ABSTRACT
The septation initiation network (SIN), comprising a GTPase and a cascade of three protein kinases, regulates cell division in fission yeast Schizosaccharomyces pombe, but questions remain about its influence on cytokinesis. Here, we made quantitative measurements of the numbers of Cdc7p kinase molecules (a marker for SIN activity) on spindle pole bodies (SPBs), and on the timing of assembly, maturation and constriction of contractile rings via six different proteins tagged with fluorescent proteins. When SIN activity is low in spg1-106 mutant cells at 32°C, cytokinetic nodes formed contractile rings ∼3 min slower than wild-type cells. During the maturation period, these rings maintained normal levels of the myosin-II mEGFP–Myo2p but accumulated less of the F-BAR protein Cdc15p–GFP than in wild-type cells. The Cdc15p–GFP fluorescence then disintegrated into spots as mEGFP–Myo2p dissociated slowly. Some rings started to constrict at the normal time, but most failed to complete constriction. When high SIN activity persists far longer than normal on both SPBs in cdc16-116 mutant cells at 32°C, contractile rings assembled and constricted normally, but disassembled slowly, delaying cell separation.
KEY WORDS: Cytokinesis, S. pombe, SIN, Cell division
Summary: Cytokinetic contractile rings assemble normally in fission yeast without the signaling cascade called the septation initiation network, but the rings are unstable, so cytokinesis fails and cells often lyse.
INTRODUCTION
The fission yeast Schizosaccharomyces pombe is an excellent model system to study cytokinesis in eukaryotic cells because it is genetically tractable and favorable for microscopic analysis. Like animal cells, it assembles a contractile ring of actin filaments and myosin-II between the poles of the mitotic spindle during cell division (Marks et al., 1986; May et al., 1997; Pollard and Wu, 2010). The position of the ring is determined during interphase by a broad band of interphase nodes around the equator (Akamatsu et al., 2014; Moseley et al., 2009) that recruit anillin Mid1p from the nucleus (Paoletti and Chang, 2000). During the 10 min prior to spindle pole body (SPB) separation, these centrally placed nodes accumulate type II myosin heavy and light chains (Myo2p, Cdc4p and Rlc1p), IQGAP Rng2p, formin Cdc12p and the F-BAR protein Cdc15p. Interactions between actin filaments assembled by Cdc12p and Myo2p in adjacent nodes drive the assembly of nodes into a homogenous, compact contractile ring structure (Wu et al., 2006). After a 20 min maturation period when tropomyosin, α-actinin and an unconventional myosin-II (Myp2p) join the ring, rings constrict at a constant rate coordinated with the centripetal growth of the primary septum into the cleavage furrow (Cortes et al., 2002; Liu et al., 2002).
A conserved cascade of protein kinases known as the septation initiation network (SIN) originates from the spindle pole bodies (SPBs) and regulates aspects of mitosis and cytokinesis (Gould and Simanis, 1997; Simanis, 2015). The SIN signal begins with the activity of the GTPase Spg1p (Balasubramanian et al., 1998; Schmidt et al., 1997), which is controlled by the bipartite GTPase-activating protein (GAP) composed of Cdc16p and Byr4p (Furge et al., 1998). Three scaffold proteins, Sid4p, Cdc11p and Ppc89p (Krapp et al., 2001; Rosenberg et al., 2006; Tomlin et al., 2002), tether Spg1p to the SPB and form a complex for recruiting other SIN components. It is not known how cells promote the exchange of GDP for GTP on Spg1p. Active Spg1p-GTP triggers a signaling cascade of protein kinases comprising Cdc7p (Fankhauser and Simanis, 1994; Sohrmann et al., 1998), the Sid1p–Cdc14p complex (Guertin et al., 2000) and the Sid2p–Mob1p complex (Hou et al., 2000; Sparks et al., 1999).
During interphase and early metaphase, Byr4p–Cdc16p GAP activity on both SPBs maintains Spg1p in the GDP-bound inactive state (Furge et al., 1998; Krapp and Simanis, 2008; Song et al., 1996). At the onset of mitosis, Byr4p–Cdc16p dissociates from SPBs, allowing Spg1p to be activated to its GTP-bound state (Li et al., 2000). Cdc7p preferentially binds Spg1p-GTP (Fankhauser and Simanis, 1994; Sohrmann et al., 1998). Upon entry into mitosis, Byr4p–Cdc16p localizes to the old SPB from which Cdc7p dissociates, terminating further SIN signaling from that SPB (Cerutti and Simanis, 1999; Li et al., 2000). Therefore, early in mitosis, Cdc7p localizes symmetrically to the two SPBs, but becomes asymmetric during late anaphase.
Cytokinesis fails without SIN signaling, generating multinucleated cells (Nurse et al., 1976). Loss of function mutations of any SIN component from Spg1p to Sid2p cause these cytokinetic defects (Hachet and Simanis, 2008). SIN signaling is not required for condensation of nodes into a contractile ring structure (Wu et al., 2003), but when mutations compromise SIN activity, the rings in many cells are less homogeneous than normal, and many rings fail to complete constriction (Hachet and Simanis, 2008; Huang et al., 2008; Mishra et al., 2004). Artificial activation of SIN signaling induces contractile ring assembly during any stage of cell cycle, producing multi-septated cells (Minet et al., 1979). Thus, the SIN is capable of driving ring assembly on its own (Schmidt et al., 1997). Overexpression of the Polo homolog Plo1p can also drive contractile ring formation and septation during interphase (Ohkura et al., 1995; Tanaka et al., 2001).
Here, we made quantitative measurements of the numbers of Cdc7p–EGFP molecules on SPBs to relate SIN activity to cell cycle events and learned that a ring assembles at the peak of Cdc7p–EGFP recruitment to the old SPB, and ring constriction begins at the peak of Cdc7p–EGFP recruitment to the new SPB. To determine how SIN activity influences the assembly, maturation and constriction of contractile rings, we measured the timing of cell cycle events in strains with spg1-106 and cdc16-116 temperature-sensitive mutants at a semi-restrictive temperature. When SIN activity was low in spg1-106 mutant cells at 32°C, cytokinetic nodes formed contractile rings slower than wild-type cells. These rings accumulated low levels of the F-BAR protein Cdc15p–GFP only transiently, and most rings disintegrated during constriction. In cdc16-116 mutant cells at 32°C, contractile rings assembled and began to constrict similar to wild-type cells, but when the SIN activity on both SPBs persisted beyond the end of mitosis, ring proteins remained at the division site, which delayed cell separation. Thus, SIN activity is required to recruit Cdc15p–GFP and build a mechanically stable contractile ring capable of full constriction, but is not required for triggering the onset of constriction. The decline in SIN activity at the end of mitosis contributes to the disassembly of the contractile ring and cell separation.
RESULTS
Quantification of SIN activity in the spg1-106 and cdc16-116 strains
We used temperature-sensitive (ts) mutant strains to vary SIN activity. At 36°C the ts allele of spg1-106 lowers the SIN activity and the ts allele of cdc16-116 increases SIN activity (Simanis, 2015). We assessed SIN activity in these strains using quantitative fluorescence microscopy to measure the numbers of molecules of the top level SIN kinase Cdc7p–EGFP on new and old SPBs (Fig. 1) and the appearance of Mob1p–GFP in the contractile ring at the end of the SIN (Fig. S1). We marked SPBs in these cells with Sad1p–tdTomato or –RFP and measured the timing of events during cytokinesis with tagged contractile ring proteins. We aligned the cells in time by setting the separation of SPBs as time zero (Wu et al., 2003).
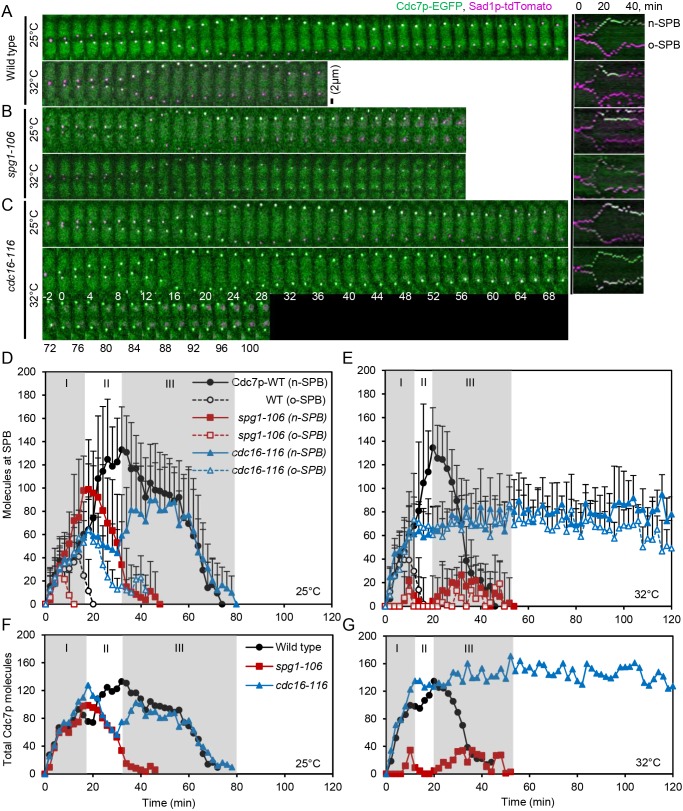
Fig. 1.
