Abstract
[Purpose] Motor function evaluation by physical therapists is considered a valuable tool to assess the progression of muscular dystrophies. Few reports have described long-term motor function assessment during the administration of corticosteroids such as prednisolone (PSL) in these patients. This study examined the importance of long-term non-invasive motor function evaluation in a series of 3 cases. [Participants and Methods] Three boys with Duchenne muscular dystrophy who were administered an identical PSL dosage regimen were retrospectively evaluated, and motor function tests were compared in them before and after an increase in PSL dosage. Regular feedback was obtained from the patients’ mothers regarding their impressions of their child’s motor function after the introduction of PSL. [Results] Motor function was conserved or significantly improved after an increase in dosage in all cases. Interestingly, subjective assessment by mothers revealed a perceived improvement only in case 1 without any changes reported in cases 2 or 3. [Conclusion] PSL was demonstrably effective for 2.5–5 years after initiating PSL treatment, although parental impressions varied. Thus, long-term non-invasive evaluation by physical therapists may provide important objective data regarding medication efficacy and disease progression. Future studies should include long-term testing results as an essential component of the discontinuation criteria for PSL.
Keywords: Duchenne muscular dystrophy, Long-term corticoid treatment, Continual motor function evaluation
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-chromosome-linked disorder that causes progressive muscle atrophy and weakness. It is the most prevalent hereditary muscle disease, occurring in 1 in every 3,600 male births1,2,3). Also the most common childhood neuromuscular disease, DMD is characterized by a complete loss of dystrophin. Whole-body muscle atrophy and weakness progress over time, and patients are typically unable to walk around 13 years of age4), with an average life expectancy of roughly 30 years generally limited by respiratory or cardiac failure1, 5).
Although therapeutic strategies such as gene therapy and regenerative medicine have been extensively developed for DMD, definite treatment recommendations for patients with DMD are lacking. Oral prednisolone (PSL) has been associated with improvements in muscle strength and prolongation of independence and is the current standard of care6, 7).
The short-term evaluation of drug efficacy in DMD includes timed tests, such as 10-meter running and rising from the floor, as well as motor function evaluation by the North Star Ambulatory Assessment (NSAA)8) as performed by physical therapists for ambulatory DMD patients. Other functional measures, including the 6-minute walking test, and timed tests like the 10-meter running test and the timed up and go test, are also effective evaluation tools for ambulatory DMD9). Studies on medication frequency have often employed 10-meter running, 4 steps stair climbing, and muscle strength assessment as efficacy indicators10). Thus, considerable evidence supports the relevance of short-term motor function assessment to evaluate PSL effectiveness.
In contrast, despite many long-term reports on the natural history of DMD11, 12), there are few on the extended assessment of drug efficacy using motor function indicators. It has already been suggested that long-term follow-up is necessary for determining the effects of PSL13). Such an understanding, if performed non-invasively, will be useful for determining future treatment policies with regard to dosage and possible side effects. Our objective was to investigate the role of long-term, non-invasive motor function evaluation by physical therapists on estimating the effect of PSL in 3 DMD cases.
PARTICIPANTS AND METHODS
The participants were 3 preschool boys with ambulatory DMD who underwent PSL treatment with the same dosage regimen starting at 1.0 mg/kg/alternate-day dose (Table 1). Based on declining muscle power, the doses were increased by 0.75 mg/kg/day at the discretion of the attending physician. Motor function indicators of 10-meter running time, time to rise from the floor, and NSAA score (maximum score of 34 points) were measured from starting motor function evaluation to the study end point. Furthermore, we regularly asked the participants’ mothers at monthly follow-up checks for their impressions of their child’s motor function after PSL commencement.
Table 1. Physical characteristics of participants.
| At prednisolone commencement | At prednisolone dosage increase | DMD gene mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years/months) | Height (cm) | BMI (kg/m2) | Serum CK (U/l) | Age (years/months) | Height (cm) | BMI (kg/m2) | Serum CK (U/l) | ||
| Case 1 | 5/7 | 103.7 | 16.3 | 18,790 | 6/6 | 108.4 | 17.6 | 34,820 | Nonsense mutation in exon 48 |
| Case 2 | 5/8 | 99.6 | 15.1 | 21,280 | 6/1 | 102.7 | 15.5 | 13,355 | Nonsense mutation in exon 18 |
| Case 3 | 5/7 | 106.0 | 15.1 | 21,510 | 6/1 | 110.0 | 19.5 | 38,290 | Nonsense mutation in exon 21 |
BMI: body mass index; CK: creatine kinase; DMD: Duchenne muscular dystrophy.
