Abstract
The hypocretins (Hcrts) are two alternatively spliced neuropeptides (Hcrt1/Ox-A and Hcrt2/Ox-B) that are synthesized exclusively in the hypothalamus. Data collected in the 20 years since their discovery have supported the view that the Hcrts play a broad role in the control of arousal with a particularly important role in the maintenance of wakefulness and sleep-to-wake transitions. While this latter point has received an overwhelming amount of research attention, a growing literature has begun to broaden our understanding of the many diverse roles that the Hcrts play in physiology and behavior. Here, we review recent advances in the neurobiology of Hcrt in three sections. We begin by surveying findings on Hcrt function within normal sleep/wake states as well as situations of aberrant sleep (that is, narcolepsy). In the second section, we discuss research establishing a role for Hcrt in mood and affect (that is, anxiety, stress, and motivation). Finally, in the third section, we briefly discuss future directions for the field and place an emphasis on analytical modeling of Hcrt neural activity. We hope that the data discussed here provide a broad overview of recent progress in the field and make clear the diversity of roles played by these neuromodulators.
Keywords: hypothalamus, vigilance, arousal, wake, sleep, addiction, memory
Introduction
In 1998, two research studies published within a month of each other described a set of novel hypothalamic peptides. The first group to describe them was led by Gregor Sutcliffe at the Scripps Research Institute in La Jolla, California. The Sutcliffe group used subtractive RNA hybridization to characterize a cDNA clone with restricted expression in the dorsal and lateral hypothalamus (LH). This cDNA clone encoded a preproprotein termed preprohypocretin. This was the putative precursor to two peptides that they named hypocretin-1 (Hcrt1/Ox-A) and hypocretin-2 (Hcrt2/Ox-B) with respective receptors OX 1R and OX 2R. Their name was a combination of hypo for their hypothalamic origin and cretin based on their sequence homology to the gut hormone secretin 1. At the same time, Masashi Yanagisawa’s group at University of Texas Southwestern was characterizing ligands for orphan G-protein-coupled receptors as a means to determine their role in various physiological processes. The group found two extracts within the hypothalamus that bound and activated two orphan receptors with unknown functions. When supraphysiological doses of peptide were injected intracerebroventricularly, these peptides promoted food intake. Owing to this effect, the group named the peptides “orexins” based on the Greek word for appetite ( orexis) 2. Indeed, the two groups were describing the same peptides, and today hypocretin and orexin are synonymous. Here, we will review some of the most recent findings in the neurobiology of Hcrt in relation to arousal, emotional processing, and motivation and finally discuss future directions for analytical modeling of Hcrt networks.
As new tools have become increasingly accessible to researchers at all levels, we have seen an explosion of studies using specific methodologies for the study of neural circuitry, namely the use of optogenetics and chemogenetics for the manipulation of neural circuits, fiber photometry and microendoscopy for the measurement of cellular activity via genetically encoded calcium indicators (for example, GCaMP6f), and precise genetic tools (for example, transcription activator-like effector nucleases [TALENs]; targeting-induced local lesions in genomes [TILLING]; and clustered regularly interspaced short palindromic repeats [CRISPR/Cas9]) and high-throughput sequencing to characterize and manipulate genes. Optogenetics is a technique in which neurons are genetically modified to express light-sensitive ion channels (for example, channelrhodopsins and archaerhodopsins). Subsequent photostimulation of these neurons can activate or inhibit cells on the basis of the wavelength and intensity of light used 3. Chemogenetics uses modified G-protein-coupled receptors (designer receptors exclusively activated by designer drugs, also known as DREADDs) that are largely activated by a metabolite of clozapine N-oxide (CNO) when injected systemically 4. Excitatory or inhibitory DREADDs can be selectively expressed in neuronal populations of interest (for example, in a Cre- or Flp-dependent manner) which then can be manipulated by injections of CNO 5. Additionally, the expression of calcium indicators allows the measurement of cell activity in relation to behavior via fiber photometry or microendoscopy 6. Most recently, genome editing via CRISPR/Cas9 systems and developmental engineering can quickly produce knock-outs or knock-ins for multiple gene targets in a single generation 7– 10. As our review focuses primarily on advances made within the past 3 years, there is an overwhelming representation of these methodologies, which already have significantly advanced our understanding of the Hcrt circuit 7, 11, 12.
