Abstract
Hydrogels provide a regenerative medicine platform with their ability to create an environment that supports transplanted or endogenous infiltrating cells and enables these cells to restore or replace the function of tissues lost to disease or trauma. Furthermore, these systems have been employed as delivery vehicles for therapeutic genes, which can direct and/or enhance the function of the transplanted or endogenous cells. Herein, we review recent advances in the development of hydrogels for cell and non-viral gene delivery through understanding the design parameters, including both physical and biological components, on promoting transgene expression, cell engraftment, and ultimately cell function. Furthermore, this review identifies emerging opportunities for combining cell and gene delivery approaches to overcome challenges to the field.
Keywords: hydrogels, cell delivery, gene delivery
Graphical Abstract
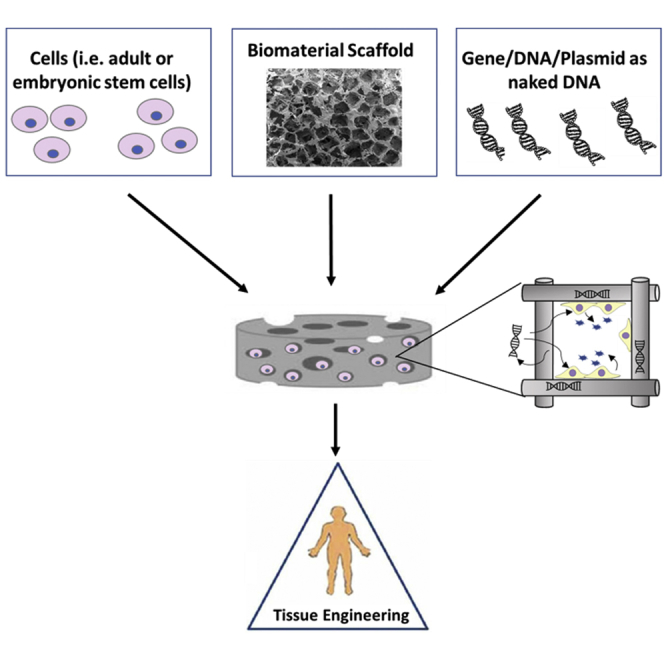
Hydrogels offer great promise in developing suitable environments for both transplanted cell survival and control over gene delivery to promote tissue repair. We review hydrogel design parameters that are available to enhance cell function and the challenges and progress in the incorporation of non-viral vectors to improve therapeutic outcomes.
Main Text
Tissue regeneration following disease or injury may require exogenous inputs to augment natural healing programs and suppress inhibitory processes. Given the essential role that the extracellular matrix (ECM) has in maintaining the physiological stability of the microenvironment and guiding tissue-specific function, biomaterial scaffolds have been designed to mimic this environment in an effort to promote tissue regeneration and to serve as vehicles for cell transplantation to promote survival, differentiation, and engraftment. Hydrogels, a class of biomaterial scaffolds, are highly hydrated crosslinked polymer networks that have been constructed from a wide range of both naturally and synthetically derived polymers.1, 2, 3, 4 Several key characteristics of hydrogels make them particularly well suited for mimicking the ECM, namely their biocompatibility;5, 6, 7 their permeability to oxygen, nutrient growth factors, and metabolic waste;8, 9 their tunable mechanical properties;10, 11, 12 and their tissue-like viscoelastic characteristics.13, 14
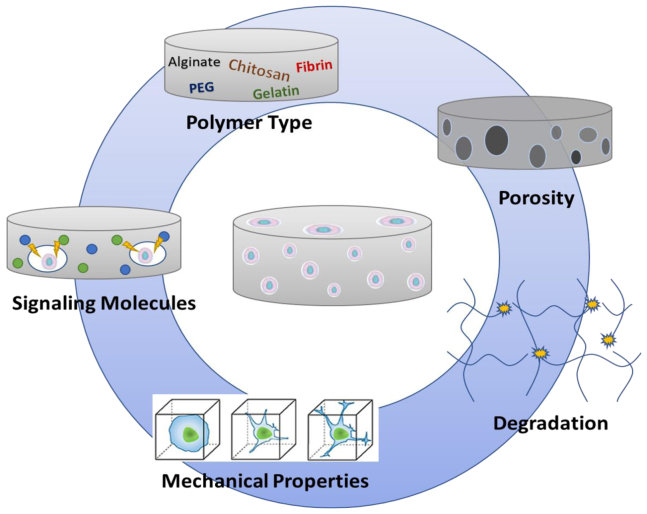
Whereas hydrogel designs largely depend on the location of implantation (e.g., tissue type to be repaired or type of stem cell to be delivered), several design parameters can be manipulated to mimic the native ECM and consequently function to promote new tissue formation and stem cell engraftment (Figure 1).15, 16 On the most fundamental level, hydrogels should define a 3D space for tissue formation, as the 3D architecture can support the infiltration and assembly of host cells into structures and induce gene expression programs associated with normal growth or development. Cell seeding and infiltration of the hydrogel scaffold can be facilitated by micron-scale porosity and/or cell-mediated degradation cues introduced into the hydrogel design. Furthermore, ECM-derived adhesion peptides, protein fragments, or native proteins can promote cell adhesion, leading to a number of specific cell processes, such as vascularization, bone regeneration, or the creation of a tissue-specific niche.17, 18 Trophic factors may also be necessary within the environment to drive cellular responses, leading to stem cell differentiation and tissue formation. Although localized and sustained release of trophic factors from biomaterials has been a research focus for nearly 20 years,19, 20 substantial challenges remain regarding formulations that retain activity without developing immune responses21 or unintended side effects due to high doses. Alternatively, the delivery of gene therapy vectors encoding for trophic factors or other tissue inductive factors may avoid some of the challenges associated with protein delivery while providing the opportunity for sustained and localized availability of the factor. Finally, tissue engineering and regenerative medicine may be nearing a tipping point based on recent investments by industry and an increasing number of clinical trials. For translational purposes, the design approach should consider manufacturing issues, such as availability, reproducibility, and processing strategies, and generally the issues that will be needed for US Food and Drug Administration (FDA) regulatory approval and commercial viability.
Figure 1.
Design Considerations
The hydrogel design for delivery in tissue engineering applications is dictated by the biocompatible polymer type, porosity, mechanical properties, degradability, and signaling factors in the microenvironment.
This review will describe recent advances in the design of hydrogels as vehicles for cell and non-viral gene delivery to promote tissue regeneration. We discuss the hydrogel design parameters that are available to enhance transplanted cell survival and appropriate interactions with the host tissue. Additionally, we discuss the challenges and progress in the incorporation of non-viral vectors within hydrogels to induce the expression through bioactive cues that promote tissue repair. The manuscript focuses on non-viral approaches due to the greater flexibility in vector design, and interested readers can find excellent reviews on viral vector delivery from biomaterials.22, 23
Hydrogels as Vehicles for Cell Delivery
The delivery of cells has most typically involved the injection of high-density cell suspensions into the target diseased or injured site. However, such direct cell injection methods often have a poor therapeutic response due to a rapid decrease in cell viability, low or modest engraftment of transplanted cells, and limited control over cell fate due to the local environment.24, 25, 26 The delivery of transplanted cells in a scaffold comprised of biocompatible materials addresses these limitations by initially providing protection to the transplanted cells that can enhance survival and prolong retention at the site.27 Moreover, hydrogels often have physical properties that are similar to the native ECM, such as their mechanical properties and water content.28 This mimicry of the niche environment can have a considerable impact on cell fate by influencing the morphology, viability, differentiation, and function.26, 29 The two main strategies in utilizing hydrogels for stem cell transplantation are non-integrating approaches that isolate cells from the host tissue through encapsulation within the hydrogel and integrating approaches that allow for the transplanted cells to directly contact host tissue either immediately through a microporous design or over time through biodegradation.3, 30, 31 Several examples of hydrogel systems designed for delivering cells in a range of regenerative medicine applications are listed in Table 1. The number of systems investigated in pre-clinical models is large and cannot be listed exhaustively; thus, we have focused on relatively recent publications that are approaching large animal or clinical translation. This section will discuss considerations in these hydrogel designs related to the survival of transplanted cells as well as their ultimate function.
Table 1.
Cell Delivery Applications in Natural and Synthetic Hydrogels
| Hydrogel Material(s) | Cells Delivered | Delivery Strategy | Target Application | Ref. | |
|---|---|---|---|---|---|
| Encapsulated hydrogels | alginate | pancreatic islets | laparoscopic implant of microcapsules | diabetes | 184 |
| adipose-derived and mesenchymal stem cells | injection co-delivered with BMP-2 | bone regeneration | 185 | ||
| CAR-programmed T cells | implanted into the peritoneal or tumor resection cavity | cancer | 186 | ||
| polyethylene glycol and alginate | ovarian follicles | encapsulated scaffold | ovarian function | 177 | |
| Tissue-integrating microporous hydrogels | gelatin | adipose-derived stromal cells | microporous microribbon hydrogel injected into cranial defect | bone regeneration | 76 |
| collagen | autogenous chondrocytes | porous scaffold matrix | cartilage repair | 82 | |
| alginate | mesenchymal stem cells | injectable, void-forming microporous hydrogel | bone regeneration | 178 | |
| Biodegradable hydrogels | collagen | iPSC-derived hepatocytes and endothelial cells | encapsulated polyelectrolyte fiber scaffold transplanted into liver | liver tissue regeneration | 180 |
| neonatal astroglial cells | transplanted gel into lesion of spinal cord | spinal cord regeneration | 181 | ||
| hyaluronic acid | cardiac progenitor cells | subcutaneous injection | angiogenesis | 182 | |
| neural progenitor cells | injection into stroke cavity | neural regeneration from stroke | 183 | ||
| gelatin | cardiac-derived stem cells | intra-myocardial injection with the controlled release of bFGF | cardiac regeneration | 172 | |
| polyethylene glycol with degradable peptide crosslinker | adipose-derived stem cells | in situ injection with encapsulated siRNA | bone regeneration | 160 | |
| iPSC-derived endothelial cells | injection co-delivered with VEGF | muscle repair | 173 | ||
| fibronectin and agarose | cardiac stem cells | in situ injection | cardiac regeneration | 174 | |
| fibrin | human embryonic stem cells | epicardial delivery of encapsulating gel | cardiac regeneration | 175 | |
| adipose-derived mesenchymal stromal cells | patch applied to surface of skin | wound healing | 187 |
Cell Delivery in Encapsulating Hydrogels
The key role of an encapsulation device is to create an environment that allows for normal cell function, while acting as an immune-regulatory barrier through isolation or modulation of the local area for better survival of the transplanted cells.32, 33, 34, 35, 36, 37, 38, 39, 40, 41 This function can be manipulated by the gelation process, the hydrogel structure, as well as material composition.30 A common encapsulation approach is illustrated by the TheraCyte device, which has a porous vascularizing outer membrane that promotes tissue integration and an inner impermeable membrane that protects the transplanted allogeneic islets.42, 43 Neonatal pancreatic tissue was implanted in non-obese diabetic mice, survived, and had a response to glucose levels for at least 50 days.44 Although this original device was not successful in clinical trials, the general strategy has evolved over the course of several companies, including Living Cell Technologies, Beta Logics, Viacyte, and Encaptra. This Encaptra device consists of a single membrane that is immunoisolating while permitting oxygen and nutrients to pass. Viacyte is currently carrying out a phase I/II clinical trial using this device with stem-cell-derived cell sources to assess the safety and efficacy in humans.45 Other encapsulation devices that have reached clinical trials have been recently reviewed in detail.46 Whereas these devices provide a clinically translational design for encapsulation delivery, hydrogels provide the same opportunity to overcome barriers, like immune cell infiltration, plus enhanced transport and more tunable properties. In a hydrogel, adhesion sites and biomechanical properties can be manipulated within the gel to enhance cell viability and therapeutic efficacy. Hydrogels are now being developed that utilize the foundational delivery approach provided by the TheraCyte design while offering tunable properties for not only the exterior but the interior of the device to enhance cell motility, viability, and function.
Alginate is a natural polymer derived from algae that has been extensively investigated for cell encapsulation due to its biocompatibility, low toxicity, relatively low cost, and mild gelation by addition of divalent cations, such as Ca2+.47, 48, 49 Alginate can also be modified to improve cell attachment and motility. A double-layered alginate hydrogel system consisting of matrix-metalloproteinases and Arg-Gly-Asp (RGD) peptide in the inner layer was designed to allow transplanted stem cells to proliferate and mobilize to the outer layer following the inflammatory storm caused from surgery.50 Following transplantation of neural stem cells (NSCs) into a rat brain trauma model, the double-layered alginate hydrogel promoted survival and differentiation of the NSCs. This overall approach focused on NSCs, which have a reduced risk of teratoma formation compared to human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), but the design could be easily adapted to other types of transplanted cells. Alginate-based biomaterials have had great success in rodent models; however, the translations to larger animal models, such as monkeys and humans, have not been immediate successes.51 Although there were no detectable inflammatory responses in human blood,52, 53, 54, 55 the limited efficacy of two clinical transplantations of human islets in barium-alginate and calcium and barium-alginate spheres has been partially attributed to a foreign body response after transplantation.56, 57, 58 Recently, fibrosis has been reported to be reduced or eliminated based on the diameter of the spheres.38 Alternatively, alginate has been functionalized with a range of chemical groups in order to screen for chemistries that would avoid a fibrotic response.39, 40 Vegas et al.41 recently identified chemically modified alginates, such as triazole-thiomorphiline dioxide (TMTD), as hydrogels that resisted fibrosis around the implant in both rodents and non-human primates. The TMTD alginate hydrogel was then used to transplant hESC-derived β cells into immune-competent streptozotocin (STZ)-treated C57BL/6J diabetic mice. The hydrogel showed no observable foreign body response and supported the engraftment and long-term glycemic correction (174 days with the mice still euglycemic at the end of the experiment) from hESC-derived β cells in immune-competent mice.43 These results lay the groundwork for studies in autoimmune animal models and future human studies using hydrogel formulations that overcome the immunological barrier inhibiting long-term cell function.
