Abstract
This 52-week, phase I/II double-blind, randomized, placebo-controlled study investigated the novel use of clenbuterol in late-onset Pompe disease (LOPD) stably treated with ERT. Eleven of thirteen participants completed the study. No serious adverse events were related to clenbuterol, and transient minor adverse events included mild elevations of creatine kinase, muscle spasms, and tremors. At week 52, the 6-min walk test distance increased by a mean of 16 m (p = 0.08), or a mean of 3% of predicted performance (p = 0.03), and the maximum inspiratory pressure increased 8% (p = 0.003) for the clenbuterol group. The quick motor function test score improved by a mean of seven points (p = 0.007); and the gait, stairs, gower, chair test improved by a mean of two points (p = 0.004). Clenbuterol decreased glycogen content in the vastus lateralis by 50% at week 52. Transcriptome analysis revealed more normal muscle gene expression for 38 of 44 genes related to Pompe disease following clenbuterol. The placebo group demonstrated no significant changes over the course of the study. This study provides initial evidence for safety and efficacy of adjunctive clenbuterol in patients with LOPD (NCT01942590).
Keywords: enzyme replacement therapy, glycogen storage disease, acid alpha-glucosidase
The response to enzyme replacement therapy in Pompe disease is limited by the low uptake of therapeutic enzyme by skeletal muscle. In this issue of Molecular Therapy, Koeberl et al. (2018) demonstrate that clenbuterol treatment improved the muscle response to enzyme replacement.
Introduction
Effective dosages for enzyme replacement therapy (ERT) in Pompe disease are up to 100-fold greater than those in other lysosomal disorders. This high-dose requirement has been attributed to the low abundance of cation-independent mannose-6-phosphate receptor (CI-MPR) in skeletal muscle. We have evaluated the impact of CI-MPR-mediated uptake of recombinant human (rh) acid-α-glucosidase (GAA) upon ERT in GAA knockout (KO) mice with Pompe disease.1, 2 These published data revealed that clenbuterol, a selective β2 agonist, enhanced CI-MPR expression and increased efficacy from ERT, thereby confirming the key role of CI-MPR with regard to replacement therapy in Pompe disease.1, 2 The clearance of stored glycogen was increased by β2 agonist treatment during ERT, as demonstrated by significantly lower glycogen content in skeletal muscle following the addition of clenbuterol or albuterol treatment to ERT (consisting of 4 weekly injections at the standard dose, 20 mg/kg) in GAA-KO mice.2 The skeletal muscles comprised primarily of type II myofibers, including the tibialis anterior muscle, responded more efficaciously to ERT when clenbuterol or albuterol therapy was added.1 However, albuterol has been less effective at lowering muscle glycogen than clenbuterol in pre-clinical experiments,2, 3 and adjunctive albuterol did not decrease muscle glycogen in a pilot clinical trial in adult patients with Pompe disease.4
Type II muscles are resistant to ERT in association with low CI-MPR expression.5, 6 The underlying mechanism for clenbuterol’s effects on muscle was demonstrated as increased expression of insulin-like growth factor (Igf) 1 and 2 and their receptors, including the Igf-2 receptor that is actually CI-MPR.7 Increased Igf-1 expression was also associated with the muscle hypertrophy following clenbuterol administration, which could be beneficial in Pompe disease.7 In addition to skeletal muscle benefits, adjunctive β2 agonist treatment with ERT or gene reversed neuromuscular involvement in GAA-KO mice as evidenced by enhanced biochemical correction in the brain and improved neuromuscular function.1, 2, 3
We currently report a 52-week randomized, double-blind placebo-controlled study of adjunctive clenbuterol in patients with late-onset Pompe disease (LOPD). Our goal was to determine safety and the muscle effects of adjunctive clenbuterol in patients with LOPD who were stably treated with ERT and expected to experience no further benefits. Safety was the primary endpoint, which was monitored by blood testing and adverse event (AE) reporting throughout the study. Secondary endpoints included muscle function tests, which were correlated with changes in skeletal muscle biopsies obtained at baseline and at week 52.
Results
Summary of Enrollment and Patient Characteristics
Thirteen participants who met all inclusion criteria were randomized, and 11 completed the study (Figure S1). Eight participants (5M:3F) were assigned to drug, and seven of those completed the study, whereas five (2M:3F) were assigned to placebo and four completed the study (Table 1). One participant withdrew after taking placebo for 6 weeks, and one was withdrawn after taking the study drug for 46 weeks following an unrelated significant AE in which the participant fell and dislocated his artificial hip. Of the four consented participants who were not randomized, one participant failed screening and three withdrew prior to randomization. In the clenbuterol group, more participants were men, and they were older (Table 1). The clenbuterol group had been treated with ERT longer (median 75 months, range 38–102) than the placebo group (median 21 months, range 18–72). The 6-min walk test (6MWT) distance and functional vital capacity (FVC) were low in both groups, consistent with LOPD. The predicted 6MWT distance trended lower (p = 0.08) and predicted FVC was lower (p = 0.007) for the clenbuterol group, in comparison with the placebo group (Table 1). These differences were consistent with more advanced impairment at later ages in the clenbuterol group. The study was double-blinded to prevent a placebo effect. However, the clenbuterol and placebo groups were not compared directly due to differing characteristics.
