Abstract
[Purpose] This study aimed to induce disuse muscle atrophy in Goto-Kakizaki rats, a type 2 diabetes model, to investigate the effects of reloading on the soleus and plantaris muscles. [Materials and Methods] Wistar and Goto-Kakizaki (GK) rats were divided into 6 groups: Wistar Control (WC), GK Control (GC), Wistar Tail suspension (WS), GK Tail suspension (GS), and Wistar Reload (WR), GK Reload (GR). [Results] Investigation of myofiber cross-sectional area in Goto-Kakizaki rat soleus muscles indicated that the GS group showed significantly lower values than the GC and GR groups. No significant differences were observed between the GC and GR groups. However, investigation of plantaris muscles in Goto-Kakizaki rats indicated that the GS and GR groups showed a significant decrease compared to the GC group. No significant differences were found between the GS and GR groups. [Conclusion] Investigation of muscle weight/body weight ratios and myofiber cross-sectional area in tail suspension groups confirmed the induction of muscular atrophy. The differences in the degree of atrophy and recovery in terms of myofiber cross-sectional area observed in Goto-Kakizaki rat plantaris muscles may be influenced by the myofiber type and diabetes.
Keywords: Tail suspension, Reloading, Diabetic rat
INTRODUCTION
Skeletal muscle atrophy is commonly encountered in rehabilitation. The main causes of muscle atrophy are neurogenic, myogenic, disuse, and age-related. Disuse muscle atrophy frequently occurs as a result of inactivity, lack of weight-bearing, and decreased physical activity1). The number of patients with diabetes in Japan is steadily increasing. The 2016 National Health and Nutrition Survey estimated that over 10 million adults have diabetes2). Patients with diabetes are likely to develop arteriosclerosis, and thus face a two- to four-fold risk of cerebral and myocardial infarction as compared to that of non-diabetic individuals3, 4). In addition, if neuropathy develops as a complication of diabetes, the risk of falling significantly increases due to sensory impairment of the feet and decreased range of motion. Furthermore, there is a high risk that patients with diabetes will develop disuse muscle atrophy due to being forced to remain at rest or supine as a consequence of cerebral infarction, myocardial infarction, or bone fractures caused by falling. However, the optimal exercise program has yet to be established for patients with diabetes and disuse muscle atrophy. Many reports of animal experiments on disuse muscle atrophy are available; reloading muscles that have atrophied using hindlimb suspension and plaster cast fixation has been shown to reverse atrophy5). Conversely, it has also been reported that skeletal muscles experiencing temporary atrophy are weakened and therefore may be damaged upon reloading6, 7). In their study on load stimuli on disuse muscle atrophy in rat soleus muscles, Zushi et al.8) reported muscle regenerative responses such as onset of increased myofiber cross-sectional area and an increase in centrally nucleated fibers associated with muscle damage. In general, such experiments utilize Wistar rats. However, the efficacy of exercise intervention in diabetic animal models with disuse muscle atrophy remains unknown. Research using diabetic animal models has shown that motor neurons in the soleus muscle weaken9). It has also been reported that myofiber transitions occur in patients with diabetes10), but it remains unknown whether the same level of recovery is obtained through weight-bearing load stimuli in diabetic animal models with disuse muscle atrophy. Thus, the objective of the present study was to create disuse muscle atrophy in Goto-Kakizaki rats (GK rats), which are a type of non-obese type 2 diabetes model rat, using tail suspension. We then investigated the effects of reloading. The target muscles were the soleus muscle, which is mostly composed of type I myofibers and has been assessed in multiple previous studies, and the plantaris muscle, which has a muscle fiber type distribution marked by a mix of type I, type IIA, and type IIB myofibers; the plantaris muscle has been found11) to both atrophy and develop fast-twitch characteristics as it is non-load bearing, although it undergoes fewer transitions than soleus myofibers.
MATERIALS AND METHODS
Twenty-four Wistar and 24 GK rats initially aged 8 weeks were included. The study was conducted after receiving approval from the Animal Experiment and Use Committee of Kanazawa University (no. AP-132919).
