ABSTRACT
Cholera toxin (CT) causes severe diarrhea by increasing intracellular cAMP leading to a PKA-dependent increase in Cl− secretion through CFTR and decreased Na+ absorption through inhibition of Na+/H+ exchanger 3 (NHE3; also known as SLC9A3). The mechanism(s) by which CT inhibits NHE3 is partially understood, although no drug therapy has been successful at reversing this inhibition. We now describe that CT phosphorylates an amino acid in the PDZ domain of SNX27, which inhibits SNX27-mediated trafficking of NHE3 from the early endosomes to the plasma membrane (PM), and contributes to reduced basal NHE3 activity through a mechanism that involves reduced PM expression and reduced endocytic recycling. Importantly, mutagenesis studies (Ser to Asp) showed that the effect of this phosphorylation of SNX27 phenocopies the effects seen upon loss of SNX27 function, affecting PM trafficking of cargo proteins that bind SNX27–retromer. Additionally, CT destabilizes retromer function by decreasing the amount of core retromer proteins. These effects of CT can be partially rescued by enhancing retromer stability by using ‘pharmacological chaperones’. Moreover, pharmacological chaperones can be used to increase basal and cholera toxin-inhibited NHE3 activity and fluid absorption by intestinal epithelial cells.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: SNX27, Retromer, NHE3, PDZ, Secretory diarrhea, Apical trafficking, Exocytosis, Cholera toxin, Early endosomes
Highlighted Article: Cholera toxin inhibits the exocytosis function of the SNX27-retromer complex, thereby inhibiting cargo trafficking to the PM.
INTRODUCTION
To infect human cells, pathogenic microorganisms have evolved numerous strategies to suppress host defenses and exploit host cellular signaling machinery. Specific pathogen virulence factors disable, subvert or even stimulate vesicular trafficking pathways to and from the host cell surface, which promotes pathogen entry, replication or escape. One prominent trafficking pathway that pathogens modulate or exploit by multiple mechanisms is the final step of endocytic recycling, at which cargo-containing vesicles dock at the cell surface. As one example, cholera toxin (CT) secreted by Vibrio cholerae compromises intestinal epithelial barrier function via cyclic AMP (cAMP)-induced disruption of Rab11- and exocyst-dependent delivery of endocytic recycling cargo to cell–cell junctions (Guichard et al., 2013).
CT is a classic AB toxin. CT translocates from the plasma membrane (PM) through the trans-Golgi network (TGN) into the endoplasmic reticulum (ER) in a retrograde fashion by binding the ganglioside GM1 via the B subunit of the native holotoxin (Orlandi and Fishman, 1998; Wernick et al., 2010). Once it is released from the ER lumen, the enzymatic moiety CT-A subunit causes a pathological increase in cellular cAMP levels and PKA activity via the induction of host PM adenylyl cyclase (De Haan and Hirst, 2004; Sack et al., 2004; Wernick et al., 2010). PKA then activates CFTR and inhibits the brush border (BB) Na+/H+ antiporter NHE3 (also known as SLC9A3), which is a major contributor to small intestinal Na+ absorption. Inhibition of NHE3 by CT and in other cAMP-related diarrheal diseases involves increased endocytosis, which reduces BB NHE3 expression (Musch et al., 2007, 2010). However, it is not known whether changes in NHE3 exocytosis are also caused by CT, particularly from the early endosome (EE) to the PM.
An early endosomal PDZ domain-containing protein, sorting nexin 27 (SNX27) binds and regulates exocytosis of NHE3 from the EE to the PM (Singh et al., 2015). SNX27 has important regulatory roles in EE-to-PM trafficking of multiple classes of proteins; this occurs via interacting with the retromer complex. The retromer complex is composed of the vacuolar protein sorting (VPS) trimer core sub-complex (VPS26–VPS29–VPS35) and a membrane-associated sorting nexin (SNX) dimer (SNX1–SNX2 or SNX5–SNX6) (Seaman, 2005). The retromer complex is important in regulating transmembrane receptor recycling from the EE either to the TGN or to the PM (Belenkaya et al., 2008; Seaman, 2007; Yang et al., 2008), with SNX27 involved in the EE-to-PM pathway (Joubert et al., 2004; Lauffer et al., 2010). In this function, SNX27 directly binds VPS26 via the SNX27 PDZ domain. In regulating this aspect of trafficking, the mammalian retromer complex binds other protein complexes including Wiskott–Aldrich protein and SCAR homolog (WASH) complex, actin, ankrin-repeat 50 domain, VPS-ankrin repeat domain protein and FAM21 proteins (Burd and Cullen, 2014).
Retromer-mediated trafficking defects has been implicated in a growing number of neurological diseases (Follett et al., 2014; Small et al., 2005; Zimprich et al., 2011). Until now, the effect of CT on retromer-mediated movement of cargo proteins in the intestine has not been described. In the present study, we demonstrate that CT increases phosphorylation of a serine residue in the PDZ domain of SNX27, which inhibits SNX27 binding to NHE3 and reduces NHE3 exocytosis to the PM. Moreover, CT destabilizes retromer function by reducing the expression of two core components of the retromer complex – VPS35 and VPS26. This previously undescribed activity of CT identifies a site at which CT affects PM transporters, including NHE3, and identifies a step in trafficking for the potential targeting of drug development to treat diarrheal diseases.
RESULTS
CT inhibits exocytosis of NHE3 in intestinal epithelial Caco-2/bbe cells
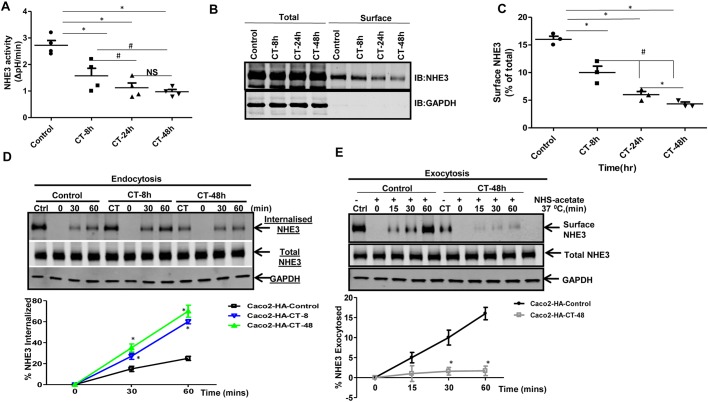
Basal NHE3 activity is known to be inhibited after CT exposure, with the effect involving reduced PM NHE3 expression and stimulation of endocytosis (Subramanya et al., 2007). However, characterization of the effects of CT on both rates of endocytosis and exocytosis of NHE3 in the same model has not been reported. To extend this characterization, we initially tested the timecourse of CT-mediated inhibition of NHE3 activity. Polarized monolayers of Caco-2/bbe cells expressing HA–NHE3 (Caco-2/bbe-HA-NHE3) were treated with CT for 8, 24 and 48 h (CT added every 12 h), and NHE3 activity was determined in the presence of 50 µM HOE694 to inhibit endogenous NHE1 and NHE2 activity (Ikuma et al., 1999). Consistent with previous studies, an 8 h CT treatment significantly decreased basal NHE3 activity (∼40%), and the effect became more pronounced at 24 h and 48 h, at which times there was a similar extent of inhibition (Fig. 1A). To further delineate the mechanism of inhibited NHE3 activity in response to CT, we determined the surface abundance of NHE3. Biotinylation-based surface abundance analysis showed that at all times studied, the level of BB NHE3 was reduced by CT exposure; however, this occurred in a time-dependent manner. Cells treated with CT for 8 h had more NHE3 on the membrane compared to cells treated for 24 h or 48 h, and the percentage of total NHE3 on the PM at all three time points of CT exposure was significantly reduced compared to the untreated control (10, 7 and 5% respectively for 8, 24 and 48 h of CT exposure, respectively, versus 15% in untreated control cells) (Fig. 1B,C). To define the effect of CT on NHE3 trafficking, we measured the rate of endocytosis and exocytosis of NHE3 in CT-treated Caco-2/bbe-HA-NHE3 cells. Endocytosis was determined using a biotinylation-based assay (Singh et al., 2015). This demonstrated similar increased rates of NHE3 endocytosis with 8 and 48 h of CT exposure (Fig. 1D) A cell surface biotinylation-based exocytosis assay showed that the rate of exocytosis of NHE3 in 8 and 48 h CT-treated cells was significantly less than that of control cells (Fig. 1E; Fig. S1). These results indicate that CT, in addition to its known effect on stimulating NHE3 endocytosis, also inhibits NHE3 exocytosis, which contributes to a lower abundance of NHE3 on the PM and to lower NHE3 activity. Since there were slightly greater CT-mediated effects on NHE3 activity and PM expression at 48 h compared to 24 h, further studies examined CT effects at early (8 h) and later times of exposure (48 h).
Fig. 1.
CT inhibits NHE3 activity, stimulates endocytosis and inhibits exocytosis in intestinal epithelial cells. (A) Initial rates of Na+/H+ exchange were measured in either untreated or CT-treated (100 ng/0.5 ml for 8 h, 24 h or 48 h) Caco-2/bbe-HA-NHE3 cells as the Na+-dependent pHi recovery by using the pH-sensitive dye BCECF. Results are means±s.e.m. with results from individual experiments shown; n=4 separate experiments. *P<0.05, comparison between control and CT-treated cells; #P<0.05, comparisons between 8 h and 24 h or 48 h CT-treated cells. NS, not significant. (B) Caco-2/bbe-HA-NHE3 cells were treated with CT (8 h, 24 h or 48 h) and surface NHE3 levels were analyzed. A representative western blot (IB) analysis is shown illustrating changes in PM expression of NHE3 in response to CT. (C) The quantification from at least three independent experiments, as in B, is shown for individual experiments expressed as the percentage of total along with the mean±s.e.m. *P<0.05, comparison between control and CT treatment; #P<0.05, comparison between 8 h and 24 h or 48 h CT-treated cells. (D) Rate of endocytosis of NHE3 in control (Ctrl) and CT-treated (100 ng/0.5 ml for 8 h or 48 h) confluent Caco-2/bbe-HA-NHE3 cells. Biotinylated cells were incubated at 37°C for 0, 30 and 60 min. The amount of endocytosis of NHE3 (internalization of surface NHE3) at the indicated time points was determined by a GSH-resistant endocytosis assay. A representative blot from three independent experiments is shown. Quantitative analysis of the amount of internalized/endocytosed NHE3 at each time point was calculated as the percentage of surface NHE3 of the corresponding control groups, which were always kept at 4°C and never exposed to GSH. Results are means±s.d., n=3. *P<0.05, comparison between control and CT-treated cells. (E) Rate of exocytosis of NHE3 in control (Ctrl) and CT-treated (100 ng/0.5 ml for 48 h) Caco-2/bbe-HA-NHE3 cells. Cells were incubated with NHS-acetate and then incubated for 0, 15, 30, and 60 min at 37°C to allow exocytosis of NHE3. Cells were then chilled to 4°C, and newly inserted NHE3 (exocytosed NHE3) was biotinylated and subjected to quantitative western blotting. The plot represent the mean±s.d. of three independent experiments. *P<0.05, comparison between control and CT-treated cells.