The number of Cdc7p–EGFP molecules at new and old SPBs depends on SIN activity. Data were collected in experiment 2 (see Materials and Methods). (A–C) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) Cdc7p–EGFP and (magenta) Sad1p–tdTomato at 25°C (top panels) and after shifting to 32°C for 60 min (lower panels). The right panel shows corresponding kymographs of new (n) and old (o) SPBs. The SPBs separated in each cell at time 0 min. Scale bar: 2 µm. (A) Wild-type cells. (B) spg1-106 mutant cells. (C) cdc16-116 mutant cells. (D–G) Measurements of SIN activity from counts of Cdc7p–EGFP molecules on SPBs versus cell cycle time in wild-type cells, spg1-106 mutant cells and cdc16-116 mutant cells. Data are means±s.d. Time 0 min is when the SPBs separated in images of Sad1p–tdTomato. Gray shading marks cell cycle stages: I, ring assembly; II, ring maturation; III, ring constriction. (D,F) Temperature: 25°C. (E,G) Temperature shifted to 32°C for 60 min before starting measurements. (D,E) Cdc7p–EGFP molecules at new and old SPBs. (F,G) Total Cdc7p–EGFP molecules at both SPBs from the data in D and E. Symbols and numbers of cells measured were: (D) Temperature: 25°C. Wild-type cells: ●, new SPB, n=14; ○, old SPB, n=14. spg1-106 cells:  , new SPB, n=26;
, new SPB, n=26;  , old SPB, n=26. cdc16-116 cells:
, old SPB, n=26. cdc16-116 cells:  , new SPB, n=14;
, new SPB, n=14;  , old SPB, n=14. (E) Temperature: 32°C. Wild-type cells: ●, new SPB, n=19; ○, old SPB, n=19. spg1-106 cells:
, old SPB, n=14. (E) Temperature: 32°C. Wild-type cells: ●, new SPB, n=19; ○, old SPB, n=19. spg1-106 cells:  , new SPB, n=10;
, new SPB, n=10;  , old SPB, n=10. cdc16-116 cells:
, old SPB, n=10. cdc16-116 cells:  , new SPB, n=10;
, new SPB, n=10;  , old SPB, n=10.
, old SPB, n=10.
Judging from fluorescent antibody staining (Cerutti and Simanis, 1999; Grallert et al., 2004; Sohrmann et al., 1998) and the fluorescence intensity of Cdc7p–GFP in live cells (Magidson et al., 2006) the SIN kinase Cdc7p first associates with both SPBs at the onset of mitosis but then disappears from the old SPB while building up to peak levels at the new SPB during anaphase B. The asymmetric distribution of Cdc7p at SPBs is required for proper SIN signaling, as shown by SIN mutants (Chan et al., 2017; García-Cortés and McCollum, 2009; Sohrmann et al., 1998). The number of molecules of Cdc7p associated with SPBs during cytokinesis was known (Wu and Pollard, 2005), but not how these numbers depend upon SIN activity. We confirmed that mitotic cells have constant total numbers of Cdc7p–EGFP molecules during mitosis: 4263±590 (mean±s.d.) copies in wild-type cells (Wu and Pollard, 2005), 3443±406 copies in spg1-106 mutant cells, and 4441±523 copies in cdc16-116 mutant cells.
The time course of SIN activation on SPBs depends on temperature in wild-type cells (Fig. 1). At 25°C, both the new and old SPBs accumulated Cdc7p–EGFP at the same rate for 14 min after SPB separation. Thereafter, Cdc7p–EGFP at the old SPB declined from a peak of 40±29 (mean±s.d.) molecules, while Cdc7p–EGFP at the new SPB increased steadily to a peak of 133±36 molecules at 32 min (Fig. 1D). At 32°C, both SPBs of wild-type cells accumulated the normal numbers of Cdc7p–EGFP more rapidly with the peak numbers occurring at 10 min and 20 min.
The spg1-106 mutant strain only accumulated a few molecules of Cdc7p–EGFP at either SPB at 32°C, indicating that the GTPase Spg1p at the top of the SIN was inactive, so the kinase Cdc7p was not bound where it could be activated on SPBs (Fig. 1E). At 25°C, the peak numbers of Cdc7p–EGFP on both SPBs were ∼30% less in spg1-106 mutant cells than the wild-type cells and this Cdc7p–EGFP dissociated prematurely from both SPBs (Fig. 1D). Thus, even at 25°C the Spg1p activity was low enough to compromise the localization of Cdc7p–EGFP at the time when contractile rings normally start to constrict.
In the cdc16-116 mutant strain at 32°C, equal numbers of Cdc7p–EGFP accumulated at both SPBs and persisted at ∼80 molecules each for the 2 h duration of the experiment. We used the difference in Cdc7p–EGFP intensity early in mitosis to distinguish the new and old SPBs, but appreciate that this was ambiguous in some cells (Fig. 1C) and was not observed in previous work (Wachowicz et al., 2015). At 25°C, these cdc16-116 mutant cells accumulated the same total numbers of Cdc7p–EGFP on the combined SPBs as wild-type cells (Fig. 1F), but the numbers of Cdc7p–EGFP at the old SPB overshot, while the accumulation of Cdc7p–EGFP at the new SPB lagged behind as Cdc7p-EGFP on the old SPB slowly declined (Fig. 1D).
Mob1p is the partner of the Sid2p kinase at the end of the SIN cascade; Mob1p–GFP began to accumulate on SPBs during interphase and later appeared in the contractile ring during mitosis in wild-type cells (Hou et al., 2000; Salimova et al., 2000), spg1-106 mutant cells and cdc16-116 mutant cells at 25°C (Fig. S1A), and wild-type cells and cdc16-116 mutant cells at 32°C (Fig. S1B). By contrast, we did not detect Mob1p–GFP in the contractile ring at 32°C in spg1-106 mutant cells. Thus, neither Cdc7p at the top, nor Mob1p at the bottom of the SIN localized at their sites of activity in spg1-106 mutant cells at 32°C. The behavior of these markers shows that the spg1-106 and cdc16-116 mutations alter both ends of the SIN at 32°C.
Phenotypes of the spg1-106 and cdc16-116 strains over a range of temperatures
We characterized the phenotypes of the spg1-106 and cdc16-116 mutant strains at 25°C, 32°C and 36°C (Fig. 2; Table 1). The spg1-106 mutant cells are not viable above 30°C (Jin et al., 2006), but we could observe their behavior after shifting cells to 32°C or 36°C. Wild-type cells essentially never fail to complete cytokinesis, but the spg1-106 mutant strain has two defects during cell division even at 25°C, where it is viable (Fig. 2A). First, spg1-106 mutant cells rarely failed to complete cytokinesis during our 2 h movies at 25°C, but 23% of the cells were elongated and had two nuclei, so they must have failed to complete a previous round of division. A total of 94% of these binucleated cells made at least one successful cleavage furrow and had separated into two binucleated daughter cells by ∼80 min after SPB separation (Fig. 2A). Second, cells with one nucleus divided normally, but 6% of the binucleated cells lysed during ingression of the cleavage furrow ∼60 min after SPB separation, accounting for the dead cells in mixed populations (Fig. 2A). To replenish the steady-state population of binucleated cells, ∼2% of uninucleate spg1-106 cells must fail to complete cytokinesis at 25°C.
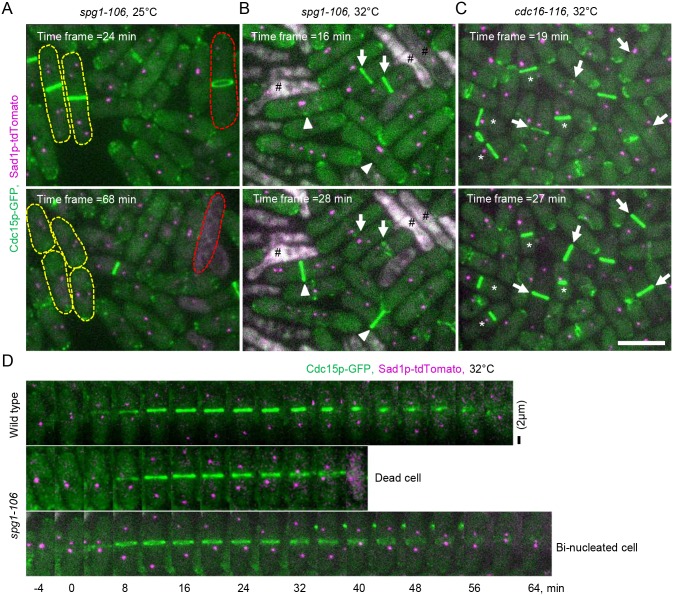
Fig. 2.
Phenotypes of SIN mutant cells at 25°C and 32°C. (A–C) Maximum intensity projections of fluorescence micrographs of asynchronous populations of cells at two times at 25°C and 60 min after shifting to 32°C. Green, Cdc15p-GFP; magenta, Sad1p–tdTomato. (A) spg1-106 mutant cells at 25°C, (B) spg1-106 mutant cells at 32°C, and (C) cdc16-116 mutant cells at 32°C. Yellow dashed lines outline two multinucleated cells that each divided into daughter cells with two nuclei. The red dashed line outlines a multinucleated cell that formed a contractile ring but lysed as the ring constricted. Arrows mark contractile rings of mitotic cells; arrowheads mark contractile rings of elongated, multinucleated cells; (#) lysed cells; (*) contractile rings in interphase cells. Scale bar: 10 µm. (D) Time series of maximum intensity projections of fluorescence micrographs at 32°C immediately shifted from 25°C to 32°C. Top panel, wild-type cells; middle panel and lower panel, spg1-106 mutant cells. Time 0 min is when the SPBs separated in each cell. Scale bar: 2 µm.
Table 1.
Probabilities of adverse outcomes during cytokinesis of spg1-106 and cdc16-116 mutant cells
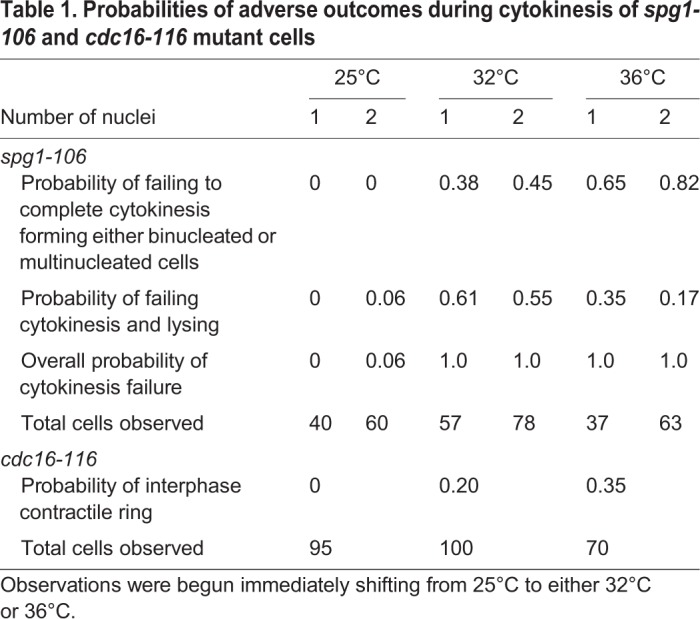
The probabilities of both of these adverse outcomes of spg1-106 mutant cells were higher at 32°C and 36°C where all of the cells failed to complete cytokinesis and either increased their number of nuclei or died (Fig. 2B,D; Table 1). The probability of lysis during cytokinesis was higher at 32°C than at 36°C, as observed previously (Jin et al., 2006), perhaps because the cells spend a little more time fruitlessly attempting to divide at 32°C. These two cytokinesis defects account for the mixtures of normal cells, elongated cells with multiple nuclei and dead cells in populations of spg1-106 mutant cells at the three temperatures. The phenotypes of spg1-B8 and sid2-250 mutant cells are stronger, as neither assembled contractile rings at 36°C (Hachet and Simanis, 2008).
