Abstract
[Purpose] To review the literature that examines rehabilitation and early mobilization and that involves different practices (effects of interventions) for the critically ill patient. [Materials and Methods] A PRISMA-Systematic review has been conducted based on different data sources: Biblioteca Virtual en Salud, CINHAL, Pubmed, Scopus, and Web of Science were used to identify randomized controlled trials, crossover trials, and case-control studies. [Results] Eleven studies were included. Early rehabilitation had no significant effect on the length of stay and number of cases of Intensive Care Unit Acquired Weaknesses. However, early rehabilitation had a significant effect on the functional status, muscle strength, mechanical ventilation duration, walking ability at discharge, and health quality of life. [Conclusion] Rehabilitation and early mobilization are associated with an increased probability of walking more distance at discharge. Early rehabilitation is associated with an increase in functional capacity and muscle strength, an improvement in walking distance and better perception of the health-related quality of life. Cycloergometer and electrical stimulation can be used to maintain muscle strength. Further research is needed to establish stronger evidences.
Keywords: Critical care, Early mobilization, Rehabilitation
INTRODUCTION
Despite the technological advances in intensive medicine, a large number of patients who survive a critical illness have their quality of life decreased1, 2). This fact is associated with a multifactorial morbidity that can cause functional, physical, cognitive and/or psychological disabilities2, 3) which persist even over 5 years after discharge4,5,6). In the critical patient management, interventions that promote long periods of immobilization are usually performed such as the use of mechanical ventilation, administration of drugs, sedatives, analgesics, drugs to control anxiety and agitation, etc7,8,9). Weakness is a common complication and is associated with a severe disability and a long rehabilitation. In this line, the intensive care unit acquired weakness (ICUAW) is associated with joint contractures, thromboembolism, resistance to insulin, microvascular alterations, pressure ulcers, atelectasis, pneumonia, extension of the weaning period, delirium, increase in the days of income, increased mortality, and development of disabilities10,11,12). Rehabilitation and early mobilization are considered therapeutic strategies to prevent the development of ICUAW13).
The concept of mobilization is large, complex, and interdisciplinary. It is energy consuming and consists of physical and psychological aspects14), including activities that produce movement such as “active limbs exercising, actively moving or rolling in bed, sitting on the edge of the bed, etc.”15). Mobilization is globally defined as “the physical activity that, performed with the appropriate intensity, produces physiological benefits for the organism”, acting on the circulation, central and peripheral perfusion, ventilation, or state of consciousness16). The term “early” refers to activities that are carried out from the initial physiological stabilisation and that continue during the ICU (Intensive Care Unit) stay17).
Early mobilization of patients is safe, feasible, and has positive results in ICU patients. However, it is not a common practice extended to all units17, 18). The literature includes various mobilization therapies and previous systematic reviews. Three of them study the effects of early rehabilitation on patients undergoing mechanical ventilation19,20,21); another one focuses on interventions aimed at preventing the ICUAW22); also, the one that examines interventions to improve physical capacity in patients who survive their disease at the ICU23). To analyse the effects of early mobilization in critically-ill patients is of vital importance, so the aim of this systematic review has been to review the literature that examines rehabilitation and early mobilization and that involves different practices (effects of interventions) in critically-ill patients.
MATERIALS AND METHODS
Methodologically the PRISMA declaration (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)24) was used to carry out the study. The search was carried out in the following online databases: Biblioteca Virtual en Salud (BVS), CINHAL, Pubmed, Scopus and Web of Science (WOS). In each of them, combinations of the following Descriptores en Ciencias de la Salud (Health Sciences Descriptors; DeCS, for its acronym in Spanish), and Medical Subject Headings (MeSH) descriptors were used: “critical care”, “rehabilitation”, “early mobilization”. The descriptors were combined using the Boolean operator “AND”. An example of the search strategy in the CINHAL database was “critical care” and “rehabilitation” and “early mobilization” that returned 14 results. The search was limited to published works from 2006 to 2016.
References of systematic reviews were analyzed to include studies of interest. The selection was made by two reviewers. The following inclusion criteria were followed:
The population was to be formed by adults over the age of 18 admitted to anintensive care unit for at least 48 hours.
Interventions had to be based on mobilization or early rehabilitation.
Studies should correspond to randomized clinical trial, crossover trial, or case-control.
The studies chosen for the analysis should be available in full text for the author, in English or in Spanish.
The studies had to be published between 1 January 2006 and 29 May 2016.
The exclusion criteria included studies in which:
A review of articles was included.