Part I: hypocretin and arousal
Hcrt cell bodies reside exclusively within the hypothalamus and project broadly throughout the brain and spinal cord 13. They receive major inputs from a diversity of afferents covering all of the major neurotransmitter systems 14. The increasing database of research on Hcrt shows that these neuropeptides may not be necessary for the generation of sleep or wakefulness per se but rather for coordinating and stabilizing these states. Hcrt activity regulates sleep-to-wake transitions via its many interactions with other neuroanatomical and neurotransmitter systems 15, 16. Thus, many of the recent findings discussed here are a combination of studies done directly on Hcrt circuitry or studies done on other systems that either coordinate activity with or are modulated by Hcrt.
Sleep and wakefulness
Hcrt deficiency underlies the majority of cases of narcolepsy 17– 20. Narcolepsy is characterized by unexpected sleep episodes during times of wakefulness, excessive daytime sleepiness, rapid eye movement (REM)-like episodes that can co-occur with conscious wakefulness, and disrupted nocturnal sleep 21, 22. Further support for aberrant state boundaries in narcolepsy was recently published showing intrusions of REM sleep during wakefulness as well as intrusions of non-REM (NREM) sleep during wakefulness 23. While it is established that Hcrt neuron degeneration contributes to the etiology of narcolepsy in many cases, recent evidence has characterized how sleep and wakefulness are impacted through the progression of Hcrt cell loss 17, 18, 24. Studies in mice at different stages of Hcrt neuron degeneration found that loss of these neurons reduces the likelihood of long wake bouts but increases the likelihood of short wake bouts (that is, wakefulness is fragmented) as a result of waking primarily during the first 30 seconds of NREM sleep and a reduced likelihood of returning to sleep within the first 60 seconds of wakefulness 24.
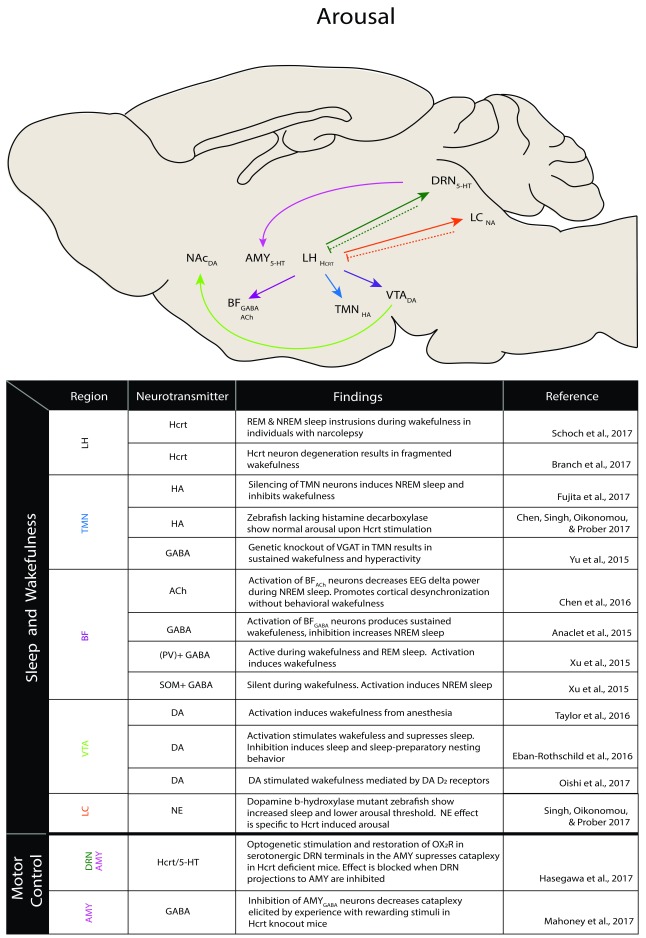
While early observations demonstrated that Hcrt deficiency underlies narcolepsy, a causal role for Hcrt in sleep-to-wake transitions was shown only in 2007 25. Optogenetic manipulations of Hcrt circuitry revealed that activation of this neuronal population induces wakefulness in mice while optogenetic inhibition promotes NREM sleep 25, 26. Likewise, chemogenetic studies targeting Hcrt neural activity have shown that injections of CNO in mice expressing excitatory (Gq) DREADDs promote wakefulness but that engagement of inhibitory (Gi) DREADDs decreases wakefulness and increases time in NREM sleep 27. Thus, Hcrt clearly plays a critical role in the regulation of sleep-to-wake transitions, but its various effects on these processes are regulated by the many brain regions and neurotransmitter systems with which it interacts. Indeed, research has demonstrated important interactions between Hcrt and histaminergic neurons within the tuberomammillary nucleus (TMN), cholinergic and GABAergic neurons of the basal forebrain (BF), dopamine (DA) neurons within the ventral tegmental area (VTA), and norepinephrine (NE) neurons of the locus coeruleus (LC), among others 28 ( Figure 1). Recent advances in our understanding of the roles of these regions in sleep/wake regulation and their possible interactions with the Hcrt system are outlined below.