These defects in cytokinesis appeared ∼1 h after shifting of spg1-106 mutant cells to 32°C. Binucleated cells and cells with single nuclei at 32°C failed to complete cytokinesis, and started to lyse during cytokinesis within 72 min or 76 min, respectively. When an asynchronous population of spg1-106 mutant cells was shifted to 36°C, some cells failed to complete cytokinesis and lysed. We first observed these defects after 64 min at 36°C in cells with two nuclei and after 44 min at 36°C in cells with single nuclei.
At 25°C, cdc16-116 mutants were indistinguishable from wild-type cells, but defects appeared at 32°C where we observed mixed populations of cells. Many cells formed contractile rings normally, but 20% of cells with a single nucleus and SPB formed a contractile ring during interphase at 32°C by ∼80 min after shifting to 32°C and 35% formed interphase contractile rings after ∼30 min at 36°C (Hachet and Simanis, 2008) (Fig. 2C; Table 1).
We characterized cytokinesis by spg1-106 and cdc16-116 mutant cells at 32°C, because incubating these ts strains at 32°C for 1 h was sufficient to inhibit or activate the SIN judging from the accumulation of Cdc7p–EGFP at the SPBs at the top of the SIN (Fig. 1) and of Mob1p–GFP in the contractile ring at the end of the SIN (Fig. S1B). To measure the timing of contractile ring assembly and constriction with altered SIN activity, we selected, from mixed populations of mutant cells, a sample of cells that appeared normal and had one nucleus and SPB. We excluded binucleated cells from our sample, because of their abnormal morphology and duplicated SPBs.
Timing of contractile ring assembly, maturation and constriction
A previous study showed that the myosin-II regulatory light chain Rlc1p–GFP did not form homogenous contractile rings when SIN activity was low in a spg1-B8 mutant strain at the restrictive temperature (36°C) (Hachet and Simanis, 2008). We extended the analysis of contractile ring assembly defects in SIN mutants through time lapse analysis of strains with GFP tags on six other contractile ring proteins, the anillin Mid1p (Mid1p–mEGFP), the conventional myosin-II Myo2p heavy chain (mEGFP–Myo2p), the IQGAP Rng2p (mEGFP–Rng2p), the formin Cdc12p (Cdc12p–3GFP), the F-BAR protein Cdc15p (Cdc15p–GFP) and the unconventional myosin-II heavy chain Myp2p (Myp2p–GFP). We tagged the two myosin-II heavy chains, because Rlc1p is a light chain for both Myo2p, which drives the assembly of the ring and contributes to its constriction, and Myp2p joins the contractile ring after it is fully formed and also contributes to its constriction (Laplante et al., 2015). Thus, Rlc1p tracks both myosin proteins. Cdc15p fused to fluorescent proteins may not be fully functional judging from synthetic interactions with domain deletions of the IQGAP Rng2p (Tebbs and Pollard, 2013). The Cdc15p–GFP construct used here functions normally in wild-type cells but may sensitize the spg1-106 and cdc16-116 mutant strains.
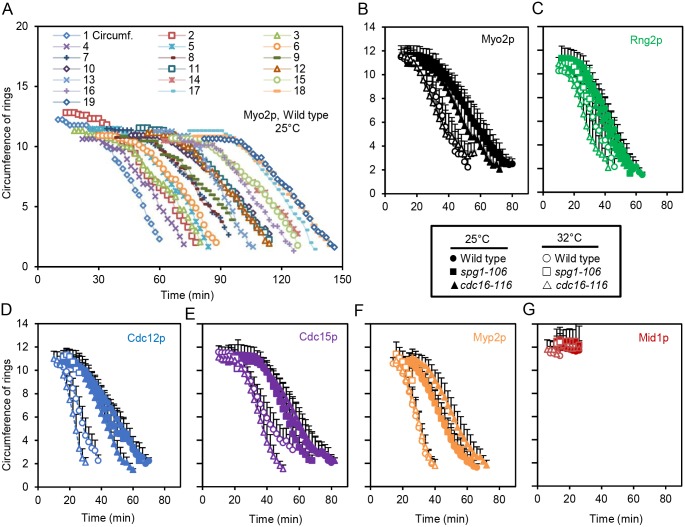
We studied these markers in wild-type cells and spg1-106 mutant cells and cdc16-116 mutant cells at 25°C (Fig. S2) and 32°C (Figs 3A–C and 5A–C). We measured the time course of cytokinesis with outcomes plots of the fractions of cells at each time point that reached three end points. We defined the time of contractile ring assembly (Fig. 3F; Table S1) as the point when nodes containing the marker proteins coalesced into a contractile ring with homogenous fluorescence intensity (Figs 3A–C and 5A–C). We identified the onset of ring constriction as time when the diameter started to decrease (Fig. 3H; Table S1). The maturation period is the interval between completion of contractile ring assembly and the onset of ring constriction (Fig. 3G; Table S2). We also measured the rate of ring constriction as the change in ring circumference over time (Fig. 4; Table S3).
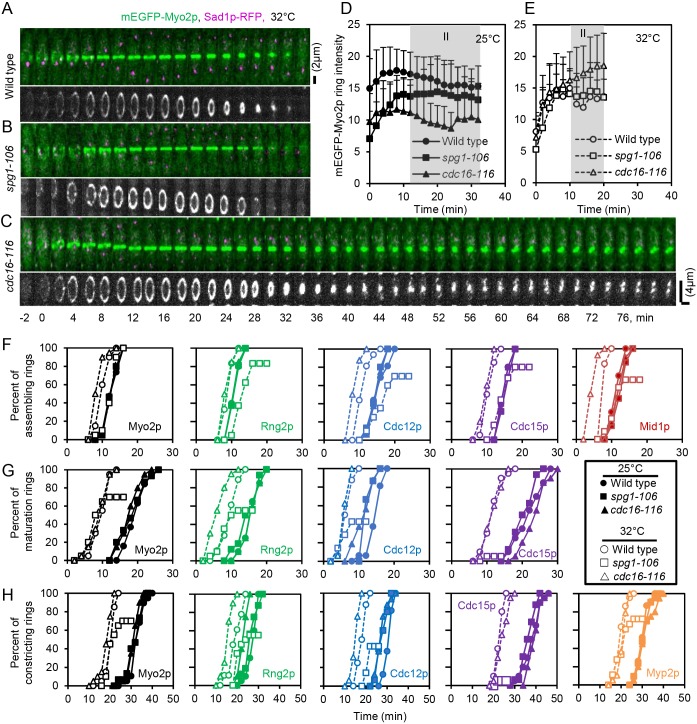
Fig. 3.
Influence of SIN activity on the time course of contractile ring assembly, maturation and constriction. (A–C) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) mEGFP–Myo2p and (magenta) Sad1p–RFP, and grayscale 3D reconstructions of rings at 32°C. (A) Wild-type cell. (B) spg1-106 mutant cell. (C) cdc16-116 mutant cell. Scale bars: 2 µm (upper panels of A–C); 4 µm (lower panels of A–C). (D,E) Outcome plots of mEGFP–Myo2p ring intensity with time 0 min set at SPB separation in wild-type cells (●, n=12; ○, n=12), spg1-106 mutant cells (■, n=10; □, n=10) and cdc16-116 mutant cells (▴, n=10; △, n=10). Gray shading marks the ring maturation phase (II) of the cell cycle. Filled symbols and solid lines are at 25°C, and open symbols and dashed lines are observations after 60 min at 32°C. Data are means±s.d. (F–H) Outcome plots of the time course of (F) cells completing assembly of a contractile ring, (G) the maturation period and (H) the onset of constriction. (●,○) Wild-type cells, (■,□) spg1-106 mutant cells and (▴,△) cdc16-116 mutant cells. Filled symbols and solid lines are at 25°C, and open symbols and dashed lines are observations after 60 min at 32°C. Symbols and numbers of cells measured were: Mid1p–mEGFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; mEGFP–Myo2p, ●, n=19; ○, n=21; ■, n=20; □, n=18; ▴, n=20; △, n=19; mEGFP–Rng2p,
, n=19; mEGFP–Myo2p, ●, n=19; ○, n=21; ■, n=20; □, n=18; ▴, n=20; △, n=19; mEGFP–Rng2p,  , n=20,
, n=20,  , n=22;
, n=22;  , n=20,
, n=20,  , n=18;
, n=18;  , n=20,
, n=20,  , n=19; Cdc12p–3GFP,
, n=19; Cdc12p–3GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; Cdc15p–GFP,
, n=19; Cdc15p–GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; Myp2p–GFP,
, n=19; Myp2p–GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19.
, n=19.
