week.
Stroke Incidence and Presentation
Individuals on dialysis were functionally more dependent at presentation than their matched non-ESRD stroke population cohort. Functional dependence is common in ESRD25 and is an independent predictor of mortality.26 Patients who received dialysis were more likely to be admitted from an acute hospital than home. There were possible explanations for this. Primarily, there is an increased likelihood of hospitalization with ESRD27 but also a temporal relationship between dialysis itself and stroke.28 In one single-center study, 39.5% of infarctions and 34.7% hemorrhages occurred during or within 30 minutes of completing dialysis. It is likely that such strokes would initially be referred to nephrology.
Inequalities in Care
As well as being less likely to be managed in acute stroke units, individuals with ESRD treated with dialysis were less likely to undergo a swallow test within the first 24 hours, less likely to receive aspirin on arrival to hospital, and less likely to receive prompt thrombolytic therapy. Interestingly, this pattern of care was not seen in renal transplant recipients. Differences in care after myocardial infarction in ESRD are previously demonstrated, but these differences were attributed to a lack of an accurate diagnosis at first assessment due to higher rates of atypical symptoms and electrocardiographic changes.12 In our cohort, presenting symptoms were similar between groups; therefore, so we believe delayed recognition of stroke did not explain the differences. It is possible that practical considerations surrounding need for dialysis make stroke unit care impractical or unattractive. In a survey of nephrologists it was felt that adverse effects of dialysis schedules, coupled with lack of stroke unit care might negatively affect recovery and would require future attention.16 An alternate explanation was that specialist stroke units might feel uncomfortable caring for dialysis patients and elect not to transfer care.
Only 280 of 486 strokes that occurred in individuals with ESRD were included in the SSCA data set. It is believed that the SSCA most accurately represents the number of strokes occurring in Scotland.29 It is acknowledged that omission of strokes from the SSCA can occur if the patient was in hospital at the time of stroke or was not referred promptly to stroke services. Such a high number of strokes not recorded in the SSCA was unexpected and suggested substantial inequality in access to gold standard stroke care for individuals with ESRD. A recent paper that addressed selection bias after stroke demonstrated consistent improvement up to 1 year after stroke from use of stroke units.17 We explored this discrepancy by examining the effects of admission to a stroke unit as a marker of optimal stroke care. Individuals with ESRD admitted to a stroke unit had a significantly lower mortality rate at follow-up than those who were not admitted. It must be acknowledged this might be in part related to selection bias at the time of stroke; those more severely affected at presentation might not be referred onward.
Discrepancies in Mortality
Mortality after stroke is declining in general population studies,30 but we found striking differences in mortality rates — both during stroke admission and on longer follow-up. This extended to individuals with ESRD and a kidney transplant who were twice as likely to die on their initial admission and had higher mortality rates at follow-up. Because our matching process controlled for hypertension, diabetes, and ischemic heart disease, an accrued risk specific to CKD not reversed by transplantation seems a likely explanation. This is an important finding because transplant recipients with normal range serum creatinine could be overlooked as having a lower risk. Unfortunately, creatinine values were not available in our non-ESRD population data.
Finally, we detected significant differences in survival based on diagnosis source; higher survival occurred in individuals diagnosed via SSCA compared with individuals in the SMR01, and there was higher survival in those admitted to a stroke unit. These findings support the conclusion that specialist stroke care was associated with a lower mortality rate in individuals with ESRD. Although this may be related to therapeutic nihilism in cases of more severe stroke, we could not provide data to support this. Therefore, serious consideration must be paid to the merits of managing stroke on a renal ward for reasons of dialysis practicalities alone.
Strengths and Limitations
Linkage of national data sets provided a large population with unique results that demonstrated issues relevant to patient care. Our main strengths included large numbers of patients in both ESRD and stroke populations. Scotland is broadly representative of both European nephrology and stroke care in terms of patient demographics and outcomes for both conditions.31, 32 Furthermore, the granularity of data allowed description of the stroke pathway from presentation to discharge, use of death records allowed a follow-up period, and since 2005, both the SRR and SSCA have been all-inclusive data sets. We supported complete capture by accessing the SMR01 data set. Despite these strengths, we acknowledge limitations. First, all data were retrospective, and therefore, we could not prove causation, only describe associations. Because it was not feasible to study the primary outcome of our study—impact of ESRD on outcomes after stroke—in a randomized fashion, we addressed the retrospective nature of the data by performing PSM. Second, missing data limited the ability to fully exclude other factors that influenced outcome. For instance, we lacked markers of stroke severity (e.g., National Institutes of Health Stroke Scale) because this was only recorded when thrombolysis was used. Finally, discrepancies in survival after stroke split by diagnosis source warrants attention. Selection bias could result in frailer, sicker patients bypassing the stroke unit (and omission from the SSCA) following deterioration on dialysis. Nonspecialist input might result in misdiagnosis. However, internal validation of SMR01 coding confirmed the approximately 90% accuracy rate,33 making this unlikely.