Table 1.
Demographic and Baseline Characteristics of the Study Population
| Clenbuterol (n = 8) | Placebo (n = 5) | |
|---|---|---|
| Age (median, range) | 52 (37–65) | 32 (19–62) |
| Gender | 5M:3F | 2M:3F |
| Race | ||
| White | 8/8 | 5/5 |
| Ethnicity | ||
| Not Hispanic or Latino | 8/8 | 5/5 |
| Weight (median), kg | 89 | 73 |
| Duration of ERT, months (median, range) | 75 (38–102) | 21 (15–72) |
| Baseline FVC, % predicted (median) | 50 | 89 |
| Baseline 6MWT, meters (median) | 350 | 450 |
| Baseline 6MWT, % predicted (median) | 51 | 72 |
Functional Effects of Clenbuterol Treatment
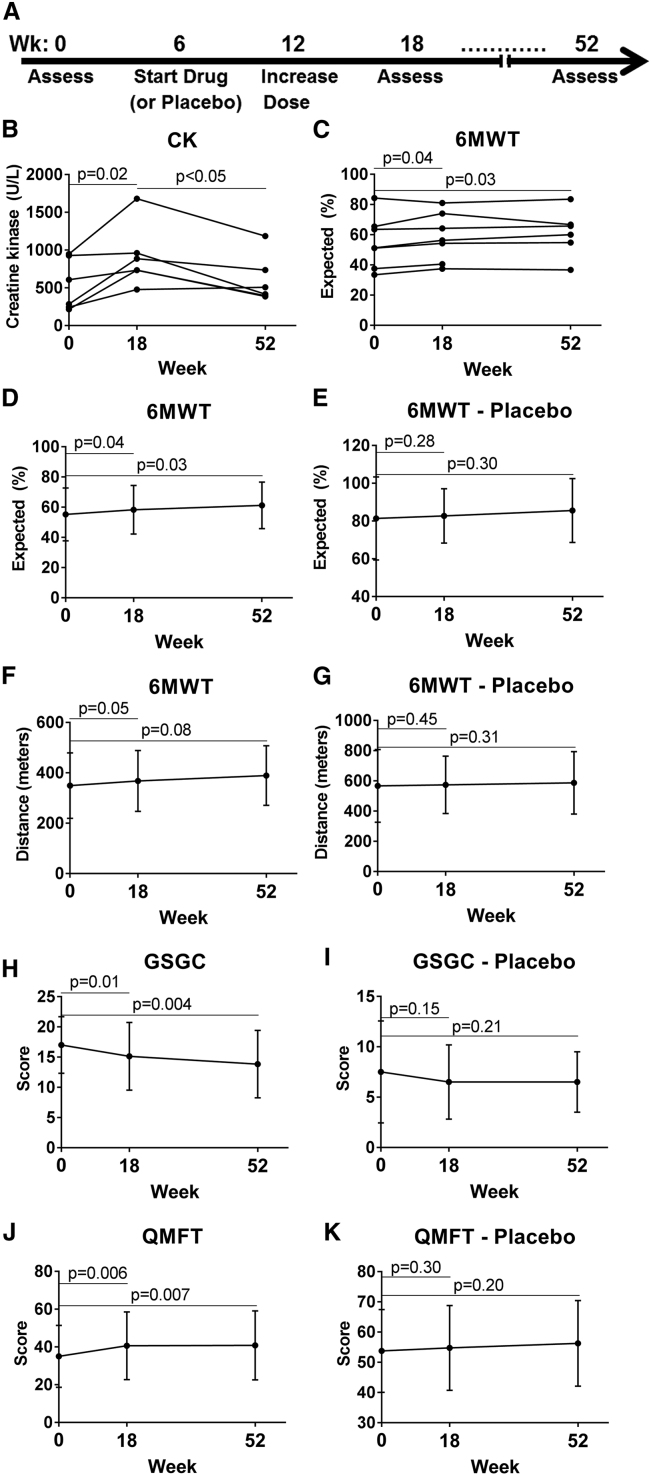
The effects of clenbuterol administration were evaluated at week 18 and week 52, in comparison with baseline (Figure 1A). Mildly elevated creatine kinase (CK) has previously been associated with clenbuterol administration.8 We observed transiently elevated CK at week 18, which returned to baseline values by week 52 (Figure 1B). No significant differences of CK from baseline were observed for the placebo group (Figure S2). The 6MWT percent-predicted values showed statistically significant and consistent increases for each individual from baseline at both week 18 and week 52 (Figure 1C), suggesting clinical as well as statistical significance. One participant stopped ERT several months before the week 52 visit, and he was not included in week 52 analyses. At week 52 6MWT increased significantly by 3% with regard to predicted performance, from 58% ± 17% to 61% ± 15% (Figure 1D; p = 0.03), whereas 6MWT performance was unchanged for the placebo group (Figure 1E). Percent-predicted 6MWT values can be used to help distinguish and highlight treatment effects from changes that may result from typical age-related changes or changes in weight over a 1-year period that could affect distance walked in the 6MWT. At week 52 the 6MWT distance increased 16 m for the clenbuterol group, from a mean of 373 m (SD ± 126 m) to a mean of 389 ± 119 m (Figure 1F; p = 0.08). At week 18, the 6MWT predicted performance increased significantly by 3%, from 55% ± 17% to 58% ± 16% (Figure 1D; p = 0.03), and 6MWT distance increased by 19 m, from 349 ± 130 m to 368 ± 121 m (Figure 1F; p = 0.05). This was an early-phase study, not powered to compare the two groups. However, 6MWT distance was unchanged from baseline to week 52 for the placebo group (Figures 1E and 1G; Table 2).
Figure 1.
Functional Testing Demonstrated Efficacy for the Clenbuterol Group
Timeline for the study (A). Participants were enrolled and completed a baseline assessment prior to randomization to clenbuterol or placebo at week 6, dose increase at week 12, and returning for assessment at weeks 18 and 52. (B) Serum CK for clenbuterol group. Each line connects the data points for one research participant. Normal range, 30–220 U/L. One participant in the clenbuterol group was excluded at week 52 due to having stopped ERT several months earlier. 6MWT for clenbuterol group is shown in XY graphs depicting (C) predicted performance (data points for each participant), (D) predicted performance (mean ± SD), and (F) distance (mean ± SD). (E) 6MWT predicted performance and (G) distance for placebo group. Functional muscle testing for clenbuterol group is shown in XY graphs depicting (H) GSGC and (J) QMFT. Functional muscle testing for placebo group: (I) GCGS and (K) QMFT for placebo group (mean ± SD). Horizontal lines indicate the data points relevant to the adjacent p value.
Table 2.
Outcomes in Clenbuterol and Placebo Groups
| Test | Clenbuterol |
Placebo |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Week 52 | Change | pa | n | Baseline | Week 52 | Change | p | |
| 6MWT (m) | 6 | 373 | 389 | 4% | 0.08 | 4 | 567 | 587 | 4% | 0.33 |
| 6MWT (%) | 6 | 58 | 61 | 5% | 0.03 | 4 | 81 | 85 | 5% | 0.3 |
| GSGC (pt) | 6 | 16 | 14 | −13% | 0.004* | 4 | 8 | 7 | −13% | 0.21 |
| QMFT (pt) | 5 | 40 | 47 | 18% | 0.007* | 4 | 54 | 56 | 4% | 0.20 |
| FEV1 (%) | 7 | 58 | 65 | 12% | 0.06 | 4 | 86 | 88 | 2% | 0.28 |
| FVC (%) | 7 | 60 | 64 | 7% | 0.11 | 4 | 83 | 90 | 8% | 0.25 |
| MEP (%) | 5 | 40 | 54 | 35% | 0.06 | 4 | 63 | 49 | −22% | 0.12 |
| MIP (%) | 5 | 51 | 69 | 35% | 0.004 | 4 | 97 | 104 | 7% | 0.28 |
| GAA (μmol/min/g tissue) | 6 | 0.14 | 0.18 | 29% | <0.05 | 4 | 0.20 | 0.15 | −25% | 0.47 |
| Glycogen | 7 | 0.75 | 0.37 | −51% | 0.007* | 4 | 0.73 | 0.88 | 21% | 0.34 |
| LAMP2 (relative to GAPDH) | 5 | 0.69 | 0.46 | −33% | 0.01 | 3 | 0.88 | 1.2 | 36% | 0.04 |
p by t test shown. If p < 0.05 by two-way ANOVA, indicated by an asterisk (*).
Standardized muscle functional assessments included the gait, stairs, gower, chair (GSGC)9 and the quick motor function test (QMFT),10 both of which have been validated in patients with LOPD. For the clenbuterol group, the score for the GSGC test improved by 2 points (pt) between baseline and week 52, from 16 ± 5 pt to 14 ± 6 pt (Figure 1H; p = 0.004; lower GSGC scores reflect improvement), while the QMFT score improved by 7 pt, from 40 ± 14 pt to 47 ± 15 pt (Figure 1J; p = 0.007) for patients on clenbuterol. Furthermore, the GSGC score improved by 2 pt between baseline and week 18 (Figure 1H; p = 0.01), while the QMFT score improved by 6 pt (Figure 1J; p = 0.006). For the placebo group, no significant changes were observed from baseline at later visits (Figures 1I and 1K). Thus, exploratory endpoints improved between baseline and the week 18 and week 52 visits for the clenbuterol group.
