The asymmetric unit of the title disubstituted 2,3′-bipyridine, contains four independent molecules (namely, A, B, C and D). The conformations of the molecules differ, as seen from the dihedral angles between the two pyridine rings in each molecule. They vary from 5.51 (9)° for molecule B to 25.25 (8)° for molecule A.
Keywords: crystal structure, dipyridyl derivative, cyano substituent, hydrogen bonds, π–π stacking interactions, C≡N⋯π interactions
Abstract
The title compound, C12H6N4, crystallizes with four independent molecules (A, B, C and D) in the asymmetric unit. The dihedral angles between the two pyridine rings in each molecule are 25.25 (8)° in A, 5.51 (9)° in B, 11.11 (9)° in C and 16.24 (8)° in D. In the crystal, molecules A and B are linked by C—H⋯N hydrogen bonds to form layers extending parallel to the ab plane, while molecules C and D are linked by C—H⋯N hydrogen bonds forming –C–D–C–D– chains propagating along the b-axis direction. The layers and the chains are stacked alternately along the c axis through offset π–π and C≡N⋯π [N-to-pyridine-centroid distance = 3.882 (2) Å] interactions, resulting in the formation of a supramolecular framework.
Chemical context
Bipyridine ligands with the C N chelating mode to transition metal ions, such as 2,3′-bipyridine, are considered to be strong candidates for the synthesis of blue phosphorescent heavy transition metal complexes because of their larger triplet energy (T
1) compared with phenylpyridine-based C
N chelating mode to transition metal ions, such as 2,3′-bipyridine, are considered to be strong candidates for the synthesis of blue phosphorescent heavy transition metal complexes because of their larger triplet energy (T
1) compared with phenylpyridine-based C N chelating ligands (Reddy & Bejoymohandas, 2016 ▸). In particular, the triplet energy of fluorine-functionalized 2,3′-bipyridine (T
1: 2.82 eV) is larger than that of alkoxy-functionalized analogue, 2′,6′-dimethoxy-2,3′-bipyridine (T
1: 2.70 eV) (Lee et al., 2017 ▸; Kim et al., 2018 ▸). Therefore, the introduction of electron-withdrawing groups into the C-coordinating pyridine group is highly desirable in order to develop blue phosphorescent metal complexes. To design a suitable ligand possessing a large triplet energy is still a main issue in the organic light-emitting diodes (OLEDs) research area because developing blue phosphorescent materials remains a problem that has not been solved so far. Although there are a number of advantages in 2,3′-bipyridine ligands, incorporating the substituents into the ligand framework is difficult owing to the low selectivity and reactivity of the pyridine ring (Oh et al., 2013 ▸). In addition, structural examples of bipyridine-bearing electron-withdrawing groups are very scarce.
N chelating ligands (Reddy & Bejoymohandas, 2016 ▸). In particular, the triplet energy of fluorine-functionalized 2,3′-bipyridine (T
1: 2.82 eV) is larger than that of alkoxy-functionalized analogue, 2′,6′-dimethoxy-2,3′-bipyridine (T
1: 2.70 eV) (Lee et al., 2017 ▸; Kim et al., 2018 ▸). Therefore, the introduction of electron-withdrawing groups into the C-coordinating pyridine group is highly desirable in order to develop blue phosphorescent metal complexes. To design a suitable ligand possessing a large triplet energy is still a main issue in the organic light-emitting diodes (OLEDs) research area because developing blue phosphorescent materials remains a problem that has not been solved so far. Although there are a number of advantages in 2,3′-bipyridine ligands, incorporating the substituents into the ligand framework is difficult owing to the low selectivity and reactivity of the pyridine ring (Oh et al., 2013 ▸). In addition, structural examples of bipyridine-bearing electron-withdrawing groups are very scarce.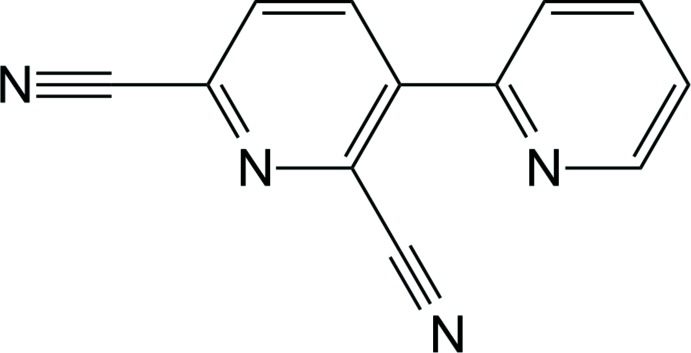
Herein, for potential applications for the development of blue phosphorescent materials, we describe the synthesis and crystal structure of the title compound, 2,3′-bipyridine-2′,6′-dicarbonitrile.
Structural commentary
As shown in Fig. 1 ▸, the asymmetric unit of the title compound contains four crystallographically independent molecules (A, B, C and D). The dihedral angles between the two pyridine rings in each molecule are 25.25 (8)° in A, 5.51 (9)° in B, 11.11 (9)° in C and 16.24 (8)° in D. In order to investigate the conformational similarity between the four molecules, the r.m.s. overlay fits of the 16 non-H atoms of each molecule were calculated using the AutoMolFit routine in PLATON (Spek, 2009 ▸). As shown in Fig. 2 ▸, and as expected in view of the values of the dihedral angles, the largest overlay fit of 0.197 Å is observed for molecules A and B, while the smallest r.m.s. overlay fit of 0.060 Å is observed for molecules C and D.
Figure 1.
The molecular structure of the four independent molecules (A, B, C and D) of the title compound, with the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Figure 2.
The overlay fits of the various molecules in the asymmetric unit of the title compound.
Supramolecular features
In the crystal, molecules A and B are linked via C—H⋯N hydrogen bonds (C3—H3⋯N3i, C3B—H3B⋯N3B
ii and C10B—H10B⋯N3, Table 1 ▸ and Fig. 3 ▸
a), forming layers extending parallel to the ab plane, while the C and D molecules are connected through C—H⋯N hydrogen bonds (C3C—H3C⋯N3D
iii and C3D–-H3D⋯N3C, Table 1 ▸ and Fig. 3 ▸
b) to from –C–D–C–D– chains propagating along the b-axis direction. The layers and chains stack alternately along the c axis, linked by intermolecular π–π stacking interactions, resulting in the formation of a supramolecular framework, as shown in Fig. 4 ▸ [Cg1⋯Cg2D
i = 3.6741 (9) Å; Cg1⋯Cg2D
iv = 3.6546 (9) Å; Cg2⋯Cg1D
iv = 3.5888 (9) Å; Cg2B⋯Cg1C
iv = 3.8196 (10) Å; Cg1 and Cg2 are the centroids of the N1/C1–C5 and N2/C6-C10 rings. Atoms and centroids labelled with suffixes B, C and D represent those of the molecules B, C and D, respectively]. In addition, intermolecular C≡N⋯π interactions between the cyano N atom of the D molecule and the N1B-containing pyridine ring of molecule B [N4D⋯Cg1B
vi = 3.882 (2) Å; Cg1B is the centroid of the N1B/C1B–C5B ring; symmetry code: (vi) −x + 1, y −  , −z +
, −z +  ], contribute to the stabilization of the framework.