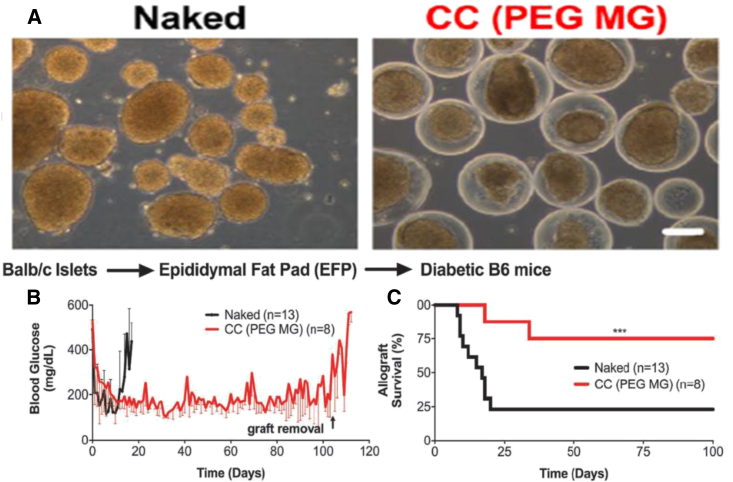
Materials derived from natural materials have had a long history as hydrogels; however, synthetic polymers have become a popular substitute because they provide a more clinically translatable model and more reproducible properties. For these purposes, non-degradable polyethylene glycol (PEG) hydrogels have been widely used for encapsulation. Also, PEG’s tunable viscoelastic properties provide a tissue-like permeable membrane with minimal inflammatory response. By applying a conformal coating around islets consisting of PEG and Matrigel, Manzoli et al.59 demonstrated a strategy for long-term reversal of diabetes with allogeneic islets transplanted in the epididymal fat pad in mice (Figure 2). The incorporation of Matrigel into the PEG coating provided important ECM interactions while keeping the permselectivity low, which resulted in a lack of immune cell penetration and T cell allogeneic priming. In addition to biocompatibility, PEG-based hydrogels are also utilized for their structural support60 and ease of functionalization.61 Studies focused on artificial ovarian tissue delivery have shown encapsulated immature ovarian follicles in PEG-RGD hydrogels can enhance primordial follicle development and graft survival compared to non-encapsulated follicles.62 After a subcutaneous transplant of encapsulated ovarian tissue, ovariectomized adult mice showed restoration of the estrous cycle within two weeks. In contrast to islets, ovarian follicles are avascular and relatively resistant to hypoxia, allowing them to maximize the benefits of immunoisolation methods.
Figure 2.
PEG-MAL Matrigel Conformed Coated Islets Transplanted in the EFP Site Reverse Diabetes Long-Term in Murine Allografts without Immunosuppression
(A) Phase contrast (scale bar, 100 μm) images of naked and PEG-Matrigel conformal coated (CC (PEG MG)) islets from Lewis rats. (B and C) Blood glucose of recipient mice (B) and survival (C) of 750–1,000 islet equivalent (IEQ) naked (black; n = 13) or CC (PEG MG) (red; n = 8) islets from BALB/c mice transplanted into fully major histocompatibility complex (MHC)-mismatched chemically induced diabetic B6 mice in the epididymal fat pad using fibrin scaffolds without any immunosuppression.190 (***p <.001.)
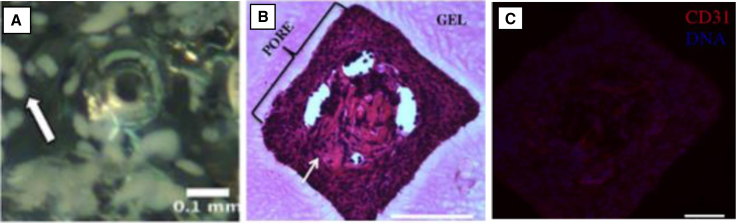
For cells that are more dependent on graft vascularization, a PEG hydrogel was designed using lithography techniques in order to develop an encapsulating gel that had microchannels for vascularization (Figure 3). This encapsulating strategy combines encapsulation with printing systems to generate microchannels in pre-defined regions of the nano-porous hydrogel.63 The scaffold architecture presented here can be designed to improve the transport of nutrients and oxygen to encapsulated cells. Furthermore, the network of larger sized channels could facilitate the invasion of the host vasculature after implantation or be employed for pre-vascularization in vitro. Their studies revealed that transplanted islets in these gels had tissue and vascular in growth within the microchannels, which promoted normoglycemia after transplantation and sustained glucose control over the two-month period of study until removal of the device. Collectively, numerous cell encapsulation systems are being developed for the treatment of various diseases. Current advances in material design, immunomodulation, and encapsulation strategies will be critical in addressing the many challenges that are involved in transitioning these cell-based therapies to the clinic.
Figure 3.
Macroencapsulating PEG Hydrogel Devices with Microchannels
(A) Encapsulated islets surrounding a microchannel in a hydrogel. Islets appear opaque, and a white arrow indicates a representative islet. (B) Cellular ingrowth (indicated by a white arrow) occurred within microchannel regions of hydrogels relative to the surrounding hydrogel. (C) CD31-positive cells are present in the microchannels with a nuclear counterstain. The scale bars represent 100 μm.84
Cell Delivery with Tissue-Integrating Hydrogels
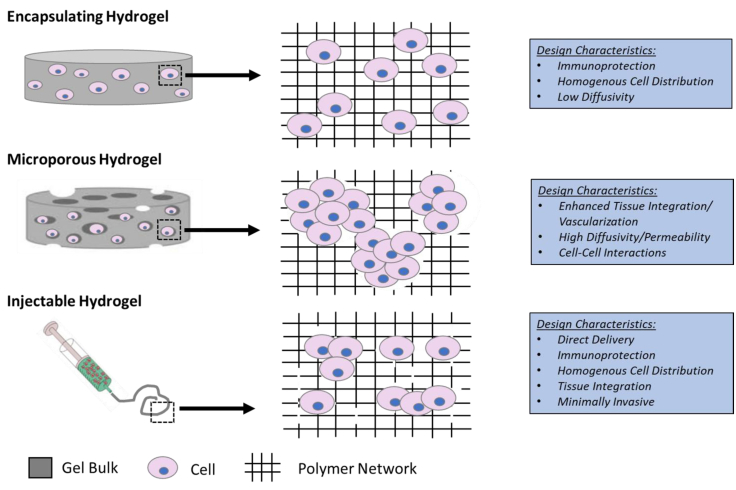
Hydrogels are also formulated into either a microporous structure or are made degradable in order to facilitate integration with the host tissue (Figure 4). The integration with the host tissue can be advantageous for vascularization to provide nutrient transport necessary for survival and appropriate cell-cell contact that can direct differentiation.64, 65, 66
Figure 4.
Schematic Representation and Design Characteristics of Cell-Laden Hydrogels
Microporous Hydrogels for Cell Delivery
Scaffolds with an interconnected microporous structure can be seeded with cells, and upon transplantation, host cells can infiltrate for integration with the transplanted cells.67 The microporous structure allows for nutrient transport and waste removal, while also providing a substantial surface for cell adhesion and space for tissue growth.68, 69 Compared to encapsulated designs, porous hydrogels can encourage cell migration and cell-cell interactions, which play an important role in regulating cell proliferation, differentiation, and the organization of some engineered tissues (e.g., cartilage and liver).58, 70 These cellular activities can be inhibited or delayed when cells are encapsulated into gel scaffolds because they are entrapped in the 3D polymer networks. As a result, the incorporation of cell living spaces that are larger than tens of microns in diameter in gel constructs can support cell spreading, migration, proliferation, and then vascularization for the establishing access to nutrients and other systemic cues.71
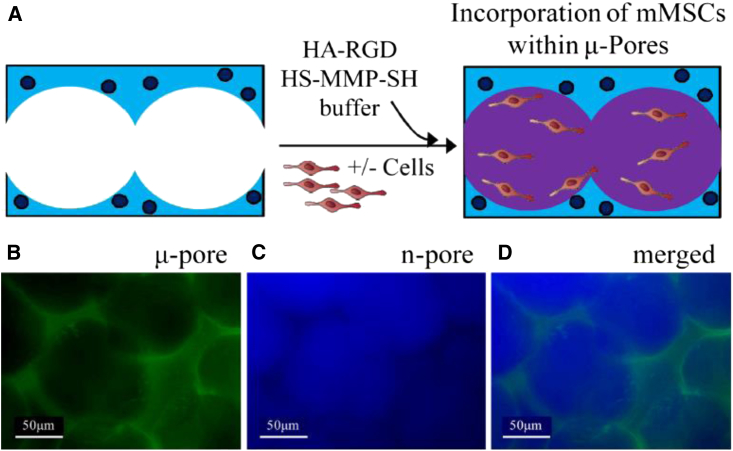
Microporous hydrogels have been developed with many materials for in vitro and in vivo use through a variety of methods, including sphere templating,72, 73, 74, 75, 76 lyophilization,77, 78, 79 and porogen leaching.80, 81, 82, 83 A challenge that comes with the prefabricated design of microporous scaffolds is uniformly distributing cells throughout the scaffold to promote effective regeneration of highly intricate tissues. Whereas encapsulation strategies allow for cells to be uniformly distributed during initial fabrication, microporous hydrogel designs with a tunnel-like network could diminish the ability to achieve heterogeneous cell distribution. Several seeding techniques have been established to improve uniform cell distribution, but many are lengthy and restrict clinical applicability.84, 85 The most common method for seeding microporous scaffolds is static seeding, in which a concentrated cell suspension is passively introduced on a scaffold. This technique, however, has several limitations that result in low seeding efficiency and poor cell penetration.86 To improve cell seeding efficiency, Tokatlian et al.87 utilized a two-phase hydrogel technique where μ-pore 3.5% hyaluronic (HA) hydrogels were formed and then seeded with mouse mesenchymal stem cells (MSCs) mixed into a thin 2.5% nano-pore HA-matrix metalloproteinase (MMP) hydrogel precursor solution (Figure 5). Due to the fluid nature of the precursor solution initially at room temperature, the gel solution flows throughout the pores of the porous hydrogel and distributes the cells uniformly. In sharp contrast to the classical approach of seeding cells on a preformed hydrogel, 3D bioprinting can simultaneously seed cells while fabricating the hydrogel to produce highly organized cellular constructs.67 This strategy helps to overcome obstacles derived from low cell densities, uncontrollable seeding positions, and a heterogeneous distribution of cells throughout the scaffold. Kolesky et al.68 recently demonstrated a new bioprinting method using cell-laden GelMA inks as a bioprintable bulk matrix to build vascularized heterogeneous tissue constructs. These 3D microengineered systems consisting of vasculature, multiple types of cells, and ECM are able to generate an environment for cell adhesion, remodeling, and migration.
Figure 5.
Enhanced Cell Seeding within Porous HA Hydrogel Using a Two-Phase Hydrogel Technique
(A) To effectively seed cells and allow for rapid cell spreading, cells were seeded within the pores of a 3.5% μ-pore HA gel directly with a soft 2.5% HA gel. (B and C) To visualize each phase separately, the μ-pore phase was stained with fluorescein isothiocyanate (FITC) (B), and the inner, n-pore phase was stained with Alexa Fluor 350 (C). (D) Merged fluorescence image of a two-phase hydrogel made using 100 μm beads.98
A common caveat of the microporous design is that these scaffolds are formed into a specific shape prior to implantation, which limits the ability to target a specific site.69 Interestingly, granular materials can be used to generate injectable yet porous materials by injecting microscale hydrogel particles and having them assemble into a bulk granular material. The first example of this approach was termed microporous annealed particle (MAP) scaffolds, where microscale hydrogel spherical beads are injected into a cavity and subsequently annealed to each other to form a bulk gel with the space between the beads serving as pores in the gel. Whereas the void fraction in MAP scaffolds is limited to approximately 20% rather than 80% for other porous hydrogels,88 MAP scaffolds have a continuous micron-sized porous structure that allows both transplanted cells and surrounding host tissue to infiltrate the scaffold without the need for material degradation. In addition, MAP gels have the ability to conform to the wound shape and promote integration to form cellular structures within days after injection.89 As a result, MAP hydrogel injection into skin and brain wounds have shown lower inflammation at the wound sites.89, 90, 91, 92 The microporous injectable hydrogel design could expand the applicability of microporous hydrogels through its injectable nature. Recently, another example of granular hydrogels utilizes particle jamming to avoid the need for particle annealing.74 This work utilizes the phenomenon that jammed granular material behaves as a solid.