Figure 1. Hypocretin arousal network.
Research of the past three years has found evidence of hypocretin-associated arousal in the illustrated circuits. Solid lines denote excitatory projections, and dashed lines denote inhibitory projections. 5-HT, serotonin; ACh, acetylcholine; AMY, amygdala; BF, basal forebrain; DA, dopamine; DRN, dorsal raphe nucleus; GABA, gamma aminobutyric acid; HA, histamine; Hcrt, hypocretin; LC, locus coeruleus; LH, lateral hypothalamus; NA, noradrenergic system; NAc, nucleus accumbens; NE, norepinephrine; NREM, non-rapid eye movement; PV, parvalbumin; REM, rapid eye movement; SOM, somatostatin; TMN, tuberomammillary nucleus; VTA, ventral tegmental area.
As we discuss below, histaminergic neurons of the TMN play a role in arousal, but the ways in which Hcrt influences TMN-mediated arousal are not clear. TMN histaminergic neurons become active during wake onset and are silent during sleep 29, 30. Optogenetic silencing of histaminergic TMN neurons induces NREM sleep and inhibits wakefulness 31. Hcrt activates TMN neurons and increases histamine release at their terminals, suggesting that Hcrt activation of TMN neurons supports wakefulness 32– 34. However, mice and zebrafish that lack the rate-limiting enzyme in histamine synthesis (histamine decarboxylase) show normal sleep-to-wake transitions upon optogenetic stimulation of Hcrt neurons 35, 36. These data suggest that histaminergic signaling in the TMN may serve a redundant function in Hcrt-mediated arousal. Recent findings also show that histaminergic regulation of wakefulness within the TMN may be via co-transmission of GABA. Small interfering RNA (siRNA)-mediated knockdown of the vesicular GABA transporter (VGAT) or genetic knockout of the VGAT gene in histaminergic neurons results in hyperactivity and sustained wakefulness 37. Future studies should characterize how manipulations of GABA transmission in the TMN impacts Hcrt-induced wakefulness specifically.
The BF is an attention- and arousal-sustaining structure containing cholinergic, GABAergic, and glutamatergic cells that are depolarized by Hcrt 38. Similarly, the region expresses both Hcrt receptors, and there is a higher density of OX 2R than OX 1R 39. This difference may be meaningful, as studies in organotypic slice cultures show that Hcrt depolarizes cholinergic cells of the BF via actions at OX 2R but not OX 1R 38. However, injections of Ox-A into the BF of rats resulted in wakefulness in regions of the BF that show stronger expression of OX 1R 40. Chemogenetic studies demonstrate that activation of cholinergic neurons of the BF decreases electroencephalogram (EEG) delta power (specifically during NREM sleep) and promotes cortical desynchronization without behavioral wakefulness 41. In contrast, activation of GABAergic neurons in this region produces sustained wakefulness whereas inhibition increases NREM sleep 42. Further genetic targeting studies show that subsets of GABAergic neurons in the region exhibit a diversity of responses across arousal states 43– 45. For example, parvalbumin-positive (PV +) GABAergic neurons are more active during wakefulness and REM sleep than during NREM sleep whereas somatostatin-positive (SOM +) GABAergic neurons are reciprocally silent during wakefulness. Predictably, optogenetic activation of PV + GABA neurons powerfully induces wakefulness whereas activation of SOM + GABAergic neurons promotes NREM sleep 46– 49. Modern genetic tools will continue to allow more detailed examinations of the impact of neuronal heterogeneity within regions in the context of Hcrt-mediated arousal.