], contribute to the stabilization of the framework.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯N3i | 0.95 | 2.42 | 3.343 (2) | 164 |
| C3B—H3B⋯N3B ii | 0.95 | 2.34 | 3.281 (2) | 169 |
| C10B—H10B⋯N3 | 0.95 | 2.57 | 3.269 (2) | 130 |
| C3C—H3C⋯N3D iii | 0.95 | 2.46 | 3.379 (2) | 164 |
| C3D—H3D⋯N3C | 0.95 | 2.56 | 3.397 (2) | 145 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Figure 3.
(a) View along the c axis of the layer formed by C—H⋯N hydrogen bonds between molecules A and B; (b) view along the c axis of the chains formed by C—H⋯N hydrogen bonds between molecules C and D [symmetry codes: (i) −x + 1, y +  , −z +
, −z +  ; (ii) −x + 2, y −
; (ii) −x + 2, y −  , −z +
, −z +  ; (iii) x, y + 1, z; colour codes: grey = carbon, blue = nitrogen and white = hydrogen].
; (iii) x, y + 1, z; colour codes: grey = carbon, blue = nitrogen and white = hydrogen].
Figure 4.
The supramolecular framework formed via intermolecular π-π stacking (black dashed lines) and C≡N⋯π (yellow dashed lines) interactions involving the four independent molecules (colour codes: gray = molecule A, red = molecule B, blue = molecule C and green = molecule D). All H atoms have been omitted for clarity.
Database survey
A search of the Cambridge Structural Database (CSD, Version 5.39, last update May 2018; Groom et al., 2016 ▸) for 2′,6′-disubstituted 2,3′-bipyridines, gave a number of hits. The majority of them involve iridium or platinum complexes of the difluoro and dimethoxy analogues of the title compound. As explained in the Chemical context, such compounds, particularly blue iridium complexes of 2′,6′-difluoro-2,3′-bipyridine, have been synthesized to study their phosphorescence (e.g. Lee et al., 2009 ▸) and electroluminescence (e.g. Xu et al., 2015 ▸) efficiency. As there are no reports of the crystal structures of either 2′,6′-difluoro-2,3′-bipyridine nor 2′,6′-dimethoxy-2,3′-bipyridine, it is not possible to compare their conformations with those of the four independent molecules of the title compound.
Synthesis and crystallization
All experiments were performed under a dry N2 atmosphere using standard Schlenk techniques. All solvents were freshly distilled over appropriate drying reagents prior to use. All starting materials were commercially purchased and used without further purification. The 1H NMR spectrum was recorded on a Bruker Avance 300 MHz spectrometer. The fluorinated bipyridine, 2′,6′-difluoro-2,3′-bipyridine, was synthesized according to previous reports (Lee et al., 2009 ▸). Then 2′,6′-difluoro-2,3′-bipyridine (2.0 g, 10.4 mmol) and sodium cyanide (1.02 g, 20.8 mmol) were dissolved in DMSO (10 ml). The reaction mixture was stirred overnight at 308 K. All the volatile components were removed under reduced pressure. The resulting mixture was poured into CH2Cl2 (20 × 3 ml), and then washed with water (3 × 50 ml) to remove any remaining sodium cyanide. Silica gel column purification with EtOAc and hexane gave a yellow powder in 60% yield. Colourless crystals suitable for X-ray crystallography analysis were obtained from a CH2Cl2/hexane solution under slow evaporation. 1H NMR (300 MHz, CDCl3, δ): 8.78 (dd, J = 3.6, 1.2 Hz, 1H), 8.40 (d, J = 8.4 Hz, 1H), 7.93–7.84 (m, 3H), 7.42 (td, J = 5.1, 1.5 Hz, 1H). IR(KBr, pellet): νCN = 2239 cm−1. Mass spectrum m/z (EI): 206 for [M]+ (calculated, 206).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms were positioned geometrically and refined using a riding model: C—H = 0.95 Å with U iso(H) = 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C12H6N4 |
| M r | 206.21 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 173 |
| a, b, c (Å) | 22.5144 (5), 13.1601 (3), 13.2652 (3) |
| β (°) | 93.4509 (11) |
| V (Å3) | 3923.24 (15) |
| Z | 16 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.40 × 0.33 × 0.29 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2014 ▸) |