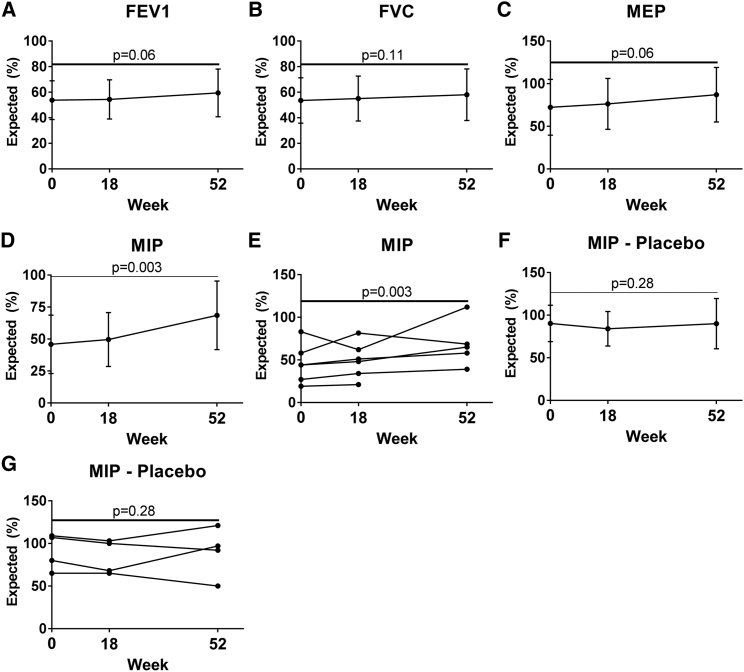
In pulmonary function testing at week 52, the predicted performance on the forced expiratory volume in 1 s (FEV1) trended greater for the clenbuterol group (Figure 2A), increasing 12% (from 58% ± 15% to 65% ± 14%; p = 0.06). The forced vital capacity (FVC) did not improve significantly, increasing from 60% ± 15% to 64% ± 15% (Figure 2B; p = 0.11). The maximum expiratory pressure (MEP) trended greater at week 52, increasing 35%, from 40% ± 11% to 54% ± 8% (Figure 2C; p = 0.06).
Figure 2.
Pulmonary Function Testing Revealed Improved Strength of Respiratory Muscles for Clenbuterol Group
Pulmonary function testing for clenbuterol group is shown in XY graphs depicting (A) FEV1, (B) FVC, (C) MEP, and (D) MIP. Mean ± SD is shown. MIP for each individual in the clenbuterol group (E). One subject was excluded at week 52 due to having stopped ERT several months earlier. MIP for placebo, mean ± SD (F). MIP for each individual in the placebo group (G).
The predicted maximum inspiratory pressure (MIP) increased by 35% for the clenbuterol group at week 52, from 51 ± 21 to 69 ± 27 (Figure 2D; p = 0.004). MIP increased for 4 of 5 individuals from baseline to week 52 (Figure 2E). MIP was unchanged from baseline at week 52 for the placebo group (Figures 2F and 2G; Table 2). Pulmonary function testing for these measures did not reveal any significant changes at week 18, in comparison with baseline. No significant differences from baseline were observed for supine FEV1 and FVC testing at the week 18 or week 52 visits (data not shown). Thus, the FEV1, MEP, and MIP demonstrated positive trends at week 52 for the clenbuterol group, although only the MIP achieved statistical significance.
Biochemical Effects of Clenbuterol Treatment
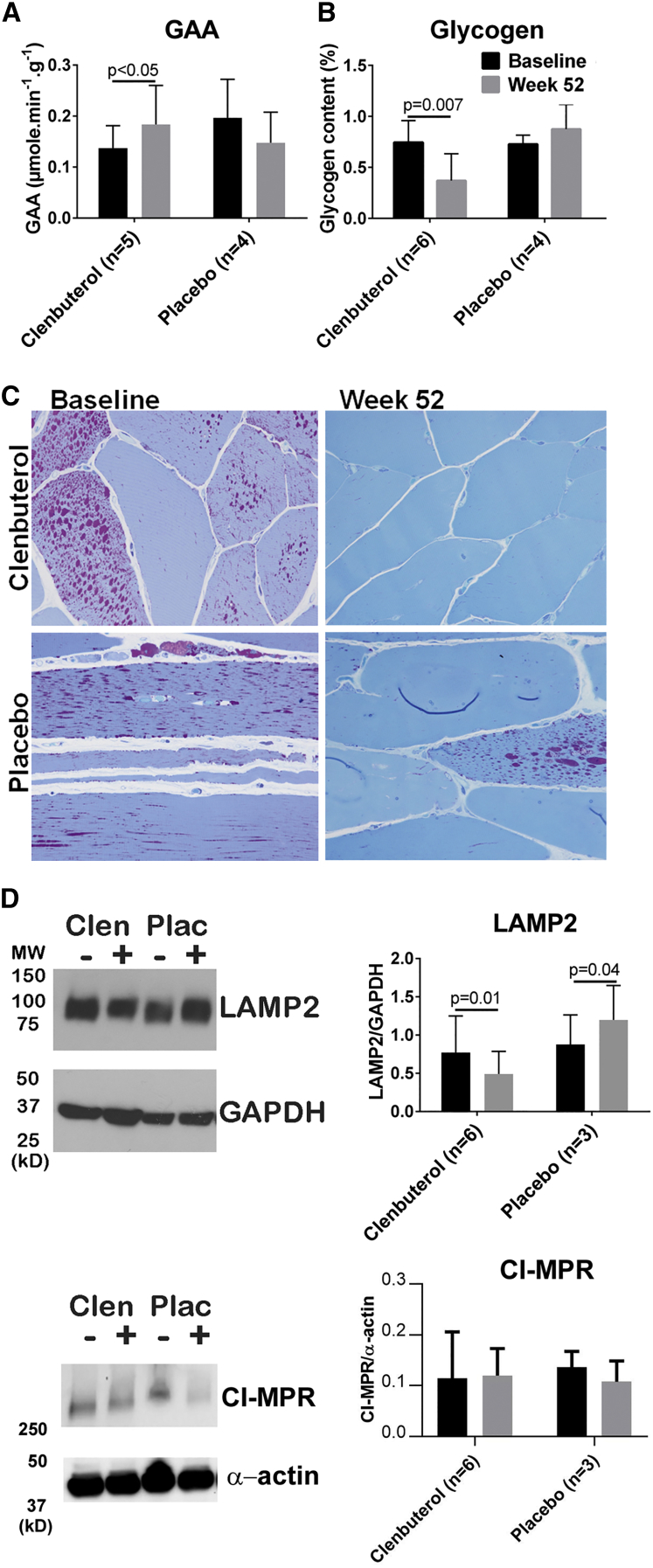
The effects of clenbuterol administration on biochemical correction of skeletal muscle were evaluated by testing of a biopsy from the vastus lateralis in the quadriceps muscle at baseline and at week 52. The vastus lateralis is comprised primarily of type 2 myofibers in adults,11 and muscles comprised of type 2 myofibers have been resistant to correction with ERT in Pompe disease.5, 6 GAA activity increased by 34% in the clenbuterol group (from 0.14 ± 0.04 to 0.18 ± 0.08 μmol/min/g tissue) (Figure 3A; p < 0.05), and glycogen content decreased by 51%, from 0.75% ± 0.21% to 0.37% ± 0.26% by biochemical assay (Figure 3B; p = 0.007). No significant changes in biochemical testing were demonstrated in the placebo-exposed group (Figure 3). Muscle biopsies were also examined histopathologically. Overall, histologic glycogen levels in these patient biopsies were very low, likely due to prior long-term treatment with ERT. Consequently, additional changes with clenbuterol treatment reflected modest improvements or stability. Three of six clenbuterol-treated patients with evaluable biopsy pairs at baseline and week 52 showed a qualitative reduction in periodic-acid Schiff (PAS)-positive glycogen. The remaining three treated patients appeared histologically stable. Four placebo patients had evaluable biopsy pairs at baseline and week 52; all four patients demonstrated stable histologic glycogen levels. For the clenbuterol group, the lysosomal associated membrane protein 2 (LAMP2) signal was decreased in western blots of patient muscle (p = 0.01), demonstrating decreased lysosomal accumulations (Figure 3D). In contrast, for the placebo group LAMP2 increased (p = 0.04). CI-MPR protein was not increased at week 52, in comparison with baseline (Figure 3D), which does not preclude an earlier increase in CI-MPR as demonstrated in pre-clinical studies.1, 2 Thus, biochemical correction of the vastus lateralis muscle, which is typically resistant to ERT, was improved only for the clenbuterol group.
