Abstract
Introduction
Urinary neutrophil gelatinase−associated lipocalin (uNGAL) and urinary kidney injury molecule−1 (uKIM-1) are established markers of subclinical acute kidney injury. In persons with reduced estimated glomerular filtration rate (eGFR) and albuminuria who are at high risk for end-stage renal disease (ESRD) and death, the associations of these urinary markers with incident ESRD or death is an area of active investigation.
Methods
Among 1472 black and white participants from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study with eGFR ≤60 ml/min per 1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] cystatin, 2012) and albumin-to-creatinine ratio (ACR) ≥30 mg/g, we evaluated the associations of baseline uNGAL and uKIM-1 with progression to ESRD and all-cause death. Cox models were sequentially adjusted for urinary creatinine, traditional risk factors, C-reactive protein, ACR, and eGFR.
Results
There were 257 ESRD events and 819 deaths over a median follow-up of 5.7 and 6.5 years, respectively. In demographic adjusted models, higher levels of uNGAL were associated with increased risk of ESRD and death, but these associations were attenuated in fully adjusted models including baseline eGFR for both ESRD (hazard ratio [HR] = 1.06 per doubling, 95% confidence interval [CI] 0.98–1.14) and death (HR = 1.04, 95% CI = 1.00–1.08). Higher levels of uKIM-1 were associated with increased risk of ESRD and death in demographic-adjusted models, and although attenuated in fully adjusted models, remained statistically significant for both ESRD (HR = 1.24 per doubling, 95% CI = 1.08–1.42) and death (HR = 1.10, 95% CI =1.03–1.19).
Conclusion
In this cohort of high-risk patients with baseline eGFR ≤60 ml/min per 1.73 m2 and albuminuria, renal tubular injury is associated with higher mortality and progression to ESRD. Further studies are necessary to investigate the mechanism underlying this increased risk.
Keywords: chronic kidney disease, end-stage renal disease, tubular injury, urinary KIM-1, urinary NGAL
Neutrophil gelatinase−associated lipocalin (NGAL) and the ectodomain of the receptor kidney injury molecule−1 (KIM-1) are rapidly released by renal tubular epithelia following ischemic or toxic acute kidney injury (AKI).1, 2 Urinary levels of NGAL (uNGAL) and KIM-1 (uKIM-1) are established as markers of acute cellular injury within kidney tubules,3 and uKIM-1 is associated with poor outcomes among patients with AKI.4 With regard to biological function, NGAL has anti-inflammatory and antibacterial effects due to its capacity to sequester iron.3 In the setting of AKI, the receptor KIM-1 facilitates phagocytosis of apoptotic cells,5, 6 but in chronic kidney disease, KIM-1 may have pathogenic effects leading to interstitial fibrosis.7 Among individuals without CKD and any known AKI, baseline uKIM-1 is associated with incident CKD,8 and both uNGAL and uKIM1 are higher in patients with CKD compared to patients with normal kidney function.9, 10, 11, 12 Taken together, these observations suggest that in the ambulatory setting, uNGAL and uKIM-1 are markers of ongoing tubular injury.
Following these observations, there have been several epidemiological investigations of uKIM1 and uNGAL as predictors of ESRD and mortality, but results have differed depending on the population studied. For example, in a study of Pima Indians with type 2 diabetes, higher uNGAL was independently associated with long-term ESRD and mortality.13 In kidney transplant recipients, uNGAL was an independent risk factor for graft failure and mortality.14 Both uNGAL and KIM-1 were associated with overall mortality in the Chronic Renal Insufficiency Cohort (CRIC).15 However, neither uNGAL nor uKIM1 were independently associated with CKD progression in CRIC16 or in the community-based Atherosclerosis Risk in Communities Study (ARIC).17 Thus, in patients with stable CKD, whether elevated levels of uNGAL and uKIM1 are associated with ESRD and all-cause mortality in large, community-based cohorts has not been well established.
We designed this study to determine the importance of tubular injury, as measured by uNGAL and uKIM-1, with short-term and long-term risk for incident ESRD and all-cause death among black and white community-dwelling persons with impaired eGFR and albuminuria. Because uNGAL and KIM1 are produced rapidly in response to AKI, we hypothesized that they would have stronger associations with short-term outcomes than with long-term outcomes. We also hypothesized that tubular marker associations would be stronger in participants with more severe albuminuria, as elevations in both markers would represent synergistic tubular and glomerular injury. In addition, we examined whether these associations were independent of baseline serum markers of inflammation and renal function, as well as traditional kidney disease risk factors.
Methods
REGARDS is a national cohort study designed to study risk factors for stroke among black and white Americans in the “stroke belt” of the United States. Between 2003 and 2007, a total of 30,239 participants aged 45 years or older were recruited to the study. Participant information was first collected via telephone interview, and a trained technician then conducted an in-home examination for the anthropometric and physical examination, specimen collection, and medication inventory.18
For these analyses, we included REGARDS participants (N = 1472) with eGFRcys ≤60 ml/min per 1.73 m2 (CKD-EPI 2012) and ACR ratio ≥30 mg/g at baseline. We specified these inclusion criteria based on our prior work in REGARDS, which has shown that these persons are at highest risk for progression to ESRD and death.19 All appropriate institutional review boards approved this study, and participants provided written informed consent.
Outcomes
Incident ESRD was ascertained by linkage to the United States Renal Data System (USRDS, www.usrds.org), which maintains data on persons who initiate dialysis or receive a kidney transplant. For these analyses, ESRD status confirmed through linkage with USRDS was updated through 2014. Deaths were reported by participants’ proxies via telephone or mail, and were then confirmed by review of death certificates or by linkage to the Social Security Death Index.20 Deaths adjudicated through February of 2016 were included in these analyses.