CT increases phosphorylation of Ser49 in the PDZ domain of SNX27
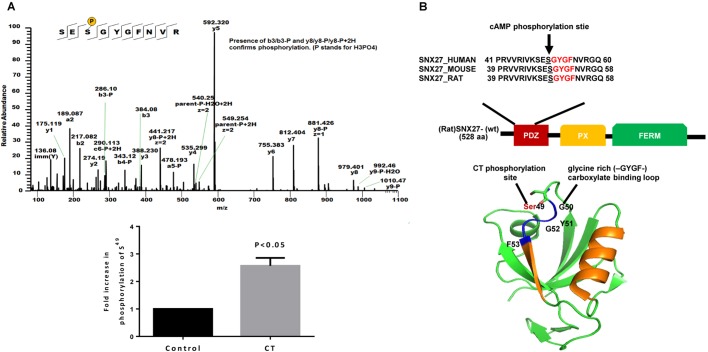
It has been previously shown that exocytosis of NHE3 from EEs to the PM is regulated by SNX27, and that SNX27 directly binds NHE3 (Singh et al., 2015). Therefore, we next investigated whether CT modified SNX27 by using two mass spectroscopy (MS)-based proteomic approaches, both based on immunoprecipitation (IP) of rat SNX27 stably expressed in HEK293 cells that were either untreated or exposed to holo-CT for 8 h. In the first approach, spectral counting from liquid chromatography tandem MS (LC-MS/MS) analysis of two experiments showed a 3.5× average increase (2.5×, 4.5×) in phosphorylation of the peptide SES49GYGFNVR at Ser49. A similar increase in phosphorylation on Ser49 was caused by forskolin (2.6×) when studied in one of the experiments. No changes in phosphorylation were detected on Ser47. Similarly, E. coli heat-stable enterotoxin (STa), which is also known to inhibit NHE3 activity by altering its trafficking, did not increase phosphorylation at Ser49 of SNX27 (data not shown), suggesting mechanistic differences between cAMP- and cGMP-mediated pathways. In the second approach, isobaric mass tags (tandem mass tags; TMT 10-plex) were used to directly quantitatively compare the IPs from three separately prepared biological replicates of unstimulated and stimulated cells (Fig. 2A). CT caused a 2.6±0.39× (mean±s.e.m.) increase in phosphorylation at Ser49 (2.3×, 2.5×, 2.9× for each replicate). A TMT-labeled phosphorylated Ser47 peptide was not detected.
Fig. 2.
CT increases phosphorylation of Ser49 in the PDZ domain of SNX27. (A) Identification of SNX27 phosphorylation site Ser49 by isobaric mass tag (TMT 10-plex) LC-MS/MS analysis of GFP-tagged rat SNX27. GFP–SNX27 (rat) was immunoprecipitated from HEK293 control and CT-treated (100 ng/0.5 ml for 8 h) cells and proteolyzed for LC-MS/MS analysis. The increase in phosphorylation of Ser49 was identified within the PDZ domain of SNX27. The MS/MS spectrum (A) shows the phosphorylation at Ser49. Phosphorylation analysis using MS was repeated in three separate experiments. The maximum Mascot score for Ser49 was 82. Results are shown below as means±s.e.m. (B) Schematic representation of SNX27 domains. The expanded box shows a sequence alignment of SNX27–PDZ domain (residues 41–60 human; 39–58 mouse and rat) of different species. Sequences were obtained from the Swiss/UniProt database. The CT-induced phosphorylation site Ser49 is underlined and the ligand-binding site GYGF is shown in red. A 3D image of the rat SNX27-PDZ domain was prepared using PyMOL computer software. The predicted structure shows that Ser49 is adjacent to the highly conserved GYGF carboxylate-binding loop.
Computational modeling of the SNX27-PDZ domain showed that Ser49 is immediately adjacent to the conserved GYGF motif at the entry to the ligand-binding site (Fig. 2B). Although basal phosphorylation of SNX27-Ser49 had been previously identified in rat and human tissues (Lundby et al., 2012; Zhou et al., 2013), no functional relevance of this phosphorylation has been described. We hypothesized that the increase in phosphorylation of Ser49 by CT might play a role in the regulation of SNX27-NHE3 binding.
Phosphorylation of SNX27 Ser49 regulates NHE3 basal activity and surface expression by inhibiting SNX27–NHE3 association
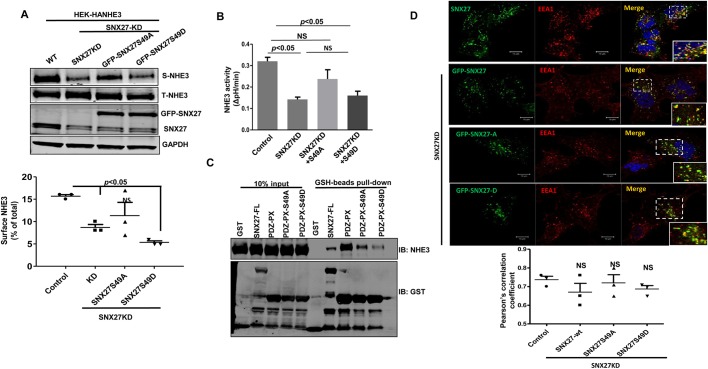
Whether CT-induced phosphorylation of Ser49 affected NHE3 basal activity and surface expression was determined. Mutants where Ser49 was replaced with a non-phosphorylatable alanine residue (S49A) or a phosphomimetic aspartic acid reside (S49D) were generated in rat-derived SNX27b (a specific splice variant) and transiently expressed in human HEK293A cells expressing HA–NH3 (HEK293A-HA-NHE3) previously transduced with shRNA lentivirus to knockdown (KD) SNX27. The SNX27 protein expression was reduced by ∼60% in SNX27 shRNA stably expressing cells. These cells were used to measure NHE3 surface expression and basal activity. Immunoblotting demonstrated expression of the rat SNX27-S49A and -S49D. Surface biotinylation assays showed that the cells containing the S49D mutant had the least NHE3 PM expression (Fig. 3A). Furthermore, the cells expressing the SNX27-S49D mutant had a similar reduction in basal NHE3 activity to that seen in SNX27KD cells (Fig. 3B). Since NHE3 surface expression largely depends upon its binding with SNX27, we next examined whether Ser49 phosphorylation altered SNX27-PDZ binding to NHE3. This was achieved by generating GST fusion proteins of the wild-type (WT) and S49A or S49D PDZ-PX domains of SNX27. An extended PDZ domain was used for this binding assay because it was previously shown that the extension of the SNX27-PDZ domain to include most of the PX domain (PDZ-PX) significantly increased SNX27 binding with NHE3 (Singh et al., 2015). Purified WT and mutant proteins were used to pulldown NHE3 from lysates of cells stably overexpressing HA–NHE3. The pulldown assay showed that the phosphomimetic S49D mutant of SNX27 pulled down less NHE3 compared to the S49A mutant or WT PDZ-PX domain of SNX27 (Fig. 3C). This indicates that the increase in phosphorylation on Ser49 decreases SNX27–NHE3 binding and also reduces NHE3 surface expression and basal activity.
Fig. 3.
Ser49 phosphorylation of SNX27 phenocopies loss of SNX27 function. (A) Cell surface proteins were biotinylated from HEK293-HA-NHE3 cells containing control shRNA or SNX27 shRNA (SNX27-KD) with or without replacement by co-transfection of rat GFP–SNX27-PDZ-S49A or -S49D mutants. Biotinylated proteins were isolated with streptavidin–Sepharose, and the amount of biotinylated NHE3 was quantified by western blotting and compared to the amount of total NHE3. NHE3 surface expression was quantified in three independent experiments (lower panel). Results are means±s.e.m. P<0.05 and NS, not significant versus control (unpaired t-test). (B) Na+/H+ exchange was measured in HEK293-HA-NHE3 cells expressing control shRNA or SNX27 shRNA with or without reconstitution with rat GFP–SNX27-PDZ-S49A or -S49D mutants. Results are means±s.e.m., n=4. P values are as indicated. (C) GST, GST–SNX27-FL, GST–SNX27-PDZ-PX, GST–SNX27-PDZ-PX-S49A or -S49D fusion proteins were mixed with HEK-HA-NHE3-SNX27KD cell lysate and then subjected to pulldown assays with GSH resin. Samples were analyzed by western blotting (IB) with antibodies against NHE3 and GST. The experiment was repeated three times with similar results, and one representative result is shown. (D) Colocalization analysis of endogenous SNX27 or GFP–SNX27 (Alexa Fluor 488) and EE marker EEA1 (Alexa Fluor 594) in HEK WT and HEK-SNX27 depleted (shRNA) cells transfected with rat GFP–SNX27-S49A or S49D mutants. Insets are magnified views of the endosomes in the dashed box. Colocalization between SNX27 and EEA1 was quantified as the Pearson's correlation coefficient over 10 images per condition containing more than 50 cells acquired from three independent experiments. Mean±s.e.m. are shown. Scale bars: 10 μm. All results were not significantly (NS) different from control (Student's t-tests).
In immunolocalization studies, we found that SNX27–GFP colocalizes prominently with EEA1-containing endosomes, as shown previously (Singh et al., 2015; Temkin et al., 2011). Similar to WT SNX27, the S49A and S49D mutants also localized to the EEA1 compartment (Fig. 3D) demonstrating that Ser49 phosphorylation does not appear to change the intracellular localization of SNX27. This was further supported by quantitative image analysis (Fig. 3D).