Figure 3.
Muscle Effects from Clenbuterol
Biochemical testing at baseline and week 52 for clenbuterol and placebo groups’ vastus lateralis muscle biopsies. (A) GAA activity. One participant from the clenbuterol group was excluded from the GAA assay due to having stopped ERT several months earlier. (B) Biochemical glycogen content. Mean ± SD are shown. (C) Histopathology revealed decreased (three patients) or stable (three patients) PAS-positive glycogen levels following clenbuterol administration; all four placebo patients with evaluable biopsy pairs demonstrated stable PAS-positive glycogen levels (high-resolution light microscopy, 1-micron epoxy resin sections, PAS-Richardsons stain, 400× magnification). Western blot quantification of (D) LAMP2 and CI-MPR in muscle biopsy samples and quantification of western blot signals. The lanes marked as + are from the post-randomization samples at week 52, whereas − indicates the baseline samples.
Transcriptome Effects of Clenbuterol Treatment
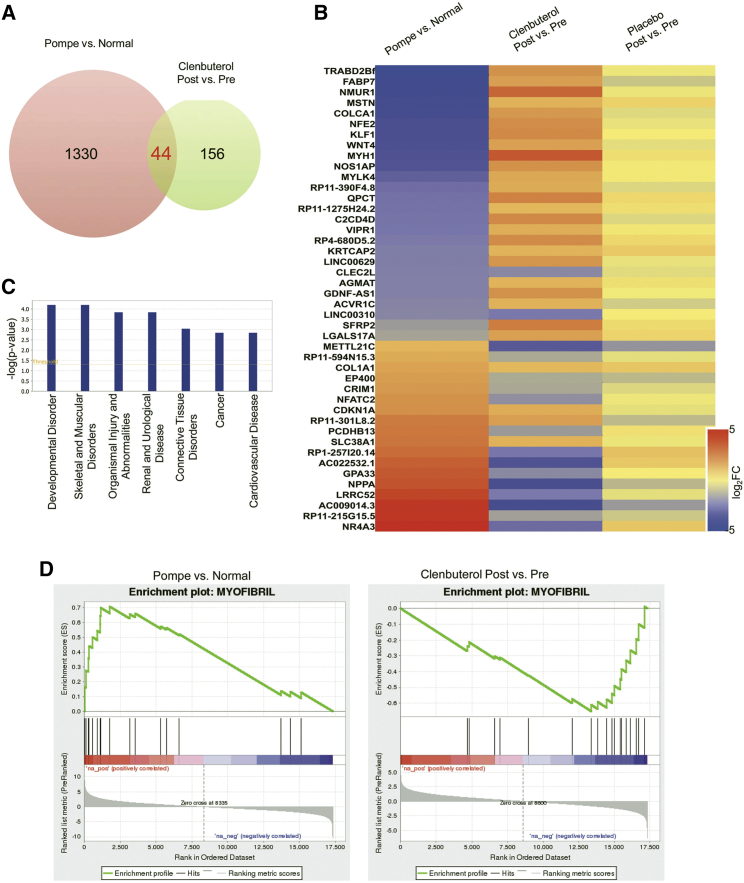
RNA-sequencing (RNA-seq) of muscle biopsies revealed that relative to pre-treatment levels, 52-week clenbuterol treatment induced differential expression (>2-fold) of 200 genes. A subset of 44 of these 200 genes (22%) overlapped with the genes significantly dysregulated by >2-fold in the biopsies from LOPD patients versus healthy age-matched individuals (Figure 4A). Notably, 38 (86%) of these 44 genes were altered in the opposite direction by disease and by clenbuterol treatment, while no genes were significantly altered >2-fold by placebo treatment (Figure 4B). Furthermore, ingenuity pathway analysis revealed that in the disease and disorders category, these 38 genes were associated in the order of significance with developmental disorder, skeletal and muscular disorders, organismal injury and abnormalities, renal and urological disease, connective tissue disorders, cancer, and cardiovascular disease (Figure 4C). This indicated that the clenbuterol treatment was specifically correcting LOPD-associated genes rather than having random effects. Finally, the gene set enrichment analysis (GSEA) of gene ontology molecular functions and biological processes of the whole transcriptome revealed that the Myofibril gene set is significantly enriched in the disease-upregulated genes and in the clenbuterol-downregulated genes, and none of the other gene sets showed this pattern (Figure 4D). Overall, the pathway analysis suggested that clenbuterol treatment was predominantly associated with ameliorating the adverse effects caused by LOPD.
Figure 4.
RNA-Seq Analyses of Differentially Expressed Genes and Pathway Analysis
RNA-seq analyses of differentially expressed genes and pathway analysis. (A) Venn diagram showing that 44 overlapping genes significantly differ (by >2-fold; p < 0.05) for both Pompe versus normal and post- versus pre-clenbuterol treatment. (B) Heatmap showing the relative differences in expression of these 44 overlapping genes (displayed as log2 fold change) for specified comparisons. Red, upregulated genes; blue, downregulated genes. (C) Ingenuity pathway analysis of diseases and disorders. (D) Gene set enrichment analysis (GSEA) plots of Myofibil gene set from the whole transcriptome of Pompe versus normal (enrichment score, 0.705) and clenbuterol post versus pre (enrichment score, −0.652).
Safety
There were no drug-related serious AEs throughout this study. There was one unrelated severe AE in which the participant fell, dislocating an artificial hip. This led to the need to withdraw the participant from the study after taking clenbuterol for 46 weeks. Expected AEs were mild and included the following: tremor, insomnia, and muscle spasms, each of which were observed in four participants (50%) and resolved spontaneously. One participant had transiently elevated CK to >3-fold the individual’s baseline concentration (to 2,430 U/L; normal range 30–220 U/L), when blood was sampled following the physical therapy evaluation instead of prior to it as normally practiced (Table 3). However, this individual’s CK returned to baseline concentrations when reassessed the following week. Insomnia (40%) and muscle spasms (20%) were observed in participants taking placebo (Table 3). Two participants required dose reductions (80 mcg morning, 40 mcg evening) related to minor AEs (jitteriness, GI upset, increased heart rate, elevated CK) but continued in the study following resolution of these symptoms.
Table 3.
AEs Possibly Related To Study Agent
| Symptom | Clenbuterol | Placebo |
|---|---|---|
| Anxiety/jitteriness | 2 (25%) | 1 (20%) |
| Decreased appetite/weight loss | 2 (25%) | 0 |
| Elevated CK (>3× baseline) | 1 (13%) | 0 |
| GI upset | 2 (25%) | 0 |
| Increased appetite/weight gain | 2 (25%) | 2 (40%) |
| Increased urination | 3 (38%) | 0 |
| Insomnia | 5 (63%) | 2 (40%) |
| Muscle spasms | 4 (50%) | 1 (20%) |
| Palpitations/increased heart rate | 2 (25%) | 1 (20%) |
| Tremors | 4 (50%) | 0 |
Classifed prior to unblinding.