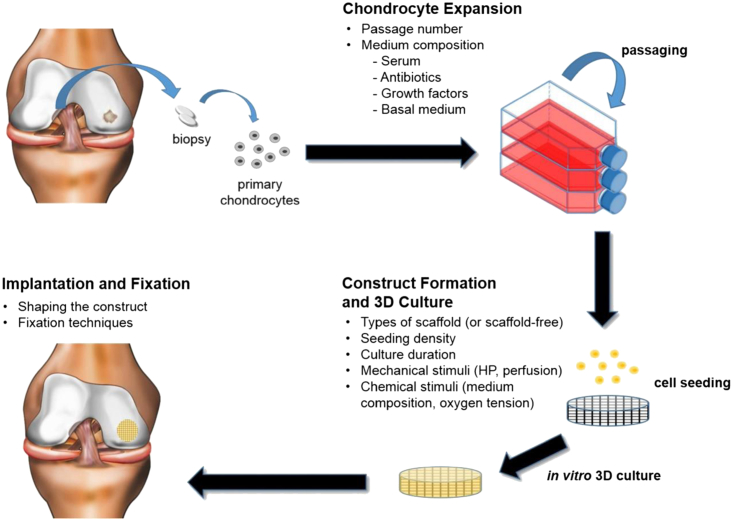
Similarly, a cytocompatible fabrication process was recently developed for generating microporous hydrogels with encapsulated cells using gelatin as a leachable porogen.93 The hydrogel was designed with a wide range of porosities and pore sizes by crosslinking oligo(poly[ethylene glycol]fumarate) in the presence of MSCs and varying sizes of gelatin microspheres. Encapsulated MSCs exhibited high viability immediately following the fabrication process, and culture of cell-laden hydrogels revealed improved cell viability with increasing porosity. An alternative technology is based on using microribbon (μRB)-like gelatin as scaffolds, in which the ribbons are building blocks forming a macroporous structure.76 Their results suggested that enhancing cell survival and proliferation using a μRBs microporous design further promoted the paracrine-signaling effects of adipose-derived stem cells for stimulating endogenous bone repair. A commercially translated microporous design has been utilized for NeoCart (Histogenics, Waltham, MA, USA), in which autologous chondrocytes are cultured on porous bovine type 1 collagen scaffolds (Figure 6) for the repair of cartilage defects in the adult knee.78 Whereas the 3D matrix bears load, its open structure allows for influx of MSCs, which ideally differentiate into chondrogenic lineage.79 A FDA phase II trial comparing NeoCart to microfracture showed significantly better results in all clinical outcome measures in the NeoCart-treated patients.82 Histogenics recently completed patient enrollment of its NeoCart Phase 3 clinical trial in accordance with the Special Protocol Assessment (SPA) agreement with the FDA. They expect to report ongoing studies next year, followed by a potential Biologics License Application (BLA) filing.83 The efficacy of these novel design methods for microporous hydrogels demonstrates the promise of cell-based therapies for enhanced long-term tissue regeneration outcomes.
Figure 6.
Tissue Engineering Strategies Used in Current Clinical Products
A schematic of the classical tissue engineering paradigm used for the fabrication of the reviewed cartilage products.108
Degradable Hydrogels for Cell Delivery
Encapsulating hydrogels that are degradable can initially function as an immunoisolation barrier similar to the encapsulating hydrogels of section Cell Delivery in Encapsulating Hydrogels, yet their degradability over time can allow for improved cell infiltration and integration with the host. Hydrogel degradation can lead to changes in mechanics and swelling over time, which will in turn affect cell behavior.94 By utilizing degradable reagents (i.e., peptides, ligands, or proteases) to form unstable bonds, hydrogels can undergo degradation through hydrolytic or enzymatic mechanisms.95 A common principle is that the degradation rate of the hydrogel should match the rate at which the tissue grows or infiltration is desired. Recently, Lima et al.96 used biodegradable alginate to fabricate beads encapsulating rat MSCs and fibronectin and implanted the particles in a calvarial bone defect in order to evaluate their potential for bone tissue regeneration.97 The hydrogel’s rate of degradation could be controlled to permit accelerated bone tissue growth while preventing cell loss and any toxic exchange of molecules with the surrounding environment.
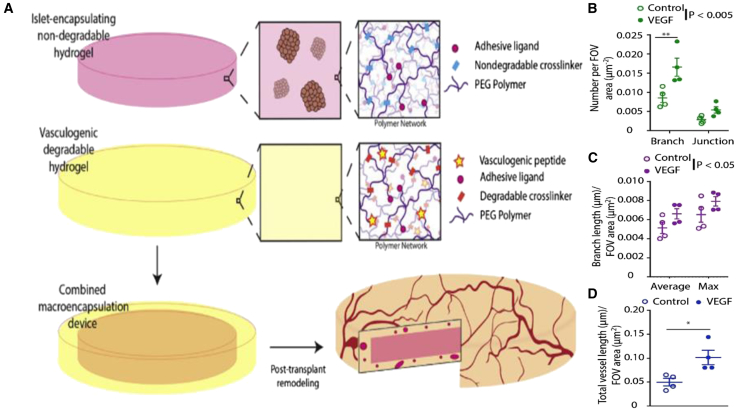
Many natural polymers are biodegradable, yet their utility in tissue engineering applications can be constrained by the intrinsic properties of the materials. In contrast, synthetic polymers provide an opportunity to control degradation through well-defined mechanisms, such as controlling the crosslinking density of such segments.98, 99 For example, the hydrogel can be crosslinked by reacting the backbone polymer with a peptide sequence that can be degraded by specific cell-secreted proteases endogenous to the wound healing microenvironment, such as matrix metalloproteinases.100, 101, 102, 103, 104 In a recent study, PEG gels co-encapsulating chondrocytes and MSCs were crosslinked with an MMP-degradable peptide in order to match the resorption of the scaffold with the rate of matrix production by cells during cartilage repair.105 Relative to non-degradable hydrogels, those that allowed for cell-mediated degradation showed significantly increased GAG and collagen deposition, which are key markers for chondrogenesis.106 Another design consideration utilizing degradative properties evaluated a two-component synthetic PEG hydrogel macrodevice for the delivery of islets to an extrahepatic transplant site (Figure 7). The hydrogel consists of an inner layer crosslinked with a non-degradable PEG dithiol and a degradable outer layer crosslinked with a proteolytically sensitive peptide to enhance localized vascularization. Encapsulated islets demonstrated high viability within the device, and implementation of a vasculogenic, degradable hydrogel layer increased the vascular density around the transplant site. Whereas normoglycemia was not achieved with the device, suggesting that parameters like islet load require further optimization, the results highlight the benefit of degradable interfaces for the promotion of engraftment.
Figure 7.
Poly(ethylene glycol)-Based Hydrogel Designed to Encapsulate Islets Shows Vascular Remodeling in the Omentum with Vasculogenic Layer
(A) Schematic for synthetic hydrogel macroencapsulation device design. A non-degradable synthetic hydrogel disk is surrounded by a degradable, vasculogenic hydrogel that remodels to promote device vascularization post-transplantation. (B–D) Surface vascularization was characterized and quantified for number of (B) vessel junctions and branches, (C) average and maximum branch length, and (D) total overall vessel length per field of view (FOV) (n = 4/condition; FOV = 5–8/n). *p < 0.05; **p < 0.005.105 Error bars, SEM.
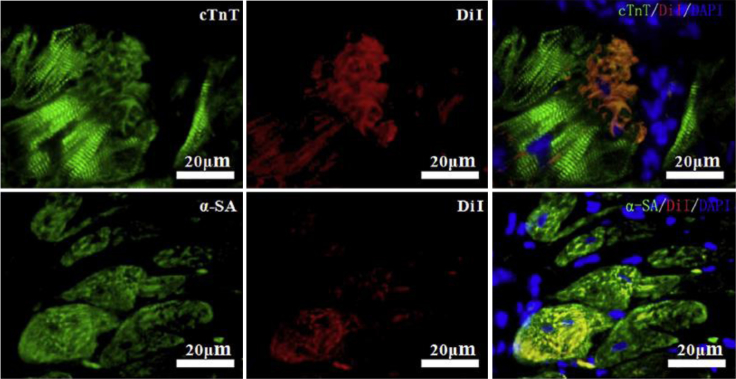
Another application for biodegradable hydrogels is as injectables, which have an unparalleled advantage for delivering cells to specific sites with minimally invasive procedures by undergoing gelation in vivo.107 Injectable hydrogel polymerization can occur as a response to temperature or pH change, ionic cross-linking, solvent exchange or crystallization, or simply thickening upon removal of the injection shear.93, 108 One of the most common polymers used for these biomedical applications is poly (N-isopropylacrylamide) (PNIPAM) due to its lower critical solution temperature being very close to body temperature.109, 110 A recent study evaluated PNIPAM-containing hydrogels used as carriers for intramyocardial delivery of brown-adipose-derived stem cells in rats with myocardial infarction.111 The hydrogel displayed rapid, subphysiological phase transition temperatures and was capable of noninvasively delivering a liquid suspension of cells that gels in situ forming a cell-loaded scaffold, essentially isolating treatment to the injection site. In addition, engraftment around the transplanted cells was significantly enhanced (Figure 8) and therapeutic efficacies were augmented in the myocardial infarction. This research provides new treatment opportunities for diseases like cardiac ischemia, which have seen limited therapeutic success in part due to poor targeting. Gaffey et al.112 addressed this concern by developing an injectable, shear-thinning HA hydrogel that delivers endothelial progenitor cells to ischemic myocardium. The hydrogel was designed to flow through a syringe with the application of shear force and then re-assemble at the injection site. In vivo improvements from this delivery strategy included enhanced cell retention and vasculogenesis, limited adverse remodeling, and improved cardiac function.
Figure 8.
Cardiac Differentiation of Brown Adipose-Derived Stem Cells in Myocardial Environment
4 weeks after transplantation, immunostaining against cardiac markers was performed on cardiac sections. Colocalization of DiI and cardiac proteins (cTnT and a-SA) was observed, indicating differentiation of transplanted cells toward cardiac lineages.191
These injectable biodegradable hydrogels may also be modified with trophic factors to enhance cell survival or function. Vascular endothelial growth factor (VEGF) has been incorporated into degradable PEG hydrogels encapsulating islets in order to promote localized vascularization.97 Transplantation of the in situ-forming injectable hydrogel at an extrahepatic site supported engraftment and reversal of diabetes, which was not achieved without VEGF. Xu et al.113 applied a similar approach by using a basic fibroblast growth factor (bFGF) release system to increase MSC survival in a thermoresponsive, biodegradable hydrogel. The incorporation of the pro-survival factor bFGF within the hydrogel improved MSC viability after intramuscular injection, as well as increased blood vessel density, limb perfusion, and muscle diameter. Whereas the functionalization of these hydrogels enhances both oxygen and nutrients to the target site, the hydrogel’s ability to promote neovascularization is still being investigated because the process of angiogenesis typically requires days to weeks. These recent studies demonstrate the critical role that local degradation seems to play within tissue engineering constructs.
Hydrogels have received increasing interest as a leading candidate for engineered tissue scaffolds due to their superior biocompatibility and inherent similarity to the natural ECM, in addition to their conducive framework for cellular proliferation and survival. These recent advances discussed above highlight important considerations for designing hydrogels for effective cell delivery. Encapsulation approaches can potentially eliminate the barriers preventing transplantation of xenogeneic or stem-cell-derived allogeneic cells. However, hypoxia can occur at the core of the gel and limit the duration that these cells function. Meanwhile, hydrogels that promote tissue integration through microporous structures or biodegradation are challenged by the immune response but can enhance vascularization and in vivo tissue growth. Also, incorporating therapeutic molecules and particles into these hydrogels is another method to guide transplanted cell behavior and function. With continued research in these areas focused on incorporating the benefits of both approaches, hydrogel-based tissue engineering will continue to make advances toward clinical restoration of tissue function.
Hydrogel-Mediated Gene Delivery in Regenerative Medicine
Cell delivery directly provides the cells necessary for tissue formation; however, the function of these cells may need to be augmented by the incorporation of additional factors that promote pro-regenerative processes. The delivery of proteins has been employed to promote repair mechanisms; however, to date, protein delivery for tissue regeneration applications has seen limited success despite several clinical trials, where most success has been observed with decellularized matrices. Therefore, alternative approaches to induce gene expression programs associated with normal growth or development are needed. One such approach is to target gene expression directly through the delivery of genes. In this approach, genes encoding for key transcription, trophic, or growth factors would be delivered from the scaffold to transfect transplanted or infiltrating host cells, which would in turn express the delivered protein to stimulate pro-regenerative behaviors. This strategy has the potential to provide more control over the duration of expression, the activity of the delivery protein, and the delivery of multiple signals from the same hydrogel. Consequently, these benefits can be applied to overcome obstacles that limit the therapeutic efficacy of cell-based therapies, like sufficient vascularization or immune rejection. Furthermore, the use of non-viral approaches avoids safety concerns surrounding the use of viral vectors, including immunogenicity associated with repeated injections and insertional mutagenesis.114, 115 However, it has been difficult to translate non-viral vectors to the clinical stage, mainly due to the primary challenge of achieving sufficient transgene expression to elicit a significant therapeutic response.