The Wistar (W) and GK rats were divided into 3 groups (n=8 each). The Control group included rats that were kept under normal conditions (WC and GC). The tail suspension group included rats that underwent tail suspension for two weeks (WS and GS). The Reload group included rats that underwent two weeks of tail suspension after which they underwent one week of normal captive conditions (WR and GR). The tail suspension method utilized in the present study was the same as the method used by Morey-Holton et al12). Under anesthesia (isoflurane), non-elastic tape was wrapped around the lateral sides of the central caudal region of the rats; they were then suspended from the roof of the cage in such a way that only their front legs were in contact with the cage floor. The period of tail suspension was two weeks, during which the rats were free to move around within the cage using their front legs. They were also allowed to freely ingest food and water (HydroGel). The room temperature was maintained at approximately 22°C and the lighting was maintained on a 12-hour light-dark cycle in order to prevent interference with the rats’ biological rhythm.
After the completion of the experiments, the rats were anesthetized (isoflurane). Their body weight was measured; blood samples were taken, and their blood glucose levels were measured. Then, samples of the soleus muscle and plantaris muscle were surgically resected; the wet weight of these samples was then measured. The resected tissue samples were rapidly frozen in isopentane solution, which was cooled using liquid nitrogen and stored at a temperature of −80°C until analysis. At a later date, thin slices (cryosections) of 10 µm near the center frozen samples along the length of the muscle were prepared using a cryostat. After allowing the frozen sections to naturally dry out at room temperature, hematoxylin-eosin (HE) staining was performed and the samples were observed using an optical microscope. As an index for muscle atrophy, we randomly selected a minimum of 150 myofibers per sample from the stained slice images. We used the image analysis software ImageJ to measure the cross-sectional areas. As an index for muscle damage, we calculated the onset rate for whole fibers that were subjected to centrally nucleated fiber formation and myofiber necrosis measurement. In this study, myofiber necrosis was identified by the presence of phagocyte infiltration or markedly lighter staining13).
The body weight, blood glucose levels, wet weights of both the soleus and plantaris muscles, muscle weight/body weight ratios, and myofiber cross-sectional areas of samples in the Control, Suspension, and Reload groups were subjected to one-way ANOVA and Tukey test to assess sub effects. The onset rates for myofiber necrosis and core fiber formation were subjected to the chi-squared test and Bonferroni correction. All statistical analyses were performed using SPSS version 23 (IBM SPSS). The significance level was set at 5%.
RESULTS
Blood glucose levels at the time of muscle sample resection were 137.7 ± 18.6 mg/dl for the Wistar rats and 224.4 ± 48.5 mg/dl for the GK rats, indicating that the GK rats utilized in the present study had significantly higher blood glucose levels than the Wistar rats.
The mean ± standard deviation (SD) values for the body weight, the muscle wet weight, the muscle weight/body weight (at the time of the resection) ratio, and the myofiber cross-sectional area are shown in Tables 1, 2. Investigation of the body weight, soleus and plantaris muscle wet weight indicated that both Wistar and GK rats in the tail suspention group showed significant decreases compared to those in the Control and Reload groups.Investigation of the muscle weight/body weight ratios for the soleus muscle samples indicated that both Wistar and GK rats in the tail suspension group showed significant decreases compared to those in the Control group and Reload group. However, no significant differences were found for the plantaris muscle samples in any of the groups or for either of the rat types. Investigation of myofiber cross-sectional area for Wistar rat soleus muscle samples indicated that the WR and WS groups had significantly lower values than the WC group. In addition, the WS had significantly lower values than the WR group. Investigation of GK rats indicated that the GS group had significantly lower values than the GC group. The GS group also had significantly lower values than the GR group. No significant differences were observed between the GC and GR groups. Investigation of plantaris muscles indicated that the Wistar rats showed no significant differences in any group. However, investigation of plantaris muscles in GK rats indicated that the GS and GR groups had significant decreases compared to those in the GC group. No significant differences were found between the GS and GR groups. The distributions for myofiber cross-sectional areas in all groups and for both types of muscle samples are shown in Figs. 1, 2, 3, 4.
Table 1. Group characteristics of soleus muscle.
| Groups | Wistar | GK | ||||
|---|---|---|---|---|---|---|
| WC | WS | WR | GC | GS | GR | |
| BW (g) | 226.1 ± 24.7 | 180.3 ± 8.1* | 212.6 ± 10.7† | 225.1 ± 7.9 | 180.9 ± 11.6* | 232.5 ± 10.7† |
| MW (mg) | 92.9 ± 6.7 | 51.9 ± 4.1* | 83.1 ± 9.5† | 99.4 ± 5.6 | 57.5 ± 8.9* | 102.1 ± 11.2† |
| MW/BW (mg/g) | 0.41 ± 0.27 | 0.29 ± 0.50* | 0.39 ± 0.89† | 0.44 ± 0.7 | 0.31 ± 0.77* | 0.44 ± 1.05† |
| MCSA (µm2) | 2,347 ± 194 | 1,320 ± 257* | 1,970 ± 351*† | 2,783 ± 337 | 1,341 ± 255* | 2,471 ± 363† |
Values are shown as mean ± SD. *Significant differences between Wistar and GK rats and the Control group (p<0.05). †Significant difference compared to the Suspension group (p<0.05).