Statistical analyses were conducted using PASW Statistics software (version 24.0, SPSS, Inc., Chicago, IL, USA), and pared t-tests were employed in all analyses.
This study was approved by the institutional ethics committee of the Shinshu University School of Medicine (no. 2340 and 2804). The experiment was orally explained to the participants and their parents, all of whom consented to participation. All study procedures were carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki.
RESULTS
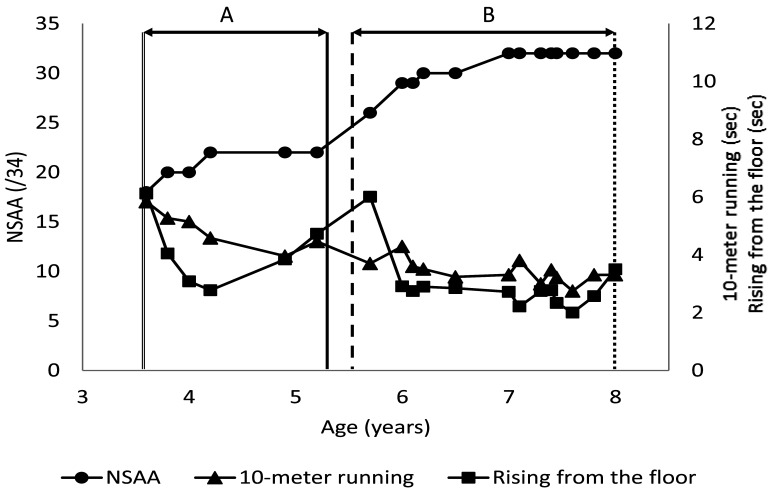
Case 1. The patient started PSL treatment at 5 years and 7 months and dosage was increased at 6 years and 6 months. The evaluation period was set from 5.5 to 10.5 years of age. He had a comorbid diagnosis of autism spectrum disorder (ASD). Motor function testing showed a significant improvement in the mean values of all outcomes in comparisons of the period from the start of treatment to the dosage increase (Section of A; Fig. 1, Table 2) with the period from the dosage increase to the observation end point (Section of B; Fig. 1, Table 2). The mother’s general impression was that he showed an improvement in movement and general expression during treatment.
Fig. 1.
Results of motor function evaluations for case 1.
The solid, dashed, and dotted lines indicate the timings of prednisolone (PSL) commencement, PSL dosage increase, and the observation end point, respectively. A: Period from start of PSL treatment to dosage increase. B: Period from PSL dosage increase to observation end point. NSAA: North Star Ambulatory Assessment.
Table 2. Motor function test outcomes.
| Test | Case 1 | Case 2 | Case 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | A vs. Ba | A | B | A vs. Ba | A | B | A vs. Ba | |
| 10-meter running (sec) | 4.4 ± 0.8 | 3.2 ± 0.3 | ** | 5.0 ± 0.4 | 4.4 ± 0.4 | * | 4.9 ± 0.6 | 3.3 ± 0.3 | ** |
| Rising from the floor (sec) | 3.4 ± 0.8 | 2.2 ± 0.4 | * | 4.2 ± 0.6 | 3.6 ± 0.5 | n.s. | 4.1 ± 1.1 | 2.7 ± 0.4 | * |
| NSAA (/34) | 27.0 ± 2.1 | 32.7 ± 0.9 | *** | 25.4 ± 2.2 | 28.4 ± 2.3 | * | 20.7 ± 1.5 | 31.4 ± 1.1 | *** |
Data are expressed as the mean ± standard deviation. apared t-test; *p<0.05; **p<0.01; *** p<0.001; n.s.: not significant.
A: the period from the start of treatment to the dosage increase (Case 1 and Case 2), the period from starting motor function evaluation to PSL commencement (Case 3) ; B: the period from the dosage increase to the study end point (Case 1 and Case 2), the period from the dosage increase to the study end point (Case 3). NSAA: North Star Ambulatory Assessment.
Case 2. The patient began PSL treatment at 5 years and 8 months and dosage was increased at 6 years and 1 month. The evaluation period was set from 5.5 to 8.5 years of age. Mean results for 10-meter running and NSAA score were significantly improved over the period from the dosage increase to the study end point (Section of B; Fig. 2, Table 2) as compared with the period from the start of treatment to the dosage increase (Section of A; Fig. 2, Table 2). There was no remarkable difference in time to rise from the floor (p=0.11). The mother’s impression was no observable improvement after the introduction of PSL.
Fig. 2.
Results of motor function evaluations for case 2.
The solid, dashed, and dotted lines indicate the timings of prednisolone (PSL) commencement, PSL dosage increase, and the observation end point, respectively. A: Period from start of PSL treatment to dosage increase. B: Period from PSL dosage increase to observation end point. NSAA: North Star Ambulatory Assessment.