Increased Ser49 phosphorylation affects SNX27 binding and trafficking of multiple cargoes
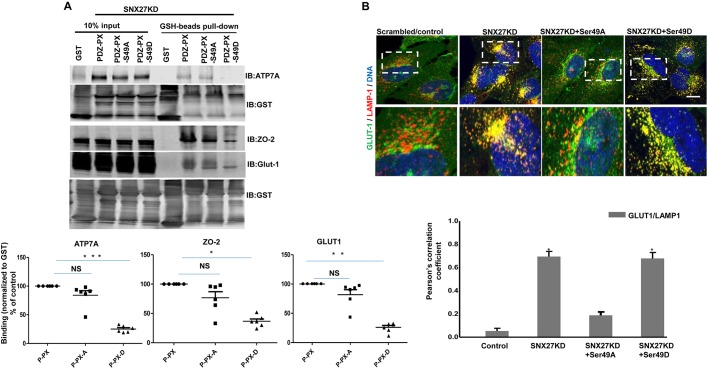
Next, we sought to investigate whether the effect of increased SNX27-Ser49 phosphorylation is limited to NHE3. To do this, we verified the binding of three other ligands previously shown to be associated with the SNX27–retromer pathway: (1) Glut-1 (also known as SLC2A1; Steinberg et al., 2013), (ii) ZO-2 (also known as TJP2; Zimmerman et al., 2013) and (3) the copper ATPase ATP7A, mutations in which causes Menkes disease (Steinberg et al., 2013). The interactions between SNX27–PDZ-PX and Glut-1, ZO-2 and ATP7A was first investigated using GST pulldown assays. GST fusion proteins of WT and SNX27 mutants (S49A/S49D) were used to pull-down cargo proteins from cell lysate prepared from Caco-2/bbe cells with SNX27KD cells. Similar to what occurred with NHE3, the unmodified SNX27 PDZ-PX domain pulled down more ATP7A, ZO-2 and Glut-1 compared to SNX27-S49A and -S49D modifications (Fig. 4A). Of note, for all three proteins, the extent of protein pulled down by WT SNX27 and SNX27-S49A were not significantly different while the phosphomimetic (D) mutation caused significantly less binding to all three proteins. Consistent with the role of SNX27 in Glut-1 trafficking, depletion of SNX27 or expression of SNX27-S49D in SNX27KD cells, led to significant mis-sorting of Glut-1 to the lysosomal compartment, whereas it is normally localized to the cell surface at the steady-state (Fig. 4B). Similarly, cell surface biotinylation studies revealed that there was decreased Glut-1 surface expression in SNX27-S49D-containing cells, which was similar to what was seen with SNX27KD, while in the presence of the SNX27-S49A, expression was similar to control (Fig. S2). These results indicate that Ser49 is critical for the interaction of multiple cargo proteins with the SNX27-PDZ domain and that CT/cAMP-mediated phosphorylation of SNX27-Ser49 disrupts these interactions and thereby regulates the PM sorting of multiple membrane proteins, including NHE3.
Fig. 4.
SNX27-PDZ domain cargo binding is regulated by Ser49 phosphorylation. (A) Caco-2/bbe-SNX27KD cell extracts (1 mg) were incubated with GST or GST fused to PDZ+PX (P-PX, amino acids 1–266), or PDZ+PX-S49A or -S49D (P-PX-A and P-PX-D) (all 1 nmol). The presence of ATP7A, ZO-2 and Glut-1 in the GST pull downs were detected by western blotting (IB, top; representative results from six independent experiments). A quantification of cargo proteins in pull downs normalized to the amount of GST from the 6 experiments is shown below as means±s.e.m. *P<0.05, **P<0.01; ***P<0.001; NS, not significant (compared to WT control). (B) HeLa cells expressing control shRNA, SNX27 shRNA and SNX27 shRNA transfected with SNX27-S49A or -S49D mutant were stained for endogenous GLUT1 (Alexa Fluor 488) and endogenous LAMP1 (Alexa Fluor 594). A representative experiment is shown above with magnified views of regions in the dashed boxes shown. Scale bars: 10 μm. Quantification of colocalization was performed across four independent experiments and is shown below. Results are shown as means±s.e.m. *P<0.05 compared to shRNA control.
Cholera toxin destabilizes the retromer by reducing the amount of core VPS proteins
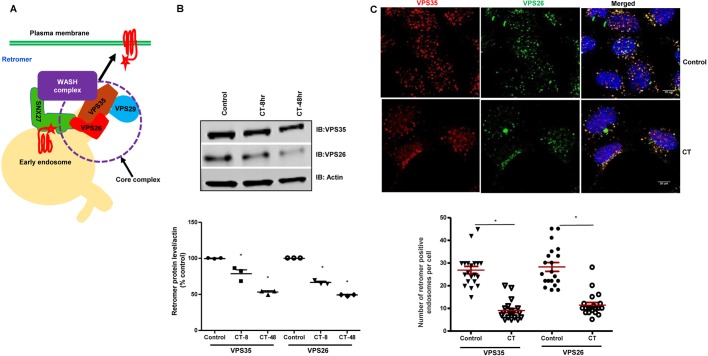
SNX27 is a component of a multi-protein assembly, the ‘retromer tubule complex’, which forms a major sorting platform on EEs (Tsvetanova et al., 2015). This complex includes the core retromer, which is composed of the VPS35–VPS29–VPS26 ternary complex. Retromer serves as a multifunctional scaffold forming an interaction hub for a wide array of endosome-associated proteins, collectively termed the retromer interactome (Fig. 5A). These interactions aid in the formation of the cargo-containing tubulovesicular membrane carriers destined for other compartments, including the Golgi and PM (Lin et al., 2015; Steinberg et al., 2013; Tsvetanova et al., 2015). Retromer-related defects have been shown to reduce trafficking of cargo out of endosomes (Bhalla et al., 2012; Vieira et al., 2010). Therefore, we investigated the effect of CT on the retromer trimeric core complex in intestinal epithelial cells and HEK293 cells. At 14 days post plating, confluent Caco-2/bbe cells were exposed to CT and the total protein levels of core retromer proteins was determined. Initially, effects on the core retromer protein VPS35 were determined, demonstrating a significant reduction in cells treated with CT for 8 h (Fig. 5B). Reduction in amount of one protein of the VPS35–VPS29–VPS26 trimeric core often leads to a secondary reduction in other retromer proteins (Fuse et al., 2015). Therefore, we also examined the levels of VPS26 and VPS29 in CT-treated Caco-2/bbe cells. VPS26 was significantly reduced while VPS29 was slightly, but not significantly, reduced (data not shown) in CT-treated Caco-2/bbe cells. This reduction was evident after 8 h of CT treatment (VPS35, 78.8±9.4%, VPS26, 66.7±2.9% versus untreated control; mean±s.e.m., P<0.05) and became more pronounced when CT was present for 48 h (VPS35, 53.3±2.9%; VPS26: 49.3±1.2% versus control; P<0.05) (Fig. 5B). However, the mRNA levels for VPS35, VPS29 and VPS26 were not affected by CT (Fig. S3). We also investigated the effects of CT (8 h) on colocalization of the retromer components in HEK293 cells via immunofluorescence. CT treatment did not appreciably alter the colocalization between VPS35 and VPS26; however, the number of VPS35-containing and VPS26-containing intracellular vesicles was significantly reduced with CT treatment compared to in untreated control cells (Fig. 5C). These results indicate that CT destabilizes retromer by reducing the levels of the core retromer proteins VPS35 and VPS26.
Fig. 5.
CT decreases retromer protein expression. (A) Schematic diagram of an EE illustrating the SNX27–retromer and the WASH complex. Branched tubules (arrow) represents discrete domains into which specific proteins are sorted and targeted to their respective destinations. (B) Expression of retromer proteins VPS35 and VPS26 in untreated and CT-treated (8 h and 4 times over 48 h) 14-day post confluent Caco-2/bbe cells. Western blot analysis of a representative blot is shown above. Quantitative analysis of the blots showing levels of VPS35 and VPS26 normalized to total levels of actin is shown below. Results are means±s.e.m., n=3. *P<0.05 compared to control. (C) Representative maximum intensity projections of HEK293 untreated and CT-treated (8 h) cells immunostained for VPS35 (Alexa Fluor 568) and VPS26 (Alexa Fluor 488). Numbers of VPS35 and VPS26 puncta in untreated (VPS35 60±8 puncta per cell and VPS26 56±12 puncta per cell) and CT-treated (VPS35 35±6 puncta per cell and VPS26 29±18 puncta per cell) cells are shown (n=20 cells studied from each of four separate experiments). Scale bars: 20 μm. Data are presented as means±s.e.m. *P<0.05.
A ‘pharmacological chaperone’ stimulates NHE3 activity in human enteroid monolayers
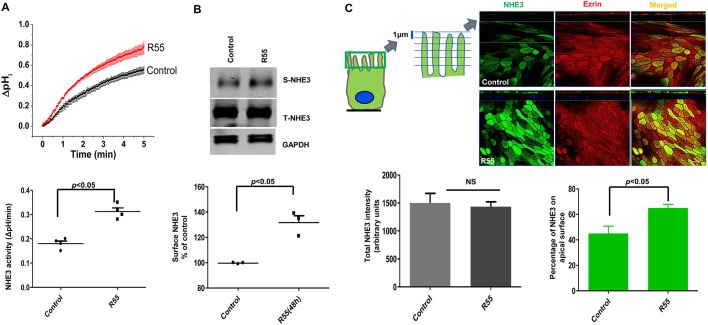
Recently, small-molecule ‘pharmacological chaperones’ that are thiophene thiourea derivatives, including R55 [thiophene-2,5-diylbis(methylene) dicarbamimidothioate dihydrochloride] have been shown to improve thermal stability of the core retromer proteins, and to increase the retromer-mediated trafficking of cargo proteins from EEs in cultured hippocampal neurons (Mecozzi et al., 2014). The effect of these compounds on intestinal ion transport physiology has not been described. Since SNX27 is part of the retromer tubule complex, we investigated the effect of R55 on NHE3 transport activity and trafficking in human intestinal stem cell-derived enteroid monolayers. For this study, we used differentiated enteroid monolayers (5 days post Wnt3A removal) derived from upper small intestine (duodenum) of healthy donors. Enteroid monolayers (Wnt3A-free; Schuijers et al., 2015; Noel et al., 2017) were treated with 5 μM R55 or vehicle control (0.2% DMSO) for 48 h and NHE3 activity was measured. The reported in vitro Kd for R55 was 5 μM (Mecozzi et al., 2014). Enteroid monolayers treated with R55 had significantly increased NHE3 basal activity (control, 0.18±0.02 ΔpH/min; R55, 0.31±0.02 ΔpH/min; P<0.05) (Fig. 6A). To determine whether the increase in basal activity was due to increased membrane trafficking, we measured NHE3 surface expression in response to R55. R55 treatment significantly increased surface expression of NHE3 (131.7±5.4%) (Fig. 6B), without changing total protein amount or NHE3 mRNA levels (Fig. S4). A similar increase in NHE3 basal activity was seen in enteroid monolayers derived from a different donor (data not shown). In addition, a similar increase in surface expression of NHE3 (142.4±3.2%) in response to R55 occurred in Caco-2/bbe cells. Consistent with this, immunofluorescence analysis also showed a significant increase in apical expression of NHE3 in response to R55 (Fig. 6C). Taken together, these findings demonstrate that R55 (a pharmacological chaperone) stimulates basal NHE3 activity by increasing PM expression of NHE3 in human intestinal epithelial cells.