The BF receives projections from midbrain DA neurons which may underlie the coupling of motivation to arousal states. Indeed, Hcrt axons project to midbrain DA neurons, and DA cell bodies express Hcrt receptors 13, 50, 51. In vitro electrophysiological recordings show that Hcrt1 and Hcrt2 treatment increases VTA DA neural firing 52. Hcrt1 injections into the VTA increase time awake and levels of DA at axonal terminals in the prefrontal cortex 53, 54. Although Hcrt neurons project to systems for all the monoamines and drugs that increase DA transmission increase wakefulness, DA was thought not to be involved in normal sleep/wake regulation until recently 55– 60. Work from our laboratory has shown a role for VTA DA neurons in promoting arousal and the initiation of sleep-preparatory behaviors 61. Optogenetic activation of VTA DA neurons induces emergence from anesthesia, and chemogenetic activation of the VTA induces and consolidates wakefulness 62, 63. Further manipulations have demonstrated that VTA effects on wakefulness are through a D 2 receptor-mediated mechanism 62, 63. Future work using projection-specific manipulations of Hcrt fibers within the VTA should better characterize their role in VTA-mediated arousal.
Noradrenergic neurons of the LC are strong promoters of arousal 64, 65. Direct administration of Hcrt1 into the LC increases firing rates while optogenetic silencing of these neurons with concurrent excitation of Hcrt cells prevents Hcrt-evoked sleep-to-wake transitions 66– 68. Additional studies have shown that noradrenergic activity is required to promote wakefulness and Hcrt-induced arousal in zebrafish. Using DA b-hydroxylase (dbh) (the rate-limiting enzyme in NE synthesis) mutant zebrafish, researchers found that these animals had dramatically increased sleep yet lower arousal thresholds 69. Additionally, wakefulness induced by genetic overexpression of Hcrt and optogenetic activation of Hcrt neurons is blocked by the inhibition or knocking out of NE in zebrafish larvae 69. However, further investigations have shown that overexpression of Hcrt or activation of Hcrt neurons has no significant effect in dbh mutant zebrafish 35. Thus, future work should continue to parse out the roles in which NE functions in sleep/wake regulation and how it may serve specifically within the Hcrt circuit to help regulate wakefulness in particular.
Motor tone
Despite evidence demonstrating innervation of motor control systems by the Hcrt neurons, the coupling of arousal states with motor control is poorly understood 70. Indeed, measures of muscle tone along with cortical activity are the most common endpoints for characterizing various arousal states. A hallmark of waking is low-amplitude, high-frequency EEG activity with high muscle activity. REM sleep, also known as paradoxical sleep, is characterized by a near complete loss of skeletal muscle activity and an EEG resembling wakefulness. Hcrt-deficient narcoleptics show cataplexy (a loss of muscle tone during wakefulness that can result in postural collapse and can be triggered by strong emotions such as happiness and fear) 22, 71– 74. Similarly, individuals with REM sleep behavior disorder (RBD) show muscle tone problems. Under normal conditions, REM sleep is devoid of skeletal muscle tone; however, in RBD, an individual acts out their dreams by moving their limbs or talking, which can be dangerous for the individual enacting their dreams as well as anyone in their surroundings 75. Noradrenergic activity is necessary for motor behavior 76. Indeed, NE depletion has been