Laboratory Methods
Urine samples were collected from participants during in-home visits. Specimens were shipped on ice to a central laboratory at the University of Vermont.21 All urine specimens were in continuous storage thereafter at −80°C. NGAL and KIM-1 were measured at the Cincinnati Children's Hospital Medical Center Biomarker Laboratory with the Bioporto Human NGAL enzyme-linked immunosorbent assay (Bioporto, Hellerup, Denmark) and with a KIM-1 assay kit developed in-house, using Duoset (R&D Systems, Minneapolis, MN) reagents.22 Coefficients of variation for uKIM-1 and uNGAL were 5.2% and 5.4%, respectively. Urine albumin was measured by nephelometry using the BNII ProSpec nephelometer (Dade-Behring, Deerfield, IL) and urine creatinine by the Jaffe method using the Modular-P chemistry analyzer (Roche/Hitachi, Basel, Switzerland) at the University of Vermont laboratory. Serum creatinine was calibrated to isotope dilution using mass spectrometry−traceable methods,23 and Cystatin C was measured by particle-enhanced immunonephelometry (N Latex Cystatin C on the BNII, Formerly Dade Behring, Now Siemens AG, Munich, Germany), as previously reported.19
Other Variables
Sociodemographic characteristics including age, race, sex, income, education, smoking status, and alcohol use were determined by self-report. Height and weight were measured by a trained technician and used to calculate body mass index (BMI). Hypertension was defined by self-reported use of antihypertensive medications or an average of 2 seated blood pressure readings (systolic blood pressure ≥140 mm Hg or diastolic pressure ≥90 mm Hg). Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting glucose ≥126 mg/dl, or nonfasting glucose ≥200 mg/dl. High-density lipoprotein and triglycerides were measured from fasting samples. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2012 equations for creatinine, cystatin, and combined creatinine−cystatin. eGFR cystatin was used as the inclusion criteria, and the combined creatinine−cystatin eGFR was used as a covariate in the multivariable models.24
Analyses
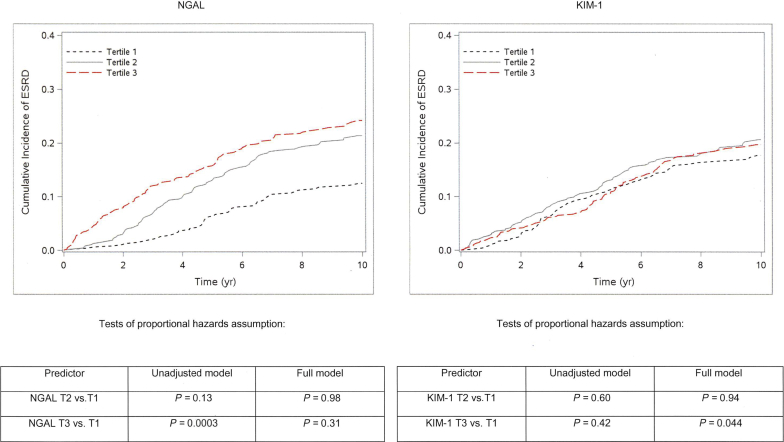
We first compared baseline characteristics by tertile of uNGAL concentration using the χ2 and Kruskal−Wallis tests for categorical and continuous variables, respectively. We then used Cox proportional hazards models to evaluate the association of each urinary biomarker with the risk of ESRD or death in separate models. Because of their right-skewed distributions, uNGAL and uKIM-1 were log transformed to normalize their distributions. We modeled uNGAL and uKIM-1 as both continuous (per doubling) and in tertiles. To determine whether each biomarker was independently associated with ESRD or mortality, multivariable models were sequentially adjusted for sociodemographic characteristics, kidney disease risk factors, C-reactive protein, ACR, and eGFR (CKD-EPI 2012 using creatinine and cystatin). We additionally adjusted for urine creatinine, to account for urine tonicity, and for ACR, to adjust for clinical evidence of glomerular damage. For ESRD outcomes, we conducted a sensitivity analysis for the competing risk of death by applying the Fine−Gray method.25 We considered both short-term (within 2 years) and long-term (within 10 years) outcomes, because we hypothesized that these established kidney injury markers, measured once at baseline, would have stronger associations with short-term outcomes than with long-term outcomes (as observed in CRIC).26 In support of this hypothesis, visual examination of the cumulative incidence of ESRD suggested that uNGAL and uKIM1 had associations with ESRD that varied over time (Figure 1). Formal tests of the proportional hazards assumption found that the effect of NGAL tertile 3 on ESRD risk varied over time in unadjusted (P = 0.0003) but not fully adjusted (P =0.31) analysis, whereas the effect of KIM tertile 3 on ESRD risk varied over time only in fully adjusted analysis (P = 0.044).
Figure 1.
Cumulative incidence of end-stage renal disease (ESRD), by tertile of urinary neutrophil gelatinase−associated lipocalin (NGAL) and kidney injury molecule−1 (KIM-1), in REasons for Geographic and Racial Differences in Stroke (REGARDS) study participants. Formal tests of the proportional hazards assumption found that the effect of NGAL tertile 3 (T3) on ESRD risk varied over time in unadjusted (P = 0.0003) but not fully adjusted (P = 0.31) analysis, whereas the effect of KIM T3 on ESRD risk varied over time only in fully adjusted analysis (P = 0.044).
Results
Baseline Characteristics
Among the 1472 participants included in these analyses, the overall mean (± SD) baseline age was 71 (±9.3) years; median (interquartile range) eGFR by CKD-EPI 2012, creatinine, and cystatin was 46 (35−55) ml/min per 1.73 m2; and median (interquartile range) ACR was 113 (55−417) mg/g. The median overall eGFRcys was 40 ml/min per 1.73 m2 versus eGFRcr of 53 ml/min per 1.73 m2. The median level (interquartile range) of uNGAL was 45 (21−127) ng/ml, and the median uKIM-1 was 886 (470−1720) pg/ml. Participants in the highest tertile of uNGAL were more likely to be female, African American, had lower income and educational levels, and had higher levels of C-reactive protein, albuminuria, and more advanced CKD, relative to those with lower levels of uNGAL. There was little difference across tertiles of NGAL for age, prevalence of diabetes, smoking, and hypertension (Table 1). Participants in the highest uKIM1 tertile were less likely to be female or African American, more likely to smoke, and had higher triglyceride levels, but did not differ in socioeconomic status. As with uNGAL, participants in the highest uKIM1 tertile had higher levels of C-reactive protein, albuminuria, and more advanced CKD (Table 2).