The intervention began at discharge from the ICU.
The intervention had begun at home prior to admittance.
TPrograms or protocols specifically designed for quality improvement projects were described.
The methodological quality of the included studies was assessed using the PEDro scale25). This scale consists of the following items: choice criteria, random assignment, concealed allocation, comparable groups, blinded participant, blinded therapists, blinded evaluators, adequate follow-up (measures of at least one of the key results obtained from more than 85% of participans), intention to treat analyses, groups’ comparison, ad hoc measures, and variability. Each criterion is scored as present or not present (Yes=1, No=0), and a maximum score of 10 was established. All criteria score 1 except for the first one, which is unscored.
The ethical considerations are considered at the declaration’s section at the end of the manuscript.
RESULTS
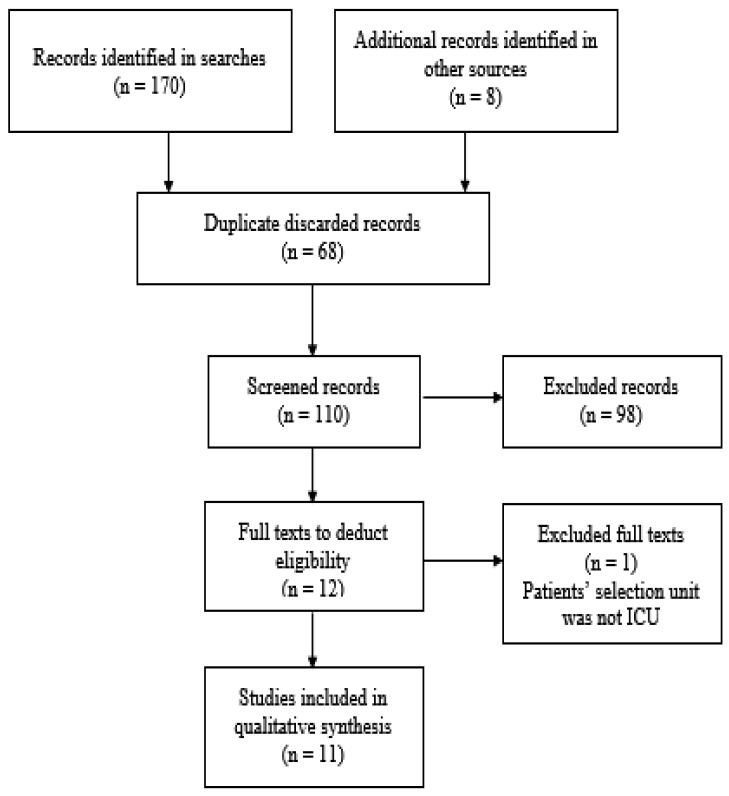
Following the search strategy, 178 results were obtained (44 in the Biblioteca Virtual en Salud, 15 in CINHAL, 59 in Pubmed, 21 in Scopus, 31 in Web of Science, and 8 in additional sources). Of them, 68 were discarded for being duplicated. After the revision of titles and summaries, 98 articles were excluded according to the exclusion criteria. Of the 12 full texts to deduct eligibility, one was excluded for failing to meet the inclusion criteria26). Finally, 11 articles were chosen for this review9, 27,28,29,30,31,32,33,34,35,36). Figure 1 shows the flowchart of the selected studies.
Fig. 1.
Study selection flowchart.
Information on the results obtained by the authors was selected from each study. The main characteristics of the studies are collected in Table 1.
Table 1. Main characteristics of the studies.