shown to have a stronger motor-impairing effect than dopaminergic lesions with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) infusions of NE-induced hyperactivity, and loss of NE neurons is associated with motor learning deficits in aged rats 77– 80. Likewise, increasing noradrenergic tone has been shown to reduce cataplectic episodes 81. As discussed above, noradrenergic neurons of the LC are powerfully regulated by Hcrt; Hcrt dysfunction predictably alters both arousal and motor control. Moreover, Hcrt neurons project to dorsal raphe nucleus (DRN) serotonergic neurons where they may further influence motor behavior. Indeed, restoration of OX 2R into serotonergic DRN neurons of dual Hcrt receptor knockout mice suppresses cataplexy-like episodes yet has no effect on sleep/wake fragmentation. Likewise, optogenetic stimulation of serotonergic DRN terminals in the amygdala (AMY) suppresses cataplexy-like arrests in Hcrt-deficient mice, and optogenetic inhibition blocks the cataplexy-reducing effect of Hcrt receptor restoration in serotonergic DRN neurons 82. Additional chemogenetic manipulations of this amygdalar circuit show that GABAergic populations of the central AMY are responsible for the production of cataplexy in mice but may not be the only circuit that can drive emotionally driven cataplexy 83. Together, these findings demonstrate a key role for amygdalar circuits in the production of cataplexy; however, they do not rule out other nuclei or circuits that may influence emotionally driven cataplexy. Indeed, the neural infrastructure exists for Hcrt activity to modulate AMY activity via its connections from the LC and DRN, and future studies should characterize the influence of Hcrt in emotion-driven cataplexy.
Part II: affect and motivation
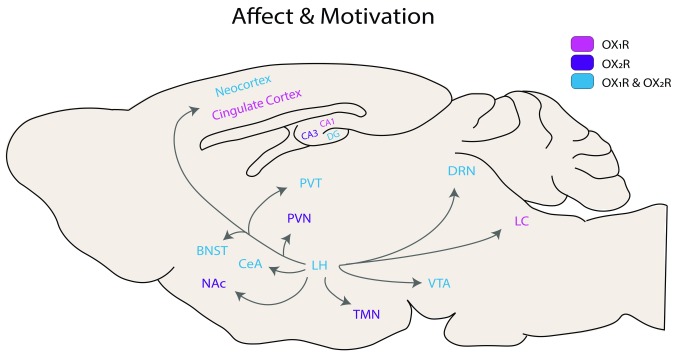
As a regulator of arousal, the Hcrt system plays additional important roles in adaptive behaviors such as the regulation of stress responses and the avoidance of punishments and seeking of rewards. Additionally, sleep supports the consolidation of memory; predictably, proper regulation of sleep and arousal is key to proper memory function. Below we discuss recent findings in the growing field of Hcrt in the regulation of emotion and motivation and place a particular focus on stress and anxiety, addiction, and memory processes. Many of the data discussed here were gathered via global manipulations of Hcrt receptor signaling and thus should be interpreted in the context of known receptor distributions, drug treatments and selectivity (as many of these drugs are known to vary in selectivity on the basis of dose 84), and drug administration schedules ( Figure 2 and Table 1).
Figure 2. Hypocretin receptor distribution in the rodent brain.
BNST, bed nucleus of the stria terminalis; CeA, central amygdala; DG, dentate gyrus; DRN, dorsal raphe nucleus; LC, locus coeruleus; LH, lateral hypothalamus; NAc, nucleus accumbens; PVN, paraventricular nucleus; PVT, paraventricular nucleus of the thalamus; TMN, tuberomammillary nucleus; VTA, ventral tegmental area.
Table 1. Summary of recent findings for hypocretin in relation to affect and motivation.
Colors match receptor representation in Figure 2: pink, OX 1R manipulation; purple, OX 2R manipulation; blue, OX 1R/OX 2R manipulation. AMY, amygdala; CO 2, carbon dioxide; CPP, conditioned place preference; DA, dopamine; DG, dentate gyrus; EtOH, ethanol; Hcrt, hypocretin; LH, lateral hypothalamus; PeF OX, perifornical area orexin; PVT, paraventricular nucleus of the thalamus; VTA, ventral tegmental area.