Table 1.
Baseline characteristics of REGARDS participants stratified by tertile of urinary NGAL
| Parameter | Tertile of NGAL |
P value | ||
|---|---|---|---|---|
| 1 (n = 490) | 2 (n = 491) | 3 (n = 491) | ||
| Range of NGAL, ng/ml | 0.1–26.4 | 26.5–81.4 | 81.5–5447 | |
| Age, yr | 71 (64–77) | 72 (64–78) | 70 (63–78) | 0.31 |
| Female gender | 145 (30%) | 223 (45%) | 310 (63%) | <.0001 |
| Race | ||||
| Black | 187 (38%) | 251 (51%) | 268 (55%) | <.0001 |
| White | 303 (62%) | 240 (49%) | 223 (45%) | |
| Income | 0.0021 | |||
| <$34k | 248 (59%) | 293 (68%) | 27 (6%) | |
| $35–74k | 133 (32%) | 109 (25%) | 99 (23%) | |
| $75k and above | 41 (10%) | 27 (6%) | 25 (6%) | |
| Diabetes mellitus | 246 (51%) | 230 (47%) | 241 (49%) | 0.55 |
| Hypertension | 419 (86%) | 414 (85%) | 422 (86%) | 0.92 |
| History of MI | 206 (43%) | 163 (34%) | 168 (35%) | 0.0091 |
| History of stroke | 59 (12%) | 82 (17%) | 80 (16%) | 0.075 |
| Cancer | 83 (25%) | 57 (17%) | 62 (20%) | 0.061 |
| Current alcohol use | 184 (38%) | 177 (36%) | 170 (35%) | 0.24 |
| Cigarette smoking | 0.73 | |||
| Current | 75 (15%) | 87 (18%) | 78 (16%) | |
| Past | 217 (44%) | 204 (42%) | 203 (41%) | |
| Never | 196 (40%) | 199 (41%) | 209 (43%) | |
| ASA | 271 (55%) | 256 (52%) | 239 (49%) | 0.11 |
| Lipid-lowering Rx | 239 (49%) | 213 (44%) | 213 (44%) | 0.17 |
| Systolic BP, mm Hg | 134 (123–145) | 137 (124–149) | 134 (123–148) | 0.14 |
| Diastolic BP, mm Hg | 78 (69–82) | 79 (70–83) | 78 (70–83) | 0.39 |
| BMI, kg/m2 | 29 (26–34) | 29 (25–34) | 30 (25–35) | 0.42 |
| Waist circumference, cm | 103 (91–114) | 102 (91–112) | 100 (91–111) | 0.092 |
| LDL, mg/dl | 99 (80–125) | 106 (81–127) | 104 (82–128) | 0.13 |
| HDL, mg/dl | 42 (34–52) | 44 (36–56) | 45 (37–57) | 0.0003 |
| TG, mg/dl | 136 (93–198) | 126 (92–188) | 130 (93–187) | 0.20 |
| CRP, mg/l | 3.0 (1.4–5.9) | 3.5 (1.5–8.6) | 4.9 (1.9–9.9) | <.0001 |
| ACR, mg/g | 94 (50–287) | 105 (50–482) | 142 (61–677) | <.0001 |
| eGFRcyscr | 49 (39–57) | 45 (34–55) | 44 (30–53) | <.0001 |
| eGFRcys | 43 (34–49) | 40 (29–48) | 37 (26–47) | <.0001 |
| eGFRcr | 55 (45–68) | 53 (38–66) | 51 (35–63) | <.0001 |
ACR, albumin-to-creatinine ratio; ASA, aspirin; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; eGFRcr, estimated glomerular filtration rate creatinine; eGFRcys, estimated glomerular filtration rate cystatin; eGFRcyscr, estimated glomerular filtration rate creatinine-cystatin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; NGAL, neutrophil gelatinase−associated lipocalin; REGARDS, REasons for Geographic and Racial Differences in Stroke; Rx, prescription; TG, triglyceride.
Data are presented as median (interquartile range) or number (percentage).
Table 2.