| Author, year | Inclusion criteria | Intervention | Control/Usual Care | Conclusions | ||
|---|---|---|---|---|---|---|
| Burtin et al.27), 2009 | Type of study: RCT | Country: Belgium | Minimum of 5 days of admittance, cardiorespiratory stability, expected admittance stay ≥ 7 days | Usual care + cycloergometer. Sedated patient: cycloergometer with passive mobilization, 20-min session following 20 cycles/min. Collaborative patient: cycloergometer with active mobilization in 2 sessions of 10 min or more intervals. | Respiratory physiotherapy + standard mobilization sessions of upper and lower limbs. Sedated patient: passive mobilization. Collaborative patient: active mobilization. | Early exercise in critical patients can improve their functional mobility capacity, their physical functioning self-perception and their quadriceps muscle strength at discharge. |
| n: 90 | Study unit: Medical and surgical ICU | |||||
| Schweickert et al.9), 2009 | Type of study: Multi-centre RCT (2) | Country: USA | MV >72 h with expected admittance of 24 more hours.Barthel >70 before (consultation to a close person on the previous 2 weeks to admittance) | Physiotherapy + occupational therapy + interruption of sedation.Passive progressive exercises, active- assisted, active; patient in supine position or sitting on the bed, transfers and walking. | Usual medical and nursing care. Physiotherapy occupational therapy care according to care team guideline. | A global rehabilitation strategy with daily interruption of sedatives, physiotherapy and occupational therapy in the first days of the critical illness is safe and well-tolerated, with better functional results at discharge, less duration of delirium and less MV days compared to the usual care. |
| n: 104 | Study unit: Medical ICU | |||||
| Dantas et al.28), 2012 | Type of study: RCT | Country: Brasil | MV, cardiorespiratory stability | Early mobilization protocol: passive progressive exercises, joint positioning, active-assisted, active-resisted, cycloergometer, transfer to sitting position, transfer to chair, orthostatic position, balance training, walking | Passive and progressive mobilization to active-resisted according to patient’s collaboration with upper and lower limbs. | The early mobilization group showed a higher inspiratory strength and higher peripheral muscle strength at discharge from ICU. |
| n: 59 | Study unit: ICU | |||||
| Denehy et al.29), 2013 | Type of study: RCT | Country: Australia | Minimum of 5 days of admittance. Residence at 50 km max from hospital. Responsible physician consent. | Individually adjusted exercises according to physical functioning test: active movements, active-resisted, transferfrom sitting to standing, walking on the spot. Rehabilitation continues at Ward and at discharge: c.v. exercises, strength, cycloergometer. | Usual care according to unit protocols | No differences in functional physical recovery are observed. |
| n: 150 | Study unit: ICU | |||||
| Collings & Cusack30), 2015 | Type of study: Crossover trial | Country: UK | MV ≥ 4 days, hemodynamic stability, capacity to mobilize > 10 m (with or without help) prior to admittance. Considered eligible by the physiotherapist. | Intervention A: passive transfer to chair + sitting on the edge of the bed versus Intervention B: sitting on the edge of the bed + passive transfer to chair | Sitting on the edge of the bed is a more demanding metabolically activity than passive transfer to chair | |
| n: 11 | Study unit: ICU | |||||
| Kho et al.32), 2015 | Type of study: RCT with simulation | Country: USA | MV at least 1 day expecting 2 more days, physiological stability | Usual care + muscular neurostimulation | Usual care based on progressive mobility interventions: in-bed exercises, transfers, standing, walking + placebo | Neuromuscular electrostimulation did not improve legs muscle strength at discharge from hospital |
| n: 36 | Study unit: Medical and surgical ICU | |||||
| Kayambu et al.31), 2015 | Type of study: RCT | Country: Australia | MV ≥ 48 h, diagnosis of sepsis | Physical rehabilitation program; progressive exercises from passive to active, muscular electrostimulation muscular, transfers, sitting out of bed, walking and others | >Usual care | >Early physical rehabilitation can improve the perceived physical function and have anti-inflammatory systemic effects |
| n: 50 | Study unit: General ICU | |||||
| Moss et al.33), 2015 | Type of study: Multi-centre RCT (5) | Country: USA | MV ≥ 4 days | Gradual physiotherapy program: proper breathing techniques, progressive mobilization, muscle strength exercises, core and elasticity, in-bed mobility, transfers, steps, balance. In the ICU, place of transfer or home up to 28 days. | Usual care with mobility and positioning exercises, functional mobility, transfers to chair and walking. In the ICU and at home under recommendation up to 28 days | The intensive physiotherapy program did not improve the physical function in the long term compared to usual care. |
| n: 50 | Study unit: ICU | |||||
| Hodgson et al.34), 2016 | Type of study: Multi-centre RCT (5) | Country: Australia and New Zealand | Patients predictably requiring MV, cardiorespiratory stability, able to walk without help before admittance. | Functional active activities protocol including: walking, standing, sitting, turning around. | Non-protocol usual care including passive mobilizations 5-10 min/day | The protocol appliance proved feasible and safe and increased the duration of the level of intensity of the active exercises. |
| n: 50 | Study unit: Medical and surgical ICU and trauma | |||||
| Fraser et al.35), 2015 | Type of study: Case-control | Country: USA | Admittance at ICU, capacity to walk without help and Barthel >60 prior to admittance. | Usual care + early mobility team care: 4 phases: passive exercises and positioning changes, sitting on the edge of the bed, getting up, chair-bed transfers, ambulation. | Usual care | Early mobilization contributes to shorter delirium periods and to an improvement in the sedation levels/functional state. |
| n: 132 | Study unit: Medical, surgical and coronary ICU | |||||
| Ota et al.36), 2015 | Type of study: Case control | Country: Japan | MV > 48 h, PSS 0–2, independent lifestyle at home prior to admittance. | Early mobilization during MV: muscular active and passive exercises, stretching, respiratory physiotherapy, head of bed 30–90 degrees, positioning changes from supine to 135 degrees in lateral decubitus position. Then, usual care. | During MV: rest; then, usual care | Early mobilization in patients requiring MV (with no NRL cause) can improve the number of discharges to home. |
| n: 111 | Study unit: ICU | |||||
RCT: Randomized Control Trials; ICU: Intensive Care Unit; n: sample size; MV: mechanical ventilation; PSS: performance status score.