Local gene delivery through implantable or injectable hydrogels has been investigated in two different manners—either as a depot that houses plasmid DNA for sustained release into surrounding tissue102 or as a DNA-loaded biomimetic scaffold that encourages cellular infiltration into the scaffold. In this second approach, cells infiltrate and degrade the scaffold, leading to transfection and local expression of the therapeutic gene. In general, the same scaffold design principles used for cell delivery can apply for gene delivery. In addition, the development of DNA-loaded hydrogels must take into account not only what gene is being delivered but also the plasmid components, hydrogel properties that affect DNA loading and release, and the interactions between cells and the scaffold that affect gene transfer, with the ultimate goal of promoting effective transgene expression and, consequently, a significant therapeutic benefit.
Delivering Pro-regenerative Genes to Enhance Therapeutic Outcomes
The selection of the transgene to be delivered is highly dependent on the target regenerative process. From the first report of scaffold-mediated non-viral gene delivery in vivo in 1996 to date, most reported regenerative medicine strategies have delivered genes encoding for native-form secreted growth factors that are functional in the extracellular microenvironment, primarily targeting vascularization and bone formation (Table 2).
Table 2.
Inductive Hydrogel Scaffold-Mediated Delivery of Therapeutic Genes In Vivo
| Gene Delivered | Year | Scaffold Material | Delivery Strategy | Target | Ref. |
|---|---|---|---|---|---|
| Bone morphogenetic protein (BMP)-4 and parathyroid hormone | 1996 | collagen | naked DNA | bone regeneration | 188 |
| Parathyroid hormone | 1999 | collagen | naked DNA | bone regeneration | 138 |
| Hypoxia-inducible factor-1α (HIF-1α) | 2006 | fibrin | DNA/poly-L-lysine (PLL) | vessel formation | 117 |
| Vascular endothelial growth factor (VEGF) | 2009 | collagen | DNA/trimethyl chitosan (TMC) | vessel formation | 147 |
| VEGF | 2010 | collagen-chitosan | DNA/TMC | vessel formation | 153 |
| Fibroblast growth factor-2, BMP-2 | 2012 | gelatin, collagen | DNA/PEI-linoleic acid | bone regeneration | 189 |
| Platelet-derived growth factor (PDGF) | 2014 | collagen | DNA/PEI | bone regeneration | 146 |
| VEGF | 2014 | gelatin | DNA/PEI-graphene oxide | vessel formation (cardiac repair) | 125 |
| VEGF | 2014 | HA | DNA/PEI | vessel formation (wound healing) | 75 |
| VEGF | 2015 | HA | DNA/PEI | vessel formation (wound healing) | 76 |
In delivering genes (e.g., VEGF) to promote vascularization in the site of scaffold implantation, researchers have evaluated changes not only in vessel count but also in indicators of vessel maturation, such as vessel thickness, length, and the presence of smooth muscle cells around endothelial structures.103, 116 Such strategies are motivated by the key role vascularization plays in repair processes, such as cutaneous wound healing and cardiac repair, in order to regenerate functional tissue. Studies investigating the application of gene-loaded scaffold implants in bone defects have evaluated the extent of cell infiltration and observed new bone formation in the implantation site.104, 105
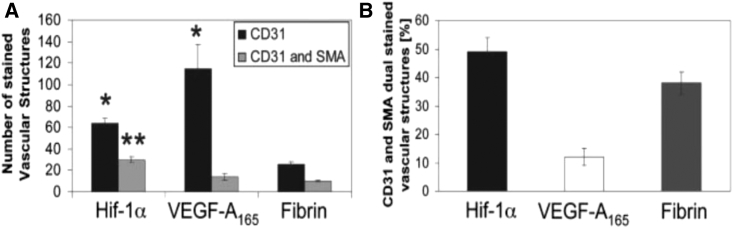
Although some of these studies have reported significant therapeutic responses, much room for improvement remains regarding transgene selection as well as of transgene optimization via engineering to enhance its potency to allow for sufficient potential for clinical translation. One less explored strategy is to deliver genes encoding for proteins other than growth factors; in fact, out of these studies, all but one have delivered genes encoding for growth factors from hydrogels.103 This study delivered 10 μg of a gene encoding for a hypoxia-insensitive variant of the transcription factor hypoxia-inducible factor 1-alpha (HIF-1α) as opposed to its native form from a fibrin matrix administered to a murine cutaneous wound healing model (Figure 9). The authors observed increased levels of angiogenesis and more mature vessel formation as compared to the delivery of 1.25 μg of the VEGF-A165 protein per wound, demonstrating the potential to enhance the potency of therapeutic genes through engineering and judicious selection of gene targets.117 Given the ease of diffusion of growth factors away from the target site, more investigation is suggested in delivering genes encoding for proteins that are active intracellularly in order to enhance the potency of the delivered payload. Expanding the range of genes for consideration may eventually lead to the development of a therapy that is therapeutically promising and clinically relevant.
Figure 9.
In Vivo Assessment of Angiogenesis 7 Days after Treatment with Fibrin Matrices Loaded with the HIF-1α Variant Gene, VEGF-A165 Protein, or Nothing
(A) Quantification of CD31-positive and CD31 and smooth muscle actin (SMA) dual-positive vascular structures 7 days after implantation of matrices. (B) Percent of CD31-positive vascular structures, which are also SMA-positive.117 (*p < 0.05 for values different from fibrin [CD31]; **p < 0.05 for values different from fibrin [CD31 and SMA].)
In addition to the target gene itself, other elements in the expression cassette, such as promoters and post-regulatory elements, can also control the transgene expression profile and therapeutic response. The magnitude of transgene expression from various constitutive and inducible promoters can vary widely both in comparison to one another and in different cell lines, suggesting that much attention should be placed in promoter selection.118 Attention to these differences is needed in in vivo scaffold-mediated gene delivery applications as well, as demonstrated by a study that compared the ubiquitin C (UbC) and cytomegalovirus (CMV) promoters in driving transgene expression from a scaffold in vivo.119, 120 In particular, although the CMV promoter is a commonly used constitutive promoter in mammalian gene expression, this study showed that replacing it with a UbC promoter resulted in higher and more sustained local transgene expression from DNA-loaded scaffolds implanted in mouse intraperitoneal fat, and expression in the CMV condition quickly decreased. Although many promoters of viral origin, such as CMV, exhibit strong initial transgene expression, they can also exhibit methylation, resulting in transcriptional silencing over time in mammalian systems.121 Furthermore, the development of hypoxia-inducible promoters to control gene expression in ischemic environments is particularly relevant to tissue regeneration applications.122, 123 Cell- or tissue-specific promoters may also be considered, especially with respect to the types of cells infiltrating the scaffold or being transplanted with the scaffold. Lastly, post-regulatory elements, such as woodchuck hepatitis virus (WHP) posttranscriptional regulatory element (WPRE), can also be added to the expression cassette to enhance mRNA stabilization to result in enhanced and longer expression; however, this effect is strongly dependent on the promoter and cell line used.124, 125 Future studies may be performed to test other plasmid components in order to further enhance transgene expression levels to achieve clinical potential.
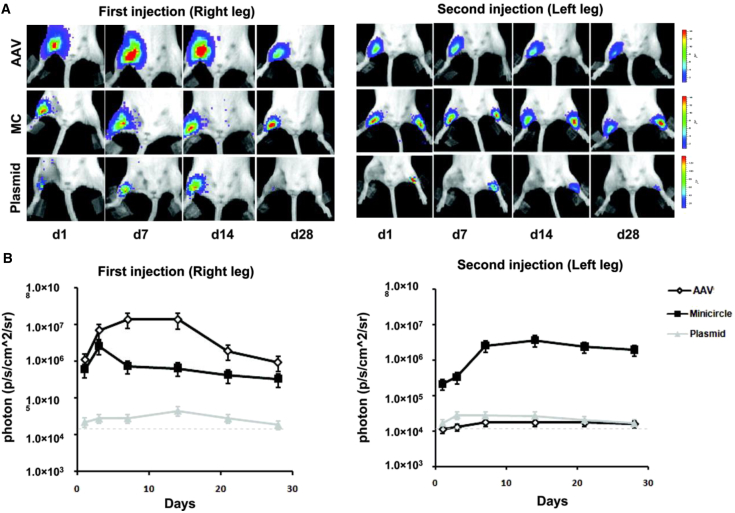
Another source of transcriptional silencing in transgene expression in mammalian systems due to DNA methylation is the presence of elements of bacterial origin, namely CpG motifs in commonly used plasmid vectors. The use of minicircle vectors, which are circular DNA molecules free of any sequences of prokaryotic origin and typically only consist of the expression cassette, instead of conventional plasmids eschews the risk of immunogenic responses to material of bacterial origin, resulting in reduced inflammation and the avoidance of transgene silencing through methylation of bacterial motifs.126, 127, 128, 129 Whereas minicircles are more complex to produce, especially on a larger scale,130 preliminary studies administering minicircle DNA have demonstrated the potential of this application to enhance and prolong transgene expression in vivo.131, 132, 133, 134 In the first study, intramyocardial and intramuscular injection of minicircle encoding for a marker gene resulted in local expression at least two orders of magnitude higher than a plasmid injection. In addition, minicircle administration was also compared to administration of an adeno-associated virus (AAV); whereas a first intramuscular injection of AAV resulted in higher expression than the minicircle injection, a repeat minicircle injection 28 days later resulted in identically strong expression and a repeat AAV injection resulted in no expression, a response attributed to both a cellular and humoral immune response against the virus (Figure 10).131, 134 This result demonstrates the potential of minicircle vectors to drive expression levels that are even higher than viral administrations. Furthermore, minicircle delivery of the HIF-1α gene via injection into the site of a myocardial infarction resulted in significantly greater improvement of ejection fraction compared to plasmid delivery.134 In the second study, implanting poly(lactic-co-glycolic-acid) (PLGA) scaffolds loaded with minicircle delivering the BMP-2 gene enhanced bone repair in a murine calvarial defect model compared to a control condition delivering a marker gene; however, no comparison to an analogous plasmid delivery system was reported.117
Figure 10.
Comparison of Minicircle, Plasmid, and Viral-Mediated Transgene Expression
(A) Representative BLI images of animals injected with AAV, minicircle (MC), and regular plasmid in the right leg (first injection), followed by the left leg 28 days later (second injection). As expected, AAV expression is more robust compared to MC and plasmids initially. However, after repeat injection, AAV expression is not detected in the contralateral leg because of host-mediated humoral immune response. Color scale bar values are expressed as photons per second per square centimeter per steradian (p/s/cm2/sr). (B) Graphical representation of longitudinal bioluminescence imaging (BLI) after first and second injections in all 3 groups. Note that day 28 of second injection in left leg would represent day 56 of first injection in right leg in the same animal.134 Error bars, SEM.
Gene Carrier Considerations and Loading of DNA Therapeutics into Hydrogels
Many DNA delivery systems have been developed for systemic administration, including delivery of naked DNA or packaging the DNA using a gene carrier into a nanostructure. Such carriers may include cationic polymers that interact with the negatively charged DNA to form nanoparticles called polyplexes, lipids to form liposomes or lipoplexes, or niosomes to form nioplexes, all of which generally significantly enhance the uptake of DNA compared to using naked DNA, resulting in greater transgene expression. For a more thorough survey of advances in developing non-viral gene carriers, we direct readers to a recent review.135, 136 However, delivering DNA as a therapeutic from hydrogel scaffolds for regenerative medicine applications concerns the local, not systemic, delivery of this therapeutic, and the design of such strategies present their own challenges and constraints. One key parameter in facilitating cell access to encapsulated DNA in the hydrogel is the cell-mediated degradation of the hydrogel by both infiltrating and transplanted cells, which may be necessary for tissue growth and also serves to release and allow access to the loaded DNA. In fact, transgene expression in cells cultured in a hydrogel scaffold has been shown to be dependent on the rate of hydrogel degradation.137
Challenges of loading DNA into hydrogel scaffolds are largely dependent on the delivery vehicle and physical nature of the final delivery formulation. Below, we describe unique challenges posed by the loading of naked and condensed plasmids into hydrogel scaffolds.
