Main Text
An estimated 500,000 people experience a spinal cord injury worldwide each year (1). At present, there is no treatment to repair spinal cord injury and restore lost function. Unlike mammals, animals such as fish, frogs, and salamanders display the amazing potential to regenerate their central nervous system through axonal regrowth and tissue regeneration (2). Since many molecular pathways are shared between zebrafish and mammals, zebrafish have emerged as a powerful model system to study central nervous system regeneration with a view toward informing therapeutic interventions in humans (3).
Historically, axonal regeneration has been attributed almost exclusively to chemical cues. A growing body of evidence now suggests that mechanical cues could—at least in part—play a critical role in guiding axonal regrowth and spinal cord repair (4). The mechanical microenvironment of living cells is increasingly recognized as an important regulator of cellular development, aging, disease, and injury healing; however, we lack technologies to reliably characterize this environment in vivo. Optical tweezers, micropipette aspiration, and microfluidics allow us to characterize the stiffness of cells in solution, but not at subcellular resolution. Atomic force microscopy provides stiffness maps at high spatial resolution, but not contact-free (5). Unfortunately, to date, there is no technique to reliably map the mechanical environment of the central nervous system contact- and label-free in vivo.
In an elegant comprehensive study in this issue of Biophysical Journal, Schlüßler et al. (6) establish a technology to map the physical properties of the spinal cord in vivo, noninvasively, and at optical resolution and demonstrate that Brillouin shifts, indicators for the stiffness of the spinal cord, increase during development and decrease transiently during spinal cord injury healing. The underlying technology, Brillouin scattering, was first proposed almost a century ago by the French physicist Léon Nicolas Brillouin (7). Brillouin scattering is a spontaneous inelastic scattering phenomenon that arises when monochromatic light waves interact with inherent density fluctuations, or acoustic phonons. Acoustic phonons are randomly present in all media, and the light scattered on them is usually extinguished by destructive interference; however, when it overlaps constructively, it induces a frequency shift in the scattered light. Brillouin spectroscopy measures this phonon-induced frequency shift, the Brillouin shift, and the phonon lifetime, the Brillouin linewidth (8). The Brillouin shift, νB = ζ × √M’, is correlated to the longitudinal modulus M’, a signature of the solid-like, elastic tissue properties, and the Brillouin linewidth, ΔB = ζ2 × M’’/ νB = 2π × ζ2 × η, is correlated to the loss modulus M’’ and the viscosity η, signatures of fluid-like, viscous tissue properties. Importantly, through the parameter ζ = 2 cos(Θ/2)/ λ × n /√ρ, both Brillouin signatures also depend on the scattering angle Θ, the imaging wavelength λ, the refractive index n, and the mass density ρ.
The original Brillouin spectroscopy is a point sampling technique that characterizes the viscoelastic properties at a material at a point. Combined with confocal sectioning, the technique is known as Brillouin microscopy and allows for a noninvasive, fully three-dimensional mapping of the longitudinal modulus and viscosity at high frequencies (8). Throughout the past decade, Brillouin microscopy has advanced to become the method of choice to characterize the mechanical properties inside living cells and tissues, contact-free, label-free, in vivo, and at high spatial resolution. Brillouin microscopy has been successfully applied to the human cornea, murine carotid arteries, rabbit bone tissue, zebrafish embryos (9), and, most recently, to ruminant retina (10); yet, to date, Brillouin microscopy has not been used to longitudinally map the mechanical environment of the central nervous system in vivo.
Schlüßler et al. (6) capitalize on the optical transparency and regenerative potential of zebrafish larvae and map the Brillouin shift and linewidth during development and regeneration in response to spinal cord transection. At three consecutive days postfertilization, they anesthetize and immobilize zebrafish larvae in low gelling point agarose, acquire brightfield microscopy images, and perform Brillouin microscopy to characterize the Brillouin signatures of the spinal cord, muscle, intestinal tissue, and notochord. At 5 days postfertilization, they sacrifice the larvae, record the Brillouin shift and linewidth of the tissue in situ, and measure the stiffness of tissue slices ex vivo using atomic force microscopy. They observe that during development, the Brillouin shift of the larval zebrafish spinal cord increases; during regeneration after spinal cord transection, the Brillouin shift of the injured region initially decreases and then gradually increases. Postmortem, the Brillouin shift in muscle tissue increases but remains constant in the spinal cord itself.
