Abstract
Introduction
Circular RNAs (circRNAs) have recently been described as novel noncoding regulators of gene expression. They might have an impact on microRNA expression by their sponging activity. The detectability in blood of these RNA transcripts has been demonstrated in patients with cancer and cardiovascular disease. We tested the hypothesis that circulating circRNAs in blood of critically ill patients with acute kidney injury (AKI) at inception of renal replacement therapy may also be dysregulated and associated with patient survival.
Methods
We performed a global circRNA expression analysis using RNA isolated from blood of patients with AKI as well as controls. This global screen revealed several dysregulated circRNAs in patients with AKI. Most highly increased circRNA-array−based transcripts as well as expression of the circRNA target miR-126-5p were confirmed in blood of 109 patients with AKI, 30 age-matched healthy controls, 25 critically ill non-AKI patients, and 20 patients on maintenance hemodialysis by quantitative real-time polymerase chain reaction.
Results
Circulating concentrations of 3 novel circRNAs were amplified in blood of patients with AKI and in controls. Circular RNA sponge of miR-126 (or ciRs-126) was most highly altered compared to healthy controls and disease controls (fold change of 52.1). ciRs-126 was shown to bioinformatically sponge miR-126-5p, which was found to be highly suppressed in AKI patients and hypoxic endothelial cells. Cox regression and Kaplan−Meier curve analysis revealed ciRs-126 as an independent predictor of 28-day survival (P < 0.01).
Conclusion
Circulating concentrations of circRNAs in patients with AKI are detectable. ciRs-126 may potentially sponge miR-126-5p and acts as a predictor of mortality in this patient cohort.
Keywords: acute kidney injury, circulating circular RNAs, mortality, renal replacement therapy
Acute kidney injury is a severe complication in critically ill patients and has been identified as an independent risk factor concerning survival.1 Mortality of patients with AKI in the intensive care unit setting is highly prevalent and unaltered over recent, years despite significant improvements in supportive care.2 As revealed by a multicenter study involving a large number of patients in the intensive care unit, the in-hospital mortality of patients with AKI exceeds 60%.3 Thus, early biomarkers enabling the clinician to detect subclinical AKI identifying patients at particular risk for both death and prolonged kidney failure after AKI in the setting of intensive care medicine and renal replacement therapy (RRT) remains an area of utmost interest. More than 90% of the human genome is transcribed into RNA transcripts without protein-coding potential.4, 5 These so called noncoding RNAs (ncRNAs) are arbitrarily separated into long ncRNAs (lncRNAs, ≥200 nucleotides) and small ncRNAs (≤200 nucleotides), based on their size. Small RNAs such as microRNAs (miRNAs) have been extensively studied over the past years.6, 7, 8 miRNA activity has been shown to be affected by the presence of miRNA sponge transcripts, the so-called competing endogenous RNA. Circular RNAs, which have recently been discovered, are likely a part of the aforementioned competing endogenous RNA class. They are endogenously expressed as single-stranded, covalently closed circular molecules.9 Most circRNAs are produced in a “back-splicing” reaction, in which a splice donor site is joined with an upstream splice acceptor site.10, 11 Circular RNAs have long been described in viroids and viruses12, 13 but have only recently come into focus in the mammalian genome.14, 15 A landmark study has proved the release into the blood of patients and elucidated the biomarker potential of this novel RNA species.16 Here, it was shown that circRNAs are remarkably stable (probably due to resistance to exonucleases through circularization) and highly expressed compared to their corresponding linear mRNAs. One of the intriguing functions of circRNAs is the sponging of microRNAs, which have been shown to serve as early and reliable biomarkers of AKI by our group.17 We here present the first study investigating the pattern of circulating circular RNAs in blood of critically ill patients with AKI requiring RRT, and their potential predictive value concerning outcome. Circulating circRNAs as well as potentially regulated miR-126-5p were assessed in RNA isolated from blood of 109 patients with AKI, 30 age-matched healthy controls, 25 non-AKI, critically ill patients and 20 patients on maintenance hemodialysis. Hsa_circ_0003266 was shown to potentially sponge miR-126-5p and is therefore referred to as circular RNA sponge of miR-126 (ciRs-126).