Table 2. Group characteristics of plantaris muscle.
| Groups | Wistar | GK | ||||
|---|---|---|---|---|---|---|
| WC | WS | WR | GC | GS | GR | |
| BW(g) | 226.1 ± 24.7 | 180.3 ± 8.1* | 212.6 ± 10.7† | 225.1 ± 7.9 | 180.9 ± 11.6* | 232.5 ± 10.7† |
| MW (mg) | 232.0 ± 11.9 | 179.3 ± 5.5* | 212.8 ± 13.4*† | 222.9 ± 9.5 | 176.1 ± 11.5* | 219 ± 11.8† |
| MW/BW (mg/g) | 1.04 ± 0.68 | 0.99 ± 0.68 | 1.00 ± 1.25 | 0.99 ± 1.2 | 0.97 ± 1.00 | 0.94 ± 1.1 |
| MCSA (µm2) | 2,165 ± 374 | 1,899 ± 250 | 1,848 ± 140 | 2,462 ± 285 | 1,738 ± 278* | 2,012 ± 307* |
Values are shown as mean ± SD. *Significant differences between Wistar and GK rats and the Control group (p<0.05). †Significant difference compared to the Suspension group (p<0.05).
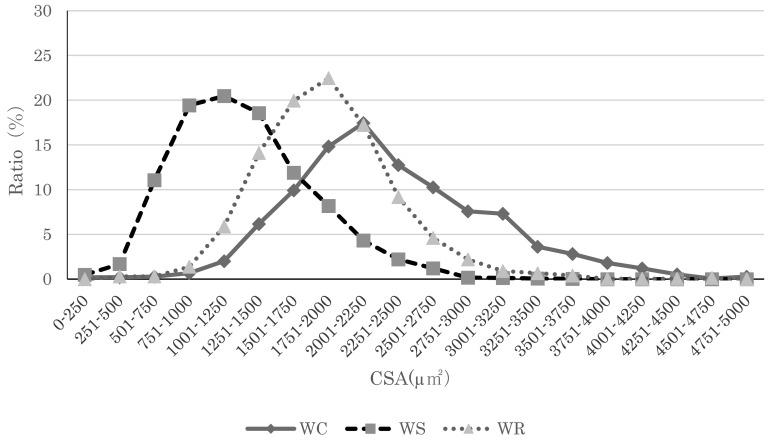
Fig. 1.
Soleus myofiber cross-sectional area distributions. Myofiber cross-sectional area distribution for Wistar rats in all groups. The peak in the Suspension group for both types of rats shifted to the left, indicating muscle atrophy. Compared to the Suspension group, the peak in the Reload group shifted left, indicating a tendency toward recovery of myofiber cross-sectional area.
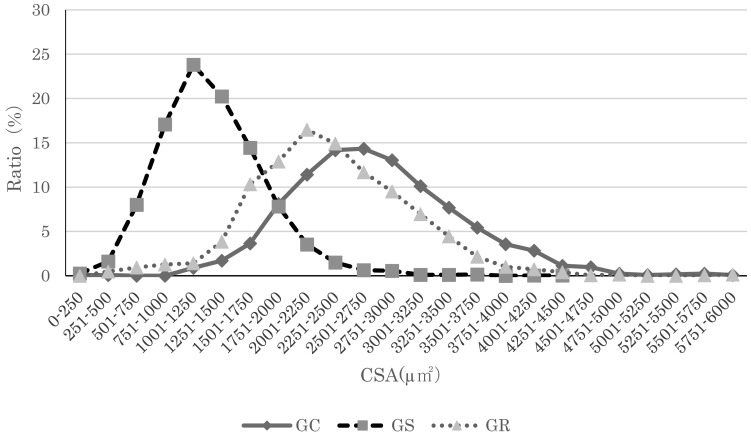
Fig. 2.
Soleus myofiber cross-sectional area distributions. Myofiber cross-sectional area distribution for GK rats in all groups. The peak in the Suspension group for both types of rats shifted to the left, indicating muscle atrophy. Compared to the Suspension group, the peak in the Reload group shifted left, indicating a tendency toward recovery of myofiber cross-sectional area.