| T min, T max | 0.696, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 39169, 9671, 6997 |
| R int | 0.034 |
| (sin θ/λ)max (Å−1) | 0.667 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.049, 0.140, 1.05 |
| No. of reflections | 9671 |
| No. of parameters | 578 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.29, −0.23 |
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989018011532/xu5937sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018011532/xu5937Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018011532/xu5937Isup3.cml
CCDC reference: 1862117
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C12H6N4 | F(000) = 1696 |
| Mr = 206.21 | Dx = 1.396 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 22.5144 (5) Å | Cell parameters from 9514 reflections |
| b = 13.1601 (3) Å | θ = 2.3–28.3° |
| c = 13.2652 (3) Å | µ = 0.09 mm−1 |
| β = 93.4509 (11)° | T = 173 K |
| V = 3923.24 (15) Å3 | Block, colourless |
| Z = 16 | 0.40 × 0.33 × 0.29 mm |
Data collection
| Bruker APEXII CCD diffractometer | 6997 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.034 |
| Absorption correction: multi-scan (SADABS; Bruker, 2014) | θmax = 28.3°, θmin = 0.9° |
| Tmin = 0.696, Tmax = 0.746 | h = −29→29 |
| 39169 measured reflections | k = −15→17 |
| 9671 independent reflections | l = −17→17 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H-atom parameters constrained |
| wR(F2) = 0.140 | w = 1/[σ2(Fo2) + (0.0597P)2 + 1.2183P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 9671 reflections | Δρmax = 0.29 e Å−3 |
| 578 parameters | Δρmin = −0.23 e Å−3 |
| 0 restraints | Extinction correction: SHELXL2014 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.00078 (18) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.49910 (5) | 0.74057 (9) | 0.26760 (10) | 0.0280 (3) | |
| N2 | 0.67745 (5) | 0.83351 (10) | 0.29072 (11) | 0.0346 (3) | |
| N3 | 0.62263 (6) | 0.61415 (10) | 0.24069 (12) | 0.0376 (3) | |
| N4 | 0.35033 (6) | 0.75276 (12) | 0.29544 (15) | 0.0533 (4) | |
| C1 | 0.55555 (6) | 0.77161 (10) | 0.26223 (11) | 0.0259 (3) | |
| C2 | 0.45846 (6) | 0.81336 (11) | 0.27582 (12) | 0.0297 (3) | |
| C3 | 0.47099 (7) | 0.91693 (11) | 0.27679 (13) | 0.0338 (3) | |
| H3 | 0.4402 | 0.9658 | 0.2815 | 0.041* | |
| C4 | 0.52920 (6) | 0.94626 (11) | 0.27070 (12) | 0.0322 (3) | |
| H4 | 0.5391 | 1.0165 | 0.2714 | 0.039* | |
| C5 | 0.57391 (6) | 0.87387 (11) | 0.26354 (11) | 0.0271 (3) | |
| C6 | 0.63739 (6) | 0.90300 (11) | 0.25798 (12) | 0.0285 (3) | |
| C7 | 0.65339 (7) | 0.99737 (11) | 0.22031 (12) | 0.0332 (3) | |
| H7 | 0.6239 | 1.0453 | 0.1983 | 0.040* | |
| C8 | 0.71324 (7) | 1.01972 (12) | 0.21571 (13) | 0.0363 (4) | |
| H8 | 0.7255 | 1.0838 | 0.1913 | 0.044* | |
| C9 | 0.75478 (7) | 0.94792 (12) | 0.24703 (13) | 0.0361 (4) | |
| H9 | 0.7961 | 0.9608 | 0.2433 | 0.043* | |
| C10 | 0.73499 (7) | 0.85668 (12) | 0.28397 (14) | 0.0376 (4) | |
| H10 | 0.7639 | 0.8075 | 0.3059 | 0.045* | |
| C11 | 0.59619 (6) | 0.68697 (11) | 0.25145 (12) | 0.0282 (3) | |
| C12 | 0.39805 (7) | 0.77851 (12) | 0.28566 (14) | 0.0374 (4) | |
| N1B | 1.00098 (5) | 0.66865 (10) | 0.25099 (11) | 0.0328 (3) | |
| N2B | 0.82233 (6) | 0.58423 (10) | 0.23869 (13) | 0.0426 (4) | |
| N3B | 0.87912 (6) | 0.79976 (11) | 0.21706 (16) | 0.0595 (5) | |
| N4B | 1.15222 (6) | 0.65194 (11) | 0.28236 (15) | 0.0522 (4) | |
| C1B | 0.94394 (6) | 0.63854 (11) | 0.24219 (12) | 0.0302 (3) | |
| C2B | 1.04188 (7) | 0.59585 (12) | 0.26116 (14) | 0.0368 (4) | |
| C3B | 1.02892 (7) | 0.49305 (13) | 0.26150 (17) | 0.0497 (5) | |
| H3B | 1.0597 | 0.4437 | 0.2677 | 0.060* | |
| C4B | 0.97019 (7) | 0.46449 (13) | 0.25256 (16) | 0.0477 (5) | |
| H4B | 0.9602 | 0.3943 | 0.2525 | 0.057* | |
| C5B | 0.92501 (7) | 0.53684 (11) | 0.24356 (13) | 0.0331 (3) | |
| C6B | 0.86123 (7) | 0.50763 (11) | 0.23825 (12) | 0.0325 (3) | |