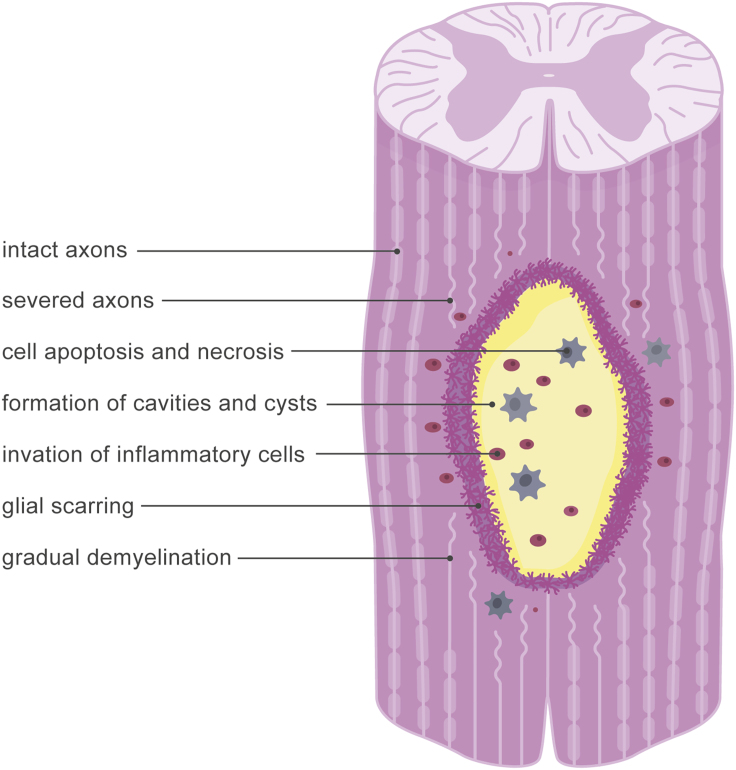
Strikingly, against our intuition that scar tissue is stiffer than healthy tissue, the Brillouin shift of the injured region decreased after spinal cord transection (6). This agrees with glial scar softening observed in atomic force microscopy studies in rats (5). An interesting question to ask is “why” does the injured region soften? Spinal cord injury typically causes the immediate death of neurons, oligodendrocytes, and astrocytes, and the formation of cavities and cysts. Secondary injury involves the invasion of inflammatory cells, glial scarring, ongoing oligodendrocyte death, and gradual demyelination (1), see Fig. 1. All these processes not only alter the chemical but also the mechanical microenvironment of the injury site (11). For example, recent studies have shown that the local myelin content is directly correlated to the local white matter stiffness (12). An integrative biochemical and biophysical analysis of the spinal cord in response to injury would be a logical next step to elucidate cause and effect of central nervous system softening.
Figure 1.
Mechanisms of spinal cord injury. Primary injury involves cell apoptosis, cell necrosis, and the formation of cavities and cysts. Secondary injury is associated with the invasion of inflammatory cells, glial scarring, and gradual demyelination. These processes not only alter the chemical but also the mechanical microenvironment of the injury site. To see this figure in color, go online.
When we think of mechanical softening, we usually associate it with a decrease in the Young’s or shear modulus. It is important to recognize that Brillouin microscopy can neither directly measure the Young’s modulus nor the shear modulus. In the quasi-static limit, Brillouin microscopy measures the longitudinal modulus, M’ = K + 4/3 G, a combination of the resistance to bulk compression through the bulk modulus K and to shear deformation through the shear modulus G (9). Because the cells and tissue of the central nervous system consist largely of water, they are quasi incompressible, and the major contribution to the recorded longitudinal modulus originates from the bulk modulus, which typically takes values on the order of gigapascals. Only a much smaller fraction, on the order of kilopascals, originates from the shear modulus (13). The Brillouin longitudinal modulus should therefore be used with caution and not be confused with the Young’s modulus or shear stiffness that we typically associate with mechanical stiffness. Aside from this limitation, Brillouin microscopy can still provide important information about changes in mechanical properties associated with hydration including cell volume regulation, intracellular phase changes, and polymerization (9).