The eligibility criteria for the selected sample were described in all studies. In two studies, there was no random allocation due to the type of case-control design35, 36). In six studies, the allocation of the participants to the groups was carried out in a concealed way9, 27, 29, 31, 32, 34). The basic characteristics of the individuals were compared in four studies9, 28, 31, 32). The participants were blinded in two studies31, 32). In none of the studies, the therapists performing the interventions were blinded. The evaluators were blinded in five studies9, 29, 31, 33, 34). The analysis was carried out by intention to treat in four studies9, 29, 31, 34). There are only three studies that can be considered of high methodological quality for meeting the criteria of concealed assignment, blinded evaluators, and intention-to-treat analysis9, 31, 34).
A total of 913 individuals participated in the studies at the time of randomization or assignment, of which 415 (45.45%) participants belonged to the intervention group and 435 (47.64%) to the control group. Of the total assigned individuals, 347 (40.82%) were women. The age and the APACHE II (Acute Physiology and Chronic Health Evaluation) score varied between the studies. The demographic characteristics of the participants are detailed in Table 2. Studies were conducted in medical9, 27, 32, 34, 35), surgical27, 32, 34, 35), and general-multivalent ICUs28,29,30,31, 33, 36) and, in some cases, patients with critical35) and coronary trauma34) were included. The investigations were conducted in Australia29, 31, 34), Belgium27), Brazil28), United States9, 32, 33, 35), Japan36), New Zealand34), and the United Kingdom30).
Table 2. Demographic characteristics of the participants.
| Author, year | group | n | Age (mean ± SD or median (IQR) or (CI)*) |
Gender, n (%) women |
APACHE IIa (mean ± SD or median (IQR) or (CI)*) |
|---|---|---|---|---|---|
| Burtin et al.27), 2009 | I | 31 | 56 ± 16 | 9 (29.03) | 26 ± 6 |
| C | 36 | 57 ± 17 | 10 (27.8) | 25 ± 4 | |
| Schweickert et al.9), 2009 | I | 49 | 57.7 (36.3–69.1) | 29 (59) | 20.0 (15.8–24.0) |
| C | 55 | 54.4 (46.5–66.4) | 23 (42) | 19.0 (13.3–23.0) | |
| Dantas et al.28), 2012 | I | 14 | 59.07 ± 15.22 | 7 (50) | 23.71 ± 8.51 |
| C | 14 | 50.43 ± 20.45 | 10 (71.43) | 21.07 ± 7.23 | |
| Denehy et al.29), 2013 | I | 74 | 61.4 ± 15.9 | 31 (41.9) | 19 ± 6 |
| C | 76 | 60.1 ± 15.8 | 24 (31.6) | 20.7 ± 7.7 | |
| Collings&Cusack30), 2015 | I: A | 5 | 61.4 (44.68–78.12)* | 1 (20) | 16.8 (15.04–26.16)* |
| I: B | 5 | 59.2 (31.43–86.97)* | 3 (60) | 20.6 (12.86–20.74)* | |
| Kayambu et al.31), 2015 | I | 26 | 62.5 (30–83) | 8 (16) | 28.0 ± 7.6 |
| C | 24 | 65.5 (37–85) | 10 (20) | 27.0 ± 6.8 | |
| Kho et al.32), 2015 | I | 16 | 54 ± 16 | 9 (56) | 25 ± 8 |
| C | 18 | 56 ± 18 | 8 (50) | 25 ± 6 | |
| Moss et al.33), 2015 | I | 59 | 56 ± 14 | 23 (39) | 17.9 ± 6.2 |
| C | 61 | 49 ± 15 | 26 (43) | 17.4 ± 5.6 | |
| Hodgson et al.34), 2015 | I | 27 | 64 ± 12 | 8 (38) | 19.8 ± 9.8 |
| C | 20 | 53 ± 15 | 12 (41) | 15.9 ± 6.9 | |
| Fraser et al.35), 2015 | I | 66 | 65.8 ± 19.6 | 32 (49) | 21.2 ± 7.5 |
| C | 66 | 63.5 ± 14.6 | 34 (52) | 20.2 ± 7.2 | |
| Ota et al.36), 2015 | I | 48 | 64 (46–73) | 14 (31) | 14 (11–20) |
| C | 60 | 72 (59–82) | 16 (27) | 16 (12–21) |
aAPACHE II: Acute Physiological and chronic health evaluation II measures illness severity and is related to mortality risk; I: Intervention; C: Control; CI: Confidence Interval; IQR: Intercuartile Range.