Methods
Patients and Procedures
This study is a post hoc measurement of prospectively collected blood samples from the Hannover Dialysis Outcome (HANDOUT) trial.18 Patients in 7 intensive care units of the tertiary care center at the Hannover Medical School with AKI were evaluated for inclusion. The study protocol was approved by the Hannover Medical School Ethics Committee and was conducted in accordance with the Declaration of Helsinki and German Federal Guidelines. The inclusion criteria were non−post-renal AKI with RRT dependence, indicated by the loss of kidney function of >30% calculated estimated glomerular filtration rate (eGFR) with either the Modification of Diet in Renal Disease (MDRD) or Cockcroft–Gault equation and/or cystatin C−glomerular filtration rate within 48 hours prior to inclusion and oliguria/anuria (<30 ml/h >6 hours prior to inclusion) or hyperkalemia (>6.5 mmol/l) or severe metabolic acidosis (pH <7.15, bicarbonate <12 mmol/l). Exclusion criteria were pre-existing chronic kidney disease as defined by eGFR <60 ml/min or a serum creatinine concentration >1.7 mg/dl more than 10 days prior to initiation of the first RRT. In addition, we considered the presence of an arteriovenous fistula or dialysis catheter as indicative of chronic kidney disease. Further exclusion criteria were participation in another study, consent denial or withdrawal, and need for extracorporeal membrane oxygenation therapy. Enrollment was performed by attending nephrologists after obtaining written informed consent from a patient or his/her legal representatives. If the patient was recovering and able to communicate, he/she was informed of the study purpose, and consent was required to further maintain his/her status as a study participant.
After inclusion, the specific medical condition leading to RRT initiation was documented from a list of 4 possible causes requiring immediate RRT. All patients received a nutritional intake of at least 25 to 30 kcal/kg per day, preferentially delivered as enteral nutrition. The prescribed protein intake was >1.2 g/kg per day. Renal replacement therapy in all patients was performed in a slow extended dialysis mode using the GENIUS dialysis system (Fresenius Medical Care, Bad Homburg, Germany) as described in detail elsewhere.19 The dose of the RRT was tailored according to the patient's individual need, starting with at least 1 treatment daily. The RRT was discontinued in patients meeting the following criteria for renal recovery: urine output >1000 ml/d and/or increased solute clearance, that is, a decline in pretreatment serum creatinine concentration with eGFR >15 ml/min (by the Modification of Diet in Renal Disease or Cockroft–Gault equation, and/or cystatin C−glomerular filtration rate. Blood was drawn immediately before the start of RRT. The first blood sample was discarded to avoid fibroblasts and activated platelets. Serum creatinine and serum C-reactive protein (CRP) concentrations were determined by an automated analyzer (Beckman Coulter, Brea, CA). The Sequential Organ Failure Assessment (SOFA) score20 and Acute Physiology and Chronic Health Evaluation II (APACHE II) score21 were obtained for each patient immediately before initiation of RRT. The presence of sepsis was defined according to the Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society International Sepsis Definitions.22 Acute kidney injury was classified post hoc by means of the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria at initiation of RRT.23 The Horowitz index was calculated as the ratio of partial pressure of oxygen in blood (PaO2) and the fraction of oxygen in the inhaled air (FIO2).
A total of 25 critically ill non-AKI patients (12 male, 13 female; age 52 years, range 42−71 years; 20 patients with acute myocardial infarction, 2 patients with liver disease, 3 patients following surgery) as well as 20 patients on maintenance hemodialysis (11 male, 9 female; age 49 years, range 39−62 years) served as disease controls. Thirty age-matched healthy controls were also included (16 male, 14 female; age 54 years, 46−66 years).
Study Outcomes and Statistical Analysis
The main objective of the study was to analyze the predictive value of circulating circRNAs concerning mortality and renal recovery of critically ill patients with AKI receiving RRT. The study endpoint was defined as survival 4 weeks after initiation of RRT and renal recovery (no RRT requirement) in survivors 4 weeks after initiation of RRT.
A detailed Supplementary Materials and Methods section can be found in the Supplementary Material.