Baseline characteristics of REGARDS participants, stratified by tertile of urinary KIM-1
| Parameter | Tertile of KIM-1 |
P value | ||
|---|---|---|---|---|
| 1 (n = 490) | 2 (n = 491) | 3 (n = 490) | ||
| Range of KIM-1, pg/ml | 15.8–587 | 589–1378 | 1380–28,631 | |
| Age, yr | 71 (64–78) | 71 (65–78) | 70 (63–77) | 0.38 |
| Female gender | 250 (51%) | 213 (43%) | 214 (44%) | 0.025 |
| Race | ||||
| Black | 273 (56%) | 231 (47%) | 201 (41%) | <.0001 |
| White | 217 (44%) | 260 (53%) | 289 (59%) | |
| Income | 0.88 | |||
| <$34k | 289 (67%) | 282 (66%) | 276 (65%) | |
| $35–74k | 109 (25%) | 117 (27%) | 115 (27%) | |
| $75k and above | 31 (7%) | 28 (7%) | 34 (8%) | |
| Diabetes mellitus | 253 (52%) | 232 (47%) | 231 (47%) | 0.29 |
| Hypertension | 427 (87%) | 411 (84%) | 416 (85%) | 0.31 |
| History of MI | 164 (34%) | 179 (37%) | 194 (40%) | 0.15 |
| History of stroke | 75 (15%) | 71 (15%) | 74 (15%) | 0.93 |
| Cancer | 69 (22%) | 78 (23%) | 54 (17%) | 0.094 |
| Current alcohol use | 163 (33%) | 172 (35%) | 196 (40%) | 0.21 |
| Cigarette smoking | <.0001 | |||
| Current | 67 (14%) | 60 (12%) | 113 (23%) | |
| Past | 199 (41%) | 227 (46%) | 197 (40%) | |
| Never | 223 (46%) | 203 (41%) | 178 (36%) | |
| ASA | 259 (53%) | 256 (52%) | 251 (51%) | 0.88 |
| Lipid-lowering Rx | 246 (51%) | 209 (43%) | 210 (43%) | 0.020 |
| Systolic BP, mm Hg | 137 (124–147) | 134 (123–146) | 134 (122–147) | 0.14 |
| Diastolic BP, mm Hg | 78 (70–83) | 78 (70–82) | 78 (70–83) | 0.91 |
| BMI, kg/m2 | 30 (26–34) | 29 (25–34) | 29 (25–35) | 0.39 |
| Waist circumference, cm | 102 (91–112) | 102 (91–112) | 102 (91–114) | 0.94 |
| LDL, mg/dl | 104 (80–127) | 101 (80–125) | 104 (83–128) | 0.48 |
| HDL, mg/dl | 45 (37–55) | 43 (35–54) | 43 (35–53) | 0.13 |
| TG, mg/dl | 124 (86–187) | 131 (91–192) | 139 (100–196) | 0.0050 |
| CRP, mg/l | 3.5 (1.6–7.4) | 3.4 (1.4–7.7) | 4.4 (1.7–9.4) | 0.0044 |
| ACR, mg/g | 94 (49–316) | 116 (56–410) | 136 (57–645) | 0.0006 |
| eGFRcyscr | 46 (34–54) | 45 (34–54) | 48 (37–57) | 0.0084 |
| eGFRcys | 40 (30–48) | 39 (29–47) | 41 (31–49) | 0.059 |
| eGFRcr | 52 (38–65) | 52 (38–64) | 56 (42–69) | 0.0029 |
ACR, albumin-to-creatinine ratio; ASA, aspirin; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; eGFRcr, estimated glomerular filtration rate creatinine; eGFRcys, estimated glomerular filtration rate cystatin; eGFRcyscr, estimated glomerular filtration rate creatinine-cystatin; HDL, high-density lipoprotein; KIM-1, kidney injury molecule−1; LDL, low-density lipoprotein; MI, myocardial infarction; REGARDS, REasons for Geographic and Racial Differences in Stroke; Rx, prescription; TG, triglyceride.
Data are presented as median (interquartile range) or number (percentage).
Correlations Among Biomarkers
In unadjusted analysis, uNGAL showed modest but statistically significant positive correlations with both uKIM-1 (r = 0.23, P < .0001) and ACR (r = 0.13, P < .0001), and was negatively correlated with eGFR (CKD-EPI 2012 cystatin and creatinine) (r = −0.18, P < .0001). By comparison, uKIM-1 was weakly positively correlated with both eGFR (r = 0.056, P = 0.03) and ACR (r = 0.092, P = 0.0004).
Urinary NGAL, KIM-1, and ESRD
A total of 257 participants progressed to ESRD over a median follow-up of 5.7 years. In demographic-adjusted models, higher levels of uNGAL were associated with an increased risk of both 2-year and 10-year ESRD (HR = 1.62 and HR = 1.25 per doubling, respectively, both P < .0001) (Table 3). These associations were attenuated and no longer statistically significant in fully adjusted models for both 2-year and 10-year ESRD (HR = 1.19, P = 0.060, and HR = 1.06, P = 0.18, respectively). Higher levels of uKIM-1 were also associated in demographic-adjusted models with increased risks of 2-year and 10-year ESRD (HR = 1.93 and 1.48 per doubling, respectively, both P < .0001). These associations were attenuated in fully adjusted models for both 2-year ESRD (HR = 1.21, P = 0.34) and 10-year ESRD (HR = 1.24, P = 0.0025). Applying the Fine−Gray analysis to account for the competing risk of death did not substantially change these results. For example, for NGAL, the HR of ESRD per doubling (95% CI) was 1.19 (0.99−1.42) and was 1.18 (0.99−1.41) using Fine−Gray analyses.
Table 3.
Associations of urinary NGAL and KIM-1 with 2-year and 10-year progression to ESRD
| ESRD events |
Model 1a HR (95% CI) |
Model 2b HR (95% CI) |
Model 3c HR (95% CI) |
|
|---|---|---|---|---|
| NGAL | ||||
| ESRD at 2-year follow-up | ||||
| NGAL T1 (reference) | 5/483 (1%) | |||
| NGAL T2 versus T1 | 14/484 (3%) | 3.25 (1.16–9.13) | 2.04 (0.73–5.71) | 1.19 (0.47–3.02) |
| NGAL T3 versus T1 | 37/484 (8%) | 10.66 (3.81–29.82) | 4.79 (1.65–13.88) | 1.84 (0.66–5.15) |
| NGAL (per doubling) | 56/1451 (4%) | 1.62 (1.43–1.83) | 1.45 (1.24–1.71) | 1.19 (0.99–1.42) |
| ESRD entire follow-up period | ||||
| NGAL T1 (reference) | 54/483 (11%) | |||
| NGAL T2 versus T1 | 95/484 (20%) | 2.36 (1.67–3.33) | 1.86 (1.29–2.68) | 1.41 (0.99–2.01) |
| NGAL T3 versus T1 | 108/484 (22%) | 2.91 (2.02–4.20) | 2.08 (1.43–3.02) | 1.30 (0.89–1.90) |
| NGAL (per doubling) | 257/1451 (18%) | 1.25 (1.17–1.34) | 1.18 (1.10–1.28) | 1.06 (0.98–1.14) |
| KIM-1 | ||||
|---|---|---|---|---|
| ESRD at 2-year follow-up | ||||
| KIM T1 (reference) | 13/483 (3%) | |||
| KIM1 T2 versus T1 | 24/484 (5%) | 3.68 (1.80–7.53) | 1.99 (0.98–4.05) | 1.50 (0.69–3.24) |
| KIM1 T3 versus T1 | 19/483 (4%) | 4.78 (2.18–10.48) | 1.41 (0.53–3.72) | 1.09 (0.38–3.15) |
| KIM-1 (per doubling) | 56/1450 (4%) | 1.93 (1.51–2.46) | 1.24 (0.86–1.79) | 1.21 (0.82–1.77) |
| ESRD entire follow-up period | ||||
| KIM T1 (reference) | 78/483 (16%) | |||
| KIM1 T2 versus T1 | 91/484 (19%) | 1.83 (1.33–2.51) | 1.33 (0.95–1.86) | 1.23 (0.88–1.72) |
| KIM1 T3 versus T1 | 88/483 (18%) | 2.54 (1.78–3.64) | 1.23 (0.83–1.84) | 1.29 (0.85–1.96) |
| KIM-1 (per doubling) | 257/1450 (18%) | 1.48 (1.33–1.66) | 1.13 (0.99–1.29) | 1.24 (1.08–1.42) |
CI, confidence interval; eGFRcr-cys, estimated glomerular filtration rate creatinine-cystatin; ESRD, end-stage renal disease; HR, hazard ratio; KIM-1, kidney injury molecule−1; NGAL, neutrophil gelatinase−associated lipocalin, T1, tertile 1; T2, tertile 2; T3, tertile 3.