The description of the interventions and the usual care applied in the control group, as well as the inclusion criteria of the participants, are reflected in Table 1. In general, therapies for progressive mobilization of passive and/or active exercises were applied to achieve ambulation. An intervention with cycloergometer was performed in three studies27,28,29), and in two of them, electrostimulation was applied31, 32). Occupational therapy was part of the intervention in one study9) and specific respiratory physiotherapy was applied in two investigations33, 35).
The frequency of early mobilization was daily9, 28, 29, 31,32,33, 36) or 5 times a week27, 35). The frequency of usual care was performed daily, 5, or 3 times a week, or was not recorded. The description of the interventions with the available data is shown in Table 3.
Table 3. Description of interventions.
| Author, year | Group | Frequency | Daily duration (minutes) | Intensity | Weekly duration (minutes/week) | Time to start the first session since admittance (days) |
|---|---|---|---|---|---|---|
| Burtin et al.27), 2009 | Intervention | 5 times/week | 20 | Individually adjusted | 100 | 14 ± 10 |
| Control | 5 times/week | Not available | Individually adjusted | Not measurable | 10 ± 8 | |
| Schweickert et al.9), 2009 | Intervention | Daily | With MV: 19.2 (10.2–28.8) | Individually adjusted | With MV: mean 134.4 | 1.5 (1–2.1) |
| Post-weaning: 12.6 (4.8–19.8) | Post-weaning: mean 88.2 | |||||
| Control | Not available | With MV: | Not available | Not measurable | 7.4 (6–10.9) | |
| Post-weaning: 11.4 (0–22.8) | ||||||
| Dantas et al.28), 2012 | Intervention | Daily 2 sessions | Not available | Individually adjusted | Not measurable | Not available |
| Control | 5 times/week | Not available | Individually adjusted | Not measurable | Not available | |
| Denehy et al.29), 2013 | Intervention | Daily | 15 min in ICU progressive to 30 min in ward and up to 60 min at discharge | Individually adjusted | 100 in ICU, 210 in ward up to 420 at discharge | |
| Control | Daily | Not available | Not available | Not measurable | Not available | |
| Kayambu et al.31), 2015 | Intervention | Daily (1 or 2 sessions) | 30–60 min | EMS defined parameters | 210–420 | 2 |
| Control | Not available | Not available | Not available | Not measurable | Not available | |
| Kho et al.32), 2015 | Intervention | Daily (1 or 2 sessions) | 60 min; mean 60 ± 31 | Adjusted through visible muscular contraction and pain assessment | 420 | 4.6 ± 1.8 |
| Control (simulation) | Daily (1 or 2 sessions) | 60 min; mean 52 ± 25 | Stimulation 0 mA | 420 | 4.4 ± 1.6 | |
| Moss et al.33), 2015 | Intervention | Daily | 30 min in ICU; 60 min in ward, transfer, or home, during 28 days | Not available | 210–420 | 1 |
| Control | 3 times/week | Not available, during 28 days | Not available | Not measurable | Not available | |
| Hodgson et al.34), 2015 | Intervention | Not available | Up to 60 min | Adjusted though IMS scale | Not measurable | 3 (2–6) |
| Control | Not available | Passive mov. 5–10 min | Not available | Not measurable | 3 (2–4) | |
| Fraser et al.35), 2015 | Intervention | 5 times/week | 30–45 min | Not available | Not measurable | Not available |
| Control | Not available | Not available | Not available | Not measurable | Not available | |
| Ota et al.36), 2015 | Intervention | Daily (twice) | Not available | Individually adjusted | Not measurable | Not available |
| Control | Not available | Not available | Not available | Not measurable | Not available | |
The start time of the intervention was collected in nine studies and varied from 133) to 14 days27).