Results
circRNA Expression Analysis in Blood
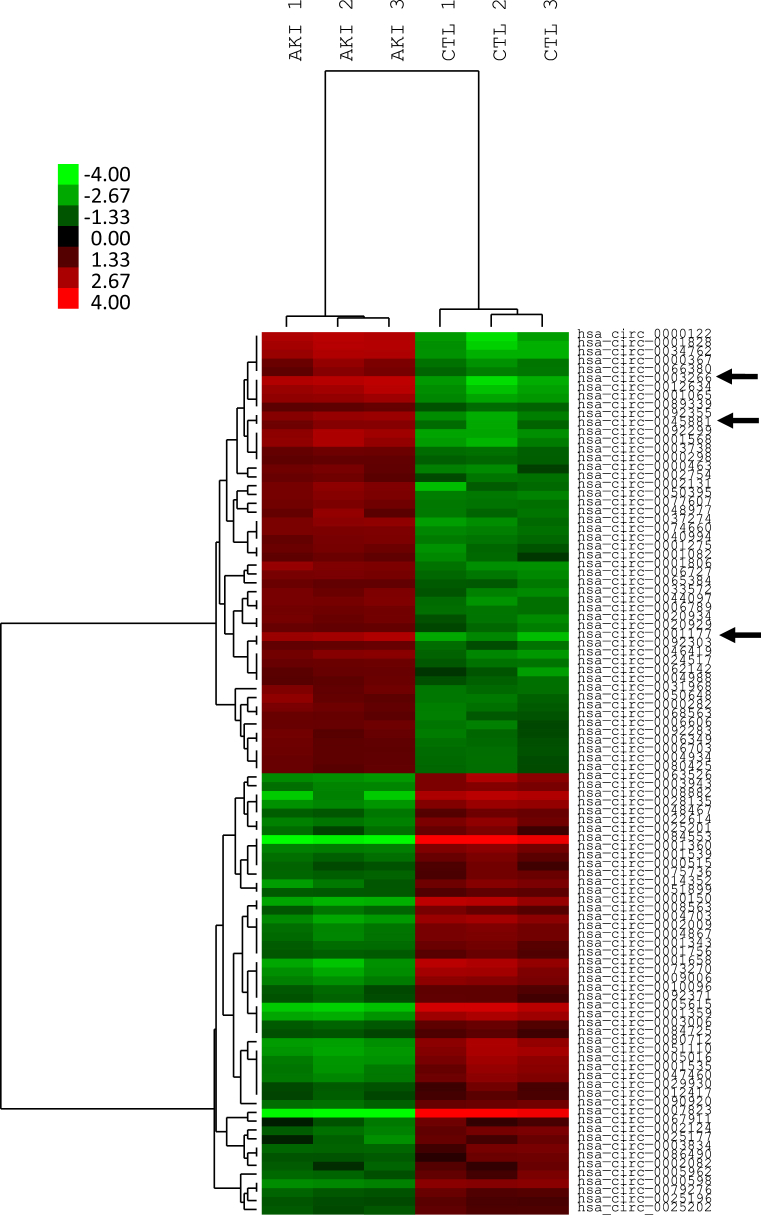
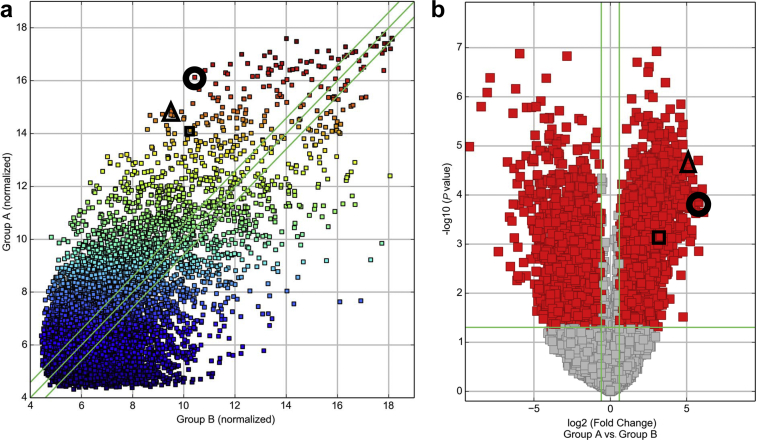
To assess an impact of AKI on circulating circRNAs, we conducted a genome-wide expression analysis in RNA isolated from whole blood of patients with AKI at inception of RRT (n = 15, 3 pools of 5 patients) and healthy age-matched controls (n = 15, 3 pools of 5 patients). Figure 1 shows that hierarchical clustering analysis clearly distinguished AKI patients from controls, as evidenced by a specific signature of significantly dysregulated circulating circRNAs. Figure 2a shows a scatter plot and Figure 2b a volcano plot analysis of identified circRNAs. circRNAs that were finally assessed in the whole cohort of patients are marked in Figure 2. The clinical characteristics of the whole cohort of AKI patients (n = 109) are shown in Table 1. A total of 4161 upregulated circulating circRNAs were detectable in all groups of the array cohort, showing a signal intensity >9 and differing between patients and healthy controls. Supplementary Table S1 displays the 50 most upregulated circRNAs in blood of AKI patients, and Supplementary Table S2 lists the top 50 downregulated circRNAs.
Figure 1.
Hierarchical cluster analysis of dysregulated circulating RNAs (circRNAs) in blood of patients with acute kidney injury (AKI) and healthy controls. Red colors represent upregulated circRNAs; green colors represent downregulated circRNAs. hsa_circ_0003266 (or ciRS-126), hsa_circ_0045881, and hsa_circ_00011776 are marked by an arrow.
Figure 2.
Scatter plot (a) and volcano plot analysis (b) of deregulated circulating RNAs (circRNAs) in blood of patients with acute kidney injury (AKI) and healthy controls. Identified circRNA are further marked: ciRS-126 (circle), hsa_circ_0045881 (triangle), and hsa_circ_0001177 (square).
Table 1.