| C7B | 0.84293 (7) | 0.40674 (12) | 0.23460 (13) | 0.0364 (4) | |
| H7B | 0.8713 | 0.3534 | 0.2336 | 0.044* | |
| C8B | 0.78279 (7) | 0.38510 (12) | 0.23251 (13) | 0.0381 (4) | |
| H8B | 0.7694 | 0.3166 | 0.2310 | 0.046* | |
| C9B | 0.74263 (7) | 0.46356 (13) | 0.23266 (13) | 0.0372 (4) | |
| H9B | 0.7011 | 0.4507 | 0.2310 | 0.045* | |
| C10B | 0.76432 (7) | 0.56175 (13) | 0.23535 (15) | 0.0421 (4) | |
| H10B | 0.7366 | 0.6162 | 0.2348 | 0.051* | |
| C11B | 0.90390 (7) | 0.72465 (12) | 0.22910 (15) | 0.0396 (4) | |
| C12B | 1.10337 (7) | 0.62904 (12) | 0.27276 (15) | 0.0408 (4) | |
| N1C | 0.25051 (5) | 0.53772 (10) | 0.02073 (10) | 0.0328 (3) | |
| N2C | 0.07122 (6) | 0.62043 (11) | −0.00555 (15) | 0.0522 (4) | |
| N3C | 0.13060 (6) | 0.40241 (12) | 0.02803 (17) | 0.0616 (5) | |
| N4C | 0.40066 (6) | 0.55328 (12) | 0.01479 (14) | 0.0520 (4) | |
| C1C | 0.19315 (6) | 0.56632 (11) | 0.02102 (12) | 0.0307 (3) | |
| C2C | 0.29079 (7) | 0.61155 (12) | 0.02084 (13) | 0.0340 (3) | |
| C3C | 0.27729 (7) | 0.71403 (13) | 0.02209 (15) | 0.0429 (4) | |
| H3C | 0.3078 | 0.7639 | 0.0238 | 0.051* | |
| C4C | 0.21814 (7) | 0.74172 (13) | 0.02080 (15) | 0.0422 (4) | |
| H4C | 0.2077 | 0.8116 | 0.0206 | 0.051* | |
| C5C | 0.17368 (7) | 0.66812 (12) | 0.01976 (12) | 0.0325 (3) | |
| C6C | 0.10958 (7) | 0.69596 (12) | 0.01452 (12) | 0.0322 (3) | |
| C7C | 0.09100 (7) | 0.79532 (13) | 0.02696 (15) | 0.0420 (4) | |
| H7C | 0.1192 | 0.8479 | 0.0415 | 0.050* | |
| C8C | 0.03076 (7) | 0.81675 (13) | 0.01785 (15) | 0.0445 (4) | |
| H8C | 0.0170 | 0.8844 | 0.0257 | 0.053* | |
| C9C | −0.00892 (7) | 0.73939 (13) | −0.00260 (14) | 0.0402 (4) | |
| H9C | −0.0505 | 0.7522 | −0.0094 | 0.048* | |
| C10C | 0.01307 (7) | 0.64306 (14) | −0.01301 (18) | 0.0527 (5) | |
| H10C | −0.0145 | 0.5893 | −0.0263 | 0.063* | |
| C11C | 0.15421 (7) | 0.47900 (12) | 0.02419 (15) | 0.0400 (4) | |
| C12C | 0.35214 (7) | 0.57814 (12) | 0.01809 (14) | 0.0396 (4) | |
| N1D | 0.25087 (5) | 0.03678 (9) | 0.02181 (10) | 0.0312 (3) | |
| N2D | 0.42958 (5) | 0.11479 (9) | −0.00983 (11) | 0.0328 (3) | |
| N3D | 0.37265 (6) | −0.09144 (10) | 0.07125 (13) | 0.0432 (4) | |
| N4D | 0.10036 (6) | 0.04871 (12) | 0.00311 (15) | 0.0545 (5) | |
| C1D | 0.30810 (6) | 0.06558 (11) | 0.02102 (11) | 0.0274 (3) | |
| C2D | 0.21010 (6) | 0.10823 (12) | −0.00026 (12) | 0.0325 (3) | |
| C3D | 0.22322 (7) | 0.20789 (12) | −0.02331 (14) | 0.0383 (4) | |
| H3D | 0.1925 | 0.2561 | −0.0382 | 0.046* | |
| C4D | 0.28232 (7) | 0.23508 (12) | −0.02402 (13) | 0.0360 (4) | |
| H4D | 0.2926 | 0.3030 | −0.0398 | 0.043* | |
| C5D | 0.32714 (6) | 0.16403 (11) | −0.00185 (11) | 0.0281 (3) | |
| C6D | 0.39103 (6) | 0.19201 (11) | −0.00522 (11) | 0.0274 (3) | |
| C7D | 0.40905 (7) | 0.29331 (11) | −0.00522 (12) | 0.0312 (3) | |
| H7D | 0.3808 | 0.3464 | −0.0003 | 0.037* | |
| C8D | 0.46879 (7) | 0.31533 (12) | −0.01251 (12) | 0.0336 (3) | |
| H8D | 0.4821 | 0.3838 | −0.0135 | 0.040* | |
| C9D | 0.50870 (7) | 0.23649 (12) | −0.01824 (12) | 0.0335 (3) | |
| H9D | 0.5499 | 0.2495 | −0.0241 | 0.040* | |
| C10D | 0.48740 (7) | 0.13777 (12) | −0.01523 (13) | 0.0354 (4) | |
| H10D | 0.5152 | 0.0835 | −0.0171 | 0.042* | |
| C11D | 0.34799 (6) | −0.01830 (11) | 0.04801 (12) | 0.0316 (3) | |
| C12D | 0.14866 (7) | 0.07449 (13) | 0.00158 (15) | 0.0408 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0254 (6) | 0.0296 (6) | 0.0288 (7) | 0.0007 (5) | 0.0007 (5) | 0.0007 (5) |
| N2 | 0.0283 (6) | 0.0308 (6) | 0.0447 (8) | −0.0002 (5) | 0.0013 (6) | 0.0033 (6) |
| N3 | 0.0288 (6) | 0.0296 (7) | 0.0544 (9) | 0.0026 (5) | 0.0009 (6) | −0.0007 (6) |
| N4 | 0.0313 (7) | 0.0405 (8) | 0.0889 (14) | 0.0008 (6) | 0.0094 (8) | 0.0011 (8) |
| C1 | 0.0251 (6) | 0.0272 (7) | 0.0252 (7) | 0.0023 (5) | 0.0006 (5) | 0.0008 (6) |
| C2 | 0.0249 (7) | 0.0305 (7) | 0.0338 (8) | 0.0020 (5) | 0.0014 (6) | 0.0012 (6) |