Naked Plasmid
The incorporation of naked plasmid is relatively straightforward, as the plasmid can be directly encapsulated within the hydrogel matrix during synthesis or in situ injection. Some of the earliest scaffolds developed for gene delivery, termed “gene-activated matrices,” combined plasmid solution at high concentrations (about 1 mg DNA per scaffold) with neutralized collagen before freezing and lyophilization to form a DNA-loaded collagen scaffold.138 The plasmid is relatively robust, and these naked encapsulation approaches have resulted in a positive therapeutic effect in vivo, though large quantities of naked DNA are required to observe gene transfer in vivo. However, there are several limitations that have limited the translation potential of naked DNA-loaded scaffolds. First, bacterial unmethylated CpG motifs present in naked plasmids are recognized by Toll-like receptor-9, resulting in a strong immunogenic response.139, 140 Given that large quantities of DNA are needed to achieve high transgene expression, this immune response is problematic. Furthermore, naked plasmids are easily degraded by serum nucleases, limiting the long-term efficacy of the delivered plasmid.141 Lastly, naked plasmids do not result in visible transfection in vitro, leaving all testing to be done in vivo.
Condensed Plasmid Using Cationic Polymer- and Lipid-Based Gene Carriers
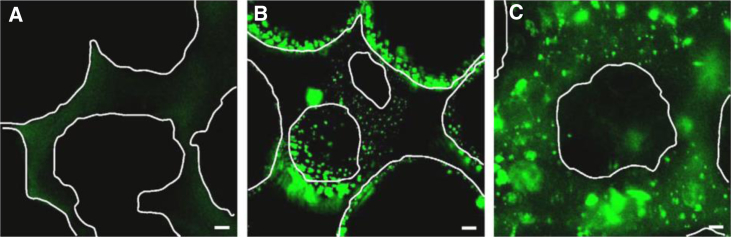
In an effort to overcome the limitations associated with naked DNA, plasmids are commonly condensed with gene carriers into nanoparticles that result in enhanced transgene expression due to facilitated transport, protect the DNA from serum nucleases,141 and can transfect cells both in vitro and in vivo. However, the materials used to condense DNA are typically highly positively charged, resulting in nanosized aggregates that are prone to aggregation, especially at the concentrations required for incorporation into a hydrogel. DNA can be complexed with cationic polymers to form polyplexes or lipid-based polymers to form lipoplexes or nioplexes. There are two general methods to incorporate condensed DNA into hydrogel scaffolds: surface coating and encapsulation (Figure 11).
Figure 11.
Loading of Porous Scaffolds with Polyplexes by Surface Coating or Encapsulation
Fluorescent microscopy images of porous fibrin scaffolds without polyplexes (A), scaffolds surface coated with polyplexes (B), and scaffolds encapsulated with polyplexes (C). White lines indicate pore boundaries.142 Scale bars indicate 20 μm.
Surface coating of condensed DNA relies on electrostatic interactions between the polyplexes and the scaffold material. Such methods often involve incubating a porous scaffold in a solution rich in condensed DNA before washing the scaffold to remove unbound particles142, 143, 144, 145 or hydrating a lyophilized hydrogel in a condensed DNA solution.146, 147, 148, 149 Via the first method, Saul et al.138 reported a maximal loading capacity of approximately 44 μg complexed DNA per 8-mm diameter by 2-mm thickness porous fibrin gel.142 The load amount varies with the DNA:PEI ratio used to form the polyplexes, which controls the surface charge of the polyplexes. In addition, this loading capacity is double that achieved through surface coating with a non-complexed plasmid solution, suggesting the important role of charge in these interactions.142 In a separate study, collagen scaffolds loaded with surface-coated polyplexes encoding for platelet-derived growth factor (PDGF)-B were implanted in a rat calvarial defect model; this implantation resulted in significantly higher bone formation than empty defects and control scaffolds without polyplexes.132, 133
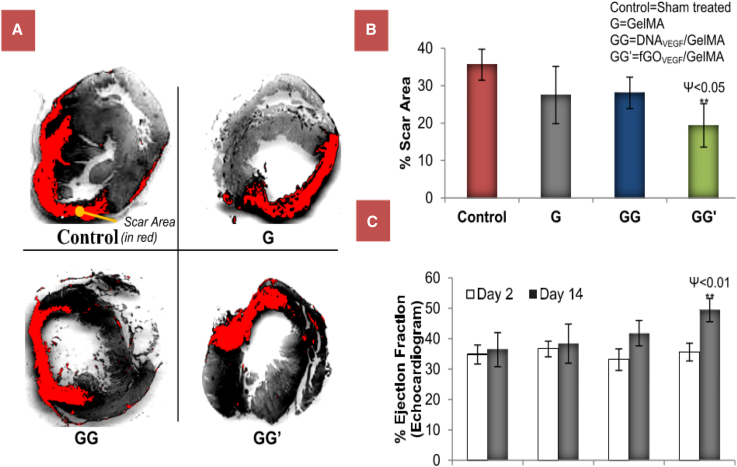
Condensed DNA can also be encapsulated within hydrogels by mixing the condensed DNA into the gel precursor solution before crosslinking. The Shea lab has encapsulated lipoplexes in PEG and fibrin hydrogels for in vitro cell culture137, 150 and coated PLGA scaffolds with lipoplexes for in vivo delivery to a spinal cord injury site.151 More recently, nioplexes, which are similar vesicular particles formed from an aminolipid and a non-ionic surfactant complexed with a nucleic acid, have been encapsulated in methylcellulose and carrageenan hydrogels, demonstrating 80% release of nioplexes over 30 hr.152 Released nioplexes were able to transfect plated HeLa cells to induce gene silencing upon delivery of anti-sense oligodeoxynucleotides and transgene expression upon delivery of pGFP plasmid. Another recent study used EDC/n-hydroxysuccinimide (NHS) coupling to functionalize low-molecular-weight PEI to graphene oxide nanosheets, which were then complexed with DNA encoding for VEGF to form nanoparticles with DNA and encapsulated in a methacrylated gelatin hydrogel. This treatment improved cardiac outcomes, such as a significant decrease in scar area and an increase in ejection fraction, after implanting in a myocardial infarction model compared to a control hydrogel containing only DNA (Figure 12).153
Figure 12.
In Vivo Assessment of Scar Area and Cardiac Function as Outcomes of Infarcted Hearts Treated with Hydrogels Loaded with Graphene Oxide-PEI-DNA (GG’) Compared to Hydrogels Loaded with Naked DNA Only (GG) and Gel Only (G)
(A) Images of left ventricle myocardial sections stained with Sirius red to show cardiac fibrosis. (B) Determination of scar area of the left ventricle. (C) Echocardiographic assessment of cardiac function through monitoring heart ejection fraction.153 Error bars, SD. (**p < 0.01 for values different from control.)
However, encapsulation poses an obstacle due to the tendency of polyplexes, lipoplexes, and nioplexes to aggregate at higher concentrations as they are incorporated into hydrogels; as a result, many studies have only been able to load lower concentrations of condensed DNA.117, 154, 155, 156, 157 PEGylating the cationic polymer to reduce surface zeta potential of polyplexes has been demonstrated as one way to decrease aggregation of polyplexes in hyaluronic acid hydrogels, though at the expense of gene transfer efficiency.144 The Segura lab has also previously developed an approach for concentrated loading of polyplexes called caged nanoparticle encapsulation (CnE), in which polyplexes are formed at low concentrations but lyophilized in the presence of agarose and sucrose to mitigate these charge-based interactions and to preserve particle integrity during the lyophilization before resuspending in low volumes of buffer.156, 158 This technique has been used to incorporate polyplexes into porous hyaluronic acid hydrogels produced using a sphere templating method and tested in a mouse wound healing model for in vivo gene transfer. However, although expression of a marker gene was observed, there was no significant difference in angiogenic outcome as measured by density and thickness of vessels within the hydrogel with the delivery of the VEGF gene as compared to a non-therapeutic gene, suggesting still insufficient levels of local transgene expression.75, 76 Similarly, trehalose, another saccharide, can also be used in the lyophilization process to preserve the stability and activity of polyplexes.159
Furthermore, condensed DNA encapsulated within hydrogels primarily relies on the gradual degradation of the hydrogel to enable DNA release and exposure to cells, resulting in transgene expression that can be orders of magnitude lower than when compared to surface-coated methods due to lower immediate availability of DNA to cells. This has been observed in two studies that loaded polyplexes via surface-coating and encapsulation methods into porous fibrin and hyaluronic acid scaffolds.142, 144 The transgene expression from the encapsulation method can be improved by tuning the hydrogel formulation to be more prone to cell-mediated degradation. However, using these two methods to load DNA simultaneously may result in the delivery and expression of transgenes following two different profiles, which may be advantageous in circumstances when different expression profiles are needed.
Material Considerations in Incorporating Genes into Cell-Laden Hydrogels for Delivery
Cell interactions with the composition and mechanical properties of the matrix substrate are critical design parameters for regenerative medicine and have been shown to modulate gene transfer, presenting opportunities to design scaffolds that enhance transfection and regeneration. Many of the studies described in this section have reported effects in one or a few cell lines, but such effects may still be cell line dependent. Also, the ideas presented in this section may be beneficial in enhancing gene transfer from hydrogel scaffolds both in co-delivered cells in the scaffold as well as infiltrating cells.
Substrate stiffness contributes to modulating proliferation, migration, and differentiation of cells yet also plays an important role in modulating gene transfer, with transgene expression increasing as a function of substrate stiffness in 2D cell culture.160 However, in 3D culture of encapsulated mouse MSCs and polyplexes in hyaluronic acid hydrogels, the inverse result was observed, with transgene expression increasing with decreasing hydrogel stiffness.161 This difference in outcomes is likely due to the dependence of cell proliferation and migration as well as polyplex release on the degradability of the hydrogel. This finding suggests that the efforts in designing biomaterials for gene delivery purposes should also take stiffness into consideration. In addition, surface charge and chemistry influence transfection efficiency, with murine NIH/3T3 fibroblasts cultured on charged hydrophilic surfaces with carboxyl groups exhibiting increased transfection and cells on uncharged methyl-coated surfaces showing decreased transfection.140 Lastly, topography was also found to influence lipoplex-mediated transfection efficiency, with human MSCs cultured on nanopillar-coated surfaces observing highest level of transfection compared to blank and micropillar-coated surfaces.162
Matrix composition can directly determine the efficiency of gene transfer through pathways related to integrin-mediated cell adhesion. For cells seeded in two dimensions, increasing concentrations of the RGD cell adhesion peptide leads to increases in transgene transfer and expression, and an increase in the physical spacing of the ligand on a substrate resulted in decreased transgene expression.163 However, in cells cultured in three dimensions in a nonporous hyaluronic acid hydrogel, an intermediate RGD concentration of 100 μM and clustering ratio of 0.4 mmol RGD/mmol HA resulted in the highest levels of transfection.161 However, these effects on both cell behavior and transfection efficiency are specific to cell type and require optimization for the transplanted cell type or for the expected range of cell types of infiltrating cells. Different ECM components as substrates also have varying effects on gene transfer. For example, in mouse MSCs administered a bolus polyplex transfection, collagen-I-coated surfaces inhibit gene transfer, although fibronectin-coated surfaces enhance gene transfer, and different ECM substrates trigger different internalization pathways by which polyplexes are trafficked into the cell.164 A separate study showed that mouse fibroblasts exhibited increased gene transfer on surfaces coated with fibronectin over collagen in surface-mediated transfection in which polyplexes were coated on the substrate, but the inverse effect was found upon bolus polyplex administration.144 Cell culture on surfaces coated with the α5β1 integrin-binding domain of fibronectin resulted in significantly enhanced expression due to polyplex-mediated transfection compared to cell culture on surfaces coated with a polymer of similar charge but lacking in cell-binding domains, suggesting that the bioactive integrin-binding function of ECM components are at least partially responsible for such effects.165
A better understanding of how cell-hydrogel interactions affect gene transfer may lend insight on how to design more effective gene delivery methods. A study has elucidated the mechanisms by which cytoskeletal dynamics and RhoGTPases control gene transfer and trafficking of polyplexes; introducing bioactive signals to manipulate these pathways may be a method of enhancing gene transfer.166 For example, in a separate study, the inhibition of polo-like kinase-1 (PLK1) was identified as a means of enhancing gene transfer.167 Microarray analysis has also been used to reveal genes that are upregulated in transfected cells as compared to non-transfected cells using both polyplexes and lipoplexes; notable targets include genes involved in integrin-mediated signaling, cytoskeletal mechanics, and membrane trafficking.168, 169, 170 However, it is important to note that different mechanisms govern gene transfer in 2D culture versus in 3D hydrogel-supported cell culture, suggesting the importance of more thorough studies in studying how a particular scaffold design influences gene transfer through cell-matrix interactions.171 Also, gene transfer may occur via different mechanisms, depending on the cell type analyzed.