Fig. 6.
A pharmacological chaperone stimulates NHE3 trafficking to the apical membrane of human enteroids and increases NHE3 activity. (A) Differentiated human enteroid monolayers (5 days post Wnt3A removal) with higher TER than undifferentiated enteroids (1458±110 Ω cm2 vs 451±19 Ω cm2, ***P<0.001) were used. Na+/H+ exchange in human enteroid monolayers was measured using the pH-sensitive dye BCECF. A single donor was used for all experiments. Basal NHE3 transport activity was measured as the initial rates of Na+-dependent intracellular alkalinization in R55- (5 μM for 48 h) or vehicle-treated monolayers. A single experiment is shown above and below are shown means±s.e.m., n=4. P values are for the comparison between control and R55 treatment. (B) A differentiated human enteroid monolayer was treated with R55 (5 μM for 48 h) and the amount of NHE3 protein in the PM was determined by surface biotinylation. GAPDH was used as a loading control. A representative experiment is shown above and below are shown results as mean±s.e.m. (n=3 separate studies on monolayers each taken from a separate passage of the same cell line). *P<0.05. (C) NHE3 expression in human enteroid monolayers was detected by immunofluorescence (confocal microscopic) analysis. A differentiated monolayer was treated with R55 as above and stained for NHE3 (Alexa Fluro 488) and phosphorylated ezrin (Alexa Fluro 568). Each panel displays an xz projection; top panels are an xy projection, bottom panels are at the level of the apical PM. The quantification of the NHE3 intensity in control and R55 treated monolayers was performed with Volocity Software. The total NHE3 amount was not increased upon R55 treatment (lower left). The percentage of apical NHE3 in the microvillar region (stacks 1–5, each stack was 1 μm in the xz dimension and 20–25 μm total cell height) normalized to total NHE3 (whole image) expression was increased with R55 treatment (lower right). At least four separate images and ten random individual areas (z sections) were analyzed for each group. Scale bar: 10 µm. Results are mean±s.e.m., n=3. P values are comparison with untreated control.
R55 prevents the CT effect on the retromer and NHE3, and increases NHE3-mediated fluid absorption in CT-treated intestinal epithelial cells
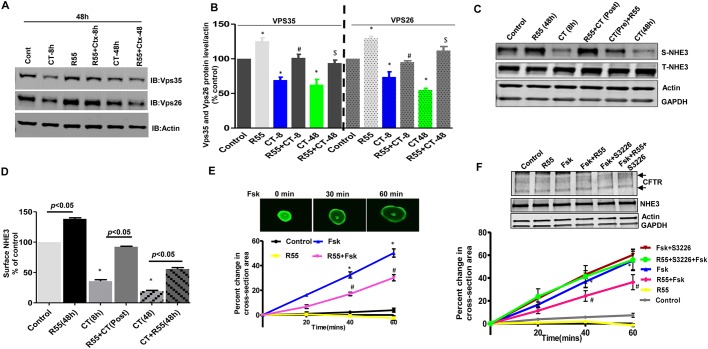
CT treatment decreased the amount of core VPS proteins (which destabilizes the trimeric core retromer), while pharmacologic chaperones stabilize the three-dimensional protein structure thereby protecting it from degradation (Ringe and Petsko, 2009). We therefore investigated the effect of R55 on the CT-induced decrease in the amounts of VPS35 and VPS26 protein. At 14 days post plating, confluent Caco-2/bbe cells were treated with R55 and/or CT for 8 h or 48 h and total changes in the level of core VPS complex proteins were measured. Compared to vehicle control, 8 h as well as 48 h of CT treatment, significantly reduced the amount of VPS35 and VPS26, while treatment with R55 alone caused a small but significant increase in the amounts of VPS35 (25±10%; mean±s.e.m., P<0.05) and VPS26 (29±7%, P<0.05). Moreover, treatment with R55 either before CT (CT-treated during last 8 h of 48 h R55 exposure) or along with CT for 48 h, prevented the reduction in the VPS35 and VPS26 amounts, leading to levels that were similar to those in untreated controls (Fig. 7A,B).
Fig. 7.
A pharmacological chaperone rescues CT-dependent retromer destabilization and stimulates fluid absorption by intestinal epithelial cells. (A) Caco-2/bbe cells were treated with either CT (8 h or 48 h) or R55 (5 μM for 48 h) or both, in pre- (CT treatment before R55 treatment) or post-combinations (CT treatment after R55 treatment) for indicated periods of time, and levels of retromer proteins VPS26 and VPS35 were analyzed using western blot analysis. A representative blot is shown. (B) Quantitative analysis of the immunoblots in A. Levels of VPS26 and VPS35 were normalized to total levels of actin (n=4 per group). *P<0.05 versus control, #P<0.05 versus CT for 8 h; $P<0.05 versus CT for 48 h. (C) Polarized Caco-2/bbe-HA-NHE3 cells were treated with CT for indicated periods of time and the effect of R55 (5 μM for 48 h) on NHE3 surface expression was analyzed in a surface biotinylation assay. S-NHE3, surface NHE3; T-NHE3, total NHE3. Results from a single experiment are shown. (D) Quantitative analysis of western blots from four separate experiments, as in C, are shown as a bar graph. The amount of surface NHE3 was normalized to total NHE3 and expressed as percentage of control. Results are means±s.e.m. P values are in comparison with the control conditions related to the individual R55 experiments. (E) FIS assay in Calcein Green-labeled human duodenal enteroids exposed to forskolin and/or R55. Upper panel, a single example of a forskolin-stimulated enteroid. Lower panel, statistical evaluation of results from four separate experiments. Surface areas, expressed as percentage increase relative to that at t=0 (100%) are indicated on the y-axis. Forskolin-stimulated (5 μM) enteroids were pre-incubated with DMSO (0.2%) or R55 (5 μM for 48 h). The surface areas of 30 enteroids were measured in each group. Results are mean±s.e.m. with average from each experiment used as n=1. *P<0.05 versus control, #P<0.05 versus forskolin. (F) Role of NHE3 in the FIS assay response to forskolin and change mediated by R55. Upper panel, a representative blot showing expression of CFTR and NHE3 in 3D enteroids used for swelling assay. Arrows indicate B and C bands of CFTR. Lower panel, normalized forskolin-induced swelling of enteroids pretreated for 1 h with DMSO or the NHE3 inhibitor S3226 (20 μM) with or without R55 (5 μM for 48 h). Contribution of NHE3 was calculated as the change induced by S3226. Results are means±s.e.m. of at least three separate experiments. *P<0.05 versus control; #P<0.05 versus forskolin.
Increasing VPS35 levels can increase retromer-mediated trafficking (Mecozzi et al., 2014; Small et al., 2005). Therefore, we tested whether R55 reversed the CT-induced decrease in NHE3 surface expression. NHE3 surface expression was measured in R55- and/or CT-treated Caco-2/bbe-HA-NHE3 cells. A biotinylation-based surface protein measurement showed that 8 h and 48 h of CT treatment significantly reduced surface NHE3 (−35.0±2.3% and −19.3±1.2%, respectively versus control; P<0.05). Importantly, CT did not change total NHE3 expression. This was further confirmed by performing degradation (half-life of total NHE3) assays from 0–24 h in control and CT-treated cells in presence of cycloheximide (100 µM) to block synthesis of new proteins. The half-life of total NHE3 was not significantly affected by CT treatment (Fig. S5). Furthermore, R55 treatment increased NHE3 (138.0±1.8%, versus control; P<0.005) surface expression. Moreover, R55 treatment either before or concurrently with CT treatment prevented the decrease in apical membrane expression of NHE3 (Fig. 7C,D). Thus, we conclude that the reduction in NHE3 PM expression mediated by CT can be restored by R55 treatment.
CT-induced diarrhea involves stimulation of Cl−/fluid secretion via increased activity of the cystic fibrosis transmembrane conductance regulator (CFTR) (Kunzelmann and Mall, 2002) and inhibition of Na+/fluid absorption mediated by NHE3 (Lin et al., 2011). We investigated the effect of R55 on NHE3-mediated fluid absorption by using the specific NHE3 inhibitor S3226. This was investigated in human 3D (closed spheroidal) enteroids using the forskolin-induced swelling (FIS) assay. Forskolin is known to stimulate CFTR-mediated fluid secretion into the lumen of small intestinal enteroids, which can be measured using live-cell microscopy (Dekkers et al., 2013). Human 3D enteroids were preincubated in HCO3−-buffered solution for 1 h and then vehicle control and R55-treated (5 μM for 48 h) enteroids were exposed to forskolin (5 μM) and a time-dependent surface area increase was measured. Forskolin caused a linear increase in the surface area of enteroids for at least 60 min as compared to vehicle control (0.2% DMSO) (P<0.005), the latter of which did not change significantly over this time. The enteroids pre-incubated with R55 (48 h) showed a small (but not significant) decrease in basal surface area over time as compared to control. In addition, enteroids treated with both R55 (48 h) and forskolin had a partially reduced FIS response compared to forskolin alone (∼30%, P<0.05 versus Fsk) (Fig. 7E,F). Previous reports have demonstrated that the FIS response is dependent on CFTR activity (Dekkers et al., 2013). We modified the assay in order to define the contribution of fluid absorption by NHE3 by including conditions in which NHE3 was inhibited with S3226 (20 μmol/l) (Schwark et al., 1998). Treatment with S3226 caused a slight increase in FIS. However, the R55 effect on FIS was totally reversed by the NHE3 inhibitor (Fig. 7F). Unlike the small effect of R55 on basal enteroid swelling, the R55 effect on forskolin-induced swelling was totally reversed by S3226 (Fig. 7F). Similar results were seen in enteroids tested from another donor (data not shown). Of note, the total amount of CFTR and NHE3 was not affect by R55 treatment (Fig. 7F). This shows that R55 stimulation of NHE3 increases intestinal fluid absorption to partially overcome the forskolin-induced fluid secretion. Cumulatively, these findings show that the retromer stabilizer R55 increases NHE3 surface expression under basal and CT-inhibited conditions and reduces forskolin-induced intestinal fluid secretion through an effect that involves a component of NHE3 stimulation.
