Abstract
We aim to examine if circulating micro-RNA and cytokine levels associate with dementia diagnosis and cognitive scores. To test our hypothesis, we use plasma donated from 48 monozygotic twin pairs in 1997 and 46 micro-RNAs and 10 cytokines were quantified using microfluidic RT-qPCR and multiplex solid-phase immunoassays, respectively. Micro-RNA and cytokine profiling were examined for associations with dementia diagnoses in a longitudinal registry study or with cognitive scores at baseline. Thirty-six micro-RNAs and all cytokines were detected consistently. Micro-RNA profiles associate with diagnoses and cognitive scores at statistically significant levels while cytokine only showed trends pointing at chronic inflammation in twins having or developing dementia. The most notable findings were decreased miR-106a and miR-210, and increased miR-106b expression in twins with a dementia diagnosis. This pioneering evaluation of micro-RNA and cytokine and dementia diagnosis suggests micro-RNA targets in vasculogenesis, lipoprotein transport, and amyloid precursor protein genes.
Keywords: Aging, Alzheimer’s disease, brain, genetics, neurodegenerative
INTRODUCTION
Numerous studies suggest that inflammatory mechanisms are involved in the development and propagation of Alzheimer’s disease (AD) [1]. Inflammation in AD is reflected by increased subpopulations of pro-inflammatory cytokines, cellular activation markers, activated complement, acute-phase proteins, and findings of activated microglia and plaque-associated inflammatory proteins by brain tissue immunohistochemistry as well as evidence of astrocytic inflammasome activation in animal studies [2–5]. Also, epidemiological studies suggest that chronic use of non-steroid antiinflammatory drugs is associated with a decreased risk of developing AD [6]. It is not settled, however, if in situ signs of inflammation are reflected by peripheral, circulating inflammatory markers [7, 8]. Peripheral markers may be of diagnostic and predictive value and informative regarding disease mechanisms. In that regard micro-RNAs (miRNAs) are emerging as interesting regulatory molecules. MiRNAs are significantly altered in inflammation including the chronic inflammatory state associated with various autoimmune diseases [9]. The miRNAs exert gene regulatory functions intracellularly, but are also released in a protected yet accessible form to the extracellular environment and thus are measurable in plasma [10]. The challenge of inflammatory biomarker studies in AD in general is the large interindividual variation of inflammatory marker (e.g., cytokines) levels in healthy people and the diversity of the genetically determined inflammatory response susceptibility [11, 12]. Also, the lack of longitudinal cohorts for establishing early and prognostic markers is a challenge.
Using the Danish aging twin cohort [13] we are here able to minimize the contribution of genetic variation by studying monozygotic twins and correlate baseline (the year 1997) miRNA and cytokine profiling with differences or similarities in cognitive performance. Cognition was evaluated over time based on composite cognitive scores at the time of blood sampling (1997) and at every second year until 2005 [14]. We hypothesize that plasma miRNA and cytokine profiles are associated with clinical diagnoses of unspecified dementia, vascular dementia, or AD from the nationwide patient registry. Additionally, we hypothesize that these profiles associate with cross-sectional and longitudinal cognitive abilities in elderly individuals. The hypotheses were tested by quantitative analysis of a panel of 46 miRNAs in samples from 48 pairs of monozygotic twins with differences or similarities in cognitive scores. The specific miRNAs were chosen based on previous work implicating them in AD, neurodegeneration or other brain functions, a subset of miRNAs were implicated in neuroinflammation or other chronic inflammatory diseases. All were previously demonstrated to be detectable in plasma samples. Additionally, a multiplex assay for cytokines in the same samples included the acute-phase marker C-reactive protein (CRP) as well as proinflammatory (TNF, IL-18), TNF-inducers (IL-12), T-cell regulators (IL-2), neuroprotective and putative plasma AD markers (MMP-9, BDNF), and anti-inflammatory (TGF-β, IL-4, IL-10) cytokines and proteins.
MATERIALS AND METHODS
Twin groups and samples
Monozygotic twin pairs eligible for the present study were identified from the Longitudinal Study of Aging Danish Twins (LSADT) of which 698 twins (126 MZ twin pairs) donated blood in 1997 [15].Twin zygosity was classified using questionnaire-based zygosity assessment [16]. Whole blood samples were drawn in 1997 into K2-EDTA tubes (BD Bioscience). All participants had scores from a five-component cognitive score (CCS) performed in 1997 [14]. CCS is an in-house assembly of representative tasks that are sensitive to normative age changes and are reliably and quickly assessed by lay interviewers. The specific tasks included a fluency test, which involved the number of animals an individual could name in a 1-min interval, forward and backward digit span, and immediate and delayed recall of a 12-item list. The CCS was computed by taking the sum of the five standardized measures [14]. Diagnoses were extracted from The Danish National Patient Registry, which contains essentially all discharges from Danish hospitals since 1977. Diagnoses from 1997 until October 2010 were classified according to the International Classification of Diseases (ICD-10). Relevant diagnoses were; AD (F00.0–9 and G30.0–9), vascular dementia (VaD) (F01.0–9), frontotemporal dementia (FTD) (F02.0), dementia with Lewy bodies (DLB) (G31.8 and F00.2), and unspecified dementia (F03.9) [17]. All diagnoses were registered after bloodwas donated except for one twin in a concordant twin pair, with a diagnosis approx. 1.5 years before. The LSADT study was approved by the Regional Committees on Health Research Ethics for Southern Denmark (S-VF-20040241). Data access: according to Danish legislation, transfer and sharing of individual-level data requires prior approval from the Danish Data Protection Agency and requires that data sharing requests be dealt with on a case-by-case basis. For this reason, the data cannot be deposited in a public database. However, we welcome any enquiries regarding collaboration and individual requests for data sharing.