Integrating genes into cell-laden hydrogels to drive differentiation is another promising strategy for enhancing tissue regeneration. Research focused on integration of cell delivery and gene delivery using hydrogels may assist in directing the maturation of those cells if the DNA encodes for a factor that promotes a certain cell lineage.172 In the field of bone tissue engineering, transforming growth factor-beta 3 and bone morphogenetic protein 2 (BMP-2) genes were complexed with nanohydroxyapatite and, along with MSCs, encapsulated in alginate hydrogels. These dual-delivery hydrogels were shown to direct the differentiation of the stem cells down the chondrogenic lineage to produce cartilage.173 Wegman et al.158 investigated this combinatorial approach by encapsulating the BMP-2 gene in alginate hydrogels along with goat multipotent stromal cells. After implantation in a goat bone model, they demonstrated enhanced osteogenic differentiation and bone formation.174 A challenge with the dual delivery of plasmid DNA and cells includes potential integration of genes into the host genome.175 The Alsberg lab has demonstrated the promise of a controlled, sustained delivery of small interfering RNA (siRNA) or microRNA (miRNA) with encapsulated MSCs that downregulate gene expression as an effective alternative tool to drive osteogenesis.50 In addition, the hydrogel’s tunable design provides controllable swelling and degradation properties that can be used for prolonged delivery of gene therapies in order to extend the regulation of the transplanted cell behavior.
Conclusions
With recent advances in design, hydrogel properties, such as porosity, cell-mediated degradability, and tethered bioactive cues, not only provide structural support for infiltrating and delivering cells but also influence and direct cell-cell interactions, proliferation, migration, and differentiation. A hydrogel delivery system no longer represents only a mere static structural scaffold for cells but rather a dynamic and versatile environment. Furthermore, therapeutics, such as genes, can be loaded into hydrogels to induce sustained transgene expression of therapeutic genes in infiltrating and transplanted cells, and hydrogel properties can be modulated to tune the extent of this transgene expression. However, several challenges remain in controlling the dynamics of the hydrogel in the complexity of the tissue environment in healthy and diseased states. Currently, no universal material fulfills all the mechanical needs to improve cell survival and functionality during the different phases of transplantation and tissue regeneration. Although some material mechanical properties, such as stiffness, may be optimal for enhanced long-term retention and differentiation, these same mechanical properties may inhibit the progression of earlier regenerative processes. Regulating the cellular processes and guiding the development of new tissue growth in parallel with the dynamic changes of the tissue environment would be pivotal in determining the outcome of the regenerative therapy. A number of hydrogel designs are being investigated to achieve this control as well, such as self-assembling peptides and peptide amphiphiles that provide for controllable gelation, degradation, and presentation of cell adhesion motifs.162, 176
A promising future research direction is the development of layered biomaterials that can recapitulate the zonal organization of native tissue in order to fulfill multiple mechanical requirements throughout the multiple stages of transplantation. A layered hydrogel consisting of different ECM proteins as well as mechanical properties could offer better delivery properties to direct cell fate and regulate processes such as wound healing and angiogenesis.163 MSCs and chondrocytes encapsulated in multi-layered scaffolds with spatially varying matrix compositions and mechanical cues have demonstrated promising results in zonal-specific differentiation.177, 178 Another useful functionality that can be incorporated is the ability to increase or decrease hydrogel stiffness after initial crosslinking and implantation. This has been achieved through stimulus-triggered secondary chemistries between functional groups or by incorporating a secondary reaction mechanism that occurs at a much larger timescale than the first primary crosslinking mechanism.179, 180, 181, 182, 183 The stiffness of the hydrogel can therefore be made time dependent and can be tuned to the different stages of the regenerative process.
Recent efforts to study the host response to implanted biomaterials can use these insights to design materials that can proactively direct the immune response upon implantation to be favorable to the regenerative process and the survival and functionality of any co-transplanted cells in the material. Examples include the tuning of material properties, which can increase the presence of pro-remodeling versus pro-inflammatory phenotypes of macrophages in implant site and can also control transplanted stem or progenitor cell behavior and differentiation.169 The delivery of genes encoding for anti-inflammatory cytokines from scaffolds represents another strategy to promote tissue repair by decreasing expression of pro-inflammatory genes and enhancing expression of pro-regenerative genes.170 Finally, with emerging technologies, such as CRISPR, transplanted cells can undergo genetic engineering to regulate the immune response as a means to prevent or avoid rejection.171
In summary, hydrogels offer great promise in designing suitable environments that provide not only structural support for cells and new tissue growth but also control over gene delivery to promote differentiation and improve therapeutic outcomes. As these hydrogel design considerations continue to be investigated, this system could provide a promising platform for dual delivery of genes and cells for a wide range of applications in tissue engineering.
Conflicts of Interest
The authors confirm that there are no known conflicts of interest associated with this publication.
Acknowledgments
This work is supported by research grants from the NIH (R01HL110592, T32GM067555, and T32GM008353).
Contributor Information
Tatiana Segura, Email: tatiana.segura@duke.edu.
Lonnie D. Shea, Email: ldshea@umich.edu.
References
- 1.Slaughter B.V., Khurshid S.S., Fisher O.Z., Khademhosseini A., Peppas N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Hollister S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 4.Drury J.L., Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 5.Jhon M.S., Andrade J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973;7:509–522. doi: 10.1002/jbm.820070604. [DOI] [PubMed] [Google Scholar]
- 6.Molinaro G., Leroux J.-C., Damas J., Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717–2722. doi: 10.1016/s0142-9612(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi T., Yamamuro T., Oka M., Kumar P., Kotoura Y., Hyon S., Ikada Y. Poly(vinyl alcohol) hydrogel as an artificial articular cartilage: evaluation of biocompatibility. J. Appl. Biomater. 1991;2:101–107. doi: 10.1002/jab.770020205. [DOI] [PubMed] [Google Scholar]
- 8.de Vos P., Lazarjani H.A., Poncelet D., Faas M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014;67-68:15–34. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 10.Ahearne M., Wilson S.L., Liu K.K., Rauz S., El Haj A.J., Yang Y. Influence of cell and collagen concentration on the cell-matrix mechanical relationship in a corneal stroma wound healing model. Exp. Eye Res. 2010;91:584–591. doi: 10.1016/j.exer.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Bott K., Upton Z., Schrobback K., Ehrbar M., Hubbell J.A., Lutolf M.P., Rizzi S.C. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–8464. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Nöth U., Schupp K., Heymer A., Kall S., Jakob F., Schütze N., Baumann B., Barthel T., Eulert J., Hendrich C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy. 2005;7:447–455. doi: 10.1080/14653240500319093. [DOI] [PubMed] [Google Scholar]
- 13.Ahearne M., Yang Y., El Haj A.J., Then K.Y., Liu K.-K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface. 2005;2:455–463. doi: 10.1098/rsif.2005.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bader R.A. Synthesis and viscoelastic characterization of novel hydrogels generated via photopolymerization of 1,2-epoxy-5-hexene modified poly(vinyl alcohol) for use in tissue replacement. Acta Biomater. 2008;4:967–975. doi: 10.1016/j.actbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Song X., Zhu C., Fan D., Mi Y., Li X., Fu R.Z., Duan Z., Wang Y., Feng R.R. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers. 2017;9:638. doi: 10.3390/polym9120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., Li S., Deng Y., He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhang Z., Soontornworajit B., Zhou J., Wang Y. Cell adhesion on an artificial extracellular matrix using aptamer-functionalized PEG hydrogels. Biomaterials. 2012;33:1353–1362. doi: 10.1016/j.biomaterials.2011.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Rape A.D., Zibinsky M., Murthy N., Kumar S. A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat. Commun. 2015;6:8129. doi: 10.1038/ncomms9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohtaram N.K., Montgomery A., Willerth S.M. Biomaterial-based drug delivery systems for the controlled release of neurotrophic factors. Biomed. Mater. 2013;8:022001. doi: 10.1088/1748-6041/8/2/022001. [DOI] [PubMed] [Google Scholar]
- 20.Burdick J.A., Mason M.N., Hinman A.D., Thorne K., Anseth K.S. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J. Control. Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 21.Anchordoquy T.J., Simberg D. Watching the gorilla and questioning delivery dogma. J. Control. Release. 2017;262:87–90. doi: 10.1016/j.jconrel.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang J.-H., Schaffer D.V., Shea L.D. Engineering biomaterial systems to enhance viral vector gene delivery. Mol. Ther. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W.W., Wang Z., Hollister S.J., Krebsbach P.H. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 24.Silva E.A., Kim E.-S., Kong H.J., Mooney D.J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl. Acad. Sci. USA. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Shansky J., Borselli C., Mooney D., Vandenburgh H. Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng. Part A. 2012;18:2000–2007. doi: 10.1089/ten.tea.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayoussef Z., Dixon J.E., Stolnik S., Shakesheff K.M. Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J. Tissue Eng. Regen. Med. 2012;6:e61–e73. doi: 10.1002/term.482. [DOI] [PubMed] [Google Scholar]
- 27.Bidarra S.J., Barrias C.C., Granja P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10:1646–1662. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 28.González-Díaz E.C., Varghese S. Hydrogels as extracellular matrix analogs. Gels. 2016;2:20. doi: 10.3390/gels2030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt N.C., Grover L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010;32:733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 30.Nicodemus G.D., Bryant S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikos A.G., Papadaki M.G., Kouvroukoglou S., Ishaug S.L., Thomson R.C. Mini-review: Islet transplantation to create a bioartificial pancreas. Biotechnol. Bioeng. 1994;43:673–677. doi: 10.1002/bit.260430717. [DOI] [PubMed] [Google Scholar]
- 32.Li R.H. Materials for immunoisolated cell transplantation. Adv. Drug Deliv. Rev. 1998;33:87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 33.Wilson J.T., Chaikof E.L. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv. Drug Deliv. Rev. 2008;60:124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber L.M., Hayda K.N., Anseth K.S. Cell-matrix interactions improve β-cell survival and insulin secretion in three-dimensional culture. Tissue Eng. Part A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber L.M., Anseth K.S. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C.-C., Anseth K.S. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic β-cells. Biomacromolecules. 2009;10:2460–2467. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuch B.E., Keogh G.W., Williams L.J., Wu W., Foster J.L., Vaithilingam V., Philips R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veiseh O., Doloff J.C., Ma M., Vegas A.J., Tam H.H., Bader A.R., Li J., Langan E., Wyckoff J., Loo W.S. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattás-Asfura K.M., Stabler C.L. Chemoselective cross-linking and functionalization of alginate via Staudinger ligation. Biomacromolecules. 2009;10:3122–3129. doi: 10.1021/bm900789a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passemard S., Szabó L., Noverraz F., Montanari E., Gonelle-Gispert C., Bühler L.H., Wandrey C., Gerber-Lemaire S. Synthesis strategies to extend the variety of alginate-based hybrid hydrogels for cell microencapsulation. Biomacromolecules. 2017;18:2747–2755. doi: 10.1021/acs.biomac.7b00665. [DOI] [PubMed] [Google Scholar]
- 41.Vegas A.J., Veiseh O., Doloff J.C., Ma M., Tam H.H., Bratlie K., Li J., Bader A.R., Langan E., Olejnik K. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang P.T.J., Shah D.K., Garcia J.A., Bae C.Y., Lim D.J., Huiszoon R.C., Alexander G.C., Jun H.W. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016;3:28. doi: 10.1186/s40580-016-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vegas A.J., Veiseh O., Gürtler M., Millman J.R., Pagliuca F.W., Bader A.R., Doloff J.C., Li J., Chen M., Olejnik K. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boettler T., Schneider D., Cheng Y., Kadoya K., Brandon E.P., Martinson L., von Herrath M. Pancreatic tissue transplanted in TheraCyte encapsulation devices is protected and prevents hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transplant. 2016;25:609–614. doi: 10.3727/096368915X688939. [DOI] [PubMed] [Google Scholar]
- 45.ViaCyte. (2015). A safety, tolerability, and efficacy study of VC-01™ combination product in subjects with type I diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT02239354.