DISCUSSION
In the current study, we provide a more complete description of the mechanism by which CT acts in producing diarrhea; we show that CT inhibits NHE3 exocytosis in addition to its already defined stimulation of endocytosis to reduce its BB expression and activity. CT also modifies the PDZ domain of SNX27 by increasing phosphorylation of Ser49. This is functionally important as the Ser49 phosphomimetic mutant of SNX27 phenocopied the effect of CT in terms of having reduced interaction with NHE3, which resulted in reduced NHE3 activity as well as reduced NHE3 surface expression. SNX27 Ser49 phosphorylation similarly reduced the binding of several other cargoes, with demonstration of effects on Glut-1, ATP7A and ZO-2. CT also destabilized the retromer by reducing the protein levels of core retromer complex components. Since the role of SNX27–retromer in NHE3 trafficking is established, we defined the effects of the pharmacologic chaperone R55 on the retromer complex and on NHE3, including the changes induced by CT. R55 stabilized the core retromer complex and increased NHE3 surface expression after CT exposure. R55 stimulated NHE3 activity by increasing its surface expression under basal conditions and in CT-treated intestinal epithelial cells. R55 also stimulated intestinal fluid absorption to partially overcome the forskolin-induced fluid secretion in human intestine, suggesting that retromer could be a potential drug target for treating diarrhea.
Regardless of the etiology of the diarrheal disease, inhibition of NHE3 occurs and contributes to the increased stool loss of Na and water (Hawker et al., 1980). As this study indicates, while we understand a great deal mechanistically about how CT contributes to the severe diarrhea by enzymatically activating adenylyl cyclase and PKA (Wernick et al., 2010), our understanding remains incomplete. Our results show that CT modifies the PDZ domain of SNX27, which is atypical in having one additional β-strand compared to most other PDZ domains (Gallon et al., 2014), by increasing phosphorylation at least one site, which in turn inhibits the physical interaction between NHE3 and SNX27. As with many other SNX27-interacting cargoes, SNX27 regulates trafficking of NHE3 from EE to PM (Singh et al., 2015). Specifically, SNX27 serves as an intermediate between the retromer and cargo proteins (Lauffer et al., 2010; Steinberg et al., 2013) and reduced SNX27–NHE3 binding was associated with a reduction in NHE3 exocytosis, BB NHE3 expression and NHE3 activity.
The CT effect on SNX27–NHE3 binding is not specific for NHE3 and also occurred for other proteins that, like NHE3, have class I PDZ domain recognition domains on their C termini; those previously identified as interacting with SNX27 and confirmed here include Glut-1, ZO-2 and ATP7A. The demonstrated consequences of the CT-induced phosphorylation of the SNX27-PDZ domain are to reduce SNX27–cargo protein binding and, at least in some cases, to consequently reduce EE to PM trafficking. These observations confirm and extend previous observations about the effects of CT. CT disrupts tight junction barrier function by inhibiting Rab11/exocyst-mediated trafficking of proteins to cell–cell junctions (Guichard et al., 2013). However, it is not known whether this pathophysiology involves failure of SNX27 binding to critical components of the exocyst. SNX27 is known to regulate trafficking of misslocalized ZO-2 from the EE back to the tight junctions (Zimmerman et al., 2013). Therefore, these data, together, provide robust evidence for a role of cAMP/PKA in regulating SNX27-mediated trafficking.
The observation that the SNX27 PDZ domain phosphorylation is regulated, in this case by CT and forskolin, adds further evidence of rapid post-translational regulation at the EE of SNX27 related to PM trafficking, particularly of proteins that contain C-terminal class I PDZ domain recognition motifs. While there is some basal phosphorylation of SNX27-Ser49, an increase in phosphorylation of this single amino acid reduced binding to multiple substrates and reduced the PM expression of each, which supports the idea that stimulation of this phosphorylation affects SNX27 function; furthermore, that the function of increased phosphorylation reduced cargo binding is supported by the effects of the S49D mutation phenocopying the effects of SNX27 KD. Conversely, that there was no significant effect with the mutation SNX27-S49A, suggests that basal SNX27 function is not dependent on the level of basal phosphorylation.
Phosphorylation of the SNX27 PDZ domain is not the only circumstance when phosphorylation affects SNX27–cargo association. Changes in phosphorylation of multiple SNX27-binding partners at their class I C-terminal PDZ domain recognition sequences also affects their binding to SNX27 (Clairfeuille et al., 2016). Some SNX27 ligands constitutively recycle from the EE to the PM while others only recycle when they are phosphorylated. NHE3 has both a C-terminal and internal PDZ domain-interacting sequence, with its interaction with SNX27 involving the C-terminal PDZ class I recognition sequence (Cha et al., 2017; Singh et al., 2015). NHE3 constitutively recycles from the EE to the PM, but whether this is dependent on basal phosphorylation of its C-terminal PDZ domain recognition sequence or correlates with its SNX27 association has not yet been defined. It is of interest that phosphorylation of the SNX27-PDZ domain-binding partners increases SNX27–cargo interactions, while phosphorylation of the SNX27-PDZ domain itself decreases SNX27–cargo interactions, at least for the ligands we have characterized. Whether the cargo and SNX27 PDZ domain phosphorylation are coordinately regulated and/or involve the same or related kinases acting together is not known.
Because SNX27-mediated trafficking of cargo proteins depends upon a stable retromer complex, we tested whether CT affects retromer function. We specifically focused on total protein changes in the core retromer complex. Deficiencies in VPS35 and VPS26 leading to defective retromer trafficking have been reported in several neurological diseases (Li et al., 2016; Small et al., 2005; Tian et al., 2015; Wang et al., 2016). Furthermore, knocking down one protein of the VPS35–VPS26–VPS29 trimeric core complex often leads to secondary reductions in the other retromer proteins (Arighi et al., 2004; Fuse et al., 2015; Vergés et al., 2004). In the present study, we observed a CT-induced decrease in the amount of core retromer proteins VPS35 and VPS26 suggesting that CT not only inhibits SNX27–NHE3 binding, but also destabilizes the retromer core complex. In an attempt to increase NHE3 activity, we used the pharmacological chaperone R55. Consistent with the previously reported effect of this class of drugs to increase protein stability (Mecozzi et al., 2014), R55 increased the levels of retromer proteins in intestinal epithelial cells under basal conditions and prevented the reduction of retromer protein levels caused by CT. Notably, R55 enhanced the function of the retromer, as indicated by increase in NHE3 basal activity in human enteroid monolayers and maintenance of PM localization of NHE3 in CT-treated intestinal epithelial cells. This is consistent with previous studies that showed that increasing retromer levels enhances retromer-mediated trafficking (MacLeod et al., 2013; Mecozzi et al., 2014; Small et al., 2005). Furthermore, R55 treatment did not increase NHE3 binding with the S49D mutant of SNX27 (Fig. S6). Therefore, these data together suggest that R55 could affect NHE3 without directly involving SNX27, since it is known that the retromer can function independently of SNX27 in the recycling of membrane proteins back to the cell surface (Steinberg et al., 2013). However, it remains to be determined whether R55 can alter trafficking of other SNX27 cargo proteins.
The finding of the current study that R55 partially reversed FIS of 3D human enteroids by stimulating NHE3-mediated fluid absorption suggests a potential additional use of the FIS assay. FIS assays have been successfully used to measure fluid secretion rates that involve CFTR as well as restoration of defective CFTR activity by drugs (Dekkers et al., 2013; Fujii et al., 2016). This assay has also been used to test the swelling response of a wide variety of compounds and to efficiently evaluate their potential to induce or otherwise inhibit the fluid secretion of intestinal epithelial cells, with all effects assumed to be based on changes in CFTR activity. By using the specific NHE3 inhibitor S3226, we are using this assay to evaluate fluid absorption related to stimulated NHE3 activity (Schwark et al., 1998). Of note, further optimization of this approach is required to allow the contribution of NHE3 to basal absorption as only slight and non-significant enteroid swelling occurred with inhibition of basal NHE3 activity. Moreover, all the swelling in the FIS assay is CFTR dependent, which likely is a further demonstration of the influence of CFTR on NHE3 activity (Bagorda et al., 2002; Mizumori et al., 2008).
The demonstration that a class of retromer stabilizers overcomes the CT reduction of retromer proteins VPS35 and VPS26, and at least partially overcomes the CT inhibition of NHE3 PM expression and activity, suggests that the retromer might be a drug target for treating severe diarrheas. In this regard, oral rehydration solution (ORS) treatment of cholera is associated with the ability to rehydrate patients without reducing cholera-related diarrhea volume or shortening the duration of the diarrhea. Part of the effect of D-glucose-containing ORS-mediated rehydration of patients with cholera appears to be due to D-glucose/SGLT1-related signaling that stimulates NHE3 activity to partially reverse the CT-inhibited NHE3 activity (Lin et al., 2011). Related to potential treatment of diarrhea, we speculate that retromer stabilizers might further increase NHE3 activity and intestinal Na+ absorption even in the presence of CT. Separately, CFTR has also been shown to bind SNX27 (McDermott et al., 2018 preprint); however, the effect of retromer stabilizers on CT-mediated stimulation of CFTR has not yet been defined.
MATERIALS AND METHODS
Glutathione–Sepharose 4B resin was from GE Healthcare Life Science (Pittsburgh, PA). BCECF-AM, nigericin and Hoechst 33342 were from Life Technologies (Grand Island, NY). Primary antibodies used in this study were: mouse anti-glyceraldehyde-3-phosphate dehydrogenase [Sigma-Aldrich, St. Louis, MO, GAPDH, #G8795; western blotting (WB) 1:3000], mouse anti-actin (Sigma-Aldrich, clone AC-74, #A2228, WB 1:3000), rabbit anti-ATP7A (Sigma-Aldrich, #HPA048107, WB 1:250), rabbit anti-GFP [Life Technologies, #A11122, WB 1:1000, immunofluorescence (IF) 1:200], mouse anti-GST (Cell Signaling Technology, Danvers, MA clone 26H1, #2624, WB 1:2000), mouse anti-GFP (Abcam, Cambridge, MA 9F9.F9, #ab1218, WB 1:3000), mouse anti-SNX27 (Abcam, clone 1C6, #AB77799, IF 1:100; WB 1:1000), goat anti-VPS29 (Abcam, #ab10160, WB 1:1000, IF 1:200), mouse anti-VPS35 (Abcam, #ab97545, WB 1:2000, IF 1:100), rabbit anti-VPS35 (Abcam, #ab97545, WB 1:1000, IF 1:100), rabbit anti-VPS26 (Abcam, #ab23892, WB 1:1000, IF 1:100), rabbit anti-Glut-1 (Abcam, #ab15309, WB 1:1000, IF 1:100), mouse monoclonal LAMP1 (Developmental Studies Hybridoma Bank, #1DB4, IF 1:500), anti ZO-2 rabbit polyclonal (Invitrogen, #PA5-27847, WB 1:1000), rabbit anti-NHE3 (Novus Biologicals, Littleton, CO, #NBP1-82574, WB 1:1000, IF 1:100). Alexa Fluor 488- and 568-conjugated goat anti-mouse-IgG and anti-rabbit-IgG secondary antibodies, were from Life Technologies. IRdye-700- and IRdye-800-conjugated goat anti-mouse-IgG and goat anti-rabbit-IgG secondary antibodies were from Rockland Immunochemicals (Gilbertsville, PA) and were used with the Odyssey system (LI-COR, Lincoln, NE) for western blot analysis. R55 TPT-260 (a thiophene thiourea derivative) was from Medkoo Biosciences (Morrisville, NC).