| C3 | 0.0298 (7) | 0.0295 (7) | 0.0421 (9) | 0.0059 (6) | 0.0019 (7) | 0.0009 (6) |
| C4 | 0.0312 (7) | 0.0253 (7) | 0.0399 (9) | 0.0008 (6) | 0.0013 (6) | 0.0000 (6) |
| C5 | 0.0284 (7) | 0.0267 (7) | 0.0259 (8) | 0.0010 (5) | 0.0000 (6) | 0.0013 (6) |
| C6 | 0.0284 (7) | 0.0266 (7) | 0.0303 (8) | −0.0017 (5) | 0.0010 (6) | −0.0022 (6) |
| C7 | 0.0327 (8) | 0.0286 (7) | 0.0381 (9) | 0.0006 (6) | 0.0014 (7) | 0.0009 (6) |
| C8 | 0.0378 (8) | 0.0307 (8) | 0.0408 (10) | −0.0074 (6) | 0.0056 (7) | −0.0003 (7) |
| C9 | 0.0280 (7) | 0.0379 (8) | 0.0425 (10) | −0.0050 (6) | 0.0035 (7) | −0.0059 (7) |
| C10 | 0.0275 (7) | 0.0352 (8) | 0.0495 (11) | −0.0003 (6) | −0.0013 (7) | 0.0013 (7) |
| C11 | 0.0247 (6) | 0.0269 (7) | 0.0328 (8) | −0.0013 (5) | −0.0003 (6) | 0.0023 (6) |
| C12 | 0.0301 (8) | 0.0306 (8) | 0.0515 (11) | 0.0036 (6) | 0.0035 (7) | 0.0012 (7) |
| N1B | 0.0271 (6) | 0.0304 (6) | 0.0407 (8) | 0.0020 (5) | 0.0013 (5) | 0.0014 (5) |
| N2B | 0.0306 (7) | 0.0301 (7) | 0.0675 (11) | −0.0003 (5) | 0.0044 (7) | 0.0042 (7) |
| N3B | 0.0304 (7) | 0.0296 (7) | 0.1174 (17) | 0.0005 (6) | −0.0046 (8) | 0.0034 (8) |
| N4B | 0.0302 (7) | 0.0396 (8) | 0.0866 (13) | 0.0031 (6) | 0.0030 (8) | −0.0004 (8) |
| C1B | 0.0276 (7) | 0.0283 (7) | 0.0346 (9) | 0.0028 (6) | 0.0008 (6) | 0.0017 (6) |
| C2B | 0.0276 (7) | 0.0343 (8) | 0.0485 (10) | 0.0038 (6) | 0.0022 (7) | 0.0033 (7) |
| C3B | 0.0324 (8) | 0.0307 (8) | 0.0859 (16) | 0.0071 (7) | 0.0016 (9) | 0.0053 (9) |
| C4B | 0.0365 (9) | 0.0276 (8) | 0.0789 (15) | 0.0030 (7) | 0.0010 (9) | 0.0052 (8) |
| C5B | 0.0309 (7) | 0.0281 (7) | 0.0403 (9) | 0.0005 (6) | 0.0010 (7) | 0.0028 (6) |
| C6B | 0.0312 (7) | 0.0296 (7) | 0.0367 (9) | −0.0006 (6) | 0.0026 (6) | 0.0033 (6) |
| C7B | 0.0369 (8) | 0.0294 (7) | 0.0426 (10) | −0.0004 (6) | 0.0009 (7) | 0.0019 (7) |
| C8B | 0.0403 (9) | 0.0318 (8) | 0.0421 (10) | −0.0063 (7) | 0.0026 (7) | 0.0005 (7) |
| C9B | 0.0324 (8) | 0.0414 (9) | 0.0381 (9) | −0.0054 (7) | 0.0048 (7) | 0.0006 (7) |
| C10B | 0.0311 (8) | 0.0365 (9) | 0.0589 (12) | 0.0004 (6) | 0.0051 (8) | 0.0026 (8) |
| C11B | 0.0258 (7) | 0.0299 (8) | 0.0628 (12) | −0.0014 (6) | −0.0004 (7) | −0.0010 (7) |
| C12B | 0.0326 (8) | 0.0313 (8) | 0.0584 (12) | 0.0066 (6) | 0.0022 (8) | 0.0019 (7) |
| N1C | 0.0265 (6) | 0.0347 (7) | 0.0373 (8) | −0.0035 (5) | 0.0023 (5) | 0.0045 (6) |
| N2C | 0.0299 (7) | 0.0340 (8) | 0.0930 (14) | −0.0011 (6) | 0.0061 (8) | −0.0108 (8) |
| N3C | 0.0309 (7) | 0.0359 (8) | 0.1179 (17) | −0.0034 (6) | 0.0041 (9) | 0.0105 (9) |
| N4C | 0.0310 (7) | 0.0441 (9) | 0.0810 (13) | −0.0046 (6) | 0.0042 (7) | 0.0110 (8) |
| C1C | 0.0274 (7) | 0.0320 (7) | 0.0327 (8) | −0.0036 (6) | 0.0021 (6) | 0.0008 (6) |
| C2C | 0.0274 (7) | 0.0362 (8) | 0.0384 (9) | −0.0044 (6) | 0.0022 (6) | 0.0024 (7) |
| C3C | 0.0317 (8) | 0.0364 (9) | 0.0607 (12) | −0.0098 (7) | 0.0035 (8) | −0.0051 (8) |
| C4C | 0.0351 (8) | 0.0315 (8) | 0.0605 (12) | −0.0036 (6) | 0.0051 (8) | −0.0065 (8) |
| C5C | 0.0302 (7) | 0.0321 (7) | 0.0353 (9) | −0.0025 (6) | 0.0029 (6) | −0.0029 (6) |
| C6C | 0.0304 (7) | 0.0322 (8) | 0.0344 (9) | −0.0009 (6) | 0.0048 (6) | −0.0023 (6) |
| C7C | 0.0356 (8) | 0.0345 (8) | 0.0554 (12) | −0.0010 (7) | −0.0009 (8) | −0.0032 (8) |
| C8C | 0.0389 (9) | 0.0346 (8) | 0.0599 (12) | 0.0052 (7) | 0.0024 (8) | −0.0014 (8) |
| C9C | 0.0315 (8) | 0.0403 (9) | 0.0491 (11) | 0.0026 (7) | 0.0050 (7) | 0.0010 (8) |
| C10C | 0.0287 (8) | 0.0405 (10) | 0.0892 (16) | −0.0020 (7) | 0.0051 (9) | −0.0084 (10) |
| C11C | 0.0261 (7) | 0.0348 (8) | 0.0592 (12) | 0.0004 (6) | 0.0035 (7) | 0.0040 (8) |
| C12C | 0.0310 (8) | 0.0367 (8) | 0.0512 (11) | −0.0067 (6) | 0.0021 (7) | 0.0064 (7) |
| N1D | 0.0237 (6) | 0.0334 (6) | 0.0364 (8) | −0.0002 (5) | 0.0019 (5) | 0.0001 (5) |
| N2D | 0.0270 (6) | 0.0299 (6) | 0.0414 (8) | −0.0011 (5) | 0.0003 (5) | 0.0011 (6) |