Then, the effects of interventions in the different studies related to functional capacity, muscle strength, mobility, quality of life, duration of mechanical ventilation, incidence of ICUAW, length of stay, and destination at discharge are described.
The functional capacity was measured by different instruments. Only two studies proved an effective intervention in improving functional capacities. One of them, at discharge from ICU35). The other one, at discharge from hospital9).
In two studies, the intervention was effective in achieving an increase in muscle strength at discharge form the ICU27, 28).
Mobility was assessed by a test or by taking into account the maximum distance walked with or without help. The intervention was effective at discharge from the hospital in three studies in a significant way9, 27, 32).
The perceived quality of life was measured in three studies using the physical function item of the health questionnaire SF-36 (Health Survey questionnaire short form 36 Physical Functioning item). The evaluation was carried out at different times: at discharge from hospital27), 3 months after discharge29), and 6 months after discharge31). In the study by Burtin et al.27), the quality of life was improved at discharge from hospital in a statistically significant manner.
The duration of mechanical ventilation was lower in the intervention group in a statistically significant way in two studies9, 36).
The proportion of ICUAW cases was collected in three studies9, 29, 34). The differences were not statistically significant among the groups.
DISCUSSION
In this review, 11 studies have been analysed by evaluating interventions based on rehabilitation and early mobilization in the ICU, with a total of 850 participants assigned to the intervention and control groups, or to habitual care groups. The characteristics of the patients, interventions, measurements and results obtained were heterogeneous, and there were conflicted positions regarding the effectiveness of the interventions. Early rehabilitation was associated with an increase in functional capacity9, 35) and muscle strength27, 28), with a shorter duration of mechanical ventilation9), and an improvement in walking distance9, 27, 32), as well as a better perception of the health-related quality of life27).
The methodological evaluation has shown a lack of high quality studies. The most valued study was that by Kayambu et al.31), although it did not reach the highest score due to the difficulty of the therapists being blinded. Other studies with medium-high methodological quality were the ones by Schweickert et al.9) and Hodgson et al.34) in which, in addition, it was not possible that participants were blinded. In the rest of the research, the low quality (3–4 scores) is mainly explained by the lack of masking, intention to treat analysis, and by the significant differences of the individuals’ characteristics, although these aspects have not been enough to consider their exclusion in the study.
In relation to the functional capacity measured through the Barthel scale, this review has found benefits, being significantly higher in the ICU intervention group at discharge from the Fraser et al. study35). This effect may be related to the significant presence of fewer patients undergoing mechanical ventilation in the intervention group.
In studies where electrostimulation31, 32) was applied, there was no significant increase in muscle strength at discharge, although successive measurements showed an improvement over the previous time interval32). In Kayambu et al.31), the positive effect of the intervention was associated with a systemic anti-inflammatory action. These results agree with a systematic review carried out in 2014, in which it was concluded that neuromuscular electrostimulation can be considered safe and effective to maintain the mass and muscle strength in the ICU37) in such a way as to favour the prevention of ICUAW.
The results of this review show a significant increase in the distance walked at hospital discharge9, 27, 32). However, patients in the study by Schweickert et al.9) walked less meters than other patients from the rest of the studies. In the study by Kho et al.32), the distance walked to the hospital was higher than the distance walked at discharge from the ICU. Likewise, in the study by Denehy et al.29), the data concerning the distance walked over the 3 months of follow-up was also higher. In parallel, the seconds used in the TUG test also decreased. In this regard, the probability of walking more meters can be explained by the use of more actively demanding interventions that focus on the musculature of the lower limbs such as the cycloergometer27, 29) or the neuromuscular electrostimulation32). Due to the lack of data on frequency, intensity, or duration, other considerations cannot be taken into account.
The perceived quality of life is related to the multifactorial morbidity suffered by patients who have survived a critical disease. This concept was valued only in three studies, so it is necessary to extend an integral conception of the patient that reflects the interest of highlighting the multidisciplinary role of the care team. The study by Burtin et al.27) showed a higher quality of life at discharge from the ICU, and Kayambu et al.31) over the 6 months after discharge. These results confirm those assessed by the review by Kayambu et al.20), where an improvement in the quality of life was also observed. However, this improvement was not only due to physical function, but also to cognitive aspects that were not valued.