Discussion
Safety and efficacy were established by this 52-week, double-blind, placebo-controlled (3:2), single-site study in which the primary endpoint of safety, as well as efficacy of adjunctive clenbuterol in motor improvements were demonstrated with up to 160 mcg daily in patients with LOPD. A transient increase in CK and other mild AEs were the only side effects in the clenbuterol group. The clenbuterol group was previously treated with ERT for at least 38 months with no further improvement expected (median of 75 months on ERT, range 38–102), while the control group was previously treated with median of 21 months (range 18–72) (Table 1). The difference in the length of time on ERT between the clenbuterol group and the placebo group prevented the direct comparison of results between the groups. However, the fact that the clenbuterol group was treated with ERT for a longer duration may further support the clinical significance of the results, because the clenbuterol group would have been expected to have been at a more stable point with respect to ERT benefit. Although some studies have reported improvement for up to 20 months,12 only the placebo group had anyone previously treated with ERT for <20 months (minimum 18 months), while the minimum duration of ERT for the clenbuterol group was 38 months. Statistically significant improvement in 7 of 11 secondary endpoints and a trend toward improvement in two other endpoints demonstrated efficacy from clenbuterol treatment in this group of patients with LOPD, who had been stably treated with ERT for >3 years (Table 2). Statistical analysis, including multiple comparisons to compare three time points for both groups, revealed statistically significant improvement for the GSGC, QMFT, and muscle glycogen tests following clenbuterol treatment (Table 2). A recent 5-year study of the benefits of ERT in patients with LOPD confirmed that benefits occurred in the first 2–3 years of treatment.13 A meta-analysis of 19 clinical studies similarly found that the FVC initially improved and then regressed to baseline over the course of 3 years of ERT in LOPD, while the 6MWT improved during the first 20 months of ERT and then stabilized.12 Improvements in pulmonary function tests and 6MWT demonstrated with clenbuterol (Table 2) in the current study of adjunctive clenbuterol are clearly over and above what would be expected following >3 years of continuous ERT in patients with LOPD.12, 13, 14
Previously published studies of ERT in Pompe disease reported improvement in 6MWT performance.14, 15, 16, 17, 18, 19, 20, 21, 22 Increased 6MWT distance and statistically significant increases in percent-predicted 6MWT over the 1-year period demonstrated efficacy in the clenbuterol group. The improvement in the predicted performance for the 6MWT (in percent) reflects a treatment effect, which can be distinguished from variation in the 6MWT distance (in meters) that might occur due to changes in age and weight over a 1-year period. The increased 6MWT performance would be unexpected in absence of any adjunctive therapy, given that no improvement was observed in the pivotal study of ERT in LOPD later than 52 weeks.14 Although the increase in FVC did not reach statistical significance, we observed a dramatic increase in MIP from pulmonary function testing in the clenbuterol group. The MIP is an index of maximal volitional inspiratory muscle strength highly correlated with invasive and/or reflexive measures of inspiratory strength reflecting diaphragmatic strength in patients with LOPD;23 therefore, the large, statistically significant increase in MIP for the clenbuterol group demonstrated efficacy. No significant improvements in any endpoints were observed in the placebo group (Table 2). Therefore, while the 6MWT distance only trended higher, the percent-predicted 6MWT improvement was statistically significant. While the FVC did not improve significantly in this study, the significant improvement in MIP reflected greater diaphragmatic strength. The endpoints currently studied, including the GSGC, QMFT, and MIP, have been validated in Pompe disease and showed statistically significant improvement with clenbuterol, reflecting clinical significance.9, 10, 23 Overall, these changes reflect improvement in more disease-specific endpoints in Pompe disease, which is important.24
Biochemical correction was demonstrated in the clenbuterol group by increased GAA activity and decreased glycogen content in the vastus lateralis. The latter effects were consistent with pre-clinical experiments demonstrating increased receptor-mediated uptake of GAA following clenbuterol administration.1, 2, 3 A previous study of adjunctive albuterol therapy in LOPD patients stably treated with ERT failed to demonstrate reduced glycogen content in the vastus lateralis.4 Indeed, the only previous study to demonstrate the clearance of accumulated glycogen in the skeletal muscle of LOPD had enrolled patients naive to ERT.25 A recent short-term study with a pharmacologic chaperone plus ERT increased GAA activity in muscle without lowering glycogen content in patients with LOPD, demonstrating the need for a sustained effect to achieve biochemical correction.26 Therefore, this is the first study to demonstrate improved biochemical correction of skeletal muscle in patients with LOPD who were previously treated with ERT. Improved biochemical correction supported our hypothesis that clenbuterol would increase CI-MPR-mediated uptake of GAA, since patients were stably treated and no greater biochemical correction was expected following >3 years of ERT.14, 27 The current study did not demonstrate elevated CI-MPR in skeletal muscle at 52 weeks, which does not exclude an increased CI-MPR expression at an earlier time during clenbuterol treatment.1, 2 Clenbuterol has been associated with transient effects consistent with tachyphylaxis following prolonged use, including transiently elevated serum CK.8 We assume that CI-MPR was elevated earlier in the study resulting in increased GAA uptake, which resulted in improved biochemical correction that persisted at week 52. Our earlier pilot study with another β2 agonist, extended release albuterol, revealed a trend toward higher CI-MPR expression in skeletal muscle at week 12, consistent with an elevation of CI-MPR early in the course of β2-agonist treatment.4 Additionally, our RNA-seq analysis showed that most of the genes that were simultaneously affected by both LOPD and clenbuterol treatment were altered in opposite directions, while this was not the case for the placebo treatment. This further demonstrated evidence for molecular correction of LOPD by clenbuterol.
The safety of β2 agonists has been evaluated in patients with muscle diseases including LOPD. Our previous study of albuterol in patients with LOPD who were stably treated with ERT revealed safety and efficacy.4 This pilot study of adjunctive albuterol demonstrated increased 6MWT distance, and albuterol was well-tolerated in eight participants.4 Prior to the availability of ERT, albuterol was administered to five patients with LOPD, who experienced mild AEs, including flushing, tremor, agitation, and palpitations, and these AEs resolved quickly when the dose of albuterol was decreased.28 In the randomized, double-blind, placebo-controlled trial of albuterol in fascioscapulhumeral muscular dystrophy, only tremors were reported at a higher frequency among albuterol-treated groups, in comparison with the placebo-treated group.29
Clenbuterol is a selective β2 agonist used for the treatment of asthma in Europe. Clenbuterol treatment has been associated with specific AEs that may include tremor, muscle cramps, nervousness, and headache, as observed in this study (Table 3). Multiple studies in asthmatics and patients with chronic airway obstruction reported no or very mild AE.30, 31, 32 Chronic administration of clenbuterol causes muscle hypertrophy in association with the upregulation of CI-MPR, which has potential advantages for the treatment of Pompe disease both through enhancing the uptake of rhGAA during ERT and by reversing muscle atrophy.2, 3, 7 Furthermore, clinical studies of clenbuterol have demonstrated increased muscle mass in other conditions, confirming its muscle effects in humans.8, 33 The known effects on muscle have led to the abuse of clenbuterol by performance athletes and to its banning by the World Anti-Doping Agency, although monitoring for clenbuterol abuse is complicated by its presence in the food supply.34
Limitations of the current study were related to the number of participants enrolled, which was small due to limited resources and other studies competing for enrollment of a limited number of patients with LOPD. Therefore, this study will need to be expanded to include a larger number of subjects in order to validate the potential benefits of clenbuterol in LOPD. The clenbuterol and placebo groups were treated with ERT for differing amounts of time, which could be avoided by more specific eligibility criteria for the duration of ERT. Furthermore, this study was not powered to reveal differences between the clenbuterol and placebo groups, and characteristics of these two groups differed at baseline due to stochastic variation. However, double blinding reduced the likelihood of artifactual responses due to a placebo effect. This study provides important preliminary data regarding the safety and efficacy of clenbuterol in participants with LOPD on ERT. The benefits demonstrated following oral clenbuterol in patients with LOPD support the further development of this adjunctive therapy for the treatment of Pompe disease.