- 46.Schweicher J., Nyitray C., Desai T.A. Membranes to achieve immunoprotection of transplanted islets. Front. Biosci. 2014;19:49–76. doi: 10.2741/4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wee S., Gombotz W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 48.Orive G., Tam S.K., Pedraz J.L., Hallé J.-P. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691–3700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 49.Günther M.I., Weidner N., Müller R., Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015;27:140–150. doi: 10.1016/j.actbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Qiao S., Liu Y., Han F., Guo M., Hou X., Ye K., Deng S., Shen Y., Zhao Y., Wei H. An intelligent neural stem cell delivery system for neurodegenerative diseases treatment. Adv. Healthc. Mater. 2018;7:e1800080. doi: 10.1002/adhm.201800080. [DOI] [PubMed] [Google Scholar]
- 51.Desai T., Shea L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 2017;16:338–350. doi: 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rokstad A.M., Brekke O.L., Steinkjer B., Ryan L., Kolláriková G., Strand B.L., Skjåk-Bræk G., Lacík I., Espevik T., Mollnes T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011;7:2566–2578. doi: 10.1016/j.actbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Rokstad A.M., Brekke O.L., Steinkjer B., Ryan L., Kolláriková G., Strand B.L., Skjåk-Bræk G., Lambris J.D., Lacík I., Mollnes T.E., Espevik T. The induction of cytokines by polycation containing microspheres by a complement dependent mechanism. Biomaterials. 2013;34:621–630. doi: 10.1016/j.biomaterials.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 54.El-Sherbiny I.M., Yacoub M.H. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob. Cardiol. Sci. Pract. 2013;2013:316–342. doi: 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn G., Park J.H., Kang T., Lee J.W., Kang H.W., Cho D.W. Effect of pore architecture on oxygen diffusion in 3D scaffolds for tissue engineering. J. Biomech. Eng. 2010;132:104506. doi: 10.1115/1.4002429. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs-Tulleneers-Thevissen D., Chintinne M., Ling Z., Gillard P., Schoonjans L., Delvaux G., Strand B.L., Gorus F., Keymeulen B., Pipeleers D., Beta Cell Therapy Consortium EU-FP7 Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56:1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 57.Malda J., Klein T.J., Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13:2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 58.Wang C., Varshney R.R., Wang D.-A. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Deliv. Rev. 2010;62:699–710. doi: 10.1016/j.addr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Manzoli V., Villa C., Bayer A.L., Morales L.C., Molano R.D., Torrente Y., Ricordi C., Hubbell J.A., Tomei A.A. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transplant. 2018;18:590–603. doi: 10.1111/ajt.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shikanov A., Smith R.M., Xu M., Woodruff T.K., Shea L.D. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011;32:2524–2531. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khansari M.M., Sorokina L.V., Mukherjee P., Mukhtar F., Shirdar M.R., Shahidi M., Shokuhfar T. Classification of hydrogels based on their source: a review and application in stem cell regulation. JOM. 2017;69:1340–1347. [Google Scholar]
- 62.David A., Day J., Shikanov A. Immunoisolation to prevent tissue graft rejection: current knowledge and future use. Exp. Biol. Med. (Maywood) 2016;241:955–961. doi: 10.1177/1535370216647129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rios P.D., Zhang X., Luo X., Shea L.D. Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice. Biotechnol. Bioeng. 2016;113:2485–2495. doi: 10.1002/bit.26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lovett M., Lee K., Edwards A., Kaplan D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rouwkema J., de Boer J., Van Blitterswijk C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–2693. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 66.Duan Y., Liu Z., O’Neill J., Wan L.Q., Freytes D.O., Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 68.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 69.Sivashanmugam A., Arun Kumar R., Vishnu Priya M., Nair S.V., Jayakumar R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015;72:543–565. [Google Scholar]
- 70.Naganuma T., Traversa E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials. 2014;35:4441–4453. doi: 10.1016/j.biomaterials.2014.01.074. [DOI] [PubMed] [Google Scholar]
- 71.Sokic S., Christenson M., Larson J., Papavasiliou G. In situ generation of cell-laden porous MMP-sensitive PEGDA hydrogels by gelatin leaching. Macromol. Biosci. 2014;14:731–739. doi: 10.1002/mabi.201300406. [DOI] [PubMed] [Google Scholar]
- 72.Galperin A., Long T.J., Ratner B.D.A. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules. 2010;11:2583–2592. doi: 10.1021/bm100521x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokatlian T., Cam C., Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv. Healthc. Mater. 2015;4:1084–1091. doi: 10.1002/adhm.201400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mealy J.E., Chung J.J., Jeong H.H., Issadore D., Lee D., Atluri P., Burdick J.A. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 2018;30:e1705912. doi: 10.1002/adma.201705912. [DOI] [PubMed] [Google Scholar]
- 75.Wang L., Lu S., Lam J., Kasper F.K., Mikos A.G. Fabrication of cell-laden macroporous biodegradable hydrogels with tunable porosities and pore sizes. Tissue Eng. Part C Methods. 2015;21:263–273. doi: 10.1089/ten.tec.2014.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han L.-H., Conrad B., Chung M.T., Deveza L., Jiang X., Wang A., Butte M.J., Longaker M.T., Wan D., Yang F. Winner of the Young Investigator Award of the Society for Biomaterials at the 10th World Biomaterials Congress, May 17-22, 2016, Montreal QC, Canada: Microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J. Biomed. Mater. Res. A. 2016;104:1321–1331. doi: 10.1002/jbm.a.35715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iyer P., Walker K.J., Madihally S.V. Increased matrix synthesis by fibroblasts with decreased proliferation on synthetic chitosan-gelatin porous structures. Biotechnol. Bioeng. 2012;109:1314–1325. doi: 10.1002/bit.24396. [DOI] [PubMed] [Google Scholar]
- 78.Huang B.J., Hu J.C., Athanasiou K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Y.H.D., Suzer F., Thermann H. Autologous matrix-induced chondrogenesis in the knee: a review. Cartilage. 2014;5:145–153. doi: 10.1177/1947603514529445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiu Y.C., Cheng M.H., Engel H., Kao S.W., Larson J.C., Gupta S., Brey E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32:6045–6051. doi: 10.1016/j.biomaterials.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 81.Chiu Y.C., Larson J.C., Isom A., Jr., Brey E.M. Generation of porous poly(ethylene glycol) hydrogels by salt leaching. Tissue Eng. Part C Methods. 2010;16:905–912. doi: 10.1089/ten.TEC.2009.0646. [DOI] [PubMed] [Google Scholar]
- 82.Crawford D.C., DeBerardino T.M., Williams R.J., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J. Bone Joint Surg. Am. 2012;94:979–989. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- 83.Histogenics. (2017). Histogenics completes enrollment for phase 3 clinical trial of Neocart® to treat knee cartilage damage. http://ir.histogenics.com/phoenix.zhtml?c=252477&p=irol-newsArticle&ID=2283474.
- 84.Parikh S.A., Edelman E.R. Endothelial cell delivery for cardiovascular therapy. Adv. Drug Deliv. Rev. 2000;42:139–161. doi: 10.1016/s0169-409x(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 85.Yow K.-H., Ingram J., Korossis S.A., Ingham E., Homer-Vanniasinkam S. Tissue engineering of vascular conduits. Br. J. Surg. 2006;93:652–661. doi: 10.1002/bjs.5343. [DOI] [PubMed] [Google Scholar]
- 86.Villalona G.A., Udelsman B., Duncan D.R., McGillicuddy E., Sawh-Martinez R.F., Hibino N., Painter C., Mirensky T., Erickson B., Shinoka T., Breuer C.K. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part B Rev. 2010;16:341–350. doi: 10.1089/ten.teb.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tokatlian T., Cam C., Siegman S.N., Lei Y., Segura T. Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs. Acta Biomater. 2012;8:3921–3931. doi: 10.1016/j.actbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sideris E., Griffin D.R., Ding Y., Li S., Weaver W.M., Di Carlo D., Hsiai T., Segura T. Particle hydrogels based on hyaluronic acid building blocks. ACS Biomater. Sci. Eng. 2016;2:2034–2041. doi: 10.1021/acsbiomaterials.6b00444. [DOI] [PubMed] [Google Scholar]
- 89.Sridhar B.V., Brock J.L., Silver J.S., Leight J.L., Randolph M.A., Anseth K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthc. Mater. 2015;4:702–713. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin D.R., Weaver W.M., Scumpia P.O., Di Carlo D., Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nih L.R., Sideris E., Carmichael S.T., Segura T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater. 2017;29:1606471. doi: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Athanasiou K.A., Darling E.M., Hu J.C. Articular cartilage tissue engineering. Synth. Lect. Tissue Eng. 2009;1:1–182. [Google Scholar]
- 93.Kondiah P.J., Choonara Y.E., Kondiah P.P., Marimuthu T., Kumar P., du Toit L.C., Pillay V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules. 2016;21:1580. doi: 10.3390/molecules21111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lima A.C., Batista P., Valente T.A., Silva A.S., Correia I.J., Mano J.F. Novel methodology based on biomimetic superhydrophobic substrates to immobilize cells and proteins in hydrogel spheres for applications in bone regeneration. Tissue Eng. Part A. 2013;19:1175–1187. doi: 10.1089/ten.TEA.2012.0249. [DOI] [PubMed] [Google Scholar]
- 97.Weaver J.D., Headen D.M., Aquart J., Johnson C.T., Shea L.D., Shirwan H., García A.J. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017;3:e1700184. doi: 10.1126/sciadv.1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu J., Marchant R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burdick J.A., Chung C., Jia X., Randolph M.A., Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lei Y., Gojgini S., Lam J., Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meilander-Lin N.J., Cheung P.J., Wilson D.L., Bellamkonda R.V. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005;11:546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 103.Trentin D., Hall H., Wechsler S., Hubbell J.A. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keeney M., Chung M.T., Zielins E.R., Paik K.J., McArdle A., Morrison S.D., Ransom R.C., Barbhaiya N., Atashroo D., Jacobson G. Scaffold-mediated BMP-2 minicircle DNA delivery accelerated bone repair in a mouse critical-size calvarial defect model. J. Biomed. Mater. Res. A. 2016;104:2099–2107. doi: 10.1002/jbm.a.35735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elangovan S., D’Mello S.R., Hong L., Ross R.D., Allamargot C., Dawson D.V., Stanford C.M., Johnson G.K., Sumner D.R., Salem A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials. 2014;35:737–747. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin J.Y., Zhang L., Clift K.L., Hulur I., Xiang A.P., Ren B.Z., Lahn B.T. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS ONE. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brooks A.R., Harkins R.N., Wang P., Qian H.S., Liu P., Rubanyi G.M. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 108.Gutowska A., Jeong B., Jasionowski M. Injectable gels for tissue engineering. Anat. Rec. 2001;263:342–349. doi: 10.1002/ar.1115. [DOI] [PubMed] [Google Scholar]
- 109.Jones D.S., Andrews G.P., Caldwell D.L., Lorimer C., Gorman S.P., McCoy C.P. Novel semi-interpenetrating hydrogel networks with enhanced mechanical properties and thermoresponsive engineered drug delivery, designed as bioactive endotracheal tube biomaterials. Eur. J. Pharm. Biopharm. 2012;82:563–571. doi: 10.1016/j.ejpb.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 110.Wintgens V., Amiel C. Physical gelation of amphiphilic poly(N-isopropylacrylamide): influence of the hydrophobic groups. Macromol. Chem. Phys. 2008;209:1553–1563. [Google Scholar]
- 111.Li X., Zhou J., Liu Z., Chen J., Lü S., Sun H., Li J., Lin Q., Yang B., Duan C. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials. 2014;35:5679–5688. doi: 10.1016/j.biomaterials.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 112.Gaffey A.C., Chen M.H., Venkataraman C.M., Trubelja A., Rodell C.B., Dinh P.V., Hung G., MacArthur J.W., Soopan R.V., Burdick J.A., Atluri P. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2015;150:1268–1276. doi: 10.1016/j.jtcvs.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Y., Fu M., Li Z., Fan Z., Li X., Liu Y., Anderson P.M., Xie X., Liu Z., Guan J. A prosurvival and proangiogenic stem cell delivery system to promote ischemic limb regeneration. Acta Biomater. 2016;31:99–113. doi: 10.1016/j.actbio.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hackett P.B., Largaespada D.A., Switzer K.C., Cooper L.J.N. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl. Res. 2013;161:265–283. doi: 10.1016/j.trsl.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shepard J.A., Huang A., Shikanov A., Shea L.D. Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three-dimensionally within hydrogels. J. Control. Release. 2010;146:128–135. doi: 10.1016/j.jconrel.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tokatlian T., Cam C., Segura T. Non-viral DNA delivery from porous hyaluronic acid hydrogels in mice. Biomaterials. 2014;35:825–835. doi: 10.1016/j.biomaterials.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bauer S., Kirschning C.J., Häcker H., Redecke V., Hausmann S., Akira S., Wagner H., Lipford G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mao Z., Shi H., Guo R., Ma L., Gao C., Han C., Shen J. Enhanced angiogenesis of porous collagen scaffolds by incorporation of TMC/DNA complexes encoding vascular endothelial growth factor. Acta Biomater. 2009;5:2983–2994. doi: 10.1016/j.actbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 119.Avilés M.O., Lin C.H., Zelivyanskaya M., Graham J.G., Boehler R.M., Messersmith P.B., Shea L.D. The contribution of plasmid design and release to in vivo gene expression following delivery from cationic polymer modified scaffolds. Biomaterials. 2010;31:1140–1147. doi: 10.1016/j.biomaterials.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.des Rieux A., Shikanov A., Shea L.D. Fibrin hydrogels for non-viral vector delivery in vitro. J. Control. Release. 2009;136:148–154. doi: 10.1016/j.jconrel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Laporte L., Yan A.L., Shea L.D. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ede C., Chen X., Lin M.-Y., Chen Y.Y. Quantitative analyses of core promoters enable precise engineering of regulated gene expression in mammalian cells. ACS Synth. Biol. 2016;5:395–404. doi: 10.1021/acssynbio.5b00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grijalvo S., Alagia A., Puras G., Zárate J., Mayr J., Pedraz J.L., Eritja R., Díaz D.D. Cationic nioplexes-in-polysaccharide-based hydrogels as versatile biodegradable hybrid materials to deliver nucleic acids. J. Mater. Chem. B. 2017;5:7756–7767. doi: 10.1039/c7tb01691c. [DOI] [PubMed] [Google Scholar]