In total 48 twin pairs were included in this study. One group of twin pairs (discordant twin pairs, n = 22) represented cases where one twin had a dementia diagnosis. For another group (concordant twin pairs, n = 5) both twins had a dementia diagnosis. In addition, twin pairs where none of the twins had a diagnosis were selected (n = 21). These 21 pairs included the following groups: 1) n =8 pairs with a difference in cognitive score of more than 5 points in 1997 equivalent to 1.5 SD; 2, n = 5 pairs with a difference in cognitive score between 3.5 and 5 points equivalent to 1.1–1.5 SD; and 3) n =8 pairs with a cognitive difference of less than 2 points equivalent to 0.6 SD. The direction of the difference in cognitive score between the twins in the pairs was consistent throughout the testing period in groups 1–3. Plasma samples were obtained and prepared in 1997 from EDTA blood by centrifugation at 1000 × g for 10 min within 12 h after sampling. Plasma was stored at −80°C until used.
Multiplex cytokine immunoassays
Quantitative multiplex immunoassays were carried out using the Meso Scale (MSD) platform (Meso Scale Diagnostics, Rockville, MD, USA). The samples (n = 96) were analyzed using in-house multiplex sandwich immunoassays based on U-plex Meso-Scale technology. We analyzed the levels of Interleukin-2 (IL-2), −4, −10, −12, −18, brain-derived neurotropic factor (BDNF), C-reactive protein (CRP), and matrix-metalloproteinase (MMP-9). In short, the different capture antibodies were first biotinylated using EZ-Link Sulfo-NHS-LC-Biotin (ThermoFisher, 21327), then bound to different linkers 1–10, mixed together at final concentrations of 10 μg/ml per antibody, added to each U-plex plate (MSD, K15235) well (50 μl/well) for all plates, and incubated for 1 h. After washing with washing buffer (PBS containing 0.05% Tween 20), the plates were stored at 4°C until use. Detection antibodies were sulfo-tagged using MSD Gold Sulfo-tag NHS-Ester (MSD, R91AO-2). All antibodies were purchased from R& D Systems (Abingdon,UK).
Samples (25 μl), calibration curves and high and low controls were pipetted to each plate. The samples were diluted 1:10 in diluent 8 (MSD, R54BA). Calibrators and controls were prepared by recombinant antigens diluted in diluent 8. The plates were sealed and incubated shaking for 2 h, washed 3 times, the corresponding detection antibodies were added (25 μl to each well, conc. 0.1 μg/ml), and incubated shaking for 2 h. Finally, the plates were washed, 150 μl 2xRead buffer T (MSD R92TC) were added per well, and the plates immediately read using the QuickPlex reader. The calibration curves were fitted with 4PL logistic regression using the MSD Discovery Workbench.
Plasma micro-RNA extraction and RT-qPCR measurement
Samples (n = 96) were thawed on ice and centrifuged at 3000 × g/10 min before total RNA extraction from 100 μL plasma aliquots according to the instructions of the manufacturer (Norgen Biotek Corp., Ontario, Canada) except for a number of small modifications listed here. Dithiothreitol (DTT, 10 mM) (Sigma-Aldrich Co. LLC, Germany) and 1.7 pM synthetic C. elegans miR-54 and -238 (Tag Copenhagen A/S, Denmark) were added into the lysis buffer. One μL of RNase inhibitor (20 U/μL) (Applied Biosystems (ABI), Foster city, CA, USA) was added to every elution tube before elution of RNA. Purified RNA samples were kept at −20°C until being used. The panel of 48 miRNAs (46 human and 2 from C. elegans for technical normalization) (Supplementary Table 1) was analyzed after reverse transcription (TaqMan microRNA Reverse Transcription Kit), preamplification (TaqMan PreAmp master mix), and quantitative PCR (qPCR) using TaqMan miRNA assays from Applied Biosystems (Applied Biosystems (ABI), Foster city, CA, USA). For the qPCR, a Fluidigm BioMark microfluidic chip system (Fluidigm Corp. USA) allowing duplicate assays for 48 miRNAs in 96 samples in one operation was used. For more technical details please refer to [18].
Data handling and analysis
In the array data analysis, the auto detectors setting (threshold cycle was set automatically) was chosen for data from chip run. All raw Cq data were exported into Excel. Average Cq values above 35 were excluded from data sets. Each remaining average Cq value was normalized with the average Cq of cel-miR-54 and cel-miR-238 for that particular sample yielding the −ΔCq (=[average Cq of cel-miR-54 and cel-miR-238] – [average Cq of hsa-miR]) values. The −ΔCq values were further normalized with the average −ΔCq of miRNAs that were detectable in all samples to further correct the variations in total input RNA. We used the average −ΔCq of 19 miRNAs (let-7b-5p, miRs-101, −106a-5p, 106b-5p, 132-3p, 146a-5p, −146b-5p, −155-5p, −16-5p, −17-5p, −191-5p, −20a-5p, − 223-3p, −24-3p, −34a-5p, −320a, −92a-3p, −93-5p, − 451a), which were detected in all samples to subtract from all miRNA −ΔCq values in each sample. These twice normalized expression values, where larger values reflect higher abundance, were used for the statistical analysis.
Statistical analysis
Cytokine data were power-transformed in an operation in the statistical computational environment R (https://www.r-project.org) followed by statistical testing for significance. For the paired analysis a factor of 20.000 was added to avoid negative values. Un-transformed data was used to estimate differences in mean values. Power analysis was performed with the R-package sizepower (Bioconductor) allowing for one false positive. With 36 miRNAs sample size a power of 81% was provided to detect expression differences of 1.25-fold for miRNA with no missing readings (Npairs = 22).