| Manipulation | Findings | Reference | ||
|---|---|---|---|---|
| Stress and Anxiety | Compound 56 | Subcutaneous treatment attenuated panic behaviors in 2 models of panic
vulnerability (PeF OX disinhibition and sodium lactate treatment). No effect on sleep duration |
Bonaventure et al., 2015 | |
| JNJ-54717793 | Attenuation of panic behavior and cardiovascular response in sodium lactate
and CO 2 panic models |
Bonaventure et al., 2017 | ||
|
Compound 56
SB-334867 |
Attenuation of CO
2 induced anxiety and cardiovascular responses. No
apparent sedative effects |
Johnson et al., 2015 | ||
| SB-334867 | Reduction in orofacial pain associated anxiety | Bahaaddini, Khatamsaz,
Esmaeili-Mahani, Abbasnejad, & Raoof, 2016 |
||
| SB-334867 | Effect on one measure of arousal (mobility in open field) in adolescent males.
No effect on anxiety related behavior |
Blume, Nam, Luz, Bangasser, &
Bhatnagar, 2018 |
||
| OX 1R Knockout | Increased anxiety, reduced social interaction, increased startle | Abbas et al., 2015 | ||
|
SORA2 JNJ-
10397049 |
No effect on anxiety or cardiovascular responses to CO
2 model of panic
induction |
Johnson et al., 2015 | ||
| DORA-12 | Attenuation of CO 2 induced anxiety responses. | Johnson et al., 2015 | ||
| OX Knockout | Increased anxiety in open field, predator scent, and light/dark box | Khalil & Fendt, 2017 | ||
| Motivation and Addiction | Cocaine | SB-334867 | Blocks cue induced reinstatement with strongest effect in animals with highest
cocaine-cue dependent behavior |
Bentzley & Aston-Jones, 2015 |
| SB-334867 | Decreased cocaine self administration and reduced cellular response to drug | Prince, Rau, Yorgason, &
España, 2015 |
||
| RTIOX-276 | Reduced responding for cocaine under high effort conditions, reduced DA
response to cocaine paired cues |
Levy et al., 2017 | ||
|
VTA OX
1R
Knockdown |
Delays acquisition of self-administration, reduces response to drug under
progressive ratio, alters DA transmission in striatum |
Bernstein, Badve, Barson, Bass
& España, 2017 |
||
| 4PT | No effect on cocaine self administration or DA respone to drug | Prince, Rau, Yorgason, &
España, 2015 |
||
| Almorexant | Reduced self administration under progressive ratio. Differential effects on DA
response to drug over time |
Prince, Rau, Yorgason, &
España, 2015 |
||
| Suvorexant | Reduces self-administration under progressive ratio, cocaine induced
ultrasonic vocalizations, and conditioned place preference. Reduces DA response to cocaine |
Gentile et al., 2018 | ||
| Hcrt Knockdown | Attenuates self administration in proggressive ratio | Schmeichel et al., 2017 | ||
| Hcrt Knockout | Blunted intake at highest dose and reduced response to drug after abstinence | Steiner et al., 2018 | ||
| EtOH | SB-334867 | Reduced EtOH intake and cue induced reinstatement in EtOH preferring rats | Moorman, James, Kilroy, &
Aston-Jones, 2017 |
|
| GSK1059865 | Reduced EtOH vapor induced EtOH drinking in dependent mice | Lopez, Moorman, Aston-Jones,
Becker, 2016 |
||
| TCS-OX2-29 | Anterior PVT injections of OX
2R antagonist reduces EtOH intake. EtOH
consumption increases OX 2R mRNA in PVT |
Barson, Tin Ho, Leibowitz, 2015 | ||
| / | In a white population, OX
2R polymorphism was associated with rate of alcohol
dependence independent of age or gender |
Klepp et al., 2017 | ||
| / | Context induced reinstatement associated with various levels of Hcrt neuron
activity across the LH |
Moorman, James, Kilroy, &
Aston-Jones, 2016 |
||
| / | Voluntary EtOH drinking in zebrafish increases Hcrt expression in
hypothalamus |
Sterling, Karatayev, Chang,
Algava, & Lebowitz, 2015 |
||
| Opioids | SB-334867 | Differentially modulates hedonic and motivational effects of remifentanyl in
high and low takers |
Porter-Stransky, Bentzley, &
Aston-Jones, 2017 |
|
| SB-334867 | Intra-VTA inections attenuate morphine CPP | Farahimanesh, Zarrabian, &
Haghparast, 2017 |
||
| SB-334867 | Intra-DG injection attenuates drug induced reinstatement of morphine CPP | Ebrahimian et al., 2016 | ||