Demographic, clinical, and laboratory characteristics of patients
| Characteristic | Total | Survivors | Nonsurvivors | P value |
|---|---|---|---|---|
| Patients, n | 109 | 69 | 40 | 0.6 |
| Male, n (%) | 64 (59) | 40 | 25 | |
| Female, n (%) | 45 (41) | 29 | 15 | |
| Discipline of ICU admission | 0.4 | |||
| Medicine, n (%) | 46 (42) | 27 (39) | 19 (48) | |
| General surgery, n (%) | 27 (25) | 16 (23) | 11 (28) | |
| Cardiac surgery, n (%) | 36 (33) | 26 (38) | 10 (24) | |
| Age, yr | 52 (40–63) | 52 (44–63) | 51 (37–63) | 0.8 |
| BMI, kg/m2 | 25 (22–28) | 25.4 (22–28) | 24.7 (22–28) | 0.6 |
| Indication for RRT | ||||
| eGFR loss >30% | 93 | 59 | 34 | 0.9 |
| Oliguria/anuria | 71 | 46 | 25 | 0.8 |
| Metabolic acidosis | 8 | 4 | 4 | 0.4 |
| Hyperkalemia | 6 | 2 | 4 | 0.1 |
| SOFA score | 13 (10–15) | 14 (11–15.5) | 13 (10–15) | 0.7 |
| Renal | 2 (1.5–3) | 2 (1–2.5) | 2 (2–3) | 0.02a |
| Coagulation | 1 (0–2) | 2 (0–2) | 1 (0–2) | 0.6 |
| Cardiovascular | 4 (0.5–4) | 4 (2–4) | 3 (0–4) | 0.2 |
| Nervous system | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.9 |
| Respiratory system | 2 (1–3) | 2 (1–3) | 2 (2–3) | 0.8 |
| Liver | 2 (0–2) | 2 (0–3) | 2 (0–2) | 0.4 |
| RIFLE class | 0.1 | |||
| Risk, n (%) | 10 (9) | 9 (13) | 1 (3) | 0.07 |
| Injury, n (%) | 15 (14) | 9 (13) | 6 (15) | 0.8 |
| Failure, n (%) | 84 (77) | 51 (74) | 33 (82) | 0.3 |
| APACHE II score | 34 (27–36) | 33 (26–36) | 35 (29–39.8) | 0.6 |
| CRP (mg/l) | 113 (56–197) | 82 (46–191) | 145.5 (67–211.5) | 0.2 |
| MAP (mm Hg) | 75.5 (67–90) | 77 (70–93) | 74 (64–86) | 0.3 |
| Heart rate (bpm) | 100 (85–110) | 99 (84–109) | 100 (89–111) | 0.3 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; MAP, mean arterial blood pressure; n, number of patients; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
Significant.
circRNA Validation in Whole Blood
The detection and amplification of circRNAs in blood is challenging due to their low abundance. To investigate the detectability of dysregulated circulating circRNAs, we assessed the detectability of the most highly dysregulated circRNAs using RNA isolated from whole blood of patients with AKI in agarose gel electrophoresis. Here, we found 3 circRNAs to display specific bands of correct size, including ciRs-126, hsa_circ_0045881, and hsa_circ_0001177. We then performed real-time polymerase chain reaction analysis in a subset of patients with AKI (n = 10). These 3 novel transcripts showed clean amplification curves, showed specific curves in melting curve analysis, and were undetectable in water controls without template. To ascertain that transripts were specifically detected in whole blood, we sequenced transcripts in these patients after polymerase chain reaction amplification, which confirmed that circRNAs were correctly amplified and detectable.
ciRs-126 Is a Biomarker of AKI
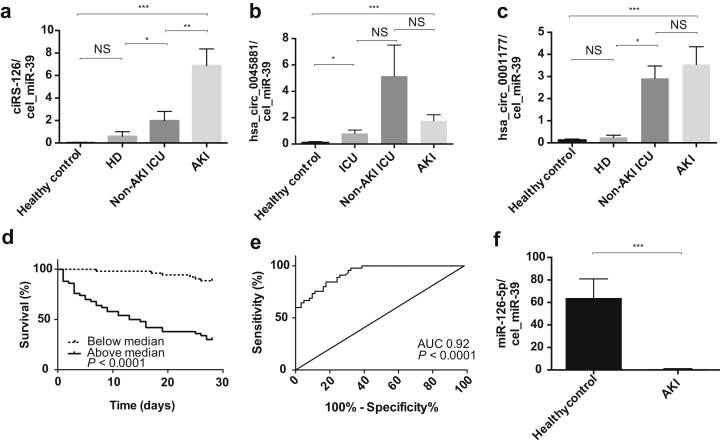
We next analyzed ciRs-126, hsa_circ_0045881, and hsa_circ_0001177 in the whole cohort of patients with AKI (n = 109) as well as disease controls (non-AKI, critically ill disease controls, n = 25; patients on maintenance hemodialysis, n = 20) and age-matched healthy individuals (n = 30). As shown in Figure 3a, ciRs-126 was significantly increased compared to that in healthy controls and disease controls, underlining the specificity as a biomarker in AKI (P < 0.001). Both hsa_circ_0045881 and hsa_circ_0001177 were significantly increased compared to those in healthy controls, but not compared to those in disease controls (Figure 3b and c). Baseline ciRs-126 correlated with SOFA score (r = 0.2, P = 0.02), length of need for respirator therapy (r = −0.4, P = 0.01), and length of stay on the intensive care unit (r = −0.5, P < 0.01).