It should be noted that the duration of mechanical ventilation was only less statistically significant in two studies9, 36). This result may be related to the daily interruption of sedation in the intervention group that could favour the weaning process. In any case, in the literature there is evidence of a shorter duration of mechanical ventilation associated with early mobilization20, 21) that cannot be widely confirmed by this review.
In relation to the length of ICU or hospital stay, the results cannot confirm positive effects as opposed to other evidence from recent multicentre studies38).
The heterogeneity of the studies is also reflected in the variability of the care given to the control group, which includes activities of different types from rest to non-standardized mobilizations or mobilization protocols. In many cases, there is no exhaustive definition of the usual care given. Although early mobilization critical patients’ care is considered important by professionals39), these data suggest the presence of barriers and difficulties that need to be analysed in order to promote an environment of mobilization culture in the critical care units.
This review has some limitations. First of all, the systematic review strategy of search can have a potential selection bias due to the established criteria. As for the methodology of the included studies, the punctuation in the PEDro scale shows an absence of high quality studies. In most of the analysed investigations, changeability exists in the characteristics of the compared patients, so this must be taken into account when generalising the whole population of critical patients. Another limitation is the scarce number of studies found.
Future studies should include patients with similar illnesses and lines of clinical treatment. It is necessary to reflect on the measurable results and the instruments to be used. The dose, intensity, frequency, and duration of the interventions must be considered, as well as trying to obtain a detailed description both of the interventions and of the usual care given. It is also necessary to keep in mind the measurement of the quality of life, the rehabilitation sessions after discharge from the ICU, as well as the long-term effects.
As for conclusions, this review has included recent scientific studies on rehabilitation and early mobilization in ICU patients. In the analysed studies, a variety of interventions have been applied according to the existing literature.
The results have showed that rehabilitation and early mobilization produce an effect on the decrease of the days of admittance both at the ICU and at the hospital. On the contrary, we can affirm that there is an effect on the progress of the functional capacity, strength, mobility, quality of life, less duration of mechanical ventilation, and a higher probability of being discharged to home.
Rehabilitation and early mobilization are related to a higher probability of traveling more distance at discharge. In the interventions, the cycloergometer and muscular electro-stimulation can be used for the maintenance of the muscle strength.
In addition, an absence of continuity is observed in patients’ follow-up after discharge from the ICU to ward, home, or in the long term. Therefore, it is necessary to make an effort to promote a culture of mobilization in the units of critical care.
The included studies have been heterogeneous and of few quality. Further research is necessary to establish more solid evidences on the effectiveness of the rehabilitation and early mobilization interventions.
Funding
Without funding.
Conflict of interest
None.
Acknowledgments
All authors have intellectually contributed to this work, meet all the authorship conditions and have approved the final version of the same.
REFERENCES
- 1.Needham DM, Dennison CR, Dowdy DW, et al. : Study protocol: the Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care, 2006, 10: R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel HJ, Needham DM, Morris PE, et al. : ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med, 2013, 41: S69–S80. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS: Long-term outcomes after critical illness. Curr Opin Crit Care, 2002, 8: 331–336. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AM, Tansey CM, Tomlinson G, et al. : Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med, 2006, 174: 538–544. [DOI] [PubMed] [Google Scholar]
- 5.Davydow DS, Katon WJ, Zatzick DF: Psychiatric morbidity and functional impairments in survivors of burns, traumatic injuries, and ICU stays for other critical illnesses: a review of the literature. Int Rev Psychiatry, 2009, 21: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan E, Dowdy DW, Colantuoni E, et al. : Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med, 2014, 42: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron S, Ball I, Cepinskas G, et al. : Early mobilization in the critical care unit: a review of adult and pediatric literature. J Crit Care, 2015, 30: 664–672. [DOI] [PubMed] [Google Scholar]
- 8.Hough CL, Needham DM: The role of future longitudinal studies in ICU survivors: understanding determinants and pathophysiology of weakness and neuromuscular dysfunction. Curr Opin Crit Care, 2007, 13: 489–496. [DOI] [PubMed] [Google Scholar]