Materials and Methods
Study Design
This was a 52-week, phase I/II double-blind, randomized, placebo-controlled study of adjunctive clenbuterol in (20 mcg Spiropent tablets) in patients with LOPD. All participants were evaluated at baseline and week 6 to establish a baseline for motor function testing. At week 6, participants were randomized 3:2 to clenbuterol or placebo and evaluated for safety and efficacy during the week 12 and 18 visits. The Investigational Drug Service performed randomization and maintained double blinding by providing either the study drug or placebo (over-encapsulated tablets) directly to participants. Unblinding, if needed related to a severe AE, was available through the Investigational Drug Service. Individual patients who experience a grade 3 (severe) or higher AE were withdrawn from the study, and the study would have been stopped if two patients developed the same grade 3 AE or for any patient that developed a grade 4 (life-threatening or debilitating) AE. The drug (or placebo) was initiated at the week 6 visit in a staged manner (see below). The study was monitored by a four-member data and safety monitoring board including of a senior medical genetics faculty member with board certification in cardiology and a biostatistician. If side effects were not tolerated following the dose increase, the participant resumed the lower dose previously tolerated for the remainder of the study. All participants returned for a final visit after a total of 52 weeks in the study. This study was approved by the Duke University Institutional Review Board, and written consent was obtained at study entry.
Study drug was initiated during the “off week,” approximately 1 week following a dose of ERT, and ERT continued throughout the duration of the study. Thereafter, study visits occurred during the “off week.” The week 6 and 12 visits were to determine the participant’s overall health status and measure early changes in motor function.
Dose of Clenbuterol or Placebo
The initial dose of clenbuterol was 40 mcg per oral each morning for 1 week, followed by 40 mcg morning and evening for the next 5 weeks until the week 12 visit. The dose was increased to 80 mcg each morning and 40 mcg each evening for 1 week, followed by 80 mcg morning and evening for the duration of the study. The participant was called to evaluate safety, 1 week following the initial administration of clenbuterol at week 7, and 1 week after the dose increase at week 13.
Patient Selection
Eligibility was determined by the following key criteria (see https://clinicaltrials.gov, NCT01942590, for detail). The criteria for inclusion is as follows: (1) diagnosis of Pompe disease by GAA enzyme assay and GAA gene sequencing and (2) receiving ERT at standard dose (20 mg/kg every 2 weeks) for at least 52 weeks. Criteria for exclusion are as follows: (1) continuous invasive ventilation (via tracheostomy or endotracheal tube), (2) cardiac involvement (myocardial infarction, arrythmia, cardiomyopathy), (3) history of hypersensitivity to β2 agonist drugs, and (4) high sustained titers of anti-GAA antibodies (>1:25,600 more than once).
Endpoints
The primary endpoint was safety of clenbuterol at up to 80 mcg twice daily, including avoidance of the following stopping rules: (1) liver toxicity, as defined by a >3-fold increase in AST or ALT from the respective baseline values and/or an increase in direct, indirect, or total bilirubin of >3-fold the upper limit of normal; (2) worsening muscle involvement, as defined by >3-fold increase in CK from baseline, twice in 1 week or >10,000 U/L. Secondary endpoints included the 6MWT and pulmonary function testing obtained at baseline and week 52. Exploratory endpoints included graded functional muscle tests (GSGC and QMFT) and muscle GAA and glycogen content.
Sample Size Determination
Due to the exploratory nature of this study and limitations in recruiting individuals with a rare disease, we estimated that 20 participants could be enrolled. Based on the treatment effect size (2.1) derived from the analysis of seven participants in an open-label study of albuterol for individuals with LOPD,4 a sample size of 20 participants resulted in insufficient power (<80%) to complete a double-blind placebo-controlled study with independent treatment and placebo groups, even if the treatment group was oversampled.
Muscle and Pulmonary Function Testing
The efficacy of clenbuterol treatment during ERT in patients with LOPD was with the 6MWT and pulmonary function testing, which are validated endpoints for Pompe disease.14 The 6MWT was performed as described at baseline, and weeks 6, 12, 18, and 52.35 Actual distance in meters and percent-predicted values were both analyzed. Percent-predicted 6MWT values can help distinguish treatment effects (i.e., actual changes in ability that may reflect changes in strength) from change that may result from typical age-related changes or changes in weight over a 1-year period that could affect distance walked in the 6MWT.36 Percent predicted was calculated in accordance with Enright et al., using a validated regression equation that includes height, weight, age, and gender, normed on a sample of 117 healthy adults and used to predict the distance typically walked by a normal adult of the same gender, height, and weight when the 6MWT is performed in accordance with ATS guidelines, as it was in this study.36 Pulmonary function testing was measured by electronic spirometer as described at baseline and weeks 18 and 52, including the FEV1, FVC, MEP, and MIP.37 FEV1 and FVC assessed in both the supine and upright positions to increase sensitivity for abnormalities detected in Pompe disease.38 Graded functional tests, which were included to allow scoring with the GSCS9 and QMFT10 tests, have been used with LOPD. The GSGC scoring is unique in that a lower score shows improvement.
Muscle Biopsy
The impact of enhanced CI-MPR-mediated uptake of GAA was analyzed by evaluating the biochemical correction of muscle in participants with LOPD treated with ERT, both prior to and during clenbuterol administration. Participants underwent a needle muscle biopsy of the quadriceps (vastus lateralis) at baseline and week 52 visits by a neuromuscular specialist with expertise in Pompe disease. The baseline biopsy was performed prior to initiating drug or placebo. Each biopsy was performed 1 week after the latest infusion to standardize the effects of ERT. The muscle biopsy was evaluated for biochemical correction and for CI-MPR expression by standard methods.1 Western blots on patient muscle samples were performed with primary antibodies, either anti-CI-MPR (Abcam, Cambridge, MA, cat # ab124767) or anti-Lamp2 (Santa Cruz Biotect, Dallas, TX, cat# sc-18822). These signals were normalized to the signal for a housekeeping gene, GAPDH or α-actin. Biopsy tissues were also processed for high-resolution light microscopy and stained for PAS-positive glycogen as described.39
RNA-Seq
Three deidentified normal muscle samples were matched to three participants in the clenbuterol group with regard to age, sex, and body mass index. These normal and Pompe disease muscle samples were subjected to RNA-seq to generate the comparisons of normal and Pompe disease skeletal muscle. Total RNA from muscle biopsies was extracted by the QIAGEN RNeasy Fibrous kit (QIAGEN; Germantown, MD; catalog number 74704) according to manufacturer’s instructions by the Duke Sequencing and Genomic Technologies Shared Resource facility. Quality of total RNA samples was checked using an Agilent 2100 Bioanalyzer. Due to the limited amount of RNA generated by the biopsies, full-length cDNA was first generated by the Clontech SMART-Seq v4 Ultra Low Input RNA Kit. Full-length cDNA was then converted into an RNA-seq library using the Kapa Hyper prep kit. Libraries were pooled and sequenced on one lane of an Illumina HiSeq 4000. Fifty-base pair single-read sequences were generated and processed using the TrimGalore toolkit40 which employs Cutadapt41 to trim low-quality bases and Illumina sequencing adapters from the 3′ end of the reads. Only reads that were 20 nucleotides or longer after trimming were kept for further analysis. Reads were mapped to the GRCh37v75 version of the human genome and transcriptome42 using the STAR RNA-seq alignment tool.43 Reads were kept for subsequent analysis if they mapped to a single genomic location. Gene counts were compiled using the HTSeq tool.44 Only genes that had at least 10 reads in any given library were used in subsequent analysis. Normalization to remove systematic differences across the samples was carried out using the EdgeR45 Bioconductor46 package from the R statistical programming environment.47 Differential expression was carried out using linear models in the voom48 and limma49 Bioconductor packages. The false discovery rate was calculated to control for multiple hypothesis testing. GSEA50 was performed to identify differentially regulated pathways and gene ontology terms for each of the comparisons performed. Ingenuity pathway analysis (IPA) was performed to identify the key biological functions based on curated disease and functions ontology in IPA knowledge database.