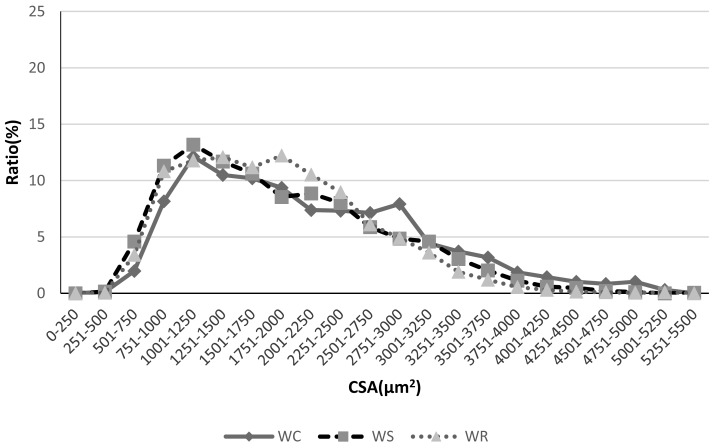
Fig. 3.
Plantaris muscle myofiber cross-sectional area distribution. Myofiber cross-sectional area distribution for Wistar rats in all groups. Although there were no major differences between the Wistar rats among groups, a higher number of rats in the WS and WR groups tends to have smaller cross-sectional areas, and the double-peaked (bimodal) distribution Reload group indicates greater dispersion than the Suspension group.
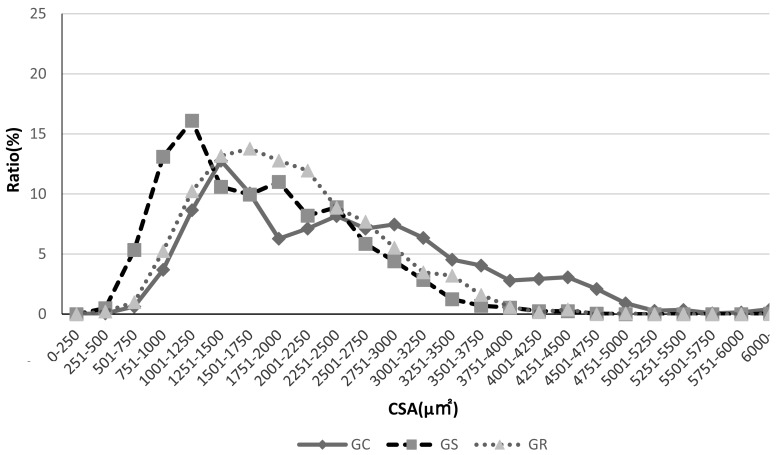
Fig. 4.
Plantaris muscle myofiber cross-sectional area distribution. Myofiber cross-sectional area distribution for GK rats in all groups.
The GK rats in the GS group showed a peak that is clearly on the left, indicating a higher number of small-bore fibers. In contrast, the GR group shows a peak shift to the right, indicating a tendency for myofiber cross-sectional area to recover.
The onset rates for myofiber necrosis and centrally nucleated myofibers in all groups for the soleus and plantaris muscles of both the Wistar and GK rats are shown in Table 3. Investigation of the plantaris muscles indicated that there were no significant differences in any group for either the Wistar or GK rats in terms of myofiber necrosis onset rate. There were also no significant differences in any of the groups for either the Wistar or GK rats regarding soleus muscles. However, the GR group—the Reload group for GK rats—showed a value of 0.34%, which was higher than that in the other groups. Investigation of centrally nucleated myofibers showed no significant differences in any group for either the soleus or plantaris muscles in Wistar or GK rats.
Table 3. Rates of myofiber necrosis and centrally nucleated myofibers (%).
| Muscle | Soleus | Plantaris | ||
|---|---|---|---|---|
| Group | Necrosis | Core fiber | Necrosis | Core fiber |
| WC | 0.20 | 0.13 | 0.12 | 0.06 |
| WS | 0.17 | 0 | 0 | 0.16 |
| WR | 0.10 | 0.15 | 0.1 | 0.05 |
| GC | 0 | 0.08 | 0.21 | 0.07 |
| GS | 0.08 | 0.23 | 0.25 | 0.10 |
| GR | 0.34 | 0.27 | 0 | 0.11 |
No significant differences were observed between soleus and plantaris muscles in the C, S, or R groups for either the Wistar or GK rats.