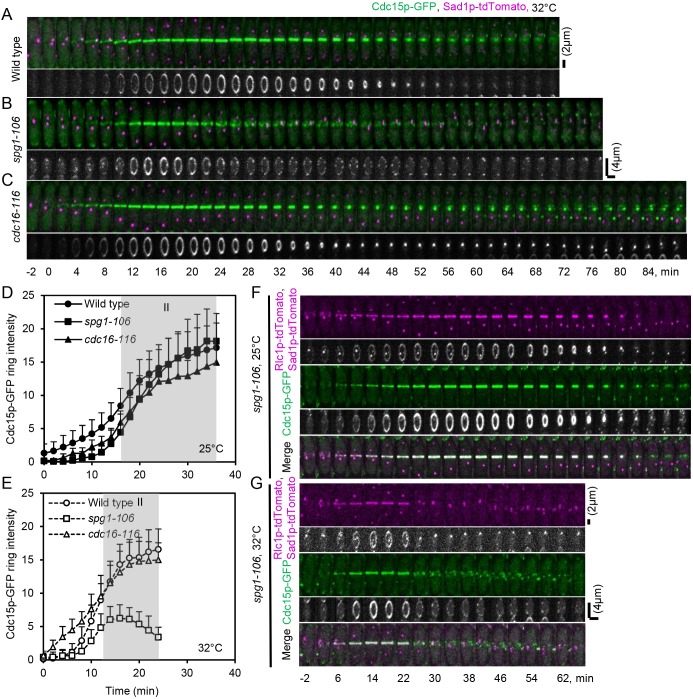
Fig. 5.
Influence of SIN activity on Cdc15p–GFP ring intensity in maturation time. (A–C) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) Cdc15p–GFP and (magenta) Sad1p–tdTomato, and grayscale 3D reconstructions of these rings at 32°C. (A) Wild-type cell. (B) spg1-106 mutant cell. (C) cdc16-116 mutant cell. Time 0 min is when SPBs separated in each cell. Scale bars: 2 µm (upper panels of A–C); 4 µm (lower panels of A–C). (D,E) Outcome plots of Cdc15p–GFP ring intensity with time 0 min at SPB separation. Filled symbols and solid lines are at 25°C, and open symbols and dashed lines are observations after 60 min at 32°C. Data are means±s.d. Gray shading marks the ring maturation phase (II) of the cell cycle. Symbols and numbers of cells were: wild-type cells, ●, n=15, ○, n=10; spg1-106 mutant cells, ■, n=10, □, n=10; and cdc16-116 mutant cells, ▴, n=15, △, n=10. (F,G) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) Cdc15p–GFP and (magenta) Rlc1p–tdTomato and Sad1p–tdTomato, and grayscale 3D reconstructions of these rings in spg1-106 mutant cells with time 0 min at SPB separation. (F) Temperature: 25°C. (G) Temperature: 32°C for 60 min. Scale bars: 2 µm (fluorescence micrographs); 4 µm (3D reconstructions).
Fig. 4.
Influence of SIN activity on the circumference of rings. (A–G) Timecourse of the constriction of the contractile ring measured as circumference with time 0 min at SPB separation. The results are mean±s.d. circumferences. We used these plots to measure the onset and rates of constriction. (●,○) wild-type cells, (■,□) spg1-106 mutant cells and (▴,△) cdc16-116 mutant cells. Filled symbols and solid lines are at 25°C, and open symbols and dashed lines are observations after 60 min at 32°C. (A) Individual rings marked with mEGFP–Myo2p in wild-type cells at 25°C, n=19. The plots are shifted by 4 min for better visualization. (B) mEGFP–Myo2p, (C) mEGFP–Rng2p, (D) Cdc12p–3GFP, (E) Cdc15p–GFP, (F) Myp2p–GFP, (G) Mid1p–mEGFP. Symbols and numbers of cells: Mid1p–mEGFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; mEGFP–Myo2p, ●, n=19; ○, n=21; ■, n=20; □, n=18; ▴, n=20; △, n=19; mEGFP–Rng2p,
, n=19; mEGFP–Myo2p, ●, n=19; ○, n=21; ■, n=20; □, n=18; ▴, n=20; △, n=19; mEGFP–Rng2p,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; Cdc12p–3GFP,
, n=19; Cdc12p–3GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; Cdc15p–GFP,
, n=19; Cdc15p–GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19; Myp2p–GFP,
, n=19; Myp2p–GFP,  , n=20;
, n=20;  , n=22;
, n=22;  , n=20;
, n=20;  , n=18;
, n=18;  , n=20;
, n=20;  , n=19.
, n=19.
Contractile ring assembly
In wild-type cells at 25°C, rings marked with Mid1p–mEGFP, mEGFP–Rng2p, mEGFP–Myo2p, Cdc12p–3GFP or Cdc15p–GFP all assembled with average times of ∼12–16 min after SPB separation. Myp2p–GFP arrived ∼9 min later in fully assembled contractile rings. At 25°C, each of these markers also assembled over the same time course in the spg1-106 and cdc16-116 mutant strains, which had the same total levels of Cdc7p–EGFP on SPBs as wild-type cells at this time (Fig. 1F).
Contractile rings assembled faster at 32°C than at 25°C in wild-type cells (Wu et al., 2003) and in cdc16-116 mutant cells with an inactive Cdc16p GAP (Fig. 3F; Table S1). Rings were complete in wild-type cells 2 to 4 min earlier than at 25°C, while the cdc16-116 mutation reduced the time further for most markers. The exceptions were mEGFP–Rng2p and Cdc15p–GFP, which assembled at the same time in wild-type cells and cdc16-116 mutant cells. The SIN activity marker Cdc7p–EGFP accumulated normally on both SPBs in this mutant during ring assembly (Fig. 1E,G).
By contrast, contractile ring assembly in spg1-106 mutant cells with low SIN activity on both SPBs at 32°C (Fig. 1E,G) differed from wild-type cells in two ways: (1) all five markers assembled into contractile rings ∼3 min slower than wild-type cells; and (2) 17–34% of the cells (depending on the marker) failed to make homogenous contractile rings (Fig. S3; Table S1), as observed previously for Rlc1p in spg1-B8 mutant cells at 36°C (Hachet and Simanis, 2008). These observations confirm that contractile ring assembly is less reliable if SIN activity is low (Fig. 1E).
At 32°C, the unconventional myosin-II Myp2p–GFP joined contractile rings at the same time in wild-type cells, cdc16-116 mutant cells and spg1-106 mutant cells. Thus, the SIN does not influence this process.
Onset of ring constriction
At 25°C, rings marked with four of the five proteins began to constrict in wild-type cells at 28–33 min after SPB separation (Fig. 3H; Table S1), the time when the numbers of Cdc7p kinase molecules peaked on the new SPB (Fig. 1D). The exception was the strain with Cdc15p–GFP, where rings in all three strains began constricting later, at time ∼38 min. This may reflect a small compromise in the function of tagged Cdc15p–GFP. The onset of ring constriction in strains with the other four markers did not differ significantly from wild-type cells in the spg1-106 and cdc16-116 mutant strains at 25°C, except for rings marked with mEGFP–Rng2p, which began to constrict earlier than the other strains in the cdc16-116 mutant. The normal constriction time in spg1-106 mutant strain is noteworthy, since the total Cdc7p–EGFP on SPBs was very low at 30 min (Fig. 1F).
At 32°C, rings in wild-type cells marked with any of five proteins constricted earlier than at 25°C (Fig. 3H; Table S1), again coincident with peak of Cdc7p–EGFP recruitment to the new SPB (Fig. 1E,G). The cdc16-116 mutant strain with persistent, symmetrical SIN activity on both SPBs (Fig. 1E) constricted rings at the same time as wild-type cells. Exceptions were strains with tagged mEGFP–Rng2p and Cdc12p–3GFP, which constricted earlier than in wild-type cells. Only a subset of spg1-106 mutant cells with very low SIN activity managed to form contractile rings at 32°C, but those that constricted began at about the same time or slightly later than wild-type cells in spite of the fact that virtually all of the Cdc7p–EGFP had dissociated from the new SPB and that most rings ultimately failed to complete constriction (Figs 3H and 4; Table S1). Thus, although the peak in recruitment of Cdc7p–EGFP to the new SPB coincides with the onset of ring constriction, and SIN activity is required assemble a ring capable of constriction, SIN activity does not appear to act as the timer for ring constriction. Instead some other aspect of the cell cycle clock may trigger ring constriction.
The SPBs migrated from the cell tips at the end of anaphase B back closer to the middle of the spg1-106 mutant cells than is the case in wild-type cells (Figs 3B and 5B). Nuclear positioning depends on the post-anaphase array of microtubules (Pardo and Nurse, 2003), which forms from γ-tubulin in a microtubule organizing center adjacent to the contractile ring. The post-anaphase microtubule array may depend on SIN activity (Heitz et al., 2001) and may be compromised in SIN mutants with ‘fragile’ contractile rings (Hachet and Simanis, 2008). This defect in SPB-mediated nuclear positioning is also seen in septation mutants (Hagan and Yanagida, 1997; Hagan and Hyams, 1988).
Ring maturation
Cells with fully assembled contractile rings pause for a maturation period before constricting. At 25°C, the average duration of this interval ranged from 15 and 22 min for the four ring markers that persist from cytokinesis nodes into the contractile ring (mEGFP–Myo2p, mEGFP–Rng2p, Cdc12p–3GFP and Cdc15p–GFP) in wild-type cells and the spg1-106 and cdc16-116 mutant strains (Fig. 3G; Table S2). Myp2p–GFP arrived after rings are fully formed (Bezanilla et al., 2000) and Mid1p–mEGFP dissociated from contractile rings during the maturation period (Sohrmann et al., 1996), so they could not be used to measure the maturation period.
The maturation period was shorter (∼6 to 11 min) at 32°C than 25°C for rings marked by mEGFP–Myo2p, mEGFP–Rng2p, Cdc12p–3GFP or Cdc15p–GFP (Fig. 3G; Table S2). At 32°C, the cdc16-116 mutant strain accumulated 60–70 copies of Cdc7p–EGFP on both SPBs at the start of maturation time rather than the normal strong bias of Cdc7p-EGFP for the new SPB (Fig. 1E). Rings marked by mEGFP–Myo2p, Cdc15p–GFP or Cdc12p–3GFP in these cdc16-116 mutant cells matured normally, but rings marked by mEGFP–Rng2p matured faster than in wild-type cells.