Statistical Analysis
Improvement in mean (±SD) values of each outcome at baseline and last study visit were evaluated using one-sided paired t tests. A p < 0.05 was considered statistically significant. Analyses were performed using Stata IC 14.2 (Stata, College Station, TX), except for two-way ANOVA with multiple comparisons that was performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA).
Author Contributions
D.D.K. performed research, analyzed data, and wrote the paper. L.E.C. performed research and muscle testing, analyzed data, and wrote the paper. E.C.S. performed research and muscle biopsies and analyzed data. C.W. served as study coordinator, performed research, and analyzed data. W.C. performed RNA-sequencing analysis and analyzed data. Y.L. performed RNA-sequencing analysis, analyzed data, and wrote the paper. S.-o.H. performed western blotting and data analysis. C.P.H. analyzed data and performed statistical analysis. K.M.H. and W.E.K. provided control muscle samples and wrote the paper. B.L.T. analyzed data, performed patient biopsy sample processing and histopathological analysis, and wrote the paper. D.L.C. performed RNA-sequencing analysis, analyzed data, and wrote the paper. D.B. and N.B. analyzed data and wrote the paper. P.S.K. designed the clinical trial, interpreted the data, and wrote the paper.
Conflicts of Interest
D.D.K. served on a data and safety monitoring board for Baxter International. He received an honorarium and grant support in the past from Genzyme Sanofi. L.E.C. has received honoraria from Genzyme Sanofi; has participated in research supported by Genzyme Sanofi, Valerion, Biomarin, and by Roivant Sciences; and is a member of the Pompe Registry North American Board of Advisors Genzyme Sanofi. B.L.T. is an employee of Genzyme Sanofi. D.B. has received research grant support and travel funds from Genzyme Sanofi, Baebies Inc., Biomarin, and Alexion Inc. P.S.K. has received research/grant support from Genzyme Sanofi and Valerion Therapeutics. She received consulting fees and honoraria from Genzyme Sanofi, Amicus Therapeutics, and Vertex. She is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Sanofi and on the Amicus Scientific advisory board. D.D.K. and P.S.K. have developed the technology that is being used in the study. If the technology is commercially successful in the future, the developers and Duke University may benefit financially. D.L.C., E.C.S., C.W., S.-o.H., Y.L., W.C., C.P.H., K.M.H., W.E.K. and N.B. report no disclosures.
Acknowledgments
This study was supported in part by the Alice and Y.-T. Chen Pediatric Genetics and Genomics Center, grant R01-FD004364 from the Food and Drug Administration (to D.D.K.), grant R01-AR065873 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to N.B. and D.D.K.), and a grant from Roivant Rare Diseases (to D.D.K.). We thank the members of the data and safety monitoring board, Dr. Stephanie Wechsler, Dr. Gordon Worley, Dr. P. Brian Smith, and Dr. C. Michael Cotton. We thank Dr. Holly Dressman and Dr. Nicolas Devos at the Duke Sequencing and Genomic Technologies Shared Resource for providing RNA-sequencing. We thank Dr. Gregory Crawford for consultation regarding transcriptomics. We would like to acknowledge outstanding technical support from Ms. Songtao Li and from Ms. Jian Dai. We thank Ms. Eleanor Geller Botha and Ms. Dawn A. Laney for reviewing literature regarding the safety of clenbuterol. We thank Dr. Gayathri Kamalakkannan and colleagues for technical advice regarding the use of clenbuterol in humans.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.06.023.
Supplemental Information
References
- 1.Koeberl D.D., Luo X., Sun B., McVie-Wylie A., Dai J., Li S., Banugaria S.G., Chen Y.T., Bali D.S. Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol. Genet. Metab. 2011;103:107–112. doi: 10.1016/j.ymgme.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeberl D.D., Li S., Dai J., Thurberg B.L., Bali D., Kishnani P.S. β2 Agonists enhance the efficacy of simultaneous enzyme replacement therapy in murine Pompe disease. Mol. Genet. Metab. 2012;105:221–227. doi: 10.1016/j.ymgme.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S., Sun B., Nilsson M.I., Bird A., Tarnopolsky M.A., Thurberg B.L., Bali D., Koeberl D.D. Adjunctive β2-agonists reverse neuromuscular involvement in murine Pompe disease. FASEB J. 2013;27:34–44. doi: 10.1096/fj.12-207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeberl D.D., Austin S., Case L.E., Smith E.C., Buckley A.F., Young S.P., Bali D., Kishnani P.S. Adjunctive albuterol enhances the response to enzyme replacement therapy in late-onset Pompe disease. FASEB J. 2014;28:2171–2176. doi: 10.1096/fj.13-241893. [DOI] [PubMed] [Google Scholar]
- 5.Raben N., Danon M., Gilbert A.L., Dwivedi S., Collins B., Thurberg B.L., Mattaliano R.J., Nagaraju K., Plotz P.H. Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Raben N., Fukuda T., Gilbert A.L., de Jong D., Thurberg B.L., Mattaliano R.J., Meikle P., Hopwood J.J., Nagashima K., Nagaraju K., Plotz P.H. Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol. Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto T., Akutsu S., Wakana N., Morito M., Shimada A., Yamane A. The expressions of insulin-like growth factors, their receptors, and binding proteins are related to the mechanism regulating masseter muscle mass in the rat. Arch. Oral Biol. 2006;51:603–611. doi: 10.1016/j.archoralbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kamalakkannan G., Petrilli C.M., George I., LaManca J., McLaughlin B.T., Shane E., Mancini D.M., Maybaum S. Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J. Heart Lung Transplant. 2008;27:457–461. doi: 10.1016/j.healun.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Angelini C., Semplicini C., Tonin P., Filosto M., Pegoraro E., Sorarù G., Fanin M. Progress in Enzyme Replacement Therapy in Glycogen Storage Disease Type II. Ther. Adv. Neurol. Disorder. 2009;2:143–153. doi: 10.1177/1756285609103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Capelle C.I., van der Beek N.A., de Vries J.M., van Doorn P.A., Duivenvoorden H.J., Leshner R.T., Hagemans M.L., van der Ploeg A.T. The quick motor function test: a new tool to rate clinical severity and motor function in Pompe patients. J. Inherit. Metab. Dis. 2012;35:317–323. doi: 10.1007/s10545-011-9388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houmard J.A., Weidner M.L., Gavigan K.E., Tyndall G.L., Hickey M.S., Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J. Appl. Physiol. 1998;85(1985):1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 12.Schoser B., Stewart A., Kanters S., Hamed A., Jansen J., Chan K., Karamouzian M., Toscano A. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J. Neurol. 2017;264:621–630. doi: 10.1007/s00415-016-8219-8. [DOI] [PubMed] [Google Scholar]
- 13.Kuperus E., Kruijshaar M.E., Wens S.C.A., de Vries J.M., Favejee M.M., van der Meijden J.C., Rizopoulos D., Brusse E., van Doorn P.A., van der Ploeg A.T., van der Beek N.A.M.E. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology. 2017;89:2365–2373. doi: 10.1212/WNL.0000000000004711. [DOI] [PubMed] [Google Scholar]