- 124.Klein R., Ruttkowski B., Knapp E., Salmons B., Günzburg W.H., Hohenadl C. WPRE-mediated enhancement of gene expression is promoter and cell line specific. Gene. 2006;372:153–161. doi: 10.1016/j.gene.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 125.Paul A., Hasan A., Kindi H.A., Gaharwar A.K., Rao V.T., Nikkhah M., Shin S.R., Krafft D., Dokmeci M.R., Shum-Tim D., Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hardee C.L., Arévalo-Soliz L.M., Hornstein B.D., Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes (Basel) 2017;8:65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Z.Y., He C.Y., Meuse L., Kay M.A. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 128.Wieland J.A., Houchin-Ray T.L., Shea L.D. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. J. Control. Release. 2007;120:233–241. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lei Y., Segura T. DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30:254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lei Y., Huang S., Sharif-Kashani P., Chen Y., Kavehpour P., Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010;31:9106–9116. doi: 10.1016/j.biomaterials.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang M., Chen Z., Hu S., Jia F., Li Z., Hoyt G., Robbins R.C., Kay M.A., Wu J.C. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120(11, Suppl):S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonadio J., Smiley E., Patil P., Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 133.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 134.Trentin D., Hubbell J., Hall H. Non-viral gene delivery for local and controlled DNA release. J. Control. Release. 2005;102:263–275. doi: 10.1016/j.jconrel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 135.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 136.Lei Y., Rahim M., Ng Q., Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J. Control. Release. 2011;153:255–261. doi: 10.1016/j.jconrel.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adolph E.J., Nelson C.E., Werfel T.A., Guo R., Davidson J.M., Guelcher S.A., Duvall C.L. Enhanced performance of plasmid DNA polyplexes stabilized by a combination of core hydrophobicity and surface PEGylation. J. Mater. Chem. B. 2014;2:8154–8164. doi: 10.1039/C4TB00352G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saul J.M., Linnes M.P., Ratner B.D., Giachelli C.M., Pun S.H. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28:4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 139.Kong H.J., Liu J., Riddle K., Matsumoto T., Leach K., Mooney D.J. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat. Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 140.Kasputis T., Pannier A.K. The role of surface chemistry-induced cell characteristics on nonviral gene delivery to mouse fibroblasts. J. Biol. Eng. 2012;6:17. doi: 10.1186/1754-1611-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teo B.K.K., Goh S.H., Kustandi T.S., Loh W.W., Low H.Y., Yim E.K. The effect of micro and nanotopography on endocytosis in drug and gene delivery systems. Biomaterials. 2011;32:9866–9875. doi: 10.1016/j.biomaterials.2011.08.088. [DOI] [PubMed] [Google Scholar]
- 142.Kong H.J., Hsiong S., Mooney D.J. Nanoscale cell adhesion ligand presentation regulates nonviral gene delivery and expression. Nano Lett. 2007;7:161–166. doi: 10.1021/nl062485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dhaliwal A., Maldonado M., Han Z., Segura T. Differential uptake of DNA-poly(ethylenimine) polyplexes in cells cultured on collagen and fibronectin surfaces. Acta Biomater. 2010;6:3436–3447. doi: 10.1016/j.actbio.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bengali Z., Rea J.C., Shea L.D. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. J. Gene Med. 2007;9:668–678. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rea J.C., Gibly R.F., Davis N.E., Barron A.E., Shea L.D. Engineering surfaces for substrate-mediated gene delivery using recombinant proteins. Biomacromolecules. 2009;10:2779–2786. doi: 10.1021/bm900628e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chiou H.C., Tangco M.V., Levine S.M., Robertson D., Kormis K., Wu C.H., Wu G.Y. Enhanced resistance to nuclease degradation of nucleic acids complexed to asialoglycoprotein-polylysine carriers. Nucleic Acids Res. 1994;22:5439–5446. doi: 10.1093/nar/22.24.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Siegman S., Truong N.F., Segura T. Encapsulation of PEGylated low-molecular-weight PEI polyplexes in hyaluronic acid hydrogels reduces aggregation. Acta Biomater. 2015;28:45–54. doi: 10.1016/j.actbio.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Truong N.F., Segura T. Sustained transgene expression via hydrogel-mediated gene transfer results from multiple transfection events. ACS Biomater. Sci. Eng. 2018;4:981–987. doi: 10.1021/acsbiomaterials.7b00957. [DOI] [PubMed] [Google Scholar]
- 149.Dhaliwal A., Maldonado M., Lin C., Segura T. Cellular cytoskeleton dynamics modulates non-viral gene delivery through RhoGTPases. PLoS ONE. 2012;7:e35046. doi: 10.1371/journal.pone.0035046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Christensen M.D., Elmer J.J., Eaton S., Gonzalez-Malerva L., LaBaer J., Rege K. Kinome-level screening identifies inhibition of polo-like kinase-1 (PLK1) as a target for enhancing non-viral transgene expression. J. Control. Release. 2015;204:20–29. doi: 10.1016/j.jconrel.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Plautz S.A., Boanca G., Riethoven J.-J.M., Pannier A.K. Microarray analysis of gene expression profiles in cells transfected with nonviral vectors. Mol. Ther. 2011;19:2144–2151. doi: 10.1038/mt.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin T.M., Plautz S.A., Pannier A.K. Network analysis of endogenous gene expression profiles after polyethyleneimine-mediated DNA delivery. J. Gene Med. 2013;15:142–154. doi: 10.1002/jgm.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo R., Xu S., Ma L., Huang A., Gao C. Enhanced angiogenesis of gene-activated dermal equivalent for treatment of full thickness incisional wounds in a porcine model. Biomaterials. 2010;31:7308–7320. doi: 10.1016/j.biomaterials.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 154.Martin T.M., Plautz S.A., Pannier A.K. Temporal endogenous gene expression profiles in response to lipid-mediated transfection. J. Gene Med. 2015;17:14–32. doi: 10.1002/jgm.2821. [DOI] [PubMed] [Google Scholar]
- 155.Dhaliwal A., Oshita V., Segura T. Transfection in the third dimension. Integr. Biol. 2013;5:1206–1216. doi: 10.1039/c3ib40086g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chung H.J., Park T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007;59:249–262. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 157.Gonzalez-Fernandez T., Tierney E.G., Cunniffe G.M., O’Brien F.J., Kelly D.J. Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. Part A. 2016;22:776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 158.Wegman F., Geuze R.E., van der Helm Y.J., Cumhur Öner F., Dhert W.J., Alblas J. Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. J. Tissue Eng. Regen. Med. 2014;8:763–770. doi: 10.1002/term.1571. [DOI] [PubMed] [Google Scholar]
- 159.Mairhofer J., Grabherr R. Rational vector design for efficient non-viral gene delivery: challenges facing the use of plasmid DNA. Mol. Biotechnol. 2008;39:97–104. doi: 10.1007/s12033-008-9046-7. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen M.K., Jeon O., Krebs M.D., Schapira D., Alsberg E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials. 2014;35:6278–6286. doi: 10.1016/j.biomaterials.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gojgini S., Tokatlian T., Segura T. Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels. Mol. Pharm. 2011;8:1582–1591. doi: 10.1021/mp200171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cui H., Webber M.J., Stupp S.I. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee J.-E., Park J.-C., Lee K.H., Oh S.H., Suh H. Laminin modified infection-preventing collagen membrane containing silver sulfadiazine-hyaluronan microparticles. Artif. Organs. 2002;26:521–528. doi: 10.1046/j.1525-1594.2002.06890.x. [DOI] [PubMed] [Google Scholar]
- 164.Nguyen L.H., Kudva A.K., Saxena N.S., Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 165.Kloxin A.M., Kasko A.M., Salinas C.N., Anseth K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kloxin A.M., Tibbitt M.W., Anseth K.S. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat. Protoc. 2010;5:1867–1887. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu H.D., Soranno D.E., Rodell C.B., Kim I.L., Burdick J.A. Secondary photocrosslinking of injectable shear-thinning dock-and-lock hydrogels. Adv. Healthc. Mater. 2013;2:1028–1036. doi: 10.1002/adhm.201200343. [DOI] [PubMed] [Google Scholar]
- 168.Zhang J., Tokatlian T., Zhong J., Ng Q.K., Patterson M., Lowry W.E., Carmichael S.T., Segura T. Physically associated synthetic hydrogels with long-term covalent stabilization for cell culture and stem cell transplantation. Adv. Mater. 2011;23:5098–5103. doi: 10.1002/adma.201103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dziki J.L., Badylak S.F. Immunomodulatory biomaterials. Curr. Opin. Biomed. Eng. 2018;6:51–57. [Google Scholar]
- 170.Park J., Decker J.T., Margul D.J., Smith D.R., Cummings B.J., Anderson A.J., Shea L.D. Local immunomodulation with anti-inflammatory cytokine-encoding lentivirus enhances functional recovery after spinal cord injury. Mol. Ther. 2018;26:1756–1770. doi: 10.1016/j.ymthe.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meissner T., Strominger J., Cowan C. The universal donor stem cell: removing the immune barrier to transplantation using CRISPR/Cas9 (TRAN1P.946) J. Immunol. 2015;194:140.28. [Google Scholar]
- 172.Takehara N., Tsutsumi Y., Tateishi K., Ogata T., Tanaka H., Ueyama T., Takahashi T., Takamatsu T., Fukushima M., Komeda M. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J. Am. Coll. Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 173.Mulyasasmita W., Cai L., Dewi R.E., Jha A., Ullmann S.D., Luong R.H., Huang N.F., Heilshorn S.C. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J. Control. Release. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mayfield A.E., Tilokee E.L., Latham N., McNeill B., Lam B.K., Ruel M., Suuronen E.J., Courtman D.W., Stewart D.J., Davis D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–142. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 176.Moore A.N., Hartgerink J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc. Chem. Res. 2017;50:714–722. doi: 10.1021/acs.accounts.6b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim J., Perez A.S., Claflin J., David A., Zhou H., Shikanov A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. NPJ Regen. Med. 2016;1 doi: 10.1038/npjregenmed.2016.10. npjregenmed201610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huebsch N., Lippens E., Lee K., Mehta M., Koshy S.T., Darnell M.C., Desai R.M., Madl C.M., Xu M., Zhao X. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015;14:1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Camarero-Espinosa S., Rothen-Rutishauser B., Weder C., Foster E.J. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials. 2016;74:42–52. doi: 10.1016/j.biomaterials.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 180.Du C., Narayanan K., Leong M.F., Wan A.C.A. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014;35:6006–6014. doi: 10.1016/j.biomaterials.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 181.Joosten E.A., Veldhuis W.B., Hamers F.P. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J. Neurosci. Res. 2004;77:127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 182.Jha A.K., Tharp K.M., Browne S., Ye J., Stahl A., Yeghiazarians Y., Healy K.E. Matrix metalloproteinase-13 mediated degradation of hyaluronic acid-based matrices orchestrates stem cell engraftment through vascular integration. Biomaterials. 2016;89:136–147. doi: 10.1016/j.biomaterials.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Moshayedi P., Nih L.R., Llorente I.L., Berg A.R., Cinkornpumin J., Lowry W.E., Segura T., Carmichael S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. doi: 10.1016/j.biomaterials.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Living Cell Technologies. (2014). Development to date. Living Cell Technologies. http://www.lctglobal.com/products/diabecell/development-to-date.
- 185.Dosier C.R., Uhrig B.A., Willett N.J., Krishnan L., Li M.T., Stevens H.Y., Schwartz Z., Boyan B.D., Guldberg R.E. Effect of cell origin and timing of delivery for stem cell-based bone tissue engineering using biologically functionalized hydrogels. Tissue Eng. Part A. 2015;21:156–165. doi: 10.1089/ten.tea.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith T.T., Moffett H.F., Stephan S.B., Opel C.F., Dumigan A.G., Jiang X., Pillarisetty V.G., Pillai S.P.S., Wittrup K.D., Stephan M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 2017;127:2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alexaki V.-I., Simantiraki D., Panayiotopoulou M., Rasouli O., Venihaki M., Castana O., Alexakis D., Kampa M., Stathopoulos E.N., Castanas E. Adipose tissue-derived mesenchymal cells support skin reepithelialization through secretion of KGF-1 and PDGF-BB: comparison with dermal fibroblasts. Cell Transplant. 2012;21:2441–2454. doi: 10.3727/096368912X637064. [DOI] [PubMed] [Google Scholar]
- 188.Jang J.-H., Bengali Z., Houchin T.L., Shea L.D. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J. Biomed. Mater. Res. A. 2006;77:50–58. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rose L.C., Kucharski C., Uludağ H. Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model. Biomaterials. 2012;33:3363–3374. doi: 10.1016/j.biomaterials.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 190.Kang H.W., Tabata Y., Ikada Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials. 1999;20:1339–1344. doi: 10.1016/s0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 191.Gaspar V.M., Maia C.J., Queiroz J.A., Pichon C., Correia I.J., Sousa F. Improved minicircle DNA biosynthesis for gene therapy applications. Hum. Gene Ther. Methods. 2014;25:93–105. doi: 10.1089/hgtb.2013.020. [DOI] [PubMed] [Google Scholar]