Twin-pair level analyses
In a subset of analyses the co-twin design [19] was used to detect the association of miRNAs with dementia and CCS, respectively, in two distinct groups of twins. The co-twin design has two advantages, firstly, it controls for genetic influence on the phenotype variation and, secondly, shared family environment is controlled for by the design. The analysis fits a linear regression model that regresses the intra-pair difference in miRNA expression on the intra-pair difference in either dementia or CCS, with intra-pair differences calculated between the twin with the higher phenotype value to the twin with the lower phenotype value, i.e., we fitted ΔmiRNA= β0+β1* Δphenotype+β2* age+β3* sex. In this model, β0 captures the overall difference in miRNA level between the twin diagnosed with dementia and the twin without diagnosis, age was centered around its median and the phenotype differences were centered at the median of the absolute differences. In the analysis of CCS β1 stands for the association of miRNA that is dependent on the level of phenotype difference (quantitative association). When miRNA expression is log transformed, the antilog of β1 is the fold change in miRNA expression for one unit increase in intra-pair phenotype difference. The effects of age and sex (women coded as 0 and men coded as 1) on intra-pair difference in miRNA expression were adjusted by including them as pair-specific variables in the regression model. MicroRNAs expressed in both twins in less than five pairs were excluded from the intra-pair analyses. The intra-pair analysis was performed using linear regression with the lm() function in R (https://www.r-project.org/). Identical models were used for the transformed cytokine data. Presented p-values were uncorrected for multiple testing.
Individual level analysis
An overall individual level analysis was performed by defining twin pairing as clusters to control for twin correlation in a linear mixed model with random intercept. The fitting was done with the lmer() function from the R-package lme4 [20]. The models detect associations between candidate miRNA and dementia by regressing miRNA expression on present/absent diagnosis. Similar to the intra-pair analysis models also were adjusted for age and sex by including them as covariates. Both regression models were fitted in R. Presented p-values were uncorrected for multiple testing.
RESULTS
Circulating miRNA and diagnoses
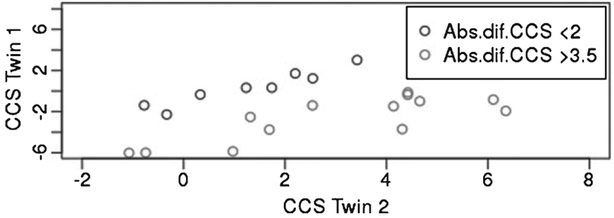
Circulating miRNA was profiled in plasma from 48 pairs of monozygotic twins. In 22 pairs one of the twins had a diagnosis of unspecified dementia, vascular dementia, or AD while in 5 pairs both twins had one of these diagnoses. On average the diagnoses were registered 7.5 (3.1 SD) years after blood was donated. In the remaining pairs (n = 21) none of the twins had any of these diagnoses. Of these 21 pairs, 13 were selected on the basis of a difference in CCS of more than 3.5 while other 8 pairs were selected on the basis of being cognitively similar (ΔCCS<2). As displayed in Fig. 1, for half (n = 7) of the 13 twin pairs with large CCS differences, twin 2 who scored best on the CCS, performed particularly well on the cognitive scale (CCS>4) equivalent to +1.2 SD. Unexpectedly, the average CCS was similar at baseline for twin pairs with and without a dementia diagnosis (Table 1).
Fig. 1.
Plots of cognitive composite score (CCS) between Twin 1, the twin performing poorest, and Twin 2, the twin performing best in a twin pair. All twins are with-out dementias diagnose. The twin pairs are grouped by the selected selection criteria, 1), to be similar (the absolute difference in CCS<2) and, 2), CCS discordant twins (the absolute difference in CCS>3.5).
Table 1.
Descriptives of participants from the Longitudinal study of Aging Danish Twins (LSADT)
| Concordant twin pairs with a dementia diagnosis |
Dementia discordant twin pairs |
Concordant twin pairs without a dementia diagnosis |
P-values concordant twin pairs versus discordant twin pairs with/without a dementia diagnosis |
|
|---|---|---|---|---|
| No. MZ twin-pairs | 5 | 22 | 21 | – |
| No. female pairs, (%) | 5 (100) | 19 (86.4) | 12 (57.1) | 1.0a/0.05a |
| Assessment year 1997: | ||||
| Age (sd) | 76.9 (2.7) | 80.2 (4.2) | 80.3 (4.4) | 0.11b/0.94b |
| Average CCS (sd) | 1.3 (3.2) | 0.1 (3.5) | 0.4 (3.0) | 0.48b/0.76b |
| ΔCCS (sd)1 | 3.8 (4.2) | 3.7 (2.2) | 3.9 (2.6) | 0.94b/0.79b |
Quantitative differences (Absolute difference) between the twins within a pair. sd, standard deviation.
:Chi2 test statistics;
:t-test statistics.
Data show that the quantitative miRNA profiles correlated with diagnoses as well as with inter- and intra-pair differences in cognitive scores. We excluded sample miRNA data where Cq > 35 and then excluded the specific miRNAs that were only detected in a third or less of the samples (see Supplementary Table 1). Thereafter, a total of 36 miRNAs remained for analysis. Additionally, one sample was inadvertently lost because of a microfluidic chip loading problem.
Twin-pair dementia discordance and miRNA
In the discordant twin analysis three miRNAs were significant associated with dementia. For miR-106a/b that belongs to paralogous miRNA gene clusters (the miR-17-92, miR-106a-363, and miR-106b-25 microRNA precursor families) [21], the two mature miRNAs were associated with dementia, i.e., miR-106b-5p was upregulated in participants who developed dementia, while miR-106a-5p was downregulated (borderline associated, p-value = 0.1). Another interesting finding was that miR-146b-5p was downregulated in twins who were diagnosed with dementia (Table 2).