Cell culture, shRNA and CT treatment
The following cell lines were studied: HEK293T, HeLa, Caco-2/bbe and human duodenal enteroids from healthy subjects (see below). All cultures were routinely tested for mycoplasma.
Human embryonic kidney-293 and HeLa cells were cultured in DMEM/F-12 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; BD), penicillin (50 mU/ml) and streptomycin (50 μg/ml), at 37°C in a 5% CO2 humidified incubator. HEK293 cells stably expressing HA–NHE3 were generated previously (Singh et al., 2015). Stable SNX27 knockdown cells (SNX27-KD; HEK293 and HeLa cells) were generated using lenti-shRNA constructs, as described previously (Singh et al., 2015). A lentivirus plasmid vector containing shRNA that does not match any known human gene (Sigma-Aldrich) was used as the transduction control. Infected cells were maintained under selection pressure of puromycin.
Caco-2/bbe cells were grown in DMEM/F12 50/50 (Gibco/Life Technologies) supplemented with 20% heat-inactivated FBS, Nu-Serum (BD #355500), and a penicillin/streptomycin mix for 14 days at 37°C with 5% CO2 and 95% humidity. Caco-2/bbe cell lines stably expressing HA–NHE3 were generated by lentivirus shRNA transduction and maintained in the presence of puromycin. HA–NHE3 was subcloned into pCDH-MCS-IRES-neo (System Biosciences Inc, Mountain View, CA). Virus was packaged in HEK293T cells by co-transfection with pMD2.G and pSPAX2. Media were collected, filtered and used to infect Caco-2/bbe cells; 1200 μg/ml G418 were used to select for the cells in which virus was integrated.
For CT treatment, cells were grown in 12-well Transwell inserts for 14 days. Purified CT (holo-CT, Sigma, St. Louis, MO) (0.1 μg/ml in 500 μl of medium) or 500 μl of control medium was present either for 8 h or added 4 times at 12 h intervals for up to 48 h, and cells were collected after the last treatment.
Tissue collection and enteroid generation
Human enteroid cultures were established from biopsies obtained after endoscopic or surgical procedures utilizing the methods developed by Sato et al. with slight modification (Noel et al., 2017; Sato et al., 2011). Biopsy tissue was obtained from healthy subjects who underwent endoscopies with no pathology identified and who provided informed consent. All experimental protocols were approved by the Johns Hopkins University Institutional Review Board (IRB# NA_00038329). All clinical investigation have been conducted according to the principles expressed in the Declaration of Helsinki. For this study, enteroids were generated and maintained from duodenal biopsies (Noel et al., 2017). Enteroid monolayers were formed onto 0.4 μm pore transparent polyester (PET) membrane 24-well Transwell cell culture inserts (Transwell; Corning or Millipore) pre-coated with human collagen IV (30 μg/ml; Sigma-Aldrich) and were maintained in Wnt3A-containing non-differentiation medium (NDM) at 37°C, 5% CO2 and 95% humidity. Upon confluency (7–14 days), monolayer differentiation was induced by removal of Wnt3A and Rspo-1 for 5 days (Foulke-Abel et al., 2014; Noel et al., 2017).
Recombinant protein purification
Plasmid pcDNA3.1-HA-NHE3, was constructed previously (Murtazina et al., 2006). Rat SNX27b (SNX27b) cDNA was kindly provided by Jae Cheng (Johns Hopkins University, Baltimore, MD). The SNX27b PDZ-S49A and -S49D point mutations were generated using the QuikChange site-directed mutagenesis kit (Agilent Technologies).
GST–SNX27-FL (full length) and PDZ-PX (GST-tagged N-terminal PDZ-PX, amino acids 1–266) were constructed and purified as previously described (Singh et al., 2015). cDNAs encoding the PDZ-PX fragment of SNX27-S49A and -S49D were generated by PCR and inserted into pGEX-4T-1 (GE Healthcare) for expression of GST-fused recombinant proteins. These constructs were transformed into the BL21(DE3) strain (EMD Millipore). When the bacterial culture reached an optical density (OD) of ∼0.8, protein expression was induced with 0.3 mM isopropyl-β-D-thiogalactoside at 16°C overnight. GST-tagged proteins were purified in a gravity-flow column following the instructions from GE Healthcare. Purified proteins were concentrated with Amicon Ultra-15 Centrifugal filter units (EMD Millipore) supplemented with 10% glycerol and 10 mM DTT, and protein concentrations were measured via a Bradford assay.
Immunoprecipitation for LC-MS/MS
Immunoprecipitation experiments were performed using lysates from control or CT-treated HEK293 cells stably expressing GFP-tagged rat SNX27 as previously described (Singh et al., 2015). Briefly, cell lysates were prepared in lysis buffer (60 mM HEPES pH 7.4, 150 mM NaCl, 3 mM KCl, 5 mM EDTA trisodium, 3 mM lysis buffer ethylene glycol tetraacetic acid, 1 mM Na3VO4, and 1% Triton X-100 with protease inhibitor cocktail; Sigma-Aldrich). Aliquots (2 mg of protein) of lysate were incubated with 20 μl of GFP–nAb agarose resin (Allele Biotechnology) at 4°C for 4 h on a rotating shaker. Beads were washed five times with the same buffer and eluted with 1× elution buffer. The input and eluted samples were separated by SDS-PAGE. After electrophoresis, the gels were stained with Super Blue Coomassie stain (Protea Biosciences). Once the gel was adequately stained, a digital image was captured and the immunoprecipitated GFP–SNX27 gel band was cut out and sent for LC-MS/MS analysis.
LC-MS/MS
Samples were proteolyzed using the ‘Filter Assisted Sample Preparation’ (FASP) method (Wiśniewski et al., 2009). Briefly, samples were reduced with 5 mM tris(2-carboxyethyl)phosphine (TCEP) at 37°C for 45 min and reduced cysteine residues were blocked using 10 mM iodoacetamide at 25°C for 15 min. Samples were then buffer exchanged using a 30 kDa Amicon Filter (EMD Millipore) three times with 9 M urea and twice with 50 mM triethyl ammonium bicarbonate. Samples were then proteolyzed with trypsin/lysC (Promega) for 12 h at 37°C. Peptides were desalted using stage-tip C18 (3 M Millipore). For TMT 10-plex comparisons, peptides were resuspended in anhydrous acetonitrile and labeled with TMT reagents according to manufacturer's recommendations (Thermo Fisher Scientific).
Protein identification by LC-MS/MS analysis of peptides was performed on a Q-Exactive mass spectrometer (Thermo Fisher Scientific) interfaced with the EasyLC 100 nanoflow LC system. Peptides were separated on a reversed-phase HPLC on a 75 μm×15 cm PicoFrit column (New Objective) packed with Magic C18AQ [5 μm, 120 Å (1 Å=0.1 nm), Michrom Bioresources]. Peptides were separated using a gradient of 0%–60% acetonitrile and 0.1% formic acid over 70 min at a flow rate of 300 nl/min. Eluting peptides were sprayed directly into Q-Exactive at 2.0 kV spray voltage. Survey scans were acquired from 350–1800 m/z with up to 15 peptide masses (precursor ions) individually isolated with a 2 Da window with 0.5 Da offset and fragmented (MS/MS) using a collision energy of 27 and 30 s dynamic exclusion. Precursor and the fragment ions were analyzed at 70,000 and 17,500 resolution, respectively. Peptide sequences were identified from isotopically resolved masses in MS and MS/MS spectra extracted with and without deconvolution using Thermo Scientific MS2 processor and Xtract software. Data were searched against human 2012 database with oxidation on methionine, deamidation on residues asparagine and glutamine, phosphorylation on serine, threonine or tyrosine residues (as different variable modifications) and carbamidomethyl on cysteine as a fixed modification using Mascot software interfaced with the Proteome Discoverer 1.4 (http://portal.thermo-brims.com/) workflow. For TMT 10-plex experiments, the TMT label on the peptide N-terminus (fixed modification) and lysine residues (variable modification) was added to the database search. Peptide identifications from Mascot searches were processed within Proteome Discoverer software to identify peptides with a confidence threshold <1% false discovery rate, based on a concatenated decoy database search to calculate the median protein and peptide ratios. Localization of serine sites was evaluated using PhosphoRS score and manual inspection. The ratios for the phosphorylated peptides in samples were calculated from TMT reporter ions after normalization on the median ratio from all peptides detected from SNX27.
GSH resin pulldown
For interaction studies, 1 nmol of recombinant GST-tagged protein was used as bait. As prey, 1 mg of cell lysate was used as indicated. The volume of the final mixture was adjusted to 500 μl with the lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 0.5% Triton X-100 and protease inhibitors). GSH resin (Glutathione–Sepharose 4B resin) was washed with lysis buffer three times. Each bait–prey mixture was mixed with 10 μl of resin and incubated at 4°C for 4 h on a rotating shaker. Resin was washed with the same lysis buffer four times and then eluted with lysis buffer supplemented with 10 mM glutathione. The input and elution samples were analyzed by SDS-PAGE and western blotting. For quantification, the densitometric measurement of bands (using ImageJ) was first normalized to the amount of GST fusion protein pulled down, and signal intensities were then normalized to control (GST–PDZ-PX) samples.
Measurement of Na+/H+ exchange activity
Cellular Na+/H+ exchange activity in HEK293-HA-NHE3 or SNX27-KD cells grown to ∼80% confluence on glass coverslips and differentiated human enteroid monolayers was determined fluorometrically by using the intracellular pH-sensitive dye 2′,7′-bis(carboxyethyl)5-6-carboxyfluorescein-acetoxymethyl ester (BCECF-AM, 5 μM) as described previously and expressed as ΔpHi/minute (Janecki et al., 1998; Levine et al., 1995).