Figure 3.
Concentrations of circulating (a) ciRS-126, (b) hsa_circ_0045881, and (c) hsa_circ_0001177 in patients with acute kidney injury (AKI) compared to healthy controls and non-AKI, critically ill disease controls and patients on maintenance hemodialysis. (d) Kaplan−Meier curve analysis and log rank testing in AKI patients above and below median during an observation of 4 weeks concerning ciRS-126. (e) Receiver operating characteristic curve (ROC) analysis identifies a cut point of 1.165 relative expression with an area under the curve (AUC) of 0.92, a sensitivity of 91%, and a specificity of 74% regarding ciRS-126. (f) Circulating levels of miR-126-5p in patients with AKI. ***P < 0.0001, **P < 0.01, *P < 0.05. ICU, intensive care unit; HD, hemodialysis; NS, not significant.
ciRs-126 Predicts Survival in AKI
Patients were grouped as survivors and nonsurvivors at 4 weeks after initiation of RRT. Patient groups were comparable with respect to baseline demographics and the proportion of RIFLE categories (Table 1). A total of 40 patients died in our cohort. Concentrations of circulating ciRs-126 were significantly (P < 0.01) increased in nonsurvivors. To investigate the prognostic potential of circulating ciRs-126 concentrations at initiation of RRT with regard to overall mortality, we performed univariate Cox proportional hazards analyses. ciRs-126 was subjected to natural logarithmic transformation. In our cohort of 109 critically ill patients with AKI, ciRs-126 concentrations, major surgery, sepsis, shock, APACHE II, SOFA, and Horowitz score showed prognostic significance at a 10% level and were subsequently subjected to multivariate Cox regression analysis (Table 2). Only APACHE II score (P = 0.06) and ciRs-126 concentrations (P < 0.001) remained independent predictors of survival in multivariate analysis. In receiver operating characteristic curve analysis ciRs-126 levels yielded an area under the receiver operating characteristic curve value of 0.92 (SEM: 0.02; 95% confidence interval: 0.88−0.97; P < 0.0001). In receiver operating characteristic curve analysis, we defined a cut point of 1.165 relative expression by ΔΔCt analysis with 91% sensitivity and 74% specificity. Figure 3d illustrates the Kaplan−Meier survival curve 4 weeks after initiation of RRT stratified to ciRs-126 concentrations above and below the median. A log rank test confirmed the statistical significance for circulating ciRs-126 concentrations (P < 0.001). Figure 3e shows receiver operating characteristic curve analysis.
Table 2.
Univariate and multivariate Cox regression analysis for survival
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| circRNA_ln | 2.678 | 2.056–3.487 | <0.001a | 2.610 | 2.012–3.385 | <0.001a |
| Sepsis (yes/no) | 2.425 | 1.341–4.388 | 0.003a | |||
| Surgery (yes/no) | 2.074 | 1.088–3.954 | 0.03a | |||
| SOFA score | 1.167 | 1.064–1.280 | 0.01a | |||
| APACHE II score | 1.046 | 1.005–1.088 | 0.03a | 1.040 | 0.999–1.083 | 0.06a |
| Horowitz score | 0.997 | 0.994–1.000 | 0.03a | |||
APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; circRNA_ln, log transformed hsa_circ_0003266; HR, hazard ratio; SOFA, Sequential Organ Failure Assessment.
P < 0.1.
ciRs-126 as a Potential miRNA Sponge
As identified by the Arraystar miRNA target prediction software (Arraystar, Rockville, MD), which is based on the TargetScan and miRanda algorithm, miRNA binding elements were identified regarding miR-126-5p in the ciRs-126 sequence (Supplementary Figure S1). To test the potential interaction between ciRs-126 and its target miRNA, miR-125-5p was amplified in the whole cohort of patients and was found to be highly suppressed in AKI patients compared to controls, indicating its potential regulation by ciRs-126 (Figure 3f). We therefore refer to hsa_circ_0003266 as circular RNA sponge for miR-126 (ciRs-126).