| N3D | 0.0279 (6) | 0.0343 (7) | 0.0671 (11) | 0.0000 (5) | 0.0006 (7) | 0.0067 (7) |
| N4D | 0.0283 (7) | 0.0475 (9) | 0.0878 (14) | 0.0010 (6) | 0.0033 (8) | 0.0088 (8) |
| C1D | 0.0248 (7) | 0.0286 (7) | 0.0286 (8) | 0.0018 (5) | −0.0001 (6) | −0.0009 (6) |
| C2D | 0.0241 (7) | 0.0347 (8) | 0.0384 (9) | 0.0023 (6) | 0.0003 (6) | −0.0026 (7) |
| C3D | 0.0285 (7) | 0.0330 (8) | 0.0526 (11) | 0.0054 (6) | −0.0042 (7) | −0.0016 (7) |
| C4D | 0.0328 (8) | 0.0280 (7) | 0.0464 (10) | 0.0016 (6) | −0.0050 (7) | 0.0006 (7) |
| C5D | 0.0268 (7) | 0.0298 (7) | 0.0276 (8) | −0.0001 (6) | −0.0006 (6) | −0.0030 (6) |
| C6D | 0.0268 (7) | 0.0294 (7) | 0.0256 (8) | −0.0007 (5) | −0.0011 (6) | −0.0008 (6) |
| C7D | 0.0323 (7) | 0.0309 (7) | 0.0304 (8) | −0.0016 (6) | 0.0010 (6) | −0.0004 (6) |
| C8D | 0.0362 (8) | 0.0314 (7) | 0.0330 (9) | −0.0073 (6) | 0.0007 (6) | 0.0010 (6) |
| C9D | 0.0284 (7) | 0.0386 (8) | 0.0332 (9) | −0.0053 (6) | 0.0000 (6) | 0.0008 (7) |
| C10D | 0.0260 (7) | 0.0361 (8) | 0.0438 (10) | −0.0009 (6) | 0.0008 (7) | 0.0009 (7) |
| C11D | 0.0234 (7) | 0.0313 (8) | 0.0401 (9) | −0.0036 (6) | 0.0011 (6) | 0.0007 (6) |
| C12D | 0.0291 (8) | 0.0388 (9) | 0.0545 (11) | 0.0043 (7) | 0.0019 (7) | 0.0028 (8) |
Geometric parameters (Å, º)
| N1—C2 | 1.3338 (18) | N1C—C2C | 1.3290 (19) |
| N1—C1 | 1.3410 (18) | N1C—C1C | 1.3454 (19) |
| N2—C6 | 1.3384 (18) | N2C—C6C | 1.333 (2) |
| N2—C10 | 1.3392 (19) | N2C—C10C | 1.341 (2) |
| N3—C11 | 1.1416 (19) | N3C—C11C | 1.142 (2) |
| N4—C12 | 1.141 (2) | N4C—C12C | 1.144 (2) |
| C1—C5 | 1.4077 (19) | C1C—C5C | 1.409 (2) |
| C1—C11 | 1.4540 (19) | C1C—C11C | 1.448 (2) |
| C2—C3 | 1.392 (2) | C2C—C3C | 1.383 (2) |
| C2—C12 | 1.449 (2) | C2C—C12C | 1.452 (2) |
| C3—C4 | 1.373 (2) | C3C—C4C | 1.380 (2) |
| C3—H3 | 0.9500 | C3C—H3C | 0.9500 |
| C4—C5 | 1.393 (2) | C4C—C5C | 1.392 (2) |
| C4—H4 | 0.9500 | C4C—H4C | 0.9500 |
| C5—C6 | 1.486 (2) | C5C—C6C | 1.486 (2) |
| C6—C7 | 1.394 (2) | C6C—C7C | 1.386 (2) |
| C7—C8 | 1.384 (2) | C7C—C8C | 1.383 (2) |
| C7—H7 | 0.9500 | C7C—H7C | 0.9500 |
| C8—C9 | 1.376 (2) | C8C—C9C | 1.371 (2) |
| C8—H8 | 0.9500 | C8C—H8C | 0.9500 |
| C9—C10 | 1.381 (2) | C9C—C10C | 1.371 (2) |
| C9—H9 | 0.9500 | C9C—H9C | 0.9500 |
| C10—H10 | 0.9500 | C10C—H10C | 0.9500 |
| N1B—C2B | 1.3301 (19) | N1D—C2D | 1.3342 (18) |
| N1B—C1B | 1.3424 (18) | N1D—C1D | 1.3437 (18) |
| N2B—C6B | 1.336 (2) | N2D—C6D | 1.3403 (19) |
| N2B—C10B | 1.337 (2) | N2D—C10D | 1.3424 (19) |
| N3B—C11B | 1.142 (2) | N3D—C11D | 1.1443 (19) |
| N4B—C12B | 1.140 (2) | N4D—C12D | 1.140 (2) |
| C1B—C5B | 1.405 (2) | C1D—C5D | 1.404 (2) |
| C1B—C11B | 1.452 (2) | C1D—C11D | 1.454 (2) |
| C2B—C3B | 1.384 (2) | C2D—C3D | 1.383 (2) |
| C2B—C12B | 1.451 (2) | C2D—C12D | 1.454 (2) |
| C3B—C4B | 1.373 (2) | C3D—C4D | 1.378 (2) |
| C3B—H3B | 0.9500 | C3D—H3D | 0.9500 |
| C4B—C5B | 1.393 (2) | C4D—C5D | 1.394 (2) |
| C4B—H4B | 0.9500 | C4D—H4D | 0.9500 |
| C5B—C6B | 1.484 (2) | C5D—C6D | 1.4879 (19) |
| C6B—C7B | 1.390 (2) | C6D—C7D | 1.394 (2) |
| C7B—C8B | 1.382 (2) | C7D—C8D | 1.385 (2) |
| C7B—H7B | 0.9500 | C7D—H7D | 0.9500 |
| C8B—C9B | 1.373 (2) | C8D—C9D | 1.378 (2) |
| C8B—H8B | 0.9500 | C8D—H8D | 0.9500 |
| C9B—C10B | 1.381 (2) | C9D—C10D | 1.386 (2) |
| C9B—H9B | 0.9500 | C9D—H9D | 0.9500 |
| C10B—H10B | 0.9500 | C10D—H10D | 0.9500 |
| C2—N1—C1 | 116.28 (12) | C2C—N1C—C1C | 116.78 (13) |
| C6—N2—C10 | 117.29 (13) | C6C—N2C—C10C | 117.86 (14) |
| N1—C1—C5 | 124.69 (13) | N1C—C1C—C5C | 124.31 (13) |
| N1—C1—C11 | 112.09 (12) | N1C—C1C—C11C | 111.17 (13) |
| C5—C1—C11 | 123.20 (12) | C5C—C1C—C11C | 124.51 (13) |
| N1—C2—C3 | 124.35 (13) | N1C—C2C—C3C | 124.24 (14) |
| N1—C2—C12 | 115.62 (13) | N1C—C2C—C12C | 115.38 (14) |
| C3—C2—C12 | 120.02 (13) | C3C—C2C—C12C | 120.38 (14) |
| C4—C3—C2 | 117.93 (13) | C4C—C3C—C2C | 118.04 (15) |
| C4—C3—H3 | 121.0 | C4C—C3C—H3C | 121.0 |
| C2—C3—H3 | 121.0 | C2C—C3C—H3C | 121.0 |