Immunofluorescence staining and confocal microscopy
HEK293 or HeLa cells seeded on coverslips or 5 day differentiated human duodenal enteroids grown on Transwell inserts were fixed with 4% paraformaldehyde for 1 h. Permeabilization and blocking were carried out simultaneously in a solution of 15% FBS, 2% BSA and 0.1% saponin (all Sigma-Aldrich) in PBS for 60 min at room temperature. Cells were rinsed with PBS and incubated overnight at 4°C with primary antibodies diluted 1:100 in PBS containing 15% FBS and 2% BSA. Stained cells were then washed three times for 10 min each with PBS followed by secondary antibodies diluted 1:100 in PBS. Secondary antibodies included goat anti-rabbit-IgG conjugated to Alexa Fluor 488, goat anti-mouse-IgG conjugated to Alexa Fluor 488, goat anti-rabbit-IgG conjugated to Alexa Fluor 568, goat anti-Mouse-IgG conjugated to Alexa Fluor 568 (all from Molecular Probes/Invitrogen, USA). Hoechst 33342 (Vector Laboratories) for nuclear labeling was used at a 1:200 dilution in PBS. After incubation, cells were washed three times for 10 min each and mounted in ProLong Gold (Vector Laboratories) overnight at 4°C.
Cells were visualized using a Zeiss LSM-510 META laser scanning confocal microscope (Zeiss, Germany) running ZEN 2012 (black edition) imaging software (Zeiss, Germany). All images were captured with a 40× (1.4 NA) oil objective. For quantitative analysis, the same settings were used to image across samples. Images were acquired and processed using ZEN 2012 (Carl Zeiss) and ImageJ software. Retromer vesicles in cells were quantified by counting the number of distinct VPS35 and VPS26 puncta in a maximal projection image. Colocalization analysis was performed with the FIJI plugin, Coloc_2 (http://fiji.sc/Colocalization_Analysis). The degree of colocalization between SNX27 (green) and EEA1 (red) was quantified and expressed as a Pearson colocalization coefficient (1, perfect correlation and −1, no correlation). The significance of colocalization was determined by Costes' randomization analysis.
Cell surface biotinylation and immunoblotting
NHS-SS-biotin was used to determine the amount of BB NHE3 (Singh et al., 2015). All the western blot analyses were performed using Odyssey imaging systems, which is known to have a linear dynamic range of at least 104. This wide dynamic range results in marked reduction of detector saturation for western blotting experiments. All western blot results were normalized to loading controls.
Exocytic insertion assays
To measure exocytic insertion of NHE3, in control and CT-treated Caco-2/bbe-HA-NHE3 cells, surface protein accessible to NHS-SS-biotin were masked by pretreatment with NHS-acetate (1.5 mg/ml) as described previously (Singh et al., 2015). Then cells were rinsed with ice-cold PBS containing 0.1 mM Ca2+ and 1 mM Mg2+ two times at 4°C and incubated for 0, 15, 30 and 60 min at 37°C to allow exocytosis of NHE3. Cells were then labeled with sulfo-NHS-SS-biotin (1.5 mg/ml) and treated with lysis buffer as described previously (Singh et al., 2015). The biotinylated fraction was precipitated with streptavidin–agarose beads. The resultant precipitate was subjected to SDS-PAGE, and biotinylated NHE3 was detected by quantitative western blot analysis as described previously (Singh et al., 2015). Biotinylated NHE3 was normalized to the total amount of NHE3 present in the lysate at each time point and expressed as percentage exocytosed.
Endocytosis internalization
Endocytosis of NHE3 in response to CT was measured by using a reduced glutathione (GSH)–resistant endocytosis assay that was described previously (Singh et al., 2015).
Total NHE3 degradation assay
Post confluent Caco-2/bbe cells (14 days; control and CT-treated) were treated with 100 µM cycloheximide for the indicated time points. Cells were lysed in PBS with 1% (v/v) Triton X-100, and NHE3 levels were determined by quantitative western blotting. β-actin fluorescence intensity was used to normalize the detected levels of NHE3. The levels of untreated controls were set to 100%, and the level of detected NHE3 was calculated as the percentage of untreated control for each time point.
Quantification of fluid secretion in human enteroids
Human enteroids from a 7–10-day-old culture were plated in 50% Matrigel (20 μl; Corning Life Sciences, Corning, NY) on glass-bottom 35-mm dishes. (P35G-1.5-10-C; MatTek, Ashland, MA), containing 50–100 enteroids and then overlaid with 1.5 ml NDM for 2–3 days. On the day of imaging, enteroids were incubated with 10 µM Calcein Green-acetoxymethyl ester (Invitrogen) in NDM for 60 min. Time-lapse z-stack images were collected every 10 min for 1 h with 488-nm excitation using a 10× phase-contrast objective on a confocal microscope system (Fluoview FV10i-LIV; Olympus) at 37°C, 5% CO2, and 95% relative humidity. To measure the effect of the pharmacological chaperone R55 on fluid absorption, enteroids were pre-incubated with 5 μM R55 for 48 h. 0.2% DMSO (control) and R55-treated enteroids were incubated with NHE3 specific inhibitor S3226 (20 µM) for 1 h treated with forskolin (5 µM), and time lapse images were collected and analyzed with MetaMorph version 7.7. The confocal section area was defined as the total area (pixels) occupied by the enteroid at a z-plane approximately halfway through the total structure height, and each successive measurement over time was made on an image from the same corresponding z-plane. The percentage change in the confocal section area is the difference in area at each time point calculated relative to the area at time=0.
Quantitative real-time PCR
Total RNA was extracted from Caco-2/bbe cell monolayers using a PureLink® RNA Mini Kit (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from 1–2 μg of RNA using SuperScript™ VILO™ MasterMix (Life Technologies, Carlsbad, CA). Quantitative real-time PCR (qRT-PCR) was performed using the Power SYBR® Green Master Mix (Life Technologies) on a QuantStudio™ 12K Flex real-time PCR system (Applied Biosystems Inc., Foster City, CA). Each sample was run in triplicate, and 5 ng RNA-equivalent cDNA was used for each reaction. The relative fold-change in the mRNA level of proteins were determined using the 2−ΔΔCT method with human 18S rRNA was used as the internal control for normalization.
Statistical analysis
All quantified western blot and confocal data are the mean of the indicated number of independent experiments. Statistical analyses were carried out using GraphPad Prism 5 software (La Jolla, CA). Either an unpaired Student's t-tests or one-way ANOVA followed by a Dunnett's post hoc test were performed to determine whether differences between conditions were statistically significant. P<0.05 is considered statistically significant.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: V.S., D.E.B., S.A.S., G.P., M.D.; Methodology: V.S., J. Yang, J. Yin, R.C., M.T., D.E.B., G.P., M.D.; Software: V.S.; Validation: V.S.; Formal analysis: V.S., J. Yin, M.T., S.A.S.; Investigation: V.S., J. Yang, S.A.S.; Resources: V.S., R.C., S.A.S., G.P., M.D.; Data curation: V.S., J. Yang, J. Yin, R.C., M.T.; Writing - original draft: V.S.; Writing - review & editing: V.S., M.T., D.E.B., M.D.; Visualization: V.S., R.C.; Supervision: V.S., M.D.; Project administration: V.S., M.D.; Funding acquisition: M.D.
Funding
This work was supported in part by NIDDK, NIAID and NCATS (National Institutes of Health) (grants R01-DK26523, R01-DK61765, R24-DK99803, P01-DK072084, P01-AI125181, UH3-TR00003, UO1-DK10316 and P30-DK89502), The Hopkins Digestive Diseases Basic and Translational Research Core Center and The Hopkins Center for Epithelial Disorders. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.218610.supplemental
References
- Arighi C. N., Hartnell L. M., Aguilar R. C., Haft C. R. and Bonifacino J. S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123-133. 10.1083/jcb.200312055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagorda A., Guerra L., Di Sole F., Hemle-Kolb C., Cardone R. A., Fanelli T., Reshkin S. J., Gisler S. M., Murer H. and Casavola V. (2002). Reciprocal protein kinase A regulatory interactions between cystic fibrosis transmembrane conductance regulator and Na+/H+ exchanger isoform 3 in a renal polarized epithelial cell model. J. Biol. Chem. 277, 21480-21488. 10.1074/jbc.M112245200 [DOI] [PubMed] [Google Scholar]
- Belenkaya T. Y., Wu Y., Tang X., Zhou B., Cheng L., Sharma Y. V., Yan D., Selva E. M. and Lin X. (2008). The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell 14, 120-131. 10.1016/j.devcel.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Bhalla A., Vetanovetz C. P., Morel E., Chamoun Z., Di Paolo G. and Small S. A. (2012). The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol. Dis. 47, 126-134. 10.1016/j.nbd.2012.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. and Cullen P. J. (2014). Retromer: a master conductor of endosome sorting. Cold Spring Harb. Perspect. Biol. 6, a016774 10.1101/cshperspect.a016774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B., Yang J., Singh V., Zachos N. C., Sarker R. I., Chen T.-E., Chakraborty M., Tse C. M. and Donowitz M. (2017). PDZ domain-dependent regulation of NHE3 protein by both internal class II and C-terminal class I PDZ-binding motifs. J. Biol. Chem. 292, 8279-8290. 10.1074/jbc.M116.774489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairfeuille T., Mas C., Chan A. S. M., Yang Z., Tello-Lafoz M., Chandra M., Widagdo J., Kerr M. C., Paul B., Mérida I. et al. (2016). A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 23, 921-932. 10.1038/nsmb.3290 [DOI] [PubMed] [Google Scholar]
- De Haan L. and Hirst T. R. (2004). Cholera toxin: a paradigm for multi-functional engagement of cellular mechanisms (Review). Mol. Membr. Biol. 21, 77-92. 10.1080/09687680410001663267 [DOI] [PubMed] [Google Scholar]
- Dekkers J. F., Wiegerinck C. L., de Jonge H. R., Bronsveld I., Janssens H. M., de Winter-de Groot K. M., Brandsma A. M., de Jong N. W. M., Bijvelds M. J. C., Scholte B. J. et al. (2013). A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939-945. 10.1038/nm.3201 [DOI] [PubMed] [Google Scholar]
- Follett J., Norwood S. J., Hamilton N. A., Mohan M., Kovtun O., Tay S., Zhe Y., Wood S. A., Mellick G. D., Silburn P. A. et al. (2014). The Vps35 D620N mutation linked to Parkinson's disease disrupts the cargo sorting function of retromer. Traffic 15, 230-244. 10.1111/tra.12136 [DOI] [PubMed] [Google Scholar]