Model 1: Age, sex, race, socioeconomic status, urine creatinine.
Model 2: 1+ diabetes, systolic blood pressure, diastolic blood pressure, hypertension, smoking, body mass index, alcohol use, albumin-to-creatinine ratio, C-reactive protein.
Model 3: 2+ eGFRcr-cys.
Urinary NGAL, KIM-1, and All-Cause Mortality
There were a total of 819 deaths over a median follow-up period of 6.5 years. In demographic-adjusted models, higher levels of uNGAL were associated with an increased risk of both 2-year and 10-year death (HR = 1.20 and HR = 1.09 per doubling, respectively, both P < .0001) (Table 4). These associations were attenuated but remained statistically significant in fully adjusted models for both 2-year and 10-year death (HR = 1.12, P = 0.0091; and HR = 1.04, P = 0.043, respectively). Higher levels of uKIM-1 were also associated in demographic-adjusted models with increased risks of 2-year and 10-year death (HR = 1.40 and 1.21 per doubling, respectively, both P < .0001). These associations were attenuated but remained statistically significant in in fully adjusted models for both 2-year and 10-year death (HR = 1.23, P = 0.016; and HR = 1.10, P = 0.0080, respectively).
Table 4.
Associations of urine NGAL and KIM-1 with 2-year and 10-year all-cause mortality
| Mortality events |
Model 1a HR (95% CI) |
Model 2b HR (95% CI) |
Model 3c HR (95% CI) |
|
|---|---|---|---|---|
| NGAL | ||||
| Mortality at 2-year follow-up | ||||
| NGAL T1 (reference) | 39/490 (8%) | |||
| NGAL T2 versus T1 | 44/491 (9%) | 1.39 (0.89, 2.17) | 1.07 (0.68, 1.69) | 1.03 (0.65, 1.62) |
| NGAL T3 versus T1 | 67/491 (14%) | 2.45 (1.61, 3.72) | 1.73 (1.12, 2.68) | 1.60 (1.03, 2.49) |
| NGAL (per doubling) | 150/1472 (10%) | 1.20 (1.11, 1.29) | 1.13 (1.05, 1.23) | 1.12 (1.03, 1.21) |
| Mortality entire follow-up period | ||||
| NGAL T1 (reference) | 252/490 (51%) | |||
| NGAL T2 versus T1 | 285/491 (58%) | 1.33 (1.12, 1.58) | 1.17 (0.98, 1.40) | 1.12 (0.93, 1.33) |
| NGAL T3 versus T1 | 282/491 (57%) | 1.45 (1.21, 1.74) | 1.20 (1.00, 1.45) | 1.13 (0.94, 1.37) |
| NGAL (per doubling) | 819/1472 (56%) | 1.09 (1.05, 1.13) | 1.05 (1.02, 1.09) | 1.04 (1.00, 1.08) |
| KIM-1 | ||||
|---|---|---|---|---|
| Mortality at 2-year follow-up | ||||
| KIM1 T1 (reference) | 41/490 (8%) | |||
| KIM1 T2 versus T1 | 48/491 (10%) | 1.27 (0.82, 1.98) | 1.09 (0.69, 1.72) | 1.05 (0.67, 1.67) |
| KIM1 T3 versus T1 | 61/490 (12%) | 2.38 (1.52, 3.73) | 1.58 (0.97, 2.57) | 1.62 (0.99, 2.66) |
| KIM-1 (per doubling) | 150/1471 (10%) | 1.40 (1.21, 1.63) | 1.22 (1.03, 1.44) | 1.23 (1.04, 1.47) |
| Mortality entire follow-up period | ||||
| KIM1 T1 (reference) | 260/490 (53%) | |||
| KIM1 T2 versus T1 | 277/491 (56%) | 1.21 (1.01, 1.44) | 1.05 (0.87, 1.26) | 1.04 (0.87, 1.25) |
| KIM1 T3 versus T1 | 281/490 (57%) | 1.58 (1.30, 1.92) | 1.19 (0.96, 1.47) | 1.23 (0.99, 1.51) |
| KIM-1 (per doubling) | 818/1471 (56%) | 1.21 (1.13, 1.29) | 1.09 (1.01, 1.17) | 1.10 (1.03, 1.19) |
CI, confidence interval; HR, hazard ratio; KIM-1, kidney injury molecule−1; NGAL, neutrophil gelatinase−associated lipocalin; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Model 1: Age, sex, race, socioeconomic status, urine creatinine.