Table 2.
miRNA associated with dementia and miRNA associated with cognitive function in the discordant co-twin design sub-study
| Dementia Discordant twins |
Discordance in quantitative difference in CCS in twins without dementia |
|||||
|---|---|---|---|---|---|---|
| miRNA | No.twin pairs | β0 coefficient | p | No. twin pairs | β1 coefficient | p |
| hsa-let-7b-5p | 22 | −0.27 | 0.71 | 20 | 0.03 | 0.78 |
| hsa-let-7f-5p | 5 | 1.51 | 0.75 | 1 | – | – |
| hsa-let-7i-5p | 16 | 2.13 | 0.42 | 14 | 0.22 | 0.34 |
| hsa-miR-101-3p | 22 | −0.10 | 0.94 | 20 | −0.07 | 0.78 |
| hsa-miR-106a-5p | 22 | −1.41 | 0.10 | 20 | 0.01 | 0.97 |
| hsa-miR-106b-5p | 22 | 3.16 | 0.03a | 20 | −0.01 | 0.98 |
| hsa-miR-128-3p | 7 | −2.14 | 0.33b | 9 | 0.55 | 0.45 |
| hsa-miR-130b-3p | 18 | −0.06 | 0.98 | 19 | −0.05 | 0.74 |
| hsa-miR-132-3p | 21 | −3.50 | 0.26 | 20 | 0.35 | 0.07 |
| hsa-miR-134-5p | 15 | −0.19 | 0.96 | 15 | 0.10 | 0.84 |
| hsa-miR-142-3p | 22 | 0.70 | 0.46 | 20 | 0.02 | 0.91 |
| hsa-miR-145-5p | 14 | 5.96 | 0.18 | 15 | 0.39 | 0.12 |
| hsa-miR-146a-5p | 22 | 0.22 | 0.85 | 20 | −0.02 | 0.82 |
| hsa-miR-146b-5p | 22 | −1.87 | 0.05 | 20 | −0.13 | 0.51 |
| hsa-miR-155-5p | 22 | −2.01 | 0.22 | 20 | 0.04 | 0.83 |
| hsa-miR-15a-5p | 1 | – | – | 2 | – | – |
| hsa-miR-15b-5p | 21 | −2.61 | 0.18 | 20 | −0.03 | 0.84 |
| hsa-miR-16-5p | 22 | −0.81 | 0.39 | 20 | 0.05 | 0.72 |
| hsa-miR-17-5p | 22 | −0.49 | 0.51 | 20 | 0.01 | 0.89 |
| hsa-miR-191-5p | 22 | 1.38 | 0.18 | 20 | 0.15 | 0.20 |
| hsa-miR-20a-5p | 22 | −0.18 | 0.80 | 20 | 0.08 | 0.39 |
| hsa-miR-210-3p | 9 | 0.11 | 0.77b | 14 | 1.19 | 0.02 |
| hsa-miR-223-3p | 22 | −0.02 | 0.97 | 20 | 0.01 | 0.80 |
| hsa-miR-23a-3p | 11 | 2.54 | 0.11 | 10 | 0.10 | 0.88 |
| hsa-miR-24-3p | 22 | −0.28 | 0.84 | 20 | −0.06 | 0.65 |
| hsa-miR-26b-5p | 22 | 1.65 | 0.12 | 18 | −0.04 | 0.73 |
| hsa-miR-27a-3p | 22 | 0.24 | 0.81 | 19 | −0.04 | 0.80 |
| hsa-miR-298 | 3 | – | – | 7 | 1.26 | 0.26 |
| hsa-miR-29a-3p | 13 | −0.07 | 0.98 | 17 | 0.20 | 0.34 |
| hsa-miR-320a | 22 | 0.28 | 0.70 | 20 | 0.09 | 0.29 |
| hsa-miR-323a-3p | 11 | −0.05 | 0.99 | 8 | 0.20 | 0.78 |
| hsa-miR-34a-5p | 22 | 1.45 | 0.83 | 20 | −0.37 | 0.63 |
| hsa-miR-423-5p | 19 | 0.34 | 0.80 | 17 | 0.02 | 0.85 |
| hsa-miR-451a | 22 | 0.78 | 0.45 | 20 | 0.11 | 0.48 |
| hsa-miR-92a-3p | 22 | −0.03 | 0.96 | 20 | 0.06 | 0.46 |
| hsa-miR-93-5p | 22 | −0.50 | 0.61 | 20 | −0.01 | 0.99 |
Significant by sexp < 0.05;
sex variable omitted due to low number of twin pairs. Coefficients β0 equals the difference between the normalized miRNA level of twin with diagnose subtracted by the equivalent level miRNA from the healthy co-twin. Coefficients β1 equals intra-pair difference in normalized miRNA level per unit intra-pair difference in CCS. All analyses were adjusted for inter-pair differences in age and sex. Entries in bold italics mark significant results (p <0.05). Number twin pairs are twin pairs where both twins have miRNA readings.