circRNAs and miR-126-5p Are Regulated in Cells
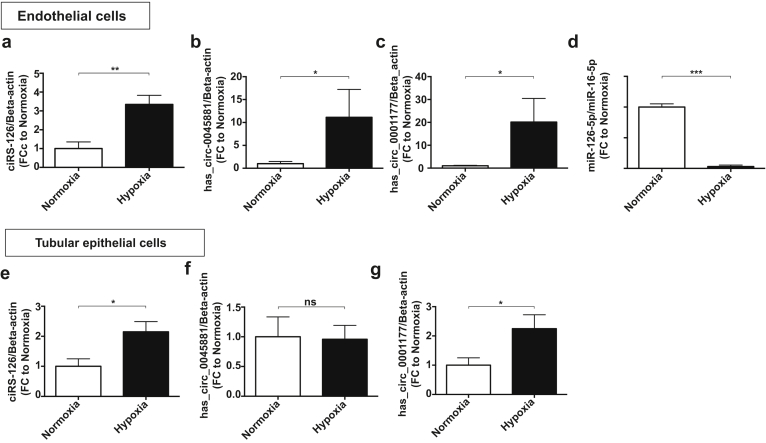
Endothelial cells and proximal tubular epithelial cells are most highly affected by AKI. Accordingly, we assessed the expression of the 3 novel circRNAs in these cells under hypoxic conditions. All 3 transcripts are significantly upregulated in hypoxic endothelial cells, whereas only ciRs-126 and hsa_circ_0001177 were induced in hypoxic proximal tubular epithelial cells (Figure 4a–c and Figure 4e–g). miR-126-5p is an endothelial-specific microRNA. Accordingly, miR-126-5p was assessed in endothelial cells in vitro and was found to be highly suppressed by hypoxia (Figure 4d).
Figure 4.
(a–c) Levels of ciRS-126, hsa_circ_0045881, and hsa_circ_0001177 in cultured endothelial cells and (e–g) proximal tubular epithelial cells subjected to hypoxia. (d) miR-126-5p in endothelial cells subjected to hypoxia. ***P < 0.0001, **P < 0.01, *P < 0.05. FC, fold change; NS, not significant.
Discussion
Our study is the first evaluation of circulating circRNAs in any cohort of patients with kidney disease. We were able to show the following: (i) circulating circRNAs in whole blood of critically ill patients with AKI were detectable; (ii) circulating circRNA concentrations specifically identified patients with AKI; (iii) baseline concentrations of ciRs-126 correlated with parameters of disease severity; (iv) baseline concentrations of circulating ciRs-126 were increased in nonsurvivors compared to survivors; (v) ciRs-126 was identified as a strong independent prognostic factor for 28-day-survival in the multivariate Cox proportional hazards regression analysis and Kaplan−Meier curve analysis; and (vi) circulating levels of miR-126-5p were highly suppressed in AKI patients and endothelial cells.
circRNAs show widespread distribution and diverse functions. They are ∼100 nucleotides in length and are highly present in the eukaryotic transcriptome and abundant in exosomes.24 As stated in the introduction, circRNAs are likely produced in a “back-splicing” reaction, in which a splice donor site is joined with an upstream splice acceptor site, thus forming a circular structure in which the 3′- and 5′-ends are covalently linked.10, 11 These circRNAs are termed “exonic” circRNAs because they arise from known exons at annotated splice sites, as opposed to “intronic” circRNAs, containing a 2′-5′ carbon linkage at the branch point stemming from introns. Owing to their circular structure and the absence of a 5′ cap, it is currently believed that circRNAs are not translated into protein.25 A characteristic element of circRNAs is the “head-to-tail” splice junction (due to backsplicing), in which exons are organized in reverse order compared to their chromosomal localization. One likely cellular function of circRNAs is the binding and sequestering of miRNAs,24, 26 but this interaction might only be observed in circRNAs with a high number of binding sites for a specific miRNA. For instance, ciRs-7 (circular RNA sponge for miR-7) has been identified in mouse brain and shown to express more than 70 conserved miR-7 target sites.26 Moreover, it is highly associated with Argonaute (AGO) proteins in a miR-7−dependent manner.26 It strongly suppresses miR-7 activity, resulting in increased levels of miR-7 targets.26 circRNAs likely exert further functions, as has been described for lncRNAs. Because circRNAs are less susceptible to exonuclease activity (due to their circular structure), they exhibit significantly longer half-lives than linear RNAs,24 suggesting their ideal role as stable biomarkers in body fluids of patients.
We identified several putative binding sites of miR-126-5p in the sequence of ciRs-126. miR-126 has previously been shown to lead to resolution of AKI in mice.27 Hematopoietic overexpression of miR-126 increased neovascularization, led to an improvement of kidney function, and increased numbers of bone marrow−derived endothelial cells. The numbers of circulating Lin(−)/Sca-1(+)/cKit(+) hematopoietic stem and progenitor cells were increased.27 Deactivation of miR-126 was shown to induce a pseudohypoxia state due to increased HIFα expression in kidney cancer. Serpin Family E Member 1 (SERPINE1) was identified as a miR-126-5p target regulating cell motility.28 Chronic heart failure was significantly associated with lower circulating levels of miR-126-5p.29 Lack of miR-126-5p reduced endothelial cell proliferation by derepression of the Notch1 inhibitor delta-like 1 homolog (Dlk1).30 Currently, we are unable to provide definitive proof that ciR-126 indeed sponges miR-126-5p. Based on bioinformatic sequence homology and level of regulation in our analyses, we hypothesize that it may be regulated by ciRs-126. Future studies will address the experimental proof of binding interaction.