Model 2: 1+ diabetes, systolic blood pressure, diastolic blood pressure, hypertension, smoking, body mass index, alcohol use, albumin-to-creatinine ratio, C-reactive protein.
Model 3: 2+ eGFRcr-cys.
Interval Hazard Ratios for Biomarkers
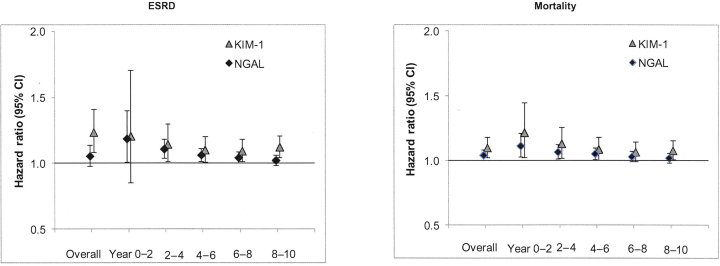
We evaluated the associations of each biomarker with the outcomes ESRD and mortality during each 2-year time interval during the period of follow-up. The hazard ratios for ESRD and mortality appeared to be strongest in the first 2 years of follow-up, with a progressive weakening over time for both biomarkers (Figure 2).
Figure 2.
Associations of neutrophil gelatinase−associated lipocalin (NGAL) and kidney injury molecule−1 (KIM-1) with risk of end-stage renal disease (ESRD) and mortality, overall and by time in REasons for Geographic and Racial Differences in Stroke (REGARDS) study participants. Figure illustrates the associations of each biomarker with the outcomes ESRD and mortality during each 2-year time interval during the period of follow-up. The hazard ratios for ESRD and mortality appeared to be strongest in the first 2 years of follow-up, with a progressive weakening over time for both biomarkers. Hazard ratios are per doubling of urinary marker, using multivariable adjusted models controlling for demographics, socioeconomic status, diabetes mellitus, systolic blood pressure, diastolic blood pressure, hypertension, smoking, body mass index, alcohol use, albumin-to-creatinine ratio, C-reactive protein, estimated glomerular filtration rate, and urine creatinine.
Interaction With Albuminuria
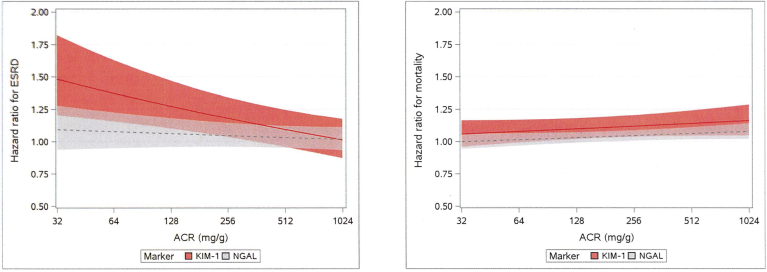
To address the hypothesis that the associations of the urine markers with ESRD and mortality might differ by severity of albuminuria, we tested interactions of KIM-1 and NGAL with ACR on each outcome. The association of KIM-1 with risk of ESRD appeared to be weaker at higher levels of ACR (P = 0.0018, test for interaction, Figure 3). For example, at an ACR of 30 mg/g, the hazard ratio was 1.49 per doubling of KIM-1; this hazard ratio fell to 1.16 at an ACR of 300 and to 1.01 at an ACR of 1000. By contrast, the association of NGAL with increased risk of ESRD appeared to be constant across the range of ACR (P = 0.40, test for interaction). We also found statistically significant interactions of KIM-1 by ACR (P = 0.0080) and NGAL by ACR (P = 0.043) on mortality, but the differences in hazard ratios at low and high levels of ACR were much more modest than for ESRD. For example, the effect of KIM-1 on mortality ranged from HR = 1.06 to 1.16 at ACR levels of 30 to 1000 mg/g, and the effect of NGAL on mortality ranged from HR = 1.00 to 1.08 at ACR levels of 30 to 1000. We performed additional tests for interaction of NGAL and KIM-1 with age, black race, diabetes, and C-reactive protein. There was a significant interaction of KIM-1 with black race for the outcome ESRD (P = 0.009, fully adjusted model), but no other significant interactions were found.
Figure 3.
Assessment of biomarker interactions for 10-year progression to end-stage renal disease (ESRD) and all-cause mortality. Hazard ratios (HRs) (with 95% confidence intervals [CIs]) are shown per doubling of kidney injury molecule−1 (KIM-1) and neutrophil gelatinase−associated lipocalin (NGAL) across the range of albumin-to-creatinine ratios (ACRs). Estimates are from Fine−Gray (ESRD) or Cox (Death) models, controlling for demographics, socioeconomic status (SES), diabetes mellitus (DM), systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension (HTN), smoking, body mass index (BMI), alcohol use, ACR, C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), urine creatinine, plus interaction terms for KIM-1−by-ACR or NGAL-by-ACR. Figure illustrates the interactions of urinary ACR with each biomarker for the outcomes ESRD and mortality. HRs are per doubling of urinary marker, using multivariable adjusted models controlling for demographics, SES, DM, SBP, DBP, HTN, smoking, BMI, alcohol use, ACR, CRP, eGFR, and urine creatinine.
Discussion
In this cohort of black and white, older, community-dwelling adults with CKD and albuminuria, uNGAL and uKIM-1 were associated with all-cause mortality independently of sociodemographic, comorbidities, and baseline eGFR. Both KIM-1 and NGAL were associated with ESRD in models that controlled for traditional risk factors. After further adjustment for eGFR, KIM1 remained associated with ESRD, whereas NGAL was no longer statistically significant. We also found that the association of KIM-1 with ESRD was weaker at higher levels of ACR.