Twin-pair difference in cognitive scores and miRNA among twins without a dementia diagnosis
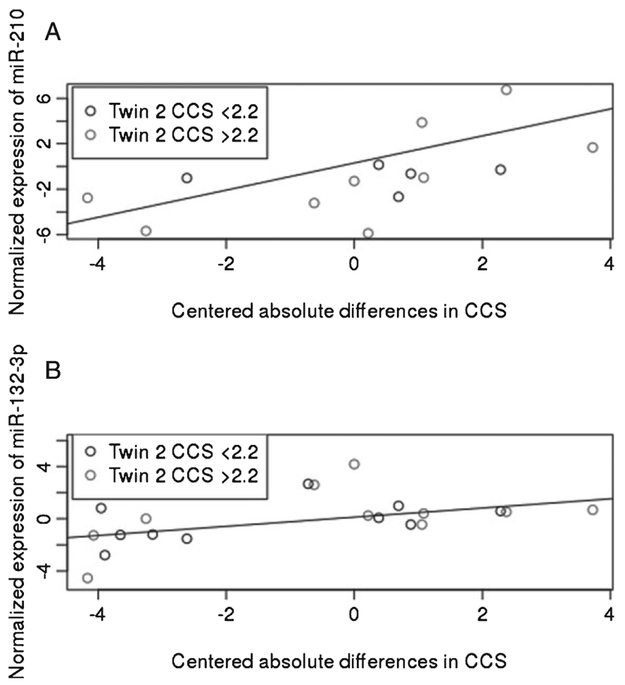
We also tested if the miRNAs were associated with CCS in the twin paired analysis. Here miR-210 was found associated positively with CCS, i.e., the degree of discordance (β1), which is the regression coefficient of the intra-pair miRNA difference and intra-pair cognitive difference, while miR-132-3p was likewise borderline associated (p-value = 0.07). Thus, for twins with good cognitive function, miRNAs were down regulated compared with the co-twin and other twins who performed poorer on the cognitive scale (Table 2). There was, however, no significant supportive evidence that any miRNAs associated with both dementia and with CCS. Noteworthy, as shown in Fig. 2A, the association between miR-210 and CCS seems to be driven by the twin pairs with a well-performing twin 2 (CCS>2.2), while the scatter of twin pairs with poorer-performing twin 2 (CCS<2.2) seems to follow a horizontal line. In contrast, miR-132-3p was positively associated with CCS, for both twin pairs with a well-performing twin 2 (CCS>2.2) and poorer-performing twin 2 (CCS<2.2) (Fig. 2B). Since not all participants had a miR-210 reading these data were investigated further. We found that in samples from all 14 twin pairs represented in Fig. 2B, miR-210 readings were missing in one of the technical replicates performed on all samples, while in samples from 17 additional twin pairs miR-210 readings were missing in both technical replicates. The surplus of miR-210 double miss-readings is unlikely due to random technical variation, and could indicate a biological cause, e.g., low levels, of miR-210. Re-analysis, now also including single readings of miR-210 (Ntwin-pairs = 16), failed to show miR-210 association at a 5% significance level although the direction of the association with CCS remained positive (β = 0.44, p = 0.28).
Fig. 2.
Plots of miRNAs miR-132-3p and miR-210 associated with cognitive composite score (CCS) in paired analysis of twins with-out dementia diagnosis. A) scatter plot of centered absolute difference in CCS and normalized expression of miR-210 with the line of regression (β1 = 1.19, p = 0.02) and B) scatter plot of centered absolute difference in CCS and normalized expression of miR-132-3p with the line of regression (β1 =0.35, p = 0.07), see Table 2 for details of regression analyses.
Individual-level association between dementia and miRNA
In addition to the paired analysis we performed an ordinary individual analysis of association with dementia for all participants. Here, let-7b-5p, miR-17-5p and miR-23a-3p (borderline associated, p = 0.08) were downregulated while miR-155-5p was upregulated in individuals with a dementia diagnosis (Table 3).
Table 3.
miRNA associated with dementia in individual analysis. All participants were included in analyses that were adjusted for age and sex. Entries in bold italics mark significant results (p < 0.05)
| miRNA | No. individuals |
Regression Coefficient |
p-value |
|---|---|---|---|
| hsa-let-7b-5p | 95 | −0.40 | 0.01 |
| hsa-let-7f-5p | 34 | 0.81 | 0.31 |
| hsa-let-7i-5p | 80 | −0.18 | 0.61 |
| hsa-miR-101-3p | 95 | 0.36 | 0.19 |
| hsa-miR-106a-5p | 95 | −0.10 | 0.47 |
| hsa-miR-106b-5p | 95 | −0.07 | 0.81 |
| hsa-miR-128-3p | 55 | −0.97 | 0.32 |
| hsa-miR-130b-3p | 90 | −0.14 | 0.59 |
| hsa-miR-132-3p | 94 | 0.45 | 0.23 |
| hsa-miR-134-5p | 80 | 0.73 | 0.31 |
| hsa-miR-142-3p | 95 | −0.16 | 0.43 |
| hsa-miR-145-5p | 75 | −0.09 | 0.84 |
| hsa-miR-146a-5p | 95 | 0.18 | 0.36 |
| hsa-miR-146b-5p | 95 | −0.09 | 0.70 |
| hsa-miR-155-5p | 95 | 0.92 | 0.01 |
| hsa-miR-15a-5p | 34 | 0.20 | 0.76 |
| hsa-miR-15b-5p | 93 | −0.18 | 0.53 |
| hsa-miR-16-5p | 95 | −0.21 | 0.26 |
| hsa-miR-17-5p | 95 | −0.25 | 0.05 |
| hsa-miR-191-5p | 95 | −0.25 | 0.13 |
| hsa-miR-20a-5p | 95 | −0.03 | 0.80 |
| hsa-miR-210-3p | 63 | 0.39 | 0.58 |
| hsa-miR-223-3p | 95 | 0.04 | 0.75 |
| hsa-miR-23a-3p | 71 | −1.02 | 0.08 |
| hsa-miR-24-3p | 95 | 0.22 | 0.35 |
| hsa-miR-26b-5p | 93 | 0.06 | 0.79 |
| hsa-miR-27a-3p | 94 | −0.20 | 0.29 |
| hsa-miR-298 | 34 | −0.31 | 0.87 |
| hsa-miR-29a-3p | 77 | −0.22 | 0.46 |
| hsa-miR-320a | 95 | −0.10 | 0.46 |
| hsa-miR-323a-3p | 67 | 0.57 | 0.32 |
| hsa-miR-34a-5p | 95 | 0.43 | 0.67 |
| hsa-miR-423-5p | 87 | −0.07 | 0.79 |
| hsa-miR-451a | 95 | −0.24 | 0.25 |
| hsa-miR-92a-3p | 95 | −0.02 | 0.88 |
| hsa-miR-93-5p | 95 | −0.20 | 0.30 |
The absence of miR-210 in duplicate readings was also pursued further in this association analysis. We observed missing miR-210 reading in half the twins in pairs who both had a dementia diagnosis (50%), while among twin pairs where one twin had a dementia diagnosis only 18% had absent miR-210 readings. Among the twins without diagnosis 13% had absent miR-210 values. These observations suggest that miR-210 absence is more likely to occur among twins with a dementia diagnosis than twins without a diagnosis (reg. −0.17, p = 0.05).