| C3—C4—C5 | 120.52 (14) | C3C—C4C—C5C | 120.61 (15) |
| C3—C4—H4 | 119.7 | C3C—C4C—H4C | 119.7 |
| C5—C4—H4 | 119.7 | C5C—C4C—H4C | 119.7 |
| C4—C5—C1 | 116.21 (13) | C4C—C5C—C1C | 115.99 (14) |
| C4—C5—C6 | 121.87 (13) | C4C—C5C—C6C | 121.63 (14) |
| C1—C5—C6 | 121.92 (12) | C1C—C5C—C6C | 122.35 (13) |
| N2—C6—C7 | 122.78 (13) | N2C—C6C—C7C | 122.05 (14) |
| N2—C6—C5 | 116.04 (13) | N2C—C6C—C5C | 116.17 (14) |
| C7—C6—C5 | 121.18 (13) | C7C—C6C—C5C | 121.75 (14) |
| C8—C7—C6 | 118.58 (14) | C8C—C7C—C6C | 118.90 (15) |
| C8—C7—H7 | 120.7 | C8C—C7C—H7C | 120.6 |
| C6—C7—H7 | 120.7 | C6C—C7C—H7C | 120.6 |
| C9—C8—C7 | 119.12 (15) | C9C—C8C—C7C | 119.33 (16) |
| C9—C8—H8 | 120.4 | C9C—C8C—H8C | 120.3 |
| C7—C8—H8 | 120.4 | C7C—C8C—H8C | 120.3 |
| C8—C9—C10 | 118.45 (14) | C8C—C9C—C10C | 118.14 (15) |
| C8—C9—H9 | 120.8 | C8C—C9C—H9C | 120.9 |
| C10—C9—H9 | 120.8 | C10C—C9C—H9C | 120.9 |
| N2—C10—C9 | 123.76 (15) | N2C—C10C—C9C | 123.71 (16) |
| N2—C10—H10 | 118.1 | N2C—C10C—H10C | 118.1 |
| C9—C10—H10 | 118.1 | C9C—C10C—H10C | 118.1 |
| N3—C11—C1 | 172.44 (15) | N3C—C11C—C1C | 170.50 (17) |
| N4—C12—C2 | 178.2 (2) | N4C—C12C—C2C | 178.75 (19) |
| C2B—N1B—C1B | 116.69 (13) | C2D—N1D—C1D | 116.55 (13) |
| C6B—N2B—C10B | 118.18 (14) | C6D—N2D—C10D | 117.67 (13) |
| N1B—C1B—C5B | 124.70 (13) | N1D—C1D—C5D | 124.58 (13) |
| N1B—C1B—C11B | 111.34 (13) | N1D—C1D—C11D | 111.24 (12) |
| C5B—C1B—C11B | 123.95 (13) | C5D—C1D—C11D | 124.17 (12) |
| N1B—C2B—C3B | 124.03 (14) | N1D—C2D—C3D | 124.30 (13) |
| N1B—C2B—C12B | 116.38 (14) | N1D—C2D—C12D | 115.07 (14) |
| C3B—C2B—C12B | 119.59 (14) | C3D—C2D—C12D | 120.63 (14) |
| C4B—C3B—C2B | 118.00 (15) | C4D—C3D—C2D | 117.84 (14) |
| C4B—C3B—H3B | 121.0 | C4D—C3D—H3D | 121.1 |
| C2B—C3B—H3B | 121.0 | C2D—C3D—H3D | 121.1 |
| C3B—C4B—C5B | 120.98 (15) | C3D—C4D—C5D | 120.75 (14) |
| C3B—C4B—H4B | 119.5 | C3D—C4D—H4D | 119.6 |
| C5B—C4B—H4B | 119.5 | C5D—C4D—H4D | 119.6 |
| C4B—C5B—C1B | 115.57 (14) | C4D—C5D—C1D | 115.97 (13) |
| C4B—C5B—C6B | 121.77 (14) | C4D—C5D—C6D | 121.10 (13) |
| C1B—C5B—C6B | 122.64 (13) | C1D—C5D—C6D | 122.91 (13) |
| N2B—C6B—C7B | 121.87 (14) | N2D—C6D—C7D | 122.40 (13) |
| N2B—C6B—C5B | 115.92 (13) | N2D—C6D—C5D | 116.34 (12) |
| C7B—C6B—C5B | 122.20 (14) | C7D—C6D—C5D | 121.25 (13) |
| C8B—C7B—C6B | 119.04 (15) | C8D—C7D—C6D | 118.96 (14) |
| C8B—C7B—H7B | 120.5 | C8D—C7D—H7D | 120.5 |
| C6B—C7B—H7B | 120.5 | C6D—C7D—H7D | 120.5 |
| C9B—C8B—C7B | 119.30 (15) | C9D—C8D—C7D | 119.06 (14) |
| C9B—C8B—H8B | 120.3 | C9D—C8D—H8D | 120.5 |
| C7B—C8B—H8B | 120.3 | C7D—C8D—H8D | 120.5 |
| C8B—C9B—C10B | 118.17 (15) | C8D—C9D—C10D | 118.44 (14) |
| C8B—C9B—H9B | 120.9 | C8D—C9D—H9D | 120.8 |
| C10B—C9B—H9B | 120.9 | C10D—C9D—H9D | 120.8 |
| N2B—C10B—C9B | 123.44 (15) | N2D—C10D—C9D | 123.43 (14) |
| N2B—C10B—H10B | 118.3 | N2D—C10D—H10D | 118.3 |
| C9B—C10B—H10B | 118.3 | C9D—C10D—H10D | 118.3 |
| N3B—C11B—C1B | 170.85 (17) | N3D—C11D—C1D | 170.90 (15) |
| N4B—C12B—C2B | 177.79 (17) | N4D—C12D—C2D | 179.5 (2) |
| C2—N1—C1—C5 | −0.7 (2) | C2C—N1C—C1C—C5C | 1.1 (2) |
| C2—N1—C1—C11 | −178.87 (13) | C2C—N1C—C1C—C11C | −178.13 (15) |
| C1—N1—C2—C3 | 1.5 (2) | C1C—N1C—C2C—C3C | 0.6 (3) |
| C1—N1—C2—C12 | −177.60 (14) | C1C—N1C—C2C—C12C | −178.59 (14) |
| N1—C2—C3—C4 | −1.2 (3) | N1C—C2C—C3C—C4C | −1.6 (3) |
| C12—C2—C3—C4 | 177.83 (15) | C12C—C2C—C3C—C4C | 177.56 (17) |
| C2—C3—C4—C5 | 0.2 (2) | C2C—C3C—C4C—C5C | 0.9 (3) |
| C3—C4—C5—C1 | 0.5 (2) | C3C—C4C—C5C—C1C | 0.5 (3) |
| C3—C4—C5—C6 | −179.31 (15) | C3C—C4C—C5C—C6C | −177.69 (17) |
| N1—C1—C5—C4 | −0.2 (2) | N1C—C1C—C5C—C4C | −1.6 (2) |
| C11—C1—C5—C4 | 177.73 (14) | C11C—C1C—C5C—C4C | 177.49 (17) |
| N1—C1—C5—C6 | 179.60 (14) | N1C—C1C—C5C—C6C | 176.61 (15) |
| C11—C1—C5—C6 | −2.5 (2) | C11C—C1C—C5C—C6C | −4.3 (3) |
| C10—N2—C6—C7 | −1.6 (2) | C10C—N2C—C6C—C7C | −0.4 (3) |