DISCUSSION
In this study, disuse muscle atrophy was induced via a tail suspension model for a two-week period using GK rats, which are a non-obese type 2 diabetic model animal. Then, we investigated the effect of subsequent reloading on the soleus and plantaris muscles, in which normal Wistar rats and GK rats were compared. The body weight loss after tail suspension was similar to other studies8, 14). The muscle weight was also analyzed using ratio of muscle and body weight due to the body loss influence. Muscle weight/body weight ratios of the soleus muscle samples in the Suspension group showed significant decreases in both the Wistar and GK rats. The Reload group exhibited no differences between the Wistar and GK rats and the Control group. We believe this was the result of muscle atrophy from tail suspension and subsequent muscle hypertrophy due to reload stimuli, as was reported in multiple previous studies. However, investigation of the plantaris muscle samples indicated no significant differences in any group of either the Wistar or GK rats. In their study of the effects of reloading on the plantaris muscles of rats using hind-leg suspension, Yamauchi et al.11) reported that there were no significant differences in muscle weight/body weight ratios after one week of suspension; however, significant differences appeared after three weeks of suspension. The plantaris muscle, which contains a high proportion of type IIB fibers, is less susceptible than the soleus muscle to the absence of load, which is the likely explanation as to why there were no significant differences after two weeks.
Myofiber cross-sectional area of soleus muscle were significantly smaller in Wistar rats in the Suspension and Reload groups than rats in the Control group; these areas in the Reload group were significantly larger than those in the Suspension group. We believe this was due to the onset of atrophy as a result of tail suspension followed by muscle hypertrophy as a result of reloading, as has been reported in previous studies8, 14). GK rats in the Suspension group also showed significantly smaller areas than those in the Control group; those in the Reload group had significantly larger areas than those in the Suspension group. In addition, there were no significant differences between the Control and Reload groups, which indicates that GK rats may have recovered more quickly from muscle atrophy. Conversely, plantaris muscles exhibited no significant differences in Wistar rats after three weeks, while the GK rats in the Reload and Suspension groups had significantly smaller values than those in the Control group. The muscles wet weight and myofiber cross-sectional areas results during atrophy and recovery could be different due to other factors such as proteins and other muscle contents that should be investigated in the future. Research into the effect of suspension on the plantaris muscles of Wistar rats have shown that, in comparison to fast-twitch type muscle, the plantaris muscle undergoes marked fiber type transitions. Thus, in the present study, the Wistar rats may have also experienced transition from type I to type IIB fibers14, 15). In contrast, GK rats exhibit a decrease in the ratio of fiber types with high oxidase activity as they age, and it has been reported that the proportion of soleus muscle type IIA fibers decreases leaving primarily type I fibers, while, in the plantaris muscle, the ratios of both type I and type IIA fibers decrease and transition to type IIB fibers16, 17). Moreover, investigation of the plantaris muscle has shown that, in type 1 diabetic rats, fast-twitch fibers atrophy before slow-twitch fibers18); furthermore, since atrophy occurs first in type IIB fibers, which are in large numbers in the plantaris muscles of GK rats, they may be strongly affected by suspension. These differences in the myofiber types in Wistar and GK rats may influence the degree of recovery of myofiber cross-sectional area resulting from reloading after tail suspension. An imbalance of protein synthesis/degradation and decreased muscle mass has been reported in patients with diabetes; it has also been reported that reduced expression of IGF-1 contributes to this process19). Further investigation into myofiber types and IGF-1 is necessary.
In our study, myofiber necrosis was not significantly different between Wistar and GK rats in any group in either soleus or plantaris muscle. A study of the effect of reloading stimuli on disuse muscle atrophy showed that overloading leads to muscle damage and that muscle damage peaks on day 2 following reloading20). In the present study, since we resected muscle tissue on day 7 following reloading, we did not observe all myofibers within each sample. This may be one reason why we observed fewer necrotic myofibers than previous studies on the recovery process from muscle damage. Nevertheless, although our investigation of GK rat soleus muscle tissue found no significant differences, there was a tendency toward the formation of higher numbers of necrotic myofibers in the Reload group, which suggests weakening of the myofibers. The soleus muscles of GK rats have reduced peripheral blood vessel volume16), and the soleus muscles of type 1 diabetic model rats are weakened due to reduced numbers of motor neurons9). Insulin acts as a protein anabolic hormone that regulates the breakdown and promotes the synthesis of muscle proteins21). This suggests the possibility that, under conditions in which insulin resistance increases as a result of diabetes, it may have a negative effect on the metabolism of skeletal muscle proteins. Thus, this suggests that the myofibers of the soleus muscles of GK rats may be weakened as a result of the effects of insulin resistance and exposure to hyperglycemia, as is seen in the case of vascular and nerve tissues.