Circulating cytokines, diagnoses and cognitive function
Circulating cytokines were also included in the study, but we found no significant association between cytokine levels and diagnosis or CCS. As displayed in Table 4, however, there was a tendency that twins with a dementia diagnosis had higher cytokine levels than their co-twin without a diagnosis.
Table 4.
Cytokines associated with dementia and cytokines associated cognitive function in the discordant co-twin design sub-study.
| Dementia Discordant twins |
Quantitative difference in CCS in twins with-out dementia |
|||||
|---|---|---|---|---|---|---|
| Cytokine | No. twin pairs | β0 coefficient | P-value | No. twin pairs | β1 coefficient | P-value |
| Bdnf | 22 | 672.6 | 0.43 | 20 | −2116.4 | 0.30 |
| IL-10 | 22 | 131.5 | 0.35 | 21 | −292.0 | 0.13 |
| IL-12 | 22 | 50.2 | 0.95b | 21 | 18.4 | 0.97b |
| IL-18 | 22 | 18.3 | 0.35a | 21 | −89.3 | 0.88 |
| IL-2 | 22 | 24.3 | 0.74a | 21 | 14.4 | 0.50 |
| IL-4 | 21 | 70.3 | 0.20 | 20 | −2.3 | 0.37 |
| MMP-9 | 22 | 4473.0 | 0.79 | 21 | 5624.5 | 0.83 |
| TGF-b | 22 | 179.7 | 0.93a | 21 | 1054.2 | 0.59 |
| TNF-a | 22 | 78.6 | 0.37 | 21 | 2.1 | 0.94 |
Significant by agep < 0.05;
p-values from analysis of untransformed data, since transformation was not applicable. Coefficients β0 equals the difference between the cytokine level of twin with diagnose subtracted by the equivalent cytokine level from the healthy co-twin. Coefficients β 1 equals intra-pair difference in cytokine level per unit intra-pair difference in CCS. All analyses were adjusted for age and sex between pairs. Coefficients β0 and β1 are from analysis of untransformed data while p-values are obtained from power transformed data.
DISCUSSION
In this study involving 48 twin pairs selected on the basis of intra-pair similarities or differences regarding dementia diagnoses and cognitive scores we aimed at correlating selected circulating miRNA and cytokine levels in baseline plasma samples with the development of the phenotypic characteristics. Candidate miRNAs were selected based on previous reports of involvement in neurodegeneration, neuroinflammation, or other chronic inflammatory diseases (Supplementary Table 1). Cytokines represented pro- and anti-inflammatory as well as immunomodulating and neuroprotective molecules. We used a longitudinal design to explore the association of circulating miRNAs in plasma among elderly twins prior to registered dementia diagnosis with 13 years of follow-up. We find that two closely related miRNAs, miR-106b-5p and miR-106a-5p and a third miRNA, miR-146b-5p, were associated with a diagnosis of dementia. Also, in a cross-sectional design, we studied cognitive performance of elderly twins without a dementia diagnosis, and found two miRNAs, miR-132-3p and miR-210, associated with cognitive function. Moreover, in an individual analysis including all twins, the expression level of four miRNAs, let-7b-5p, miR-17-5p, miR-23a-3p and miR-155-5p, and the absence of miR-210 expression were associated with dementia. Overall the analyses did not indicate significant association of miRNAs with dementia or cognitive score performance on the cross-sectional level and in the paired and individual longitudinal level. Rather, the findings may reflect the molecular biology predisposing to dementia. Still, the data provide consistent evidence, e.g., we find that low expression level of miR-210 is associated with low cognitive performance and that undetectable levels of miR-210 are more frequently found among twins with a dementia diagnosis. Interestingly, other studies have demonstrated an association of low levels of miR-210 in spinal fluid and serum with both AD and mild cognitive impairment [22]. Also, miR-210 has been implicated in inflammation, both as a regulator of apoptosis, an inhibitor of proinflammatory cytokine expression after LPS stimulation, and by being regulated by the cytokines TNF and IL-6 [23, 24].
Further, we observed that miR-132-3p was positively associated with CCS among twins without a dementia diagnosis. Interestingly, this is one of the most frequently appearing miRNAs in AD studies, as it was found upregulated in AD patients in body fluids in three independent studies [25] and is dysregulated in post-mortem brains [26]. On the molecular level, the influence of miR-132-3p on the transcription factor, nuclear factor κB (NF-κB), i.e., induction of NF-κB translocation to the nucleus as a preamble for cytokine (IL-8 and MCP-1 production) [27], is of potential relevance for the association of increased miR-132-3p with decreased CCS in the present study. The miR-146b-5p, which we observed associated with dementia in the discordant twin analysis, is an anti-inflammatory miRNA [28] and was downregulated in twins with a diagnosis of dementia. We also found miR-155-5p, which is pro-inflammatory [29], upregulated in twins with a diagnosis of dementia in analysis at the individual level. Likewise, the association of let-7b-5p, miR-17-5p, and miR-23a-3p with the absence of a dementia diagnosis on the individual level also putatively fits a paradigm of inflammation as an integral part of dementia development. In cell lines and gastric biopsies, let-7b targets toll-like receptor 4 (TLR4) expression inhibits NF-κB activation and inflammation during infection (H. pylori) [30]. Control of inflammation is also exerted by miR-17 [31, 32], and miR-23a-3p is important for cytokine-mediated apoptosis [33].
The deliberations illustrate an enrichment of miRNA with an inflammatory/anti-inflammatory role among the significant miRNAs. That supports the notion [34] that inflammatory processes may be involved in the development and progression of dementia in affected twins and that these processes to some extent act independently of genetic factors. Although, the data from the individual analysis in the present study did not replicate in the paired analysis, which suggests at least for some of the miRNAs there could be a genetic/heritable contribution. Our present contributions of what could be early biomarkers of dementia add to the main literature on post-diagnostic biomarkers. Also, the present literature may lead to a new understanding of pathways and new early warning diagnostic tools. We need larger, preferential longitudinal studies to achieve this goal and preferential supportive functional studies.