There are several distinct possible sources of circulating circRNAs. It was previously shown that circRNAs are physiological components of neutrophils, B-cells, and hematopoietic stem cells, confirming that they may be derived from these circulating cells.31 It is moreover conceivable that they might also be secreted into the extracellular space and transported in exosomes or small vesicles, as has been shown for microRNAs. This has been suggested recently.32 Platelets may be another important source of circRNAs, as has recently been described.33, 34 CircRNAs have been shown to serve as noninvasive diagnostic tools in patients with atherosclerosis,15 disorders of the central nervous system,35 degenerative diseases,10 and cancers.32, 36
As has been previously discussed for microRNAs and lncRNAs, a “housekeeping” circRNA has not been identified in bodily fluids of patients, which is why a strategy was chosen to normalize circulating circRNAs to recombinant Caenorhabditis elegans miR-39 (cel_miR-39).6, 7, 17, 37, 38, 39 Cel_miR-39 was supplemented to samples during the RNA isolation process.
We focused on the novel circRNA transcript ciRs-126, which we identified as a specific biomarker of AKI. A specific role for this circRNA has not yet been described. We herein provide evidence of ciRs-126 as an independent prognostic survival factor in AKI. Of note, miR-126-5p might be a downstream interaction partner mediating the effects of our newly identified circRNA.
Intriguingly, the linear counterpart Leucine-rich repeats and Ig-like domains protein 1 (LRIG1) of ciRs-126 might have an important role in acute kidney injury. It encodes a transmembrane protein that has been shown to interact with receptor tyrosine kinases of the EGFR family,40 tyrosine-protein kinase Met,41 and RET proto-oncogene.42 Met has been shown to be highly important for early cytoprotection and regeneration in ischemic AKI.43 Activation of EGFR was demonstrated to accelerate recovery from acute nephrotoxic kidney injury.44 Because miR-126 has been shown to control endothelial cell homeostasis30 as well as hypoxia signaling28 and has also been shown to be an important protective microRNA in AKI,27 we hypothesize that ciRs-126 may be part of signaling cascade involving LRIG and miR-126, which may have implications as a cytoprotective mechanism in AKI, which is released into the circulation to counteract progressive kidney injury.
Important limitations to our study should be mentioned. First, we do not outline insights into associated molecular mechanisms of ciRs-126 release. In-depth basic science studies are warranted to assess the specific function of ciRs-126 in the kidney. Second, our study represents merely a single-center experience with a limited number of patients. Larger independent cohorts are highly desirable to validate our findings. Finally, most patients recruited in our study were patients with severe kidney injury (RIFLE stage 3). It would be desirable to include more patients with mild and moderate kidney injury (RIFLE stages 1 and 2) in future studies.
In conclusion, our study is the first to provide insights into the concentrations of circulating circRNAs in critically ill patients with AKI. A large number of circulating circRNAs can be reliably detected in blood of AKI patients. We identified the novel circRNA transcript ciRs-126 as a strong and independent predictor of mortality in critically ill patients with AKI. Circulating ciRs-126 may therefore be an intriguing RNA-based biomarker that might reflect miRNA dysregulation noninvasively.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We kindly acknowledge support of the Fresenius Foundation in form of the Memorial Scholarship to JML as well as the National Competence Center in Research Kidney (NCCR Kidney.CH) in form of the junior grant to JML and the Nephro Physician-Scientist grant to MK.
Footnotes
Supplementary Methods.
Table S1. Top 50 upregulated circRNAs in blood of AKI patients versus healthy controls, analyzed circRNAs in the whole cohort are marked in red.
Table S2. Top 50 downregulated circRNAs in blood of AKI patients versus healthy controls.
Table S3. Primer pairs.
Figure S1. Sequence base pairing between ciRs-126 and miR-126-5p (B). Bp = base pairs.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Top 50 upregulated circRNAs in blood of AKI patients versus healthy controls, analyzed circRNAs in the whole cohort are marked in red.
Top 50 downregulated circRNAs in blood of AKI patients versus healthy controls.
Primer pairs.
Sequence base pairing between ciRs-126 and miR-126-5p (B). Bp = base pairs.