Conclusions
Stroke is common in individuals with ESRD with poor outcomes. Individuals on dialysis are functionally more dependent at presentation and are less likely to be managed on a stroke unit. Individuals with a renal transplantation are similar at presentation and provided with equivalent care to the general population, but retain a significantly higher mortality rate. ESRD should not exclude individuals from ideal care after stroke, and efforts should be made to deliver such care to all.
Disclosure
All the authors declared no competing interests.
Acknowledgments
MDF is funded by a Kidney Research UK Training Fellowship and is supported by a grant from Darlinda’s Charity for Renal Research. Funding was provided by Kidney Research UK.
The funders had no role in study design, data collection, analysis or interpretation, or writing the manuscript. MDF, RM, PBM, and JD had full access to data, and all authors had final responsibility for the decision to submit for publication.
MDF, JD, and PBM had the original idea. MDF and RM analyzed the data. JPT, MJM, and WM verified the source data and assisted with the analysis. MDF constructed the first draft, and all authors contributed to the final draft.
Footnotes
Table S1. Cause of death as per International Classification of Diseases (ICD-10) rules, in all end-stage renal disease patients versus the general population.
Table S2. Cause of death as per International Classification of Diseases (ICD-10) rules, in dialysis patients versus the general population.
Table S3. Cause of death as per International Classification of Diseases (ICD-10) rules, in all renal transplant patients versus the general population.
Table S4. Baseline demographics of all dialysis patients, no stroke versus stroke.
Table S5. Baseline demographics of all renal transplant patients, no stroke versus stroke.
Table S6. Auditable standards in all end-stage renal disease patients matched to the general population.
Table S7. Auditable standards in dialysis patients matched to the general population.
Table S8. Auditable standards in renal transplant patients matched to the general population.
Table S9. Multivariable Cox regression, effect of admission to a stroke unit on mortality after stroke.
Figure S1. Flow diagram demonstrating construction of the data set to calculate stroke incidence in patients with end-stage renal disease (ESRD), 2005 to 2013.
Figure S2. Flow diagram demonstrating the construction of the data set to compare presentation, management, and outcomes following stroke and propensity score matching.
Figure S3. Kaplan-Meier survival estimator demonstrating time to death following stroke, split by matched stroke population without end-stage renal disease (ESRD), kidney transplant recipients, peritoneal dialysis patients, and hemodialysis patients. Log-rank test; P < 0.0001.
Figure S4. Strobe checklist.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Cause of death as per International Classification of Diseases (ICD-10) rules, in all end-stage renal disease patients versus the general population.
Cause of death as per International Classification of Diseases (ICD-10) rules, in dialysis patients versus the general population.
Cause of death as per International Classification of Diseases (ICD-10) rules, in all renal transplant patients versus the general population.
Baseline demographics of all dialysis patients, no stroke versus stroke.
Baseline demographics of all renal transplant patients, no stroke versus stroke.
Auditable standards in all end-stage renal disease patients matched to the general population.
Auditable standards in dialysis patients matched to the general population.
Auditable standards in renal transplant patients matched to the general population.
Multivariable Cox regression, effect of admission to a stroke unit on mortality after stroke.
Flow diagram demonstrating construction of the data set to calculate stroke incidence in patients with end-stage renal disease (ESRD), 2005 to 2013.
Flow diagram demonstrating the construction of the data set to compare presentation, management, and outcomes following stroke and propensity score matching.
Figure S3.
Kaplan-Meier survival estimator demonstrating time to death following stroke, split by matched stroke population without end-stage renal disease (ESRD), kidney transplant recipients, peritoneal dialysis patients, and hemodialysis patients. Log-rank test; P < 0.0001.
Strobe checklist.
References
- 1.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 3.JBS3 Board Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100:ii1–ii67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 4.Foley R.N., Parfrey P.S., Sarnak M.J. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 5.de Jager D.J., Grootendorst D.C., Jager K.J. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre C.W. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76:371–375. doi: 10.1038/ki.2009.207. [DOI] [PubMed] [Google Scholar]
- 7.Boots J.M.M., Christiaans M.H.L., van Hooff J.P. Effect of immunosuppressive agents on long-term survival of renal transplant recipients. Drugs. 2004;64:2047–2073. doi: 10.2165/00003495-200464180-00004. [DOI] [PubMed] [Google Scholar]
- 8.Seliger S., Gillen D.L., Longstreth W.T. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Ocak G., Van Stralen K.J., Rosendaal F.R. Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J Thromb Haemost. 2012;10:2484–2493. doi: 10.1111/j.1538-7836.2012.04921.x. [DOI] [PubMed] [Google Scholar]
- 10.Martirosyan H.A., Brass E.P., Mehrotra R. Differential management of cardiovascular disease in ESRD by nephrologists and cardiologists. Am J Kidney Dis. 2004;44:309–321. doi: 10.1053/j.ajkd.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Fox C.S., Muntner P., Chen A.Y. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network Registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog C.A., Littrell K., Arko C. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: a collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation. 2007;116:1465–1472. doi: 10.1161/CIRCULATIONAHA.107.696765. [DOI] [PubMed] [Google Scholar]
- 13.Fellström B.C., Jardine A.G., Schmieder R.E. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 14.Shah M., Avgil Tsadok M., Jackevicius C. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. doi: 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 15.Zannad F., Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation. 2017;135:1769–1771. doi: 10.1161/CIRCULATIONAHA.117.027338. [DOI] [PubMed] [Google Scholar]
- 16.Power A., Fogarty D., Wheeler D.C. Acute stroke thrombolysis in end-stage renal disease: a national survey of nephrologist opinion. Nephron Clin Pract. 2013;124:167–172. doi: 10.1159/000357155. [DOI] [PubMed] [Google Scholar]
- 17.Turner M., Barber M., Dodds H. The impact of stroke unit care on outcome in a Scottish stroke population, taking into account case mix and selection bias. J Neurol Neurosurg Psychiatry. 2015;86:314–318. doi: 10.1136/jnnp-2013-307478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Information Divisions Scotland (ISD). The Scottish Renal Registry. Available at: http://www.srr.scot.nhs.uk. Accessed August 30, 2017.