Although we observed novel findings in our study we acknowledge that dementia diagnoses from registers are typically under-reported and not as accurate classified as validated dementia diagnoses, thus results are likely biased toward the null hypothesis. Also register studies are limited by the fact that patients may have been improperly clinically diagnosed. However, Dementia registers in Denmark has high validity (85.8% are correctly classified) [35], while subtypes such as Alzheimer’s or vascular dementia are poorly classified, thus such subset diagnoses were not included in the analysis and the present study suffer to some extent from heterogeneity of the different types of dementia.
The five-component CCS has been used in a variety of our aging studies and is sensitive to normative age changes [14, 15, 36–39]. CCS is a relative narrow cognitive measure of the elderly individuals working memory, i.e., their ability to recall animals and digits. However, CCS overlaps to some extend with memory loss, which is a key symptom for patients with a Dementia diagnosis, and even associate with AD related genes such as APOE, CLU and PICALM [37–39]. Unexpectedly, the average CCS was similar at baseline for twin pairs with and without a dementia diagnosis, yet both were lower than the average CCS of LSADT blood donors (CCS: 0.95) [36]. A potential explanation for low CCS in the concordant twin pairs without diagnosis could be that twin pairs are selected for large and small CCS differences. On average, twin pairs concordant for dementia diagnoses had higher CCS, but they were also younger and CCS is inaccurately estimated due to the small sample size (n = 10). In general, the sample size in the present work is limited even for miRNA expressed in all samples, e.g., in the dementia discordance design 22 pair of twins were included, and larger studies are needed to replicate our explorative findings. Power calculations suggested adequate power (e.g., power of approximately 80%) to detect expression differences of 1.25-fold that being a moderate effect size. However, the presented results were uncorrected for multiple testing and to obtain desirable significant association after multiple testing larger samples are needed.
There is some variation to how the blood samples were handled as they were donated by the participants in their homes during the population-based cohort study. Samples were prior analysis stored for more than 15 years and thus the miRNA level, may be inflated by the level of hemolysis and the amount of leukocytes, yet, likely to affect the level of association toward the null, since the variation is likely to have occurred irrespective of the donors cognitive abilities.
In conclusion, we find a consistent but not exhaustive pattern of anti-inflammatory and pro-inflammatory miRNA differences in twins with dementia. A similar pattern might have been expected in the cytokine data, but, despite trends pointing to the involvement of anti-inflammatory and pro-inflammatory cytokines as early biomarkers of dementia, no significant associations of any cytokine levels with dementia or CCS were observed. A cautious suggestion would be that both pro-inflammatory and anti-inflammatory markers are higher in twins with a dementia diagnosis, but a larger sample size is needed to support the cytokine alteration hypothesis. The most consistent findings were decreased miR-106a, increased miR-106b and decreased miR-210 in dementia. In addition to its proposed roles in regulation of apoptosis and cytokine production, the regulation of vascularization by miR-210 might be of relevance for the findings of dysregulation of this miRNA in accordance with other studies [22]. In the case of miRs-106a and 106b, both target amyloid-β precursor protein (AβPP) mRNA and both are decreased in the temporal cortex of AD patients [40]. Both are also targeting expression of proteins involved in transport of apolipoprotein E as has been shown experimentally for miR-106b [41]. It is generally not possible to predict the correspondence between intracellular alterations in miRNA levels and changes in circulating miRNA levels, but it is interesting that in addition to regulatory functions in inflammation, vascular growth factors, lipoprotein transporters, and AβPP mRNA are among the targets of the prominent miRNAs that are altered in twins with dementia diagnoses.
Supplementary Material
ACKNOWLEDGMENTS
The Danish Aging Research Center is supported by a grant from the VELUX Foundation. The study was supported by The European Union’s Seventh Framework Programme (FP7/2007-2011) under grant agreement n° 259679, The National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology and Innovation and the US National Institutes of Health (P01 AG08761), The Danish Council for Independent Research (DFF – 6110-00016) and by Odense University Hospital Free Research Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1163r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-171163.
REFERENCES
- [1].Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- [2].Barber R (2011) Inflammatory signaling in Alzheimer disease and depression. Cleve Clin J Med 78(Suppl 1), S47–49. [DOI] [PubMed] [Google Scholar]
- [3].Couturier J, Stancu IC, Schakman O, Pierrot N, Huaux F, Kienlen-Campard P, Dewachter I, Octave JN (2016) Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J Neuroinflammation 13, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rozemuller AJ, van Gool WA, Eikelenboom P (2005) The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer’s disease: Therapeutic implications. Curr Drug Targets CNS Neurol Disord 4, 223–233. [DOI] [PubMed] [Google Scholar]
- [5].van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, Thongtang N, Wolf PA, Schaefer EJ (2012) Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: Results from the Framingham Heart Study. Arch Neurol 69, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH (2001) Non-steroidal antiinflammatory drugs and the riskof Alzheimer’s disease. N Engl J Med 345, 1515–1521. [DOI] [PubMed] [Google Scholar]
- [7].Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH (2009) Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord 28, 281–287. [DOI] [PubMed] [Google Scholar]
- [8].van Exel E, Eikelenboom P, Comijs H, Frolich M, Smit JH, Stek ML, Scheltens P, Eefsting JE, Westendorp RG (2009) Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry 66, 1263–1270. [DOI] [PubMed] [Google Scholar]
- [9].Heegaard NHH, Carlsen AL, Skovgaard K, Heegaard PMH (2015) Circulating extracellular microRNA in systemic autoimmunity. EXS 106, 171–195. [DOI] [PubMed] [Google Scholar]