References
- 1.Barrantes F., Tian J., Vazquez R. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 2.Mehta R.L., Kellum J.A., Shah S.V. Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S., Kellum J.A., Bellomo R. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.ENCODE Project Consortium. Bernstein B.E., Birney E. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djebali S., Davis C.A., Merkel A. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzen J.M., Thum T. Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol. 2012;7:1528–1533. doi: 10.2215/CJN.01170212. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzen J.M., Haller H., Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol. 2011;7:286–294. doi: 10.1038/nrneph.2011.26. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzen J.M., Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360–373. doi: 10.1038/nrneph.2016.51. [DOI] [PubMed] [Google Scholar]
- 9.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwal-Fluss R., Meyer M., Pamudurti N.R. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Starke S., Jost I., Rossbach O. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Sanger H.L., Klotz G., Riesner D. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kos A., Dijkema R., Arnberg A.C. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986;323:558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 14.Zaphiropoulos P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A. 1996;93:6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burd C.E., Jeck W.R., Liu Y. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memczak S., Papavasileiou P., Peters O., Rajewsky N. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS One. 2015;10:e0141214. doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzen J.M., Kielstein J.T., Hafer C. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–1546. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 18.Faulhaber-Walter R., Hafer C., Jahr N. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2009;24:2179–2186. doi: 10.1093/ndt/gfp035. [DOI] [PubMed] [Google Scholar]
- 19.Fliser D., Kielstein J.T. Technology insight: treatment of renal failure in the intensive care unit with extended dialysis. Nat Clin Pract Nephrol. 2006;2:32–39. doi: 10.1038/ncpneph0060. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Knaus W.A., Draper E.A., Wagner D.P. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 22.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R., Ronco C., Kellum J.A. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memczak S., Jens M., Elefsinioti A. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 25.Li X.F., Lytton J. A circularized sodium-calcium exchanger exon 2 transcript. J Biol Chem. 1999;274:8153–8160. doi: 10.1074/jbc.274.12.8153. [DOI] [PubMed] [Google Scholar]
- 26.Hansen T.B., Jensen T.I., Clausen B.H. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 27.Bijkerk R., van Solingen C., de Boer H.C. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J Am Soc Nephrol. 2014;25:1710–1722. doi: 10.1681/ASN.2013060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Chen H., Wong N. Pseudohypoxia induced by miR-126 deactivation promotes migration and therapeutic resistance in renal cell carcinoma. Cancer Lett. 2017;394:65–75. doi: 10.1016/j.canlet.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang A., Kwee L.C., Grass E. Whole blood sequencing reveals circulating microRNA associations with high-risk traits in non-ST-segment elevation acute coronary syndrome. Atherosclerosis. 2017;261:19–25. doi: 10.1016/j.atherosclerosis.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Schober A., Nazari-Jahantigh M., Wei Y. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salzman J., Gawad C., Wang P.L. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Zheng Q., Bao C. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhasan A.A., Izuogu O.G., Al-Balool H.H. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood. 2016;127:e1–e11. doi: 10.1182/blood-2015-06-649434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preußer C., Hung L.H., Schneider T. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1424473. doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukiw W.J. Circular RNA (circRNA) in Alzheimer's disease (AD) Front Genet. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P., Chen S., Chen H. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzen J.M., Schauerte C., Kölling M. Long noncoding RNAs in urine are detectable and may enable early detection of acute T cell-mediated rejection of renal allografts. Clin Chem. 2015;61:1505–1514. doi: 10.1373/clinchem.2015.243600. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzen J.M., Schauerte C., Kielstein J.T. Circulating long noncoding RNATapSaki is a predictor of mortality in critically ill patients with acute kidney injury. Clin Chem. 2015;61:191–201. doi: 10.1373/clinchem.2014.230359. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzen J.M., Volkmann I., Fiedler J. Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant. 2011;11:2221–2227. doi: 10.1111/j.1600-6143.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 40.Gur G., Rubin C., Katz M. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004;23:3270–3281. doi: 10.1038/sj.emboj.7600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shattuck D.L., Miller J.K., Laederich M. LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol. 2007;27:1934–1946. doi: 10.1128/MCB.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledda F., Bieraugel O., Fard S.S. Lrig1 is an endogenous inhibitor of Ret receptor tyrosine kinase activation, downstream signaling, and biological responses to GDNF. J Neurosci. 2008;28:39–49. doi: 10.1523/JNEUROSCI.2196-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason S., Hader C., Marlier A. Met activation is required for early cytoprotection after ischemic kidney injury. J Am Soc Nephrol. 2014;25:329–337. doi: 10.1681/ASN.2013050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z., Chen J.K., Wang S.W. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14:3147–3154. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Top 50 upregulated circRNAs in blood of AKI patients versus healthy controls, analyzed circRNAs in the whole cohort are marked in red.
Top 50 downregulated circRNAs in blood of AKI patients versus healthy controls.
Primer pairs.
Sequence base pairing between ciRs-126 and miR-126-5p (B). Bp = base pairs.