Case 3. The patient started PSL treatment at 5 years and 7 months and dosage was increased at 6 years and 1 month. The evaluation period was set from 3.5 to 8 years of age. He had comorbid diagnoses of attention deficit/hyperactivity disorder (ADHD) and ASD. The mean values of all outcomes during the period from starting motor function evaluation to PSL commencement (Section of A; Fig. 3, Table 2) and the period from the dosage increase to the study end point (Section of B; Fig. 3, Table 2) were significantly improved. The mother felt that there was no significant motor function improvement before and after introduction of PSL as the child had apparently displayed an elevated amount of movement even before the introduction of PSL.
Fig.3.
Results of motor function evaluations for case 3.
The double, single, dashed, and dotted lines indicate the timings of motor function evaluation, prednisolone (PSL) commencement, PSL dosage increase, and the observation end point, respectively. A: Period from start of motor function evaluation to PSL commencement. B: Period from PSL dosage increase to observation end point. NSAA: North Star Ambulatory Assessment.
DISCUSSION
It was conducted a long-term evaluation of PSL efficacy on 10-meter running time, time to rise from the floor, and NSAA score in 3 ambulatory patients with DMD who received the same dosage regimen. Apart from the time to rise from the floor for case 2, all participants’ motor function outcomes improved significantly between the periods before and after a PSL increase. Thus, steroid treatment proved efficacious over 2.5–5 years after the initial administration.
In interviews with the patients’ mothers on their impression of treatment outcomes, those for patients 2 and 3 differed from the objective testing results. Particularly in case 3, there was an underlying diagnosis of ADHD that could have limited the parent’s ability to notice improvements in motor function. Accordingly, long-term, non-invasive evaluation by physical therapists plays an important role in objectively evaluating motor function in daily life and may assist in consultations with patients and their parents.
Regarding the use of PSL for DMD, there are currently no standard criteria for medication protocols in Japan13). However, we could conclude non-invasively that the effects of PSL improved or maintained motor function over several years, which highlighted the importance of long-term monitoring of motor function.
There were several limitations to this study. First, it was performed on a small number of cases that restricted the generalizability of the results. Secondly, the evaluation period differed among participants: for cases 1 and 2, we were unable to perform longer evaluation from the start of motor function evaluation to the beginning of PSL treatment, while in case 3, there was a shorter time from the beginning of PSL administration to the dosage increase.
Future studies in larger cohorts are needed to verify our findings on long-term motor function evaluations to establish PSL commencement, increasing, and discontinuation guidelines.
Conflict of interest
The authors declare that they have no competing interests.
REFERENCES
- 1.Bushby K, Finkel R, Birnkrant DJ, et al. DMD Care Considerations Working Group: Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol, 2010, 9: 77–93. [DOI] [PubMed] [Google Scholar]
- 2.Bushby K, Finkel R, Birnkrant DJ, et al. DMD Care Considerations Working Group: Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol, 2010, 9: 177–189. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EP, Brown RH, Jr, Kunkel LM: Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell, 1987, 51: 919–928. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Campbell KA, Fox DJ, et al. MD STARnet: Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol, 2015, 30: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenichel GM, Florence JM, Pestronk A, et al. : Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology, 1991, 41: 1874–1877. [DOI] [PubMed] [Google Scholar]
- 6.Eagle M, Baudouin SV, Chandler C, et al. : Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord, 2002, 12: 926–929. [DOI] [PubMed] [Google Scholar]
- 7.Gloss D, Moxley RT, 3rd, Ashwal S, et al. : Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the guideline development subcommittee of the American Academy of Neurology. Neurology, 2016, 86: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercuri E, Coratti G, Messina S, et al. : Revised North Star Ambulatory Assessment for Young Boys with Duchenne Muscular Dystrophy. PLoS One, 2016, 11: e0160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzone E, Martinelli D, Berardinelli A, et al. : North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord, 2010, 20: 712–716. [DOI] [PubMed] [Google Scholar]
- 10.Escolar DM, Hache LP, Clemens PR, et al. : Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology, 2011, 77: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pane M, Mazzone ES, Sivo S, et al. : Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes. PLoS One, 2014, 9: e108205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald CM, Henricson EK, Abresch RT, et al. Cinrg Investigators: The cooperative international neuromuscular research group Duchenne natural history study—a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve, 2013, 48: 32–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M, Komaki H, Takeshita E, et al. : Long-term outcomes of steroid therapy for Duchenne muscular dystrophy in Japan. Brain Dev, 2016, 38: 785–791. [DOI] [PubMed] [Google Scholar]