At 32°C, the spg1-106 mutant cells accumulated only small numbers of Cdc7p–EGFP on either SPB during the maturation time (Fig. 1E), so the SIN activity was low, but rings maintained normal numbers of mEGFP–Myo2p (Fig. 3E), mEGFP–Rng2p and Cdc12p–3GFP (data not shown). By contrast, maturing rings in spg1-106 mutant cells accumulated less of the F-BAR protein Cdc15p–GFP than wild-type cells (Fig. 5E). After time 18 min, the Cdc15p–GFP fluorescence declined and broke into spots similar to nodes (Fig. 5B), as observed by Hachet and Simanis (2008). These spots of Cdc15p–GFP then disappeared from the middle plane over ∼60 min. Some rings marked with mEGFP–Myo2p (Table S1), mEGFP–Rng2p or Cdc12p–3GFP started to constrict at the normal time of ∼23 min, but these proteins dissociated prematurely from rings (Fig. 3B) and many rings failed to constrict (mEGFP–Myo2p, 30%; mEGFP–Rng2p, 45%; Cdc12p–3GFP, 57%; and Cdc15p–GFP, 95%) (Fig. 3G). To understand how the disintegration of Cdc15p–GFP into spots relates to the disappearance of other markers from the ring, we co-expressed Cdc15p–GFP and Rlc1p–tdTomato in spg1-106 mutant cells. At 32°C, with low SIN activity Cdc15p–GFP fluorescence broke into spots at 22 min after SPB separation, concurrent with the uniform disappearance of Rlc1p–tdTomato (Fig. 5G). These observations confirm that SIN activity is required for the maturation and stability of contractile rings. The maintenance of Cdc12p–3GFP, mEGFP–Rng2p and mEGFP–Myo2p in maturing rings is less sensitive to low SIN activity than the accumulation of Cdc15p–GFP, perhaps due to its subtle loss of function.
Mid1p–mEGFP dissociates from fully formed contractile rings during the maturation period. This transition was earlier in cdc16-116 mutants but normal in spg1-106 mutants (Table S2). Thus, SIN activity has only a minor influence on the dissociation of Mid1p–mEGFP from the contractile ring during the maturation period.
Rate of ring constriction
Rings marked with one of five of the fluorescent fusion proteins constricted at the same rates at 25°C in wild-type cells and the mutant spg1-106 and cdc16-116 strains (Fig. 4; Table S3). At 32°C, rings in wild-type cells constricted at about twice the rate seen at 25°C. The exception was rings in cells with Cdc15p–GFP, which constricted slower than others. Rings in the cdc16-116 mutant strain with persistent SIN activity on both SPBs (Fig. 1E) constricted at about the same rates as wild-type cells (Fig. 4; Table S3).
By contrast, ring constriction was severely compromised in spg1-106 mutant cells at 32°C with very low SIN activity during anaphase (Fig. 1E). Constriction failed in all cells with rings marked with five different fusion proteins. Between 28% (Myp2p–GFP) and 95% (Cdc15p–GFP) of the cells showed no sign of constriction and disassembled in place (Cdc15p–GFP, Fig. 5B). The rest began to constrict at approximately the same time as wild-type cells, but the rings fell apart after constricting partially at normal rates (Fig. 4B–F; Table S3).
Influence of SIN activity on Bgs1p–mEGFP recruitment in cell cycle events
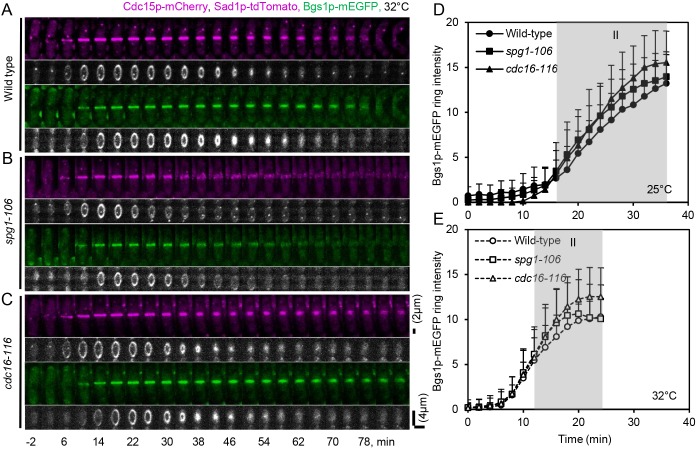
During contractile ring maturation, SIN initiates the recruitment of the β-glucan synthetase Bgs1p to the plasma membrane next to the contractile ring to synthesize the primary septum (Cortes et al., 2002), and Cdc15p may also help to anchor the ring to the plasma membrane (Roberts-Galbraith et al., 2009). When Cdc15p is mutated or depleted from cells, the contractile ring slides along the cortex (Arasada and Pollard, 2014) and Bgs1p is transported slowly from the trans-Golgi network to the plasma membrane (Arasada and Pollard, 2014). We found that maturing rings in spg1-106 mutant cells accumulated less of the F-BAR protein Cdc15p–GFP than wild-type cells (Fig. 5E). To understand how Bgs1p recruitment to the division site depends on SIN activity and Cdc15p, we co-expressed Cdc15p–mCherry and Bgs1p–mEGFP in both spg1-106 and cdc16-116 mutant cells (Fig. 6).
Fig. 6.
Influence of SIN activity on Bgs1p-mEGFP recruitment to the division site. (A-C) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) Bgs1p-mEGFP and (magenta) Sad1p-tdTomato and Cdc15p-mCherry, and gray-scale 3D reconstructions of the fluorescence at the cleavage furrows of these cells after 60 min at 32°C. (A) Wild-type cell. (B) spg1-106 mutant cell. (C) cdc16-116 mutant cell. Time 0 min is when SPBs separated at each cell. Scale bars: 2 µm (fluorescence micrographs of A–C) and 4 µm (3D reconstructions of A-C). (D,E) Outcome plots of Bgs1p-mEGFP ring intensity with time 0 min at SPB separation. Symbols and numbers of cells: wild-type cells, ●, n=16, ○, n=19; spg1-106 mutant cells, ■, n=19, □, n=19; and cdc16-116 mutant cells, ▴, n=11, △, n=19. Filled symbols and solid lines are at 25°C, and open symbols and dashed lines are observations after 60 min at 32°C. Data points are means±s.d. Gray shading marks the ring maturation phase (II) of the cell cycle.
At both 25°C and 32°C spg1-106 mutant cells accumulated the same numbers of Bgs1p–mEGFP molecules at the division site (Fig. 6D,E) in same time (Fig. S4D) as wild-type cells, but, at 32°C, these Bgs1p–mEGFP molecules started to disperse at same time that the Cdc15p–mCherry fluorescence in rings broke into spots and dispersed (Fig. 6B). These results show that spg1-106 mutant cells with low SIN activity transported Bgs1p–mEGFP normally from the Golgi to the plasma membrane, in spite of the fact that less Cdc15p–mCherry accumulated in the contractile ring. However, Bgs1p-mEGFP around the equator dispersed when the contractile ring fragmented (Fig. 6B).
At both 25°C and 32°C cdc16-116 mutant cells accumulated same numbers of Bgs1p–mEGFP molecules at the division site (Fig. 6D,E) but faster than wild-type cells (Fig. S4D). However, at 32°C, with persistent high SIN activity, Bgs1p–mEGFP along with Mob1p–GFP (Fig. S1B), Cdc15p–mCherry (Fig. 6C) and other contractile ring proteins (Figs 3C and 5C) remained at the division site longer than normal and cell separation was delayed. Disappearance of Bgs1p–mEGFP from next to the contractile ring may be necessary to ensure proper separation into daughter cells.
DISCUSSION
Quantitative analysis of SIN activity across the cell cycle
Our quantitative measurements of Cdc7p–EGFP on SPBs show how SIN activity is linked to cell cycle events (Fig. 1D,E). In wild-type cells at both 25°C and 32°C, the peak numbers of Cdc7p–EGFP on the two SPBs coincide with two critical cytokinesis events: Cdc7p–EGFP peaks on the old SPB just as nodes finish condensing into a contractile ring, whereas Cdc7p–EGFP peaks at a much higher level on the new SPB (∼3% of total in our hands) as the ring starts to constrict (Fig. 1D,E).
We detected only small fluctuating numbers of ∼20 molecules of Cdc7p–EGFP associated with both SPBs in the spg1-106 strain at 32°C (Fig. 1) consistent with no Cdc7p being detected with fluorescent antibodies on SPBs in the spg1-B8 mutant strain (Sohrmann et al., 1998). This behavior of Cdc7p–EGFP at the top of the SIN and the lack of appearance of Mob1p–GFP at the contractile ring at the bottom of the SIN establish that the spg1-106 mutation attenuates both ends of the SIN at 32°C. Even at 25°C, the spg1-106 mutation reduced the accumulation of Cdc7p–EGFP on both SPBs, although cytokinesis was normal. The outcome is different when the terminal SIN kinase Sid2p is mutated. Normal numbers of Cdc7p localized symmetrically to both SPBs in the sid2-250 mutant strain at both 25°C and 36°C (Feoktistova et al., 2012; Wachowicz et al., 2015), presumably because active Spg1p at the top of the network anchors Cdc7p to the SPBs.
The temperature-sensitive cdc16-116 mutation activates the Spg1 GTPase at the top of the SIN by inhibiting the GAP activity that inactivates Spg1p. Even at 25°C, the cdc16-116 mutation subtly altered the balance of Cdc7p–EGFP on the SPBs without affecting cytokinesis (Fig. 1). At 32°C, the cdc16-116 mutant cells accumulated equal numbers of Cdc7p–EGFP molecules on both SPBs, and these persisted for more than 2 h at total numbers approximately equal to the peak number on the new SPB in wild-type cells during anaphase. The symmetrical, persistent activation of Cdc7p–EGFP on both SPBs had a minimal impact on the assembly or initial constriction of the contractile ring, although disassembly of the contractile ring and cell separation were delayed.