- 9.Schweickert WD, Pohlman MC, Pohlman AS, et al. : Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet, 2009, 373: 1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans G, Van den Berghe G: Clinical review: intensive care unit acquired weakness. Crit Care, 2015, 19: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleton RT, Kinsella J, Quasim T: The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc, 2015, 16: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhan H, Moreno-Duarte I, Latronico N, et al. : Acquired muscle weakness in the surgical intensive care unit: nosology, epidemiology, diagnosis, and prevention. Anesthesiology, 2016, 124: 207–234. [DOI] [PubMed] [Google Scholar]
- 13.Fan E: Critical illness neuromyopathy and the role of physical therapy and rehabilitation in critically ill patients. Respir Care, 2012, 57: 933–944, discussion 944–946. [DOI] [PubMed] [Google Scholar]
- 14.Amidei C: Mobilisation in critical care: a concept analysis. Intensive Crit Care Nurs, 2012, 28: 73–81. [DOI] [PubMed] [Google Scholar]
- 15.Stiller K: Physiotherapy in intensive care: an updated systematic review. Chest, 2013, 144: 825–847. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Avila AC, Serón P, Fan E, et al. : Effect of early rehabilitation during intensive care unit stay on functional status: systematic review and meta-analysis. PLoS One, 2015, 10: e0130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey P, Thomsen GE, Spuhler VJ, et al. : Early activity is feasible and safe in respiratory failure patients. Crit Care Med, 2007, 35: 139–145. [DOI] [PubMed] [Google Scholar]
- 18.Morris PE, Goad A, Thompson C, et al. : Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med, 2008, 36: 2238–2243. [DOI] [PubMed] [Google Scholar]
- 19.Adler J, Malone D: Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J, 2012, 23: 5–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Kayambu G, Boots R, Paratz J: Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med, 2013, 41: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Peng X, Zhu B, et al. : Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil, 2013, 94: 551–561. [DOI] [PubMed] [Google Scholar]
- 22.Hermans G, De Jonghe B, Bruyninckx F, et al. : Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev, 2014, (1): CD006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo-Ayala E, Khan BA, Farber MO, et al. : Interventions to improve the physical function of ICU survivors: a systematic review. Chest, 2013, 144: 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urrútia G, Bonfill X: Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin (Barc) [Internet], 2010. 135(11): 507–511. http://linkinghub.elsevier.com/retrieve/pii/S0025775310001454 (Accessed Jul. 17, 2017) [DOI] [PubMed]
- 25.Maher CG, Sherrington C, Herbert RD, et al. : Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther, 2003, 83: 713–721. [PubMed] [Google Scholar]
- 26.Cumming TB, Thrift AG, Collier JM, et al. : Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke, 2011, 42: 153–158. [DOI] [PubMed] [Google Scholar]
- 27.Burtin C, Clerckx B, Robbeets C, et al. : Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med, 2009, 37: 2499–2505. [DOI] [PubMed] [Google Scholar]
- 28.Dantas CM, Silva PF, Siqueira FH, et al. : Influence of early mobilization on respiratory and peripheral muscle strength in critically ill patients. Rev Bras Ter Intensiva, 2012, 24: 173–178 (In Portuguese). [PubMed] [Google Scholar]
- 29.Denehy L, Skinner EH, Edbrooke L, et al. : Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care, 2013, 17: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collings N, Cusack R: A repeated measures, randomised cross-over trial, comparing the acute exercise response between passive and active sitting in critically ill patients. BMC Anesthesiol, 2015, 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayambu G, Boots R, Paratz J: Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med, 2015, 41: 865–874. [DOI] [PubMed] [Google Scholar]
- 32.Kho ME, Truong AD, Zanni JM, et al. : Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care, 2015, 30: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss M, Nordon-Craft A, Malone D, et al. : A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med, 2016, 193: 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodgson CL, Bailey M, Bellomo R, et al. Trial of Early Activity and Mobilization Study Investigators: A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Crit Care Med, 2016, 44: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 35.Fraser D, Spiva L, Forman W, et al. : Original research: implementation of an early mobility program in an ICU. Am J Nurs, 2015, 115: 49–58. [DOI] [PubMed] [Google Scholar]
- 36.Ota H, Kawai H, Sato M, et al. : Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci, 2015, 27: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wageck B, Nunes GS, Silva FL, et al. : Application and effects of neuromuscular electrical stimulation in critically ill patients: systematic review. Med Intensiva, 2014, 38: 444–454. [DOI] [PubMed] [Google Scholar]
- 38.Wahab R, Yip NH, Chandra S, et al. : The implementation of an early rehabilitation program is associated with reduced length of stay: a multi-ICU study. J Intensive Care Soc, 2016, 17: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber EA, Everard T, Holland AE, et al. : Barriers and facilitators to early mobilisation in intensive care: a qualitative study. Aust Crit Care, 2015, 28: 177–182, quiz 183. [DOI] [PubMed] [Google Scholar]