- 19.Hemke AC, Heemskerk MBA, van Diepen M, et al. Performance of an easy-to-use prediction model for renal patient survival: an external validation study using data from the ERA-EDTA Registry [epub ahead of print]. Nephrol Dial Transplant. 10.1093/ndt/gfx348. Accessed June 20, 2018. [DOI] [PubMed]
- 20.Findlay M.D., Donaldson K., Doyle A. Factors influencing withdrawal from dialysis: a national registry study. Nephrol Dial Transplant. 2016;31:2041–2048. doi: 10.1093/ndt/gfw074. [DOI] [PubMed] [Google Scholar]
- 21.Ho D.E., Imai K., King G. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 22.Willicombe M., Kumar N., Goodall D. Incidence, risk factors, and outcomes of stroke post-transplantation in patients receiving a steroid sparing immunosuppression protocol. Clin Transplant. 2015;29:18–25. doi: 10.1111/ctr.12476. [DOI] [PubMed] [Google Scholar]
- 23.Findlay M.D., Thomson P.C., Fulton R.L. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke. 2015;46:2477–2481. doi: 10.1161/STROKEAHA.115.009095. [DOI] [PubMed] [Google Scholar]
- 24.Information Services Division. Scotland. Data Tables available via Stroke Publications. Available at: http://www.isdscotland.org/Health-Topics/Stroke/Publications. Accessed March 13, 2017.
- 25.Kavanagh N.T., Schiller B., Saxena A.B. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodial Int. 2015;19:593–600. doi: 10.1111/hdi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jassal S.V., Karaboyas A., Comment L.A. Functional dependence and mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2016;67:283–292. doi: 10.1053/j.ajkd.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mix T.-C.H., St peter W.L., Ebben J. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis. 2003;42:972–981. doi: 10.1016/j.ajkd.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda K., Fujii K., Fujimi S. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis. 2005;45:1058–1066. doi: 10.1053/j.ajkd.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Turner M., Barber M., Dodds H. Agreement between routine electronic hospital discharge and Scottish Stroke Care Audit (SSCA) data in identifying stroke in the Scottish population. BMC Health Serv Res. 2015;15:583. doi: 10.1186/s12913-015-1244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackland D.T., Roccella E.J., Deutsch A.F. Factors influencing the decline in stroke mortality. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steenkamp R., Rao A., Fraser S. UK Renal Registry 18th annual report (December 2015) chapter 5: survival and causes of death in UK adult patients on renal replacement therapy in 2014: national and centre-specific analyses. Nephron. 2016;132:111–144. doi: 10.1159/000444819. [DOI] [PubMed] [Google Scholar]
- 32.Malmivaara A., Meretoja A., Peltola M. Comparing ischaemic stroke in six European countries. The EuroHOPE register study. Eur J Neurol. 2015;22:284–291. doi: 10.1111/ene.12560. [DOI] [PubMed] [Google Scholar]
- 33.Information Services Division Scotland (ISD). Assessment of SMR01 Data Scotland 2014-2015. Available at: http://www.isdscotland.org/Products-and-Services/Data-Quality/Assessments. Accessed August 30, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cause of death as per International Classification of Diseases (ICD-10) rules, in all end-stage renal disease patients versus the general population.
Cause of death as per International Classification of Diseases (ICD-10) rules, in dialysis patients versus the general population.
Cause of death as per International Classification of Diseases (ICD-10) rules, in all renal transplant patients versus the general population.
Baseline demographics of all dialysis patients, no stroke versus stroke.
Baseline demographics of all renal transplant patients, no stroke versus stroke.
Auditable standards in all end-stage renal disease patients matched to the general population.
Auditable standards in dialysis patients matched to the general population.
Auditable standards in renal transplant patients matched to the general population.
Multivariable Cox regression, effect of admission to a stroke unit on mortality after stroke.
Flow diagram demonstrating construction of the data set to calculate stroke incidence in patients with end-stage renal disease (ESRD), 2005 to 2013.
Flow diagram demonstrating the construction of the data set to compare presentation, management, and outcomes following stroke and propensity score matching.
Strobe checklist.