Influence of SIN activity on contractile ring assembly and constriction
Our quantitative measurements with multiple markers in live cells confirmed the pioneering observations made with the Rlc1p marker showing that the low level of SIN activity early in mitosis (Fig. 1) is not required for cytokinesis nodes to condense around the equator of the cell but that contractile rings have imperfections without SIN activity (Hachet and Simanis, 2008). The spg1-106 mutant cells with low SIN activity at 32°C recruited Mid1p–mEGFP, mEGFP–Myo2p, mEGFP–Rng2p, Cdc12p–3GFP and Cdc15p–GFP to nodes that condensed into contractile rings in just a few minutes slower than wild-type cells. As in sid2-250 mutant cells with low SIN activity at 36°C (Hachet and Simanis, 2008), spg1-106 mutant cells recruited less Cdc15p–GFP to the contractile ring, although the numbers of mEGFP–Rng2p, Cdc12p–3GFP and mEGFP–Myo2p molecules were normal.
Although rings assemble normally in cells with low SIN activity, they have mechanical defects. Rings were less homogenous than normal in up to one third of cells with tagged Mid1p–mEGFP, mEGFP–Rng2p, Cdc12p–3GFP or Cdc15p–GFP, although they appeared normal in all cells with tagged mEGFP–Myo2p. Hachet and Simanis (2008) concluded that SIN activity is not required “to maintain a properly assembled ring”, but regardless of the marker, we found that rings are less stable in spg1-106 mutant cells at 32°C than in wild-type cells. Fully formed rings marked with Cdc15p–GFP broke up into spots similar to nodes before fading away, similar to what was found by Hachet and Simanis (2008), while mEGFP–Myo2p, mEGFP–Rng2p and Cdc12p–3GFP dissociated from fully formed rings over ∼30 min without constricting. Thus, our data show that SIN activity is required to stabilize fully formed contractile rings, as shown previously (Alcaide-Gavilan et al., 2014). This differs from other mutations that prevent ring constriction, such as cps1-191 cells with defective Bgs1p, where these markers persist in non-constricting rings (Arasada and Pollard, 2014).
In agreement with previous work with other mutants with low SIN activity (Lee et al., 2012), ring constriction was compromised in spg1-106 mutant cells at 32°C (Figs 3H and 4B–F). Ring constriction was delayed in some cells and failed completely in all cells with tagged Cdc15p–GFP and a subset of cells in the other tagged strains (Cdc15p–GFP>Cdc12p–3GFP >mEGFP–Rng2p >mEGFP–Myo2p=Myp2p–GFP), indicative of synthetic interactions between the fluorescent protein tags and the spg1-106 mutation. Nevertheless, rings constricted at the same rate as wild-type cells in spg1-106 mutant cells at 32°C until they disintegrated (Table S3). Therefore, other cell cycle regulators appear to time the onset of ring constriction. Depletion of Cdc15p also delays the onset of ring constriction (Arasada and Pollard, 2014).
Possible mechanisms
The mechanisms by which the SIN regulates contractile ring assembly, stability and constriction have been unclear, in part, because of limited knowledge about target substrates of the SIN kinases. However, the terminal kinase Sid2p has two established substrates relevant to cytokinesis (Chen et al., 2008) and our findings.
The Sid2p kinase phosphorylates the Cdc14p-like Clp1p phosphatase and regulates its localization to the contractile ring. Dephosphorylation by Clp1p during mitosis allows Cdc15p to accumulate in the contractile ring during the last minutes of ring assembly and the maturation period (Chen et al., 2008; Clifford et al., 2008; Fankhauser et al., 1995; Roberts-Galbraith et al., 2010; Wachtler et al., 2006) and to interact with the C2 domain protein Fic1p, paxillin Pxl1p and another F-BAR protein Imp2p (Ge and Balasubramanian, 2008; Roberts-Galbraith et al., 2009; Wachtler et al., 2006). These interactions of dephosphorylated Cdc15p may stabilize the contractile ring, the most important function of SIN activity during cytokinesis revealed by our observations. The mechanical instability of rings in cells with low SIN activity can explain both the small delay in the onset of ring constriction in some cells and the disintegration of rings during ring constriction.
A second function of Cdc15p is to direct the β-glucansynthase Bgs1p from the Golgi to the equator, where this transmembrane enzyme helps to anchor the contractile ring (Arasada and Pollard, 2014; Cortes et al., 2002; Liu et al., 2002). This translocation of Bgs1p–mEGFP is normal in cells with low SIN activity (Fig. 6). This makes sense, because phosphorylated Cdc15p participates in clathrin-mediated vesicle formation during endocytosis (Arasada and Pollard, 2011) and presumably also during vesicle budding from the Golgi. Deletion of the Clp1p phosphatase produces similar, but milder, cytokinesis defects than depletion of Cdc15. The lack of dephosphorylation of Cdc15p can explain most of these defects, but the slow Bgs1p transport to the plasma membrane may arise because Clp1p also acts on proteins involved in vesicle transport (Chen et al., 2013).
The unstable contractile rings in cells with low SIN activity differ from the stable but poorly anchored contractile rings that slide from the center of cells with compromised or deficient Cdc15p, Clp1p or Bgs1p (Arasada and Pollard, 2014; Pardo and Nurse, 2003; Roberts-Galbraith et al., 2009). Therefore, low SIN activity compromises the mechanical properties of contractile rings rather than their anchoring to the membrane.
Sid2p also phosphorylates the formin Cdc12p and allows it to bundle actin filaments (Bohnert et al., 2013), as required to form a compact ring (Li et al., 2016; Vavylonis et al., 2008; Wu et al., 2001). Interactions between Cdc15p and Cdc12p (Carnahan and Gould, 2003) may also be relevant to explaining the SIN phenotypes, given that both interphase SIN activation and overexpression of Cdc15p causes the production of rings assembled from extended filamentous structures independently from spatial cues provided by Mid1p (Hachet and Simanis, 2008).
One must consider the transient activation of the cell cycle kinases Cdk1–cyclin B and the polo kinase Plo1p to understand the influence of the SIN on cytokinesis. When they are active, these kinases not only push the cell into mitosis but also cooperatively phosphorylate Byr4p, removing it and the Cdc16p GAP from the mitotic SPBs and allowing Cdc7p to localize to both SPBs. Contractile ring constriction and septation depend on degradation of cyclin B and loss of Cdk1 activity during anaphase (Chang et al., 2001; Dischinger et al., 2008; Guertin et al., 2000). Cyclin proteolysis does not depend on inactivation the SIN (Guertin et al., 2000), but SIN may mediate the response of cells to the inactivation of Cdk1–cyclin B (Balasubramanian et al., 2004) by allowing Byr4p–Cdc16p to return to the old SPB (Rachfall et al., 2014) and promoting the association of Sid1p–Cdc14p with the new SPB (Guertin et al., 2000) to establish SIN asymmetry. Low GAP activity in cdc16-116 cells at 32°C results in Cdc7p localizing on both SPBs during anaphase. High Spg1p activity may limit the return of the Byr4p–Cdc16p GAP complex to the old SPB.
Other contractile ring proteins are phosphorylated during mitosis, including the anillin Mid1p (Sohrmann et al., 1996), the Myo2p heavy chain (Motegi et al., 2004; Mulvihill et al., 2001) and the myosin-II regulatory light chain Rlc1p (Loo and Balasubramanian, 2008; Pollard et al., 2017), but SIN signaling has not been linked to phosphorylation of these proteins.
Cytokinetic events not influenced by SIN activity
We confirmed that localization of Myp2p–GFP to fully formed contractile rings does not depend on SIN activity (Wu et al., 2003), and found that high SIN activity does not influence the timing. This was unexpected, since Myp2p–GFP joins the ring about the same time that SIN activity peaks at the old SPB. Therefore, another mechanism controls the association of this unconventional myosin-II with actin filaments in maturing contractile rings.
The anillin Mid1p–mEGFP disappears from contractile rings as they mature before the time that Cdc7p–EGFP increases to a peak on the new SPB and artificially induced high SIN activity disperses Cdr2p and Mid1p from interphase nodes (Pu et al., 2015). We found that Mid1p–mEGFP dissociates from the ring earlier in cdc16-116 mutant cells with high SIN activity on both SPBs, so asymmetrical distribution of Cdc7p–EGFP on SPBs is not required. Surprisingly, Mid1p-mEGFP leaves maturing contractile rings at the normal time in spg1-106 mutants with low SIN activity. Therefore, other cell cycle regulators must contribute to the dissociation of Mid1p–mEGFP from rings.
MATERIALS AND METHODS
Strains and growth conditions
Table S4 lists the strains used in this study. All fluorescent protein tags were integrated at native chromosomal loci, so fusion proteins were expressed under the control of endogenous promoters (Bähler et al., 1998). All strains were obtained from laboratory stocks or by crossing laboratory stock strains. Cells were grown in YE5S medium and maintained in mid-log phase [optical density at 595 nm (OD595)<0.6] for 36 h before time lapse analysis at room temperature.
Microscopy and image analysis
Cells for live-cell microscopy were collected from liquid cultures, centrifuged at 5000 rpm, and then washed twice with EMM5S medium. Cells were resuspended in EMM5S medium and spotted on an agar pads prepared in EMM5S liquid medium at room temperature. For imaging at elevated temperatures, cells were incubated at 32°C for 1 h in liquid suspension prior to imaging on agar pads with the exception of Fig. 2D and Table 1 where the cells were imaged immediately after shifting from 25°C to either 32°C or 36°C. We carried out separate experiments on two different microscopes. For experiment 1, agar pads were placed in a temperature block to maintain the temperature at 32°C or on a glass slide at 25°C. Images were acquired on a Nikon TE2000E inverted microscope (Nikon, Melville, NY), a 60×1.4 NA objective with a 1.5× optical magnifier, an Andor Revolution spinning disk confocal system (Andor, Belfast, UK) equipped with a CSU-X1 confocal head (Yokogawa, Tokyo, Japan) and an Andor iXon EM+888 EM CCD camera. Confocal illumination was with 488 nm and 640 nm laser lines controlled with an Andor Laser Combiner. Emission wavelengths were selected with bandpass filters from Chroma Technology (Bellows Falls, VT) mounted in a Sutter LB10W-2800 filter wheel. All hardware and image acquisition were controlled with µ-Manager software (Edelstein et al., 2014). For experiment 2, agar pads were placed on a glass slide at 25°C or the slide was mounted under a therminator block to maintain the temperature of the cells at 32°C (Davies et al., 2017). We used an inverted Olympus IX-71 microscope (Olympus America, Center Valley, PA) with a 100×1.4 NA objective, solid-state lasers (Coherent Inc., Santa Clara, CA), a spinning disk confocal head (Yokogawa CSU- X1) and an electron multiplying CCD camera (EMCCD iXon 897, Andor) using µ-Manager software. Time-lapse images were collected as a z-series of 21 confocal slices at 0.5 µm intervals encompassing the entire cell every 2 min at both 25°C and 32°C. Images were visualized as sum projections of all the confocal slices using ImageJ (National Institutes of Health, Bethesda, MD). We defined SPB separation as time zero of the cellular clock (Wu et al., 2003). We measured the diameter of a ring by drawing a line across the ring after sum projections of an image with maximum intensity by using ImageJ, and calculated the circumference. The rate of ring constriction was measured with linear fits to plots of the circumference versus time. The fluorescence of a ring measured by using the formula, corrected total cell fluorescence (CTCF)=integrated density−(area of selected cell×mean fluorescence of background readings) by ImageJ (McCloy et al., 2014). We used a log rank test [MedCalc software for Windows 10 (version 18), MedCalc Software, Ostend, Belgium] to compare time courses of outcome plots with wild-type cells and calculated a P-value for each pair of curves. We used a Student's t-test to calculate the statistical the significance of differences in mean times for contractile ring assembly, maturation and onset of constriction and for the rate of contractile ring constriction.