Urinary Markers and ESRD
uKIM1 was associated with 10-year ESRD after adjustment for traditional CKD risk factors and eGFR(creatinine-cystatin [cr-cys]). In contrast, uNGAL was associated with ESRD in models adjusted for sociodemographics and comorbidities, but not after adjustment for eGFR(cr-cys). Prior studies have differed in regard to associations of these markers with ESRD. Neither uNGAL nor uKIM1 was independently associated with CKD progression in the CRIC16 or ARIC.17 In the Pima Indian study, uNGAL was associated with ESRD, but uKIM1 was not.13 However, uKIM1 has been shown to associate with progression to ESRD in studies limited to specific CKD populations: for example, in a cohort of diabetic patients undergoing cardiopulmonary bypass27 and also a cohort of patients with IgA nephropathy.28
The differences in associations of uKIM1 and uNGAL with ESRD that we observed could be accounted for by the different biological mechanisms of production of uNGAL and uKIM1. Although uNGAL and uKIM-1 are both produced as a result of renal tubular injury, they differ in biological production, function, and metabolism. uNGAL is secreted from both proximal and distal renal tubular epithelial cells, whereas KIM-1 is released from primarily proximal tubular cells.29 Expression of renal epithelial KIM-1 causes interstitial fibrosis,7 whereas uNGAL is not known to directly cause renal damage.
The associations of both uNGAL and KIM1 with ESRD were strongest for events that occurred within the first 2 years of biomarker measurement. The finding of stronger associations for shorter-term ESRD may reflect the within-subject variability of these markers. A potential clinical application of this finding is that it may prove useful to use repeated measures of these markers to evaluate short-term risk. Our findings are in accordance with several studies of uNGAL in populations with prevalent renal disease. In the CRIC study, uNGAL had stronger associations with short-term26 than long-term ESRD.16
It is also notable that in this REGARDS subcohort of individuals with eGFR ≤60 ml/min per 1.73 m2 and albuminuria, as well as in CRIC, the association of uNGAL with ESRD was partially attenuated after adjustment for comorbidities, ACR, and eGFR. There are several potential explanations for this finding. One interpretation is that uNGAL and eGFR convey overlapping information in relation to this outcome. In support of this, uNGAL and eGFR showed a significant, albeit modest, correlation. Another potential explanation is that uNGAL reflects systemic NGAL levels, and because systemic levels are filtered at the glomerulus, the resultant uNGAL level may be explained by both systemic levels and eGFR. It is noteworthy that some studies have reported an association of uNGAL with ESRD that was independent of comorbidities and eGFR, including the Folic Acid for Vascular Outcome Reduction in Transplantation Trial (FAVORIT) in kidney transplant recipients,14 the Pima Indian study,13 and studies of pediatric patients with normal baseline kidney function undergoing cardiac surgery.30 In contrast to this REGARDS study subgroup and the CRIC cohort, participants in these studies had relatively normal eGFR at baseline. uNGAL, a 25-kDa molecule, is freely filtered at the glomerulus in the setting of normal kidney function31; thus urinary levels may be determined largely by serum levels in patients with normal renal function. uNGAL, a byproduct of neutrophil activation, is higher in the setting of systemic inflammation,32 for example, bacterial infection.33 In nonrenal, noninfectious disease, systemic inflammation likely accounts for the associations of serum NGAL with disease severity in inflammatory bowel disease34 or metastasis of colorectal cancer.35 For this reason, we included C-reactive protein in our multivariable models. Although the association of uNGAL with ESRD was independent of C-reactive protein in our study, we cannot exclude the possibility that systemic inflammation may contribute to elevations in uNGAL.
Urinary Markers and Overall Mortality
In this REGARDS subcohort, uNGAL and uKIM-1 both were associated with all-cause mortality, independently of ACR and eGFR. This finding is in accordance with the independent associations of uNGAL with mortality among kidney transplant recipients,14 and the associations of both uNGAL and uKIM-1 with mortality in CRIC.15 Studies in the Framingham cohort,36 and in the Healthy Aging, Body and Composition (Health ABC) cohort37 also demonstrated an association of uKIM-1 with mortality independent of eGFR. One way to interpret these observations is that uNGAL or KIM-1 adds information about renal disease that is distinct from eGFR (e.g., sodium handling or water balance), and that these associations confirm the adverse effects of renal dysfunction on the heart and other organ systems. However, in the case of uNGAL, it is also possible that the associations with cardiovascular events or overall mortality, independently of eGFR, are explained by the influence of systemic inflammation on serum NGAL, which in turn is filtered at the glomerulus. It is also worth noting that the cohort comprised mostly participants more than 65 years of age, and there was a fairly high frequency of death over 10 years (56%). Taken together with the fact that eGFR estimates by cystatin were substantially lower than eGFR by creatinine, and that we did not measure “change in eGFR” as an outcome, it is possible that in this relatively older, frail population that death events may have preceded CKD progression. However, we did not find substantive changes in the hazard ratios when using Fine−Gray models to account for the competing risk of death.
Interactions With Albuminuria
We found that the association of KIM-1 with ESRD was weaker at higher levels of ACR. This was contrary to our hypothesis of the potential synergistic effect of the 2 markers. This may be due to the well-known associations between albuminuria and CKD progression, rendering KIM-1 levels less relevant at higher levels of ACR. Although the associations of both uNGAL and uKIM-1 with mortality were somewhat stronger at higher levels of ACR, the differences in hazard ratios were very small. This was consistent with our hypothesis that concomitant glomerular and tubular damage may be additive in leading to adverse outcomes.
Our study represents a large, diverse, community-dwelling sample of adults with moderate CKD. The REGARDS study performed in-home visits in 48 states across the United States, which increases the generalizability of the findings to the community. The cohort represents a well-characterized population with 2 serum markers of renal function (i.e., creatinine and cystatin C), urinary ACR, and 2 novel urinary markers of tubular injury. There is long-term follow-up for the clinical outcomes ESRD and mortality.