- 14.van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 15.Ravaglia S., Pichiecchio A., Ponzio M., Danesino C., Saeidi Garaghani K., Poloni G.U., Toscano A., Moglia A., Carlucci A., Bini P. Changes in skeletal muscle qualities during enzyme replacement therapy in late-onset type II glycogenosis: temporal and spatial pattern of mass vs. strength response. J. Inherit. Metab. Dis. 2010;33:737–745. doi: 10.1007/s10545-010-9204-5. [DOI] [PubMed] [Google Scholar]
- 16.Strothotte S., Strigl-Pill N., Grunert B., Kornblum C., Eger K., Wessig C., Deschauer M., Breunig F., Glocker F.X., Vielhaber S. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 17.de Vries J.M., van der Beek N.A., Kroos M.A., Ozkan L., van Doorn P.A., Richards S.M., Sung C.C., Brugma J.D., Zandbergen A.A., van der Ploeg A.T., Reuser A.J. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol. Genet. Metab. 2010;101:338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Merk T., Wibmer T., Schumann C., Krüger S. Glycogen storage disease type II (Pompe disease)—influence of enzyme replacement therapy in adults. Eur. J. Neurol. 2009;16:274–277. doi: 10.1111/j.1468-1331.2008.02377.x. [DOI] [PubMed] [Google Scholar]
- 19.Bembi B., Pisa F.E., Confalonieri M., Ciana G., Fiumara A., Parini R., Rigoldi M., Moglia A., Costa A., Carlucci A. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J. Inherit. Metab. Dis. 2010;33:727–735. doi: 10.1007/s10545-010-9201-8. [DOI] [PubMed] [Google Scholar]
- 20.Ishigaki K., Murakami T., Nakanishi T., Oda E., Sato T., Osawa M. Close monitoring of initial enzyme replacement therapy in a patient with childhood-onset Pompe disease. Brain Dev. 2012;34:98–102. doi: 10.1016/j.braindev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Angelini C., Semplicini C., Ravaglia S., Bembi B., Servidei S., Pegoraro E., Moggio M., Filosto M., Sette E., Crescimanno G., Italian GSDII Group Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J. Neurol. 2012;259:952–958. doi: 10.1007/s00415-011-6293-5. [DOI] [PubMed] [Google Scholar]
- 22.van Capelle C.I., van der Beek N.A., Hagemans M.L., Arts W.F., Hop W.C., Lee P., Jaeken J., Frohn-Mulder I.M., Merkus P.J., Corzo D. Effect of enzyme therapy in juvenile patients with Pompe disease: a three-year open-label study. Neuromuscul. Disord. 2010;20:775–782. doi: 10.1016/j.nmd.2010.07.277. [DOI] [PubMed] [Google Scholar]
- 23.Prigent H., Orlikowski D., Laforêt P., Letilly N., Falaize L., Pellegrini N., Annane D., Raphael J.C., Lofaso F. Supine volume drop and diaphragmatic function in adults with Pompe disease. Eur. Respir. J. 2012;39:1545–1546. doi: 10.1183/09031936.00169011. [DOI] [PubMed] [Google Scholar]
- 24.Lachmann R., Schoser B. The clinical relevance of outcomes used in late-onset Pompe disease: can we do better? Orphanet J. Rare Dis. 2013;8:160. doi: 10.1186/1750-1172-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Ploeg A., Carlier P.G., Carlier R.Y., Kissel J.T., Schoser B., Wenninger S., Pestronk A., Barohn R.J., Dimachkie M.M., Goker-Alpan O. Prospective exploratory muscle biopsy, imaging, and functional assessment in patients with late-onset Pompe disease treated with alglucosidase alfa: The EMBASSY Study. Mol. Genet. Metab. 2016;119:115–123. doi: 10.1016/j.ymgme.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Kishnani P., Tarnopolsky M., Roberts M., Sivakumar K., Dasouki M., Dimachkie M.M., Finanger E., Goker-Alpan O., Guter K.A., Mozaffar T. Duvoglustat HCl Increases Systemic and Tissue Exposure of Active Acid α-Glucosidase in Pompe Patients Co-administered with Alglucosidase α. Mol. Ther. 2017;25:1199–1208. doi: 10.1016/j.ymthe.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Ploeg A.T., Barohn R., Carlson L., Charrow J., Clemens P.R., Hopkin R.J., Kishnani P.S., Laforêt P., Morgan C., Nations S. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol. Genet. Metab. 2012;107:456–461. doi: 10.1016/j.ymgme.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Angelini C., Pegoraro E., Marsala R.Z., Vergani L., Nascimbeni A.C., Fulizio L., Fanin M. Adult acid maltase deficiency: an open trial with albuterol and branched-chain aminoacids. Basic Appl. Myol. 2004;14:71–78. [Google Scholar]
- 29.Kissel J.T., McDermott M.P., Mendell J.R., King W.M., Pandya S., Griggs R.C., Tawil R., FSH-DY Group Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57:1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 30.Di Gioacchino M., Mezzetti A., Mancini M., Guglielmi M.D., Lo Medico E., Proietti Franceschilli G., Marzio L., Cuccurullo F. Study of the cardiovascular effects of clenbuterol in exercise-induced asthma. Respiration. 1987;51:205–213. doi: 10.1159/000195203. [DOI] [PubMed] [Google Scholar]
- 31.Tschan M., Perruchoud A., Herzog H. Dose response relationship of clenbuterol (NAB 365) as a solution for inhalation. Eur. J. Clin. Pharmacol. 1979;15:159–162. doi: 10.1007/BF00563099. [DOI] [PubMed] [Google Scholar]
- 32.Pasotti C., Capra A., Vibelli C. NAB 365 (clenbuterol) and salbutamol in asthmatics: a double-blind clinical trial. Int. J. Clin. Pharmacol. Biopharm. 1979;17:176–180. [PubMed] [Google Scholar]
- 33.Maltin C.A., Delday M.I., Watson J.S., Heys S.D., Nevison I.M., Ritchie I.K., Gibson P.H. Clenbuterol, a beta-adrenoceptor agonist, increases relative muscle strength in orthopaedic patients. Clin. Sci. (Lond.) 1993;84:651–654. doi: 10.1042/cs0840651. [DOI] [PubMed] [Google Scholar]
- 34.Geyer H., Schänzer W., Thevis M. Anabolic agents: recent strategies for their detection and protection from inadvertent doping. Br. J. Sports Med. 2014;48:820–826. doi: 10.1136/bjsports-2014-093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 36.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 37.Brooke M.H., Fenichel G.M., Griggs R.C., Mendell J.R., Moxley R., Miller J.P., Province M.A. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 38.van der Beek N.A., van Capelle C.I., van der Velden-van Etten K.I., Hop W.C., van den Berg B., Reuser A.J., van Doorn P.A., van der Ploeg A.T., Stam H. Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol. Genet. Metab. 2011;104:129–136. doi: 10.1016/j.ymgme.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Lynch C.M., Johnson J., Vaccaro C., Thurberg B.L. High-resolution light microscopy (HRLM) and digital analysis of Pompe disease pathology. J. Histochem. Cytochem. 2005;53:63–73. doi: 10.1177/002215540505300108. [DOI] [PubMed] [Google Scholar]
- 40.Babraham Bioinformatics. (2012). Trim galore. http://www.bioinformatics.babraham.ac.uk/projects/trim_galore.
- 41.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. [Google Scholar]
- 42.Kersey P.J., Staines D.M., Lawson D., Kulesha E., Derwent P., Humphrey J.C., Hughes D.S., Keenan S., Kerhornou A., Koscielny G. Ensembl Genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res. 2012;40:D91–D97. doi: 10.1093/nar/gkr895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders, S. (2010). HTSeq. http://www-huber.embl.de/users/anders/HTSeq/.
- 45.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huber W., Carey V.J., Gentleman R., Anders S., Carlson M., Carvalho B.S., Bravo H.C., Davis S., Gatto L., Girke T. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The R Foundation. (2004). The R project for statistical computing. https://www.r-project.org.
- 48.Law C.W., Chen Y., Shi W., Smyth G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.