Tables S1 and S2 summarize data from a biological replicate for four contractile ring markers. Minor differences in timing in the two experiments are probably due to small differences in temperature on the two microscopes. The small but statistically significant differences for the timing of some markers in a given strain likely arise from effects of the fluorescent protein tags.
Counting numbers of molecules of Cdc7p–EGFP at SPBs
We measured the local numbers of Cdc7p-EGFP molecules from time-lapse images, collected from z-series of 17 confocal slices at 0.36 µm intervals encompassing the entire cell every 2 min at both 25°C and 32°C. After sum projections of slices, the local number was calculated from the images by taking the measuring a circle 7 pixels (0.92 µm) in diameter in the regions of both SPBs. Images were corrected for camera noise and uneven illumination. The intensity of wild-type cells was subtracted to remove auto-fluorescence and background from the measurement. The number of molecules was calculated from the fluorescence intensities by using a calibration curve constructed using mEGFP- or GFP-tagged proteins (capping protein subunit, Acp2p; α-actinin, Ain1p; actin related protein 2, Arp2p; actin related protein 3, Arp3p; actin related protein C5, ArpC5p; fimbrin, Fim1p; and type II myosin, Myo2p) (Wu and Pollard, 2005). Time courses of the average numbers of molecules were produced by aligning individual time courses to time zero, the separation of spindle pole bodies, and averaging the number of molecules at each time point.
Supplementary Material
Acknowledgements
The authors thank Paul Forscher for sharing his confocal system for imaging and Samatha Dundon, Alexander Epstein, Harry Doernberg and other members of the Pollard lab for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: T.D.P.; Validation: S.K.D., T.D.P.; Formal analysis: S.K.D.; Investigation: S.K.D.; Resources: T.D.P.; Writing - original draft: S.K.D., T.D.P.; Writing - review & editing: S.K.D., T.D.P.; Visualization: S.K.D., T.D.P.; Supervision: T.D.P.; Project administration: T.D.P.; Funding acquisition: T.D.P.
Funding
This work was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM026132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.216895.supplemental
References
- Akamatsu M., Berro J., Pu K.-M., Tebbs I. R. and Pollard T. D. (2014). Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. J. Cell Biol. 204, 977-988. 10.1083/jcb.201307174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide-Gavilan M., Lahoz A., Daga R. R. and Jimenez J. (2014). Feedback regulation of SIN by Etd1 and Rho1 in fission yeast. Genetics 196, 455-470. 10.1534/genetics.113.155218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R. and Pollard T. D. (2011). Distinct roles for F-BAR proteins Cdc15p and Bzz1p in actin polymerization at sites of endocytosis in fission yeast. Curr. Biol. 21, 1450-1459. 10.1016/j.cub.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R. and Pollard T. D. (2014). Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 8, 1533-1544. 10.1016/j.celrep.2014.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Wu J.-Q., Longtine M. S., Shah N. G., McKenzie A. III, Steever A. B., Wach A., Philippsen P. and Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S. and Gould K. L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E. and Glotzer M. (2004). Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14, R806-R818. 10.1016/j.cub.2004.09.022 [DOI] [PubMed] [Google Scholar]
- Bezanilla M., Wilson J. M. and Pollard T. D. (2000). Fission yeast myosin-II isoforms assemble into contractile rings at distinct times during mitosis. Curr. Biol. 10, 397-400. 10.1016/S0960-9822(00)00420-6 [DOI] [PubMed] [Google Scholar]
- Bohnert K. A., Grzegorzewska A. P., Willet A. H., Vander Kooi C. W., Kovar D. R. and Gould K. L. (2013). SIN-dependent phosphoinhibition of formin multimerization controls fission yeast cytokinesis. Genes Dev. 27, 2164-2177. 10.1101/gad.224154.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan R. H. and Gould K. L. (2003). The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 162, 851-862. 10.1083/jcb.200305012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti L. and Simanis V. (1999). Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J. Cell Sci. 112, 2313-2321. [DOI] [PubMed] [Google Scholar]
- Chan K. Y., Alonso-Nunez M., Grallert A., Tanaka K., Connolly Y., Smith D. L. and Hagan I. M. (2017). Dialogue between centrosomal entrance and exit scaffold pathways regulates mitotic commitment. J. Cell Biol. 216, 2795-2812. 10.1083/jcb.201702172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Morrell J. L., Feoktistova A. and Gould K. L. (2001). Study of cyclin proteolysis in anaphase-promoting complex (APC) mutant cells reveals the requirement for APC function in the final steps of the fission yeast septation initiation network. Mol. Cell. Biol. 21, 6681-6694. 10.1128/MCB.21.19.6681-6694.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. T., Feoktistova A., Chen J. S., Shim Y. S., Clifford D. M., Gould K. L. and McCollum D. (2008). The SIN kinase Sid2 regulates cytoplasmic retention of the S. pombe Cdc14-like phosphatase Clp1. Curr. Biol. 18, 1594-1599. 10.1016/j.cub.2008.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-S., Broadus M. R., McLean J. R., Feoktistova A., Ren L. and Gould K. L. (2013). Comprehensive proteomics analysis reveals new substrates and regulators of the fission yeast clp1/cdc14 phosphatase. Mol. Cell. Proteomics 12, 1074-1086. 10.1074/mcp.M112.025924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D. M., Wolfe B. A., Roberts-Galbraith R. H., McDonald W. H., Yates J. R. III and Gould K. L. (2008). The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin-related Mid1. J. Cell Biol. 181, 79-88. 10.1083/jcb.200709060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J. C. G., Ishiguro J., Duran A. and Ribas J. C. (2002). Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115, 4081-4096. 10.1242/jcs.00085 [DOI] [PubMed] [Google Scholar]
- Davies T., Sundaramoorthy S., Jordan S. N., Shirasu-Hiza M., Dumont J. and Canman J. C. (2017). Using fast-acting temperature-sensitive mutants to study cell division in Caenorhabditis elegans. Methods Cell Biol. 137, 283-306. 10.1016/bs.mcb.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Dischinger S., Krapp A., Xie L., Paulson J. R. and Simanis V. (2008). Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network. J. Cell Sci. 121, 843-853. 10.1242/jcs.021584 [DOI] [PubMed] [Google Scholar]
- Edelstein A. D., Tsuchida M. A., Amodaj N., Pinkard H., Vale R. D. and Stuurman N. (2014). Advanced methods of microscope control using muManager software. J. Biol. Methods 1, e10 10.14440/jbm.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C. and Simanis V. (1994). The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 13, 3011-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Reymond A., Cerutti L., Utzig S., Hofmann K. and Simanis V. (1995). The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell 82, 435-444. 10.1016/0092-8674(95)90432-8 [DOI] [PubMed] [Google Scholar]
- Feoktistova A., Morrell-Falvey J., Chen J.-S., Singh N. S., Balasubramanian M. K. and Gould K. L. (2012). The fission yeast septation initiation network (SIN) kinase, Sid2, is required for SIN asymmetry and regulates the SIN scaffold, Cdc11. Mol. Biol. Cell 23, 1636-1645. 10.1091/mbc.e11-09-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge K. A., Wong K., Armstrong J., Balasubramanian M. and Albright C. F. (1998). Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr. Biol. 8, 947-954. 10.1016/S0960-9822(98)70394-X [DOI] [PubMed] [Google Scholar]
- García-Cortés J. C. and McCollum D. (2009). Proper timing of cytokinesis is regulated by Schizosaccharomyces pombe Etd1. J. Cell Biol. 186, 739-753. 10.1083/jcb.200902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W. and Balasubramanian M. K. (2008). Pxl1p, a paxillin-related protein, stabilizes the actomyosin ring during cytokinesis in fission yeast. Mol. Biol. Cell 19, 1680-1692. 10.1091/mbc.e07-07-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L. and Simanis V. (1997). The control of septum formation in fission yeast. Genes Dev. 11, 2939-2951. 10.1101/gad.11.22.2939 [DOI] [PubMed] [Google Scholar]
- Grallert A., Krapp A., Bagley S., Simanis V. and Hagan I. M. (2004). Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev. 18, 1007-1021. 10.1101/gad.296204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D. A., Chang L., Irshad F., Gould K. L. and McCollum D. (2000). The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 19, 1803-1815. 10.1093/emboj/19.8.1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet O. and Simanis V. (2008). Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes Dev. 22, 3205-3216. 10.1101/gad.1697208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. M. and Hyams J. S. (1988). The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89, 343-357. [DOI] [PubMed] [Google Scholar]
- Hagan I. and Yanagida M. (1997). Evidence for cell cycle-specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 110, 1851-1866. [DOI] [PubMed] [Google Scholar]
- Heitz M. J., Petersen J., Valovin S. and Hagan I. M. (2001). MTOC formation during mitotic exit in fission yeast. J. Cell Sci. 114, 4521-4532. [DOI] [PubMed] [Google Scholar]
- Hou M.-C., Salek J. and McCollum D. (2000). Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr. Biol. 10, 619-622. 10.1016/S0960-9822(00)00492-9 [DOI] [PubMed] [Google Scholar]
- Huang Y., Yan H. and Balasubramanian M. K. (2008). Assembly of normal actomyosin rings in the absence of Mid1p and cortical nodes in fission yeast. J. Cell Biol. 183, 979-988. 10.1083/jcb.200806151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q.-W., Zhou M., Bimbo A., Balasubramanian M. K. and McCollum D. (2006). A role for the septation initiation network in septum assembly revealed by genetic analysis of sid2-250 suppressors. Genetics 172, 2101-2112. 10.1534/genetics.105.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A. and Simanis V. (2008). An overview of the fission yeast septation initiation network (SIN). Biochem. Soc. Trans. 36, 411-415. 10.1042/BST0360411 [DOI] [PubMed] [Google Scholar]