Our study also has certain limitations. The majority of the cohort comprises patients more than 65 years of age, and the discrepancy between cystatin- and creatinine-based eGFR suggests that members of the cohort were frail. Our findings may not generalize to a younger, less frail population. We acknowledge that the event rates for 2-year ESRD are lower than for other outcomes, and thus confidence intervals are wider for this outcome. We cannot determine the extent to which systemic disease could influence this association via serum NGAL filtered by the glomerulus, and we lacked information about infectious disease in the cohort. Although the urinary specimens were not processed immediately after voiding, and although NGAL was measured after thaw, errors from collection would bias our findings toward the null. Kidney biopsy samples were not obtained, so we cannot definitively ascertain the specific cause of kidney disease. Incident ESRD was ascertained after the patient had been on dialysis for 3 months; although this increased our ability to discern ESRD from AKI requiring dialysis, we may have missed ESRD events in patients who died shortly after starting dialysis.
In summary, uNGAL and KIM-1 were associated with all-cause mortality independently of traditional risk factors, ACR, and eGFR. Moreover, higher levels of KIM-1 were associated with progression to ESRD. These findings suggest that tubular injury may represent an important risk factor for complications, including death and ESRD, for patients with CKD. Future studies are warranted to elucidate mechanisms to explain the observed associations.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. RFD is funded by U01DK108809 and R03 DK104013 from the National Institute of Diabetes and Digestive and Kidney Diseases and RO3.
References
- 1.Andreucci M., Faga T., Pisani A. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur J Intern Med. 2017;39:1–8. doi: 10.1016/j.ejim.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre J.V. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293–299. discussion 299. [PMC free article] [PubMed] [Google Scholar]
- 3.Schrezenmeier E.V., Barasch J., Budde K. Biomarkers in acute kidney injury—pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219:554–572. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca S.G., Garg A.X., Thiessen-Philbrook H. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25:1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichimura T., Asseldonk E.J., Humphreys B.D. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Investig. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L., Brooks C.R., Xiao S. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Investig. 2015;125:1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys B.D., Xu F., Sabbisetti V. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Investig. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peralta C.A., Katz R., Bonventre J.V. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60:904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolignano D., Coppolino G., Campo S. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–378. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 10.Bolignano D., Coppolino G., Campo S. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant. 2008;23:414–416. doi: 10.1093/ndt/gfm541. [DOI] [PubMed] [Google Scholar]
- 11.Waanders F., Vaidya V.S., van Goor H. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53:16–25. doi: 10.1053/j.ajkd.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo K.S., Choi J.L., Kim B.R. Urinary neutrophil gelatinase-associated lipocalin levels in comparison with glomerular filtration rate for evaluation of renal function in patients with diabetic chronic kidney disease. Diabetes Metab J. 2012;36:307–313. doi: 10.4093/dmj.2012.36.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fufaa G.D., Weil E.J., Nelson R.G. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia. 2015;58:188–198. doi: 10.1007/s00125-014-3389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal N., Carpenter M.A., Weiner D.E. Urine injury biomarkers and risk of adverse outcomes in recipients of prevalent kidney transplants: the Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol. 2016;27:2109–2121. doi: 10.1681/ASN.2015030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park M., Hsu C.Y., Go A.S. Urine kidney injury biomarkers and risks of cardiovascular disease events and all-cause death: the CRIC Study. Clin J Am Soc Nephrol. 2017;12:761–771. doi: 10.2215/CJN.08560816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu C.Y., Xie D., Waikar S.S. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int. 2017;91:196–203. doi: 10.1016/j.kint.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster M.C., Coresh J., Bonventre J.V. Urinary biomarkers and risk of ESRD in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015;10:1956–1963. doi: 10.2215/CJN.02590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard V.J., Cushman M., Pulley L. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Peralta C.A., Shlipak M.G., Judd S. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnock D.G., Muntner P., McCullough P.A. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2010;56:861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillett S.R., Boyle R.H., Zakai N.A. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47:243–246. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi S., Farmer T., Kapke G.F. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurella Tamura M., Wadley V. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine J.P.G.R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;04:496–509. [Google Scholar]
- 26.Liu K.D., Yang W., Anderson A.H. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83:909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabbisetti V.S., Waikar S.S., Antoine D.J. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters H.P., Waanders F., Meijer E. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:3581–3588. doi: 10.1093/ndt/gfr135. [DOI] [PubMed] [Google Scholar]
- 29.Bonventre J.V., Vaidya V.S., Schmouder R., Feig P., Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nature Biotech. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh C.R., Devarajan P., Zappitelli M. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zang X., Zheng F., Hong H.J. Neutrophil gelatinase-associated lipocalin protects renal tubular epithelial cells in hypoxia-reperfusion by reducing apoptosis. Int Urol Nephrol. 2014;46:1673–1679. doi: 10.1007/s11255-014-0749-3. [DOI] [PubMed] [Google Scholar]
- 32.Kjeldsen L., Johnsen A.H., Sengelov H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 33.Fjaertoft G., Foucard T., Xu S., Venge P. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr. 2005;94:661–666. doi: 10.1111/j.1651-2227.2005.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 34.Oikonomou K.A., Kapsoritakis A.N., Theodoridou C. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol. 2012;47:519–530. doi: 10.1007/s00535-011-0516-5. [DOI] [PubMed] [Google Scholar]
- 35.Ozemir I.A., Aslan S., Eren T. The diagnostic and prognostic significance of serum neutrophil gelatinase-associated lipocalin levels in patients with colorectal cancer. Chirurgia. 2016;111:414–421. doi: 10.21614/chirurgia.111.5.414. [DOI] [PubMed] [Google Scholar]
- 36.O'Seaghdha C.M., Hwang S.J., Larson M.G. Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. J Am Soc Nephrol. 2013;24:1880–1888. doi: 10.1681/ASN.2013010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarnak M.J., Katz R., Newman A. Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol. 2014;25:1545–1553. doi: 10.1681/ASN.2013070713. [DOI] [PMC free article] [PubMed] [Google Scholar]