Taken together, various cell types and cellular processes involved in spinal cord repair display a mechanosensitive signature, both in vitro and in vivo. Although several techniques exist to characterize cell and tissue stiffnesses in vitro, Brillouin microscopy is currently emerging as a powerful technology to map the mechanical properties of the central nervous system contact- and label-free in vivo. As such, it can provide valuable insight into spatiotemporal changes in the mechanical environment of the spinal cord in response to injury. Although we are only at the beginning of understanding the importance of these mechanical alterations and further studies are needed to interpret the underlying biochemical and biophysical mechanisms, there is every reason to believe that injury-induced stiffness changes could provide important signaling cues for mechanosensitive cells in the spinal cord. Manipulating the mechanical environment and recreating developmental conditions could hold the key to enhance axonal sprouting, trigger axonal regrowth, and stimulate remyelination. A better understanding of the mechanical microenvironment in regenerative species like the zebrafish is a valuable first step toward informing these new treatment strategies for neuronal regeneration in humans.
Acknowledgments
We acknowledge the illustrations by Lucy Reading-Ikkanda and support by the National Science Foundation grant CMMI 1727268 “Understanding Neurodegeneration across the Scales.”
Editor: Christopher Yip.
References
- 1.Holmes D. Repairing the neural highway. Nature. 2017;552:S50–S51. doi: 10.1038/d41586-017-07551-8. [DOI] [PubMed] [Google Scholar]
- 2.Diaz Quiroz J.F., Echeverri K. Spinal cord regeneration: where fish, frogs and salamanders lead the way, can we follow? Biochem. J. 2013;451:353–364. doi: 10.1042/BJ20121807. [DOI] [PubMed] [Google Scholar]
- 3.Becker C.G., Becker T. Adult zebrafish as a model for successful central nervous system regeneration. Restor. Neurol. Neurosci. 2008;26:71–80. [PubMed] [Google Scholar]
- 4.Franze K., Janmey P.A., Guck J. Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 2013;15:227–251. doi: 10.1146/annurev-bioeng-071811-150045. [DOI] [PubMed] [Google Scholar]
- 5.Moeendarbary E., Weber I.P., Franze K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017;8:14787. doi: 10.1038/ncomms14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlüßler R., Möllmert S., Guck J. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by brillouin imaging. Biophys. J. 2018;115:911–923. doi: 10.1016/j.bpj.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brillouin L. Diffusion de la lumière et des rayons X par un corps transparent homogène – Influence de l’aitation thermique. Ann. Phys. (Paris) 1922;9:88–122. [Google Scholar]
- 8.Scarcelli G., Yun S.H. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Photonics. 2007;2:39–43. doi: 10.1038/nphoton.2007.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarcelli G., Polacheck W.J., Yun S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods. 2015;12:1132–1134. doi: 10.1038/nmeth.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber I.P., Yun S.H., Franze K. The role of cell body density in ruminant retina mechanics assessed by atomic force and Brillouin microscopy. Phys. Biol. 2017;14:065006. doi: 10.1088/1478-3975/aa6d18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rooij R., Kuhl E. Microtubule polymerization and cross-link dynamics explain axonal stiffness and damage. Biophys. J. 2018;114:201–212. doi: 10.1016/j.bpj.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weickenmeier J., de Rooij R., Kuhl E. The mechanical importance of myelination in the central nervous system. J. Mech. Behav. Biomed. Mater. 2017;76:119–124. doi: 10.1016/j.jmbbm.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Wu P.J., Kabakova I., Overly D.R. Brillouin microscopy, what is it really measuring? arXiv. 2018 arXiv:1711.03312. [Google Scholar]