- Foulke-Abel J., In J., Kovbasnjuk O., Zachos N. C., Ettayebi K., Blutt S. E., Hyser J. M., Zeng X.-L., Crawford S. E., Broughman J. R. et al. (2014). Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp. Biol. Med. 239, 1124-1134. 10.1177/1535370214529398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Suzuki K., Kawamoto A., Ishibashi F., Nakata T., Murano T., Ito G., Shimizu H., Mizutani T., Oshima S. et al. (2016). PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci. Rep. 6, 36795 10.1038/srep36795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse A., Furuya N., Kakuta S., Inose A., Sato M., Koike M., Saiki S. and Hattori N. (2015). VPS29-VPS35 intermediate of retromer is stable and may be involved in the retromer complex assembly process. FEBS Lett. 589, 1430-1436. 10.1016/j.febslet.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Gallon M., Clairfeuille T., Steinberg F., Mas C., Ghai R., Sessions R. B., Teasdale R. D., Collins B. M. and Cullen P. J. (2014). A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. USA 111, E3604-E3613. 10.1073/pnas.1410552111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A., Cruz-Moreno B., Aguilar B., van Sorge N. M., Kuang J., Kurkciyan A. A., Wang Z., Hang S., Pineton de Chambrun G. P., McCole D. F. et al. (2013). Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe 14, 294-305. 10.1016/j.chom.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker P. C., McKay J. S. and Turnberg L. A. (1980). Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology 79, 508-511. [PubMed] [Google Scholar]
- Ikuma M., Kashgarian M., Binder H. J. and Rajendran V. M. (1999). Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am. J. Physiol. 276, G539-G549. 10.1152/ajpgi.1999.276.2.G539 [DOI] [PubMed] [Google Scholar]
- Janecki A. J., Montrose M. H., Zimniak P., Zweibaum A., Tse C. M., Khurana S. and Donowitz M. (1998). Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Protein kinase C-mediated inhibition of the exchanger. J. Biol. Chem. 273, 8790-8798. 10.1074/jbc.273.15.8790 [DOI] [PubMed] [Google Scholar]
- Joubert L., Hanson B., Barthet G., Sebben M., Claeysen S., Hong W., Marin P., Dumuis A. and Bockaert J. (2004). New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J. Cell Sci. 117, 5367-5379. 10.1242/jcs.01379 [DOI] [PubMed] [Google Scholar]
- Kunzelmann K. and Mall M. (2002). Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 82, 245-289. 10.1152/physrev.00026.2001 [DOI] [PubMed] [Google Scholar]
- Lauffer B. E. L., Melero C., Temkin P., Lei C., Hong W., Kortemme T. and von Zastrow M. (2010). SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565-574. 10.1083/jcb.201004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. A., Nath S. K., Yun C. H. C., Yip J. W., Montrose M., Donowitz M. and Tse C. M. (1995). Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J. Biol. Chem. 270, 13716-13725. 10.1074/jbc.270.23.13716 [DOI] [PubMed] [Google Scholar]
- Li C., Shah S. Z., Zhao D. and Yang L. (2016). Role of the Retromer complex in neurodegenerative diseases. Front. Aging Neurosci. 8, 42 10.3389/fnagi.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Murtazina R., Cha B., Chakraborty M., Sarker R., Chen T. E., Lin Z., Hogema B. M., de Jonge H. R., Seidler U. et al. (2011). D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 140, 560-571. 10.1053/j.gastro.2010.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-B., Lai C.-Y., Hsieh M.-C., Wang H.-H., Cheng J.-K., Chau Y.-P., Chen G.-D. and Peng H.-Y. (2015). VPS26A-SNX27 interaction-dependent mGluR5 recycling in dorsal horn neurons mediates neuropathic pain in rats. J. Neurosci. 35, 14943-14955. 10.1523/JNEUROSCI.2587-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A., Secher A., Lage K., Nordsborg N. B., Dmytriyev A., Lundby C. and Olsen J. V. (2012). Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat. Commun. 3, 876-885. 10.1038/ncomms1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B. D., Marder K. S., Honig L. S., Clark L. N., Small S. A. et al. (2013). RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron 77, 425-439. 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. I., Thelin W. R., Chen Y., Lyons P. T., Reilly G., Gentzsch M., Lei C., Hong W., Stutts M. J., Playford M. P. and Bankaitis V. A. (2018). Sorting nexin 27 (SNX27): a novel regulator of cystic fibrosis transmembrane conductance regulator (CFTR) trafficking. bioRxiv, 304717 10.1101/304717 [DOI] [Google Scholar]
- Mecozzi V. J., Berman D. E., Simoes S., Vetanovetz C., Awal M. R., Patel V. M., Schneider R. T., Petsko G. A., Ringe D. and Small S. A. (2014). Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 10, 443-449. 10.1038/nchembio.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori M., Choi Y., Guth P. H., Engel E., Kaunitz J. D. and Akiba Y. (2008). CFTR inhibition augments NHE3 activity during luminal high CO2 exposure in rat duodenal mucosa. Am. J. Physiol. Gastrointestinal Liver Physiol. 294, G1318-G1327. 10.1152/ajpgi.00025.2008 [DOI] [PubMed] [Google Scholar]
- Murtazina R., Kovbasnjuk O., Donowitz M. and Li X. (2006). Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J. Biol. Chem. 281, 17845-17855. 10.1074/jbc.M601740200 [DOI] [PubMed] [Google Scholar]
- Musch M. W., Arvans D. L., Walsh-Reitz M. M., Uchiyama K., Fukuda M. and Chang E. B. (2007). Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP- and Ca2+-induced endocytosis by recruitment of AP2 and clathrin. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1549-G1558. 10.1152/ajpgi.00388.2006 [DOI] [PubMed] [Google Scholar]
- Musch M. W., Arvans D. L., Wang Y., Nakagawa Y., Solomaha E. and Chang E. B. (2010). Cyclic AMP-mediated endocytosis of intestinal epithelial NHE3 requires binding to synaptotagmin 1. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G203-G211. 10.1152/ajpgi.00379.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel G., Baetz N. W., Staab J. F., Donowitz M., Kovbasnjuk O., Pasetti M. F. and Zachos N. C. (2017). A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 7, 45270 10.1038/srep45270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A. and Fishman P. H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905-915. 10.1083/jcb.141.4.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe D. and Petsko G. A. (2009). What are pharmacological chaperones and why are they interesting? J. Biol. 8, 80 10.1186/jbiol186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B., Nair G. B. and Siddique A. K. (2004). Cholera. Lancet 363, 223-233. 10.1016/S0140-6736(03)15328-7 [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D. E., Ferrante M., Vries R. G., Van Es J. H., Van den Brink S., Van Houdt W. J., Pronk A., Van Gorp J., Siersema P. D. et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762-1772. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Schuijers J., Junker J. P., Mokry M., Hatzis P., Koo B.-K., Sasselli V., van der Flier L. G., Cuppen E., van Oudenaarden A. and Clevers H. (2015). Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158-170. 10.1016/j.stem.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Schwark J.-R., Jansen H. W., Lang H.-J., Krick W., Burckhardt G. and Hropot M. (1998). S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch. 436, 797-800. 10.1007/s004240050704 [DOI] [PubMed] [Google Scholar]
- Seaman M. N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15, 68-75. 10.1016/j.tcb.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Seaman M. N. J. (2007). Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 120, 2378-2389. 10.1242/jcs.009654 [DOI] [PubMed] [Google Scholar]
- Singh V., Yang J., Cha B., Chen T.-E., Sarker R., Yin J., Avula L. R., Tse M. and Donowitz M. (2015). Sorting nexin 27 regulates basal and stimulated brush border trafficking of NHE3. Mol. Biol. Cell 26, 2030-2043. 10.1091/mbc.e14-12-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S. A., Kent K., Pierce A., Leung C., Kang M. S., Okada H., Honig L., Vonsattel J.-P. and Kim T.-W. (2005). Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann. Neurol. 58, 909-919. 10.1002/ana.20667 [DOI] [PubMed] [Google Scholar]
- Steinberg F., Gallon M., Winfield M., Thomas E. C., Bell A. J., Heesom K. J., Tavaré J. M. and Cullen P. J. (2013). A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15, 461-471. 10.1038/ncb2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanya S. B., Rajendran V. M., Srinivasan P., Nanda Kumar N. S., Ramakrishna B. S. and Binder H. J. (2007). Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G857-G863. 10.1152/ajpgi.00462.2006 [DOI] [PubMed] [Google Scholar]
- Temkin P., Lauffer B., Jäger S., Cimermancic P., Krogan N. J. and von Zastrow M. (2011). SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 13, 715-721. 10.1038/ncb2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Tang F.-L., Sun X. D., Wen L., Mei L., Tang B.-S. and Xiong W.-C. (2015). VPS35-deficiency results in an impaired AMPA receptor trafficking and decreased dendritic spine maturation. Mol. Brain 8, 70 10.1186/s13041-015-0156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova N. G., Irannejad R. and von Zastrow M. (2015). G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J. Biol. Chem. 290, 6689-6696. 10.1074/jbc.R114.617951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergés M., Luton F., Gruber C., Tiemann F., Reinders L. G., Huang L., Burlingame A. L., Haft C. R. and Mostov K. E. (2004). The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 6, 763-769. 10.1038/ncb1153 [DOI] [PubMed] [Google Scholar]
- Vieira S. I., Rebelo S., Esselmann H., Wiltfang J., Lah J., Lane R., Small S. A., Gandy S., da Cruz e Silva E. F. and da Cruz e Silva O. A. B. (2010). Retrieval of the Alzheimer's amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol. Neurodegener. 5, 40-60. 10.1186/1750-1326-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Niu M., Zhou Z., Zheng X., Zhang L., Tian Y., Yu X., Bu G., Xu H., Ma Q. et al. (2016). VPS35 regulates cell surface recycling and signaling of dopamine receptor D1. Neurobiol. Aging 46, 22-31. 10.1016/j.neurobiolaging.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernick N. L. B., Chinnapen D. J.-F., Cho J. A. and Lencer W. I. (2010). Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins 2, 310-325. 10.3390/toxins2030310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski J. R., Zougman A., Nagaraj N. and Mann M. (2009). Universal sample preparation method for proteome analysis. Nat. Methods 6, 359-361. 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- Yang P.-T., Lorenowicz M. J., Silhankova M., Coudreuse D. Y. M., Betist M. C. and Korswagen H. C. (2008). Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev. Cell 14, 140-147. 10.1016/j.devcel.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Zhou H., Di Palma S., Preisinger C., Peng M., Polat A. N., Heck A. J. R. and Mohammed S. (2013). Toward a comprehensive characterization of a human cancer cell phosphoproteome. J. Proteome Res. 12, 260-271. 10.1021/pr300630k [DOI] [PubMed] [Google Scholar]
- Zimmerman S. P., Hueschen C. L., Malide D., Milgram S. L. and Playford M. P. (2013). Sorting nexin 27 (SNX27) associates with zonula occludens-2 (ZO-2) and modulates the epithelial tight junction. Biochem. J. 455, 95-106. 10.1042/BJ20121755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A., Benet-Pagès A., Struhal W., Graf E., Eck S. H., Offman M. N., Haubenberger D., Spielberger S., Schulte E. C., Lichtner P. et al. (2011). A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89, 168-175. 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.