- [10].Pritchard CC, Cheng HH, Tewari M (2012) MicroRNA profiling: Approaches and considerations. Nat Rev Genet 13, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roth-Isigkeit A, Hasselbach L, Ocklitz E, Bruckner S, Ros A, Gehring H, Schmucker P, Rink L, Seyfarth M (2001) Inter-individual differences in cytokine release in patients undergoing cardiac surgery with cardiopulmonary bypass. Clin Exp Immunol 125, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS (2008) Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev 17, 3450–3456. [DOI] [PubMed] [Google Scholar]
- [13].Christensen K, Holm NV, McGue M, Corder L, Vaupel JW (1999) A Danish population-based twin study on general health in the elderly. J Aging Health 11, 49–64. [DOI] [PubMed] [Google Scholar]
- [14].McGue M, Christensen K (2001) The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 years and older. Psychol Aging 16, 272–280. [DOI] [PubMed] [Google Scholar]
- [15].Christensen K, Gaist D, Vaupel JW, McGue M (2002) Genetic contribution to rate of change in functional abilities among Danish twins aged 75 years or more. Am J Epidemiol 155, 132–139. [DOI] [PubMed] [Google Scholar]
- [16].Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K, Kyvik K (2003) Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res 6, 275–278. [DOI] [PubMed] [Google Scholar]
- [17].Salem LC, Andersen BB, Nielsen TR, Stokholm J, Jorgensen MB, Waldemar G (2014) Inadequate diagnostic evaluation in young patients registered with a diagnosis of dementia: A nationwide register-based study. Dement Geriatr Cogn Dis Extra 4, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heegaard NH, Carlsen AL, Lilje B, Ng KL, Ronne ME, Jorgensen HL, Sennels H, Fahrenkrug J (2016) Diurnal variations of human circulating cell-free micro-RNA. PLoS One 11,e0160577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan Q, Christiansen L, von Bornemann Hjelmborg J, Christensen K (2015) Twin methodology in epigenetic studies. J Exp Biol 218, 134–139. [DOI] [PubMed] [Google Scholar]
- [20].Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67, 1–48. [Google Scholar]
- [21].Khuu C, Utheim TP, Sehic A (2016) The three paralogous MicroRNA clusters in development and disease, miR-17-92, miR-106a-363, and miR-106b-25. Scientifica (Cairo) 2016, 1379643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhu Y, Li C, Sun A, Wang Y, Zhou S (2015) Quantification of microRNA-210 in the cerebrospinal fluid and serum: Implications for Alzheimer’s disease. Exp Ther Med 9, 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo L, Zhang Y, Zhang L, Huang F, Li J, Wang S (2016) MicroRNAs, TGF-beta signaling, and the inflammatory microenvironment in cancer. Tumour Biol 37, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C (2012) microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS Lett 586, 1201–1207. [DOI] [PubMed] [Google Scholar]
- [25].Wu HZ, Ong KL, Seeher K, Armstrong NJ, Thalamuthu A, Brodaty H, Sachdev P, Mather K (2016) Circulating micro RNAs as biomarkers of Alzheimer’s disease: A systematic review. J Alzheimers Dis 49, 755–766. [DOI] [PubMed] [Google Scholar]
- [26].Pichler S, Gu W, Hartl D, Gasparoni G, Leidinger P, Keller A, Meese E, Mayhaus M, Hampel H, Riemenschneider M (2017) The miRNome of Alzheimer’s disease: Consistent downregulation of the miR-132/212 cluster. Neurobiol Aging 50, 167 e161–167 e110. [DOI] [PubMed] [Google Scholar]
- [27].Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martinez JA, Marti A (2015) Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J 29, 3595–3611. [DOI] [PubMed] [Google Scholar]
- [28].Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT (2014) MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307, L727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O’Connell RM, Rao DS, Baltimore D (2012) microRNA regulation of inflammatory responses. Annu Rev Immunol 30, 295–312. [DOI] [PubMed] [Google Scholar]
- [30].Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J (2013) Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One 8, e56709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Concepcion CP, Bonetti C, Ventura A (2012) The microRNA-17-92 family of microRNA clusters in development and disease. Cancer J 18, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Philippe L, Alsaleh G, Bahram S, Pfeffer S, Georgel P (2013) The miR-17 approximately 92 cluster: A key player in the control of inflammation during rheumatoid arthritis. Front Immunol 4, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grieco FA, Sebastiani G, Juan-Mateu J, Villate O, Marroqui L, Ladriere L, Tugay K, Regazzi R, Bugliani M, Marchetti P, Dotta F, Eizirik DL (2017) MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p Regulate the expression of proapoptotic BH3-only proteins DP5 and PUMA in human pancreatic beta-cells. Diabetes 66, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ (2002) Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 52, 168–174. [DOI] [PubMed] [Google Scholar]
- [35].Phung TK, Andersen BB, Hogh P, Kessing LV, Mortensen PB, Waldemar G (2007) Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 24, 220–228. [DOI] [PubMed] [Google Scholar]
- [36].Mengel-From J, Thinggaard M, Lindahl-Jacobsen R, McGue M, Christensen K, Christiansen L (2013) CLU genetic variants and cognitive decline among elderly and oldest old. PLoS One 8, e79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lindahl-Jacobsen R, Tan Q, Mengel-From J, Christensen K, Nebel A, Christiansen L (2013) Effects of the APOE epsilon2 allele on mortality and cognitive function in the oldest old. J Gerontol A Biol Sci Med Sci 68, 389–394. [DOI] [PubMed] [Google Scholar]
- [38].Mengel-From J, Christensen K, Thinggaard M, McGue M, Christiansen L (2011) Genetic variants in the choline acetyltransferase (ChAT) gene are modestly associated with normal cognitive function in the elderly. Genes Brain Behav 10, 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mengel-From J, Christensen K, McGue M, Christiansen L (2011) Genetic variations in the CLU and PICALM genes are associated with cognitive function in the oldest old. Neurobiol Aging 32, 554 e557–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Femminella GD, Ferrara N, Rengo G (2015) The emerging role of microRNAs in Alzheimer’s disease. Front Physiol 6, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C, Kim J (2012) MiR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Exp Neurol 235, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.