- Krapp A., Schmidt S., Cano E. and Simanis V. (2001). S. pombe cdc11p, together with sid4p, provides an anchor for septation initiation network proteins on the spindle pole body. Curr. Biol. 11, 1559-1568. 10.1016/S0960-9822(01)00478-X [DOI] [PubMed] [Google Scholar]
- Laplante C., Berro J., Karatekin E., Hernandez-Leyva A., Lee R. and Pollard T. D. (2015). Three myosins contribute uniquely to the assembly and constriction of the fission yeast cytokinetic contractile ring. Curr. Biol. 25, 1955-1965. 10.1016/j.cub.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-J., Coffman V. C. and Wu J.-Q. (2012). Contractile-ring assembly in fission yeast cytokinesis: recent advances and new perspectives. Cytoskeleton 69, 751-763. 10.1002/cm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Furge K. A., Cheng Q.-C. and Albright C. F. (2000). Byr4 localizes to spindle-pole bodies in a cell cycle-regulated manner to control Cdc7 localization and septation in fission yeast. J. Biol. Chem. 275, 14381-14387. 10.1074/jbc.275.19.14381 [DOI] [PubMed] [Google Scholar]
- Li Y., Christensen J. R., Homa K. E., Hocky G. M., Fok A., Sees J. A., Voth G. A. and Kovar D. R. (2016). The F-actin bundler alpha-actinin Ain1 is tailored for ring assembly and constriction during cytokinesis in fission yeast. Mol. Biol. Cell 27, 1821-1833. 10.1091/mbc.e16-01-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H., Oliferenko S. and Balasubramanian M. K. (2002). The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 989-1000. 10.1091/mbc.01-12-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T.-H. and Balasubramanian M. (2008). Schizosaccharomyces pombe Pak-related protein, Pak1p/Orb2p, phosphorylates myosin regulatory light chain to inhibit cytokinesis. J. Cell Biol. 183, 785-793. 10.1083/jcb.200806127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson V., Chang F. and Khodjakov A. (2006). Regulation of cytokinesis by spindle-pole bodies. Nat. Cell Biol. 8, 891-893. 10.1038/ncb1449 [DOI] [PubMed] [Google Scholar]
- Marks J., Hagan I. M. and Hyams J. S. (1986). Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J. Cell Sci. 1986 Suppl. 5, 229-241. 10.1242/jcs.1986.Supplement_5.15 [DOI] [PubMed] [Google Scholar]
- May K. M., Watts F. Z., Jones N. and Hyams J. S. (1997). Type II myosin involved in cytokinesis in the fission yeast, Schizosaccharomyces pombe. Cell Motil. Cytoskeleton 38, 385-396. [DOI] [PubMed] [Google Scholar]
- McCloy R. A., Rogers S., Caldon C. E., Lorca T., Castro A. and Burgess A. (2014). Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13, 1400-1412. 10.4161/cc.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P. and Mitchison J. M. (1979). Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 137, 440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Trautmann S., Wang H., McCollum D. and Balasubramanian M. K. (2004). The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 117, 3897-3910. 10.1242/jcs.01244 [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Mayeux A., Paoletti A. and Nurse P. (2009). A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 459, 857-860. 10.1038/nature08074 [DOI] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K. and Mabuchi I. (2004). Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 165, 685-695. 10.1083/jcb.200402097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill D. P., Barretto C. and Hyams J. S. (2001). Localization of fission yeast type II myosin, Myo2, to the cytokinetic actin ring is regulated by phosphorylation of a C-terminal coiled-coil domain and requires a functional septation initiation network. Mol. Biol. Cell 12, 4044-4053. 10.1091/mbc.12.12.4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P. and Nasmyth K. (1976). Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146, 167-178. 10.1007/BF00268085 [DOI] [PubMed] [Google Scholar]
- Ohkura H., Hagan I. M. and Glover D. M. (1995). The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9, 1059-1073. 10.1101/gad.9.9.1059 [DOI] [PubMed] [Google Scholar]
- Paoletti A. and Chang F. (2000). Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell 11, 2757-2773. 10.1091/mbc.11.8.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M. and Nurse P. (2003). Equatorial retention of the contractile actin ring by microtubules during cytokinesis. Science 300, 1569-1574. 10.1126/science.1084671 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. and Wu J.-Q. (2010). Understanding cytokinesis: lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 11, 149-155. 10.1038/nrm2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard L. W., Bookwalter C. S., Tang Q., Krementsova E. B., Trybus K. M. and Lowey S. (2017). Fission yeast myosin Myo2 is down-regulated in actin affinity by light chain phosphorylation. Proc. Natl. Acad. Sci. USA 114, E7236-E7244. 10.1073/pnas.1703161114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu K.-M., Akamatsu M. and Pollard T. D. (2015). The septation initiation network controls the assembly of nodes containing Cdr2p for cytokinesis in fission yeast. J. Cell Sci. 128, 441-446. 10.1242/jcs.160077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfall N., Johnson A. E., Mehta S., Chen J.-S. and Gould K. L. (2014). Cdk1 promotes cytokinesis in fission yeast through activation of the septation initiation network. Mol. Biol. Cell 25, 2250-2259. 10.1091/mbc.e14-04-0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith R. H., Chen J.-S., Wang J. and Gould K. L. (2009). The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J. Cell Biol. 184, 113-127. 10.1083/jcb.200806044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith R. H., Ohi M. D., Ballif B. A., Chen J.-S., McLeod I., McDonald W. H., Gygi S. P., Yates J. R. III and Gould K. L. (2010). Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol. Cell 39, 86-99. 10.1016/j.molcel.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. A., Tomlin G. C., McDonald W. H., Snydsman B. E., Muller E. G., Yates J. R. III and Gould K. L. (2006). Ppc89 links multiple proteins, including the septation initiation network, to the core of the fission yeast spindle-pole body. Mol. Biol. Cell 17, 3793-3805. 10.1091/mbc.e06-01-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimova E., Sohrmann M., Fournier N. and Simanis V. (2000). The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J. Cell Sci. 113, 1695-1704. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Sohrmann M., Hofmann K., Woollard A. and Simanis V. (1997). The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 11, 1519-1534. 10.1101/gad.11.12.1519 [DOI] [PubMed] [Google Scholar]
- Simanis V. (2015). Pombe's thirteen - control of fission yeast cell division by the septation initiation network. J. Cell Sci. 128, 1465-1474. 10.1242/jcs.094821 [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser C., Brodbeck C. and Simanis V. (1996). The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 10, 2707-2719. 10.1101/gad.10.21.2707 [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Schmidt S., Hagan I. and Simanis V. (1998). Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 12, 84-94. 10.1101/gad.12.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Mach K. E., Chen C. Y., Reynolds T. and Albright C. F. (1996). A novel suppressor of ras1 in fission yeast, byr4, is a dosage-dependent inhibitor of cytokinesis. J. Cell Biol. 133, 1307-1319. 10.1083/jcb.133.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C. A., Morphew M. and McCollum D. (1999). Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J. Cell Biol. 146, 777-790. 10.1083/jcb.146.4.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Petersen J., MacIver F., Mulvihill D. P., Glover D. M. and Hagan I. M. (2001). The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe. EMBO J. 20, 1259-1270. 10.1093/emboj/20.6.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbs I. R. and Pollard T. D. (2013). Separate roles of IQGAP Rng2p in forming and constricting the Schizosaccharomyces pombe cytokinetic contractile ring. Mol. Biol. Cell 24, 1904-1917. 10.1091/mbc.e12-10-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin G. C., Morrell J. L. and Gould K. L. (2002). The spindle pole body protein Cdc11p links Sid4p to the fission yeast septation initiation network. Mol. Biol. Cell 13, 1203-1214. 10.1091/mbc.01-09-0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D., Wu J.-Q., Hao S., O'Shaughnessy B. and Pollard T. D. (2008). Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319, 97-100. 10.1126/science.1151086 [DOI] [PubMed] [Google Scholar]
- Wachowicz P., Chasapi A., Krapp A., Cano Del Rosario E., Schmitter D., Sage D., Unser M., Xenarios I., Rougemont J. and Simanis V. (2015). Analysis of S. pombe SIN protein association to the SPB reveals two genetically separable states of the SIN. J. Cell Sci. 128, 741-754. 10.1242/jcs.160150 [DOI] [PubMed] [Google Scholar]
- Wachtler V., Huang Y., Karagiannis J. and Balasubramanian M. K. (2006). Cell cycle-dependent roles for the FCH-domain protein Cdc15p in formation of the actomyosin ring in Schizosaccharomyces pombe. Mol. Biol. Cell 17, 3254-3266. 10.1091/mbc.e05-11-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-Q. and Pollard T. D. (2005). Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310-314. 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Bähler J. and Pringle J. R. (2001). Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol. Biol. Cell 12, 1061-1077. 10.1091/mbc.12.4.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-Q., Kuhn J. R., Kovar D. R. and Pollard T. D. (2003). Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723-734. 10.1016/S1534-5807(03)00324-1 [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Sirotkin V., Kovar D. R., Lord M., Beltzner C. C., Kuhn J. R. and Pollard T. D. (2006). Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 174, 391-402. 10.1083/jcb.200602032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.