There are some limitations of this study. This study analyzed muscle conditions after one week of reloading following a two-week period of caudal suspension; further study of the effects on the second day following reloading, which is considered the time when muscle damage is most pronounced, and analysis over a longer period until complete muscle recovery remain necessary. In addition, since it is possible that myofiber types undergo transition in diabetic rats, further study by myofiber type may provide a foundation for future study of effective exercise protocols in humans.
Conflict of interest
None.
REFERENCES
- 1.Yamazaki T: The efficacy of physiotherapy on muscle atrophy from the perspective of animal experiment data. Phys Ther Rev, 2013, 40: 63–67. [Google Scholar]
- 2.2016. National Health and Nutrition Survey.
- 3.Tanaka S, Tanaka S, Iimuro S, et al. Japan Diabetes Complications Study Group: Cohort profile: the Japan diabetes complications study: a long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int J Epidemiol, 2014, 43: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujishima M, Kiyohara Y, Kato I, et al. : Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama Study. Diabetes, 1996, 45: S14–S16. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell PO, Pavlath GK: A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol, 2001, 281: C1706–C1715. [DOI] [PubMed] [Google Scholar]
- 6.St Pierre BA, Tidball JG: Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol 1985, 1994, 77: 290–297. [DOI] [PubMed] [Google Scholar]
- 7.Wanek LJ, Snow MH: Activity-induced fiber regeneration in rat soleus muscle. Anat Rec, 2000, 258: 176–185. [DOI] [PubMed] [Google Scholar]
- 8.Zushi K, Yamazaki T: The effect of reloading on disuse muscle atrophy: time course of hypertrophy and regeneration focusing on the myofiber cross-sectional area and myonuclear change. J Jpn Phys Ther Assoc, 2012, 15: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamaki T, Ikutomo S, Muramatsu K, et al. : Motor neurons that support the soleus muscle weaken as a result of hyperglycemia. JMDD, 2016, 26: 99–102. [Google Scholar]
- 10.Oberbach A, Bossenz Y, Lehmann S, et al. : Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care, 2006, 29: 895–900. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi H, Tabata J, Endo C, et al. : Histochemical features and changes caused by lack of load in rat plantaris muscle: Comparison of sites along the long axis. Rehabil Med, 2001, 38: 832–838. [Google Scholar]
- 12.Morey-Holton ER, Globus RK: Hindlimb unloading rodent model: technical aspects. J Appl Physiol 1985, 2002, 92: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 13.Nonaka I: Clinical Muscle Pathology, 2nd ed. Nonaka I (ed.), Tokyo: Japan Medical Journal, 1993, pp 36–39. [Google Scholar]
- 14.Nishikawa M, Yamazaki T, Zushi K, et al. : The effect of reloading on the process of recovery from soleus muscle disuse atrophy in rats: differences between muscle sites. J Rehabil Sci, 2011, 26: 133–137. [Google Scholar]
- 15.Diffee GM, Caiozzo VJ, Herrick RE, et al. : Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am J Physiol, 1991, 260: C528–C534. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda K, Nishikawa W, Iwanaka N, et al. : Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol, 2002, 29: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 17.Murakami S, Fujita N, Kondo H, et al. : Abnormalities in the fiber composition and capillary architecture in the soleus muscle of type 2 diabetic Goto-Kakizaki rats. Sci World J, 2012, 2012: 680189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Sanchez M, Rodriguez-Sanchez C, Vega-Alvarez JA, et al. : Proximal skeletal muscle alterations in streptozotocin-diabetic rats: a histochemical and morphometric analysis. Am J Anat, 1991, 191: 48–56. [DOI] [PubMed] [Google Scholar]
- 19.Dehoux M, Van Beneden R, Pasko N, et al. : Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology, 2004, 145: 4806–4812. [DOI] [PubMed] [Google Scholar]
- 20.Tidball JG, Berchenko E, Frenette J: Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol, 1999, 65: 492–498. [PubMed] [Google Scholar]
- 21.Fujita S, Rasmussen BB, Cadenas JG, et al. : Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab, 2006, 291: E745–E754. [DOI] [PMC free article] [PubMed] [Google Scholar]