| C10—N2—C6—C5 | 178.21 (14) | C10C—N2C—C6C—C5C | −178.60 (18) |
| C4—C5—C6—N2 | 154.51 (15) | C4C—C5C—C6C—N2C | 167.51 (17) |
| C1—C5—C6—N2 | −25.3 (2) | C1C—C5C—C6C—N2C | −10.6 (2) |
| C4—C5—C6—C7 | −25.7 (2) | C4C—C5C—C6C—C7C | −10.7 (3) |
| C1—C5—C6—C7 | 154.52 (15) | C1C—C5C—C6C—C7C | 171.18 (17) |
| N2—C6—C7—C8 | 0.6 (2) | N2C—C6C—C7C—C8C | −0.3 (3) |
| C5—C6—C7—C8 | −179.14 (14) | C5C—C6C—C7C—C8C | 177.86 (17) |
| C6—C7—C8—C9 | 0.9 (2) | C6C—C7C—C8C—C9C | 0.4 (3) |
| C7—C8—C9—C10 | −1.3 (3) | C7C—C8C—C9C—C10C | 0.1 (3) |
| C6—N2—C10—C9 | 1.1 (3) | C6C—N2C—C10C—C9C | 1.0 (3) |
| C8—C9—C10—N2 | 0.4 (3) | C8C—C9C—C10C—N2C | −0.8 (3) |
| C2B—N1B—C1B—C5B | 0.3 (2) | C2D—N1D—C1D—C5D | −0.5 (2) |
| C2B—N1B—C1B—C11B | −178.58 (15) | C2D—N1D—C1D—C11D | 178.94 (14) |
| C1B—N1B—C2B—C3B | 1.1 (3) | C1D—N1D—C2D—C3D | 0.0 (2) |
| C1B—N1B—C2B—C12B | −178.75 (15) | C1D—N1D—C2D—C12D | −179.83 (15) |
| N1B—C2B—C3B—C4B | −1.2 (3) | N1D—C2D—C3D—C4D | 0.3 (3) |
| C12B—C2B—C3B—C4B | 178.61 (19) | C12D—C2D—C3D—C4D | −179.86 (16) |
| C2B—C3B—C4B—C5B | −0.1 (3) | C2D—C3D—C4D—C5D | −0.2 (3) |
| C3B—C4B—C5B—C1B | 1.3 (3) | C3D—C4D—C5D—C1D | −0.2 (2) |
| C3B—C4B—C5B—C6B | −177.33 (18) | C3D—C4D—C5D—C6D | 178.30 (15) |
| N1B—C1B—C5B—C4B | −1.5 (3) | N1D—C1D—C5D—C4D | 0.6 (2) |
| C11B—C1B—C5B—C4B | 177.32 (18) | C11D—C1D—C5D—C4D | −178.73 (15) |
| N1B—C1B—C5B—C6B | 177.12 (15) | N1D—C1D—C5D—C6D | −177.91 (14) |
| C11B—C1B—C5B—C6B | −4.1 (3) | C11D—C1D—C5D—C6D | 2.8 (2) |
| C10B—N2B—C6B—C7B | −0.1 (3) | C10D—N2D—C6D—C7D | −0.4 (2) |
| C10B—N2B—C6B—C5B | −179.15 (16) | C10D—N2D—C6D—C5D | 178.54 (14) |
| C4B—C5B—C6B—N2B | 174.30 (17) | C4D—C5D—C6D—N2D | −162.62 (15) |
| C1B—C5B—C6B—N2B | −4.2 (2) | C1D—C5D—C6D—N2D | 15.8 (2) |
| C4B—C5B—C6B—C7B | −4.7 (3) | C4D—C5D—C6D—C7D | 16.4 (2) |
| C1B—C5B—C6B—C7B | 176.74 (16) | C1D—C5D—C6D—C7D | −165.21 (15) |
| N2B—C6B—C7B—C8B | −0.7 (3) | N2D—C6D—C7D—C8D | 1.5 (2) |
| C5B—C6B—C7B—C8B | 178.25 (16) | C5D—C6D—C7D—C8D | −177.44 (14) |
| C6B—C7B—C8B—C9B | 0.9 (3) | C6D—C7D—C8D—C9D | −0.8 (2) |
| C7B—C8B—C9B—C10B | −0.3 (3) | C7D—C8D—C9D—C10D | −0.8 (2) |
| C6B—N2B—C10B—C9B | 0.8 (3) | C6D—N2D—C10D—C9D | −1.3 (2) |
| C8B—C9B—C10B—N2B | −0.6 (3) | C8D—C9D—C10D—N2D | 1.9 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···N3i | 0.95 | 2.42 | 3.343 (2) | 164 |
| C3B—H3B···N3Bii | 0.95 | 2.34 | 3.281 (2) | 169 |
| C10B—H10B···N3 | 0.95 | 2.57 | 3.269 (2) | 130 |
| C3C—H3C···N3Diii | 0.95 | 2.46 | 3.379 (2) | 164 |
| C3D—H3D···N3C | 0.95 | 2.56 | 3.397 (2) | 145 |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2; (ii) −x+2, y−1/2, −z+1/2; (iii) x, y+1, z.
Funding Statement
This work was funded by National Research Foundation of Korea grants NRF-2016R1D1A1B01012630 and 2018R1D1A3A03000716.
References
- Brandenburg, K. (2010). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (2014). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kim, M., Kim, J., Park, K.-M. & Kang, Y. (2018). Bull. Korean Chem. Soc. 39, 703–706.
- Lee, C., Kim, J., Choi, J. M., Lee, J. Y. & Kang, Y. (2017). Dyes Pigments, 137, 378–383.
- Lee, S. J., Park, K.-M., Yang, K. & Kang, Y. (2009). Inorg. Chem. 48, 1030–1037. [DOI] [PubMed]
- Oh, H., Park, K.-M., Hwang, H., Oh, S., Lee, J. H., Lu, J.-S., Wang, S. & Kang, Y. (2013). Organometallics, 32, 6427–6436.
- Reddy, M. L. P. & Bejoymohandas, K. S. (2016). J. Photochem. Photobiol. Photochem. Rev. 29, 29–47.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Xu, Q.-L., Liang, X., Jiang, L., Zhao, Y. & Zheng, Y.-X. (2015). RSC Adv. 5, 89218–89225.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989018011532/xu5937sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018011532/xu5937Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018011532/xu5937Isup3.cml
CCDC reference: 1862117
Additional supporting information: crystallographic information; 3D view; checkCIF report