| TCS-OX2-29 | Intra-VTA injections attenuates morphine CPP | Farahimanesh, Zarrabian, &
Haghparast, 2017 |
||
| TCS-OX2-29 | Intra-DG injection attenuates drug induced reinstatement of morphine CPP | Ebrahimian et al., 2016 | ||
| NBI-80713 | Reduced heroin self administration in long access paradigm and increase in
OX 2R mRNA in the AMY |
Schmeichel et al., 2015 | ||
| / | Morphine CPP increases Hcrt1 release in DG | Guo et al., 2016 |
Stress and anxiety
Hcrt plays a role in the coordination of stress responses. Plasticity in the Hcrt system is thought to contribute to long-term dysregulation of arousal seen in certain psychiatric disorders 85, 86. This may be an adaptive response to repeated stress, where heightened arousal and vigilance are needed under conditions of instability or high threat 87. Recent literature has supported the idea that activation of OX 1R promotes anxiety-like behavior. For example, in rodent models of panic, an extreme form of anxiety, animals with panic vulnerability treated with the OX 1R antagonist compound 56 reduced panic-like behaviors in a sodium lactate model of panic induction 88. Similarly, treatment with the OX 1R antagonist JNJ-54717793 attenuates panic-like behavior and cardiovascular responses in both the sodium lactate model of panic and a carbon dioxide (CO 2) model of panic provocation 89. Additional studies within the CO 2 model that screened selective Hcrt receptor antagonists (SORAs) and dual Hcrt receptor antagonists (DORAs) found that both a SORA1 (compound 56) and a DORA-12 attenuate anxiety-like behaviors but that a SORA2 did not 90. Importantly, these data provide a promising treatment route, as animals treated with SORA1 and DORA-12 showed no significant changes in sleep 90. Currently, the levels of benzodiazepines needed to achieve anxiolytic effects are also sedating; as discussed here, OX 1R antagonists can have anxiolytic effects without impacting sleep 90.
Although the mechanism of action of the wake-promoting drug modafinil is mainly through activation of DA circuitry, it also activates Hcrt neurons and is used for the treatment of narcolepsy. Treatment with modafinil after a traumatic experience reduces the incidence of post-traumatic stress disorder (PTSD), a disorder characterized by anxiety and hyperarousal. The anxiolytic effect of this treatment may be due to its interference with normal sleep-dependent memory processes 91. However, the benefits of modafinil treatment may go beyond this, as it has been shown to stimulate adaptive stress responses in an animal model of PTSD 92, 93. In a model of orofacial pain-induced anxiety, rats given injections of capsaicin into the upper lip showed increased anxiety-like responses on the elevated plus maze. Administration of Hcrt exacerbates this response while treatment with OX 1R antagonists inhibits orofacial pain-associated anxiety 94. In another study, differential effects of OX 1R antagonism were observed. The OX 1R antagonist SB-334867 influenced arousal (mobility/immobility in an open field) but not anxiety-like behavior (center exploration) in conditions of mild stress in male rats 95. Yet Hcrt knockout mice show increased anxiety in the open-field test, light-dark box test, and predator scent avoidance test despite intact fear learning 96. Likewise, OX 1R receptor knockout mice show increased anxiety and reduced social interaction, increased startle responses, and altered depressive-like behavior 97. Although genetic knockout results do not completely contradict findings from pharmacological studies, they do showcase the necessity to use the newest genetic techniques to parse out the role of Hcrt in anxiety. Two points must be made with regard to these findings: first, knockout models may result in compensatory mechanisms that may explain how Hcrt-null or OX 1R-deficient mice display lower anxiety. Second, models of stress discussed here vary greatly, and the conclusions drawn from these works may reflect the differences in the circuits underlying different types of anxiety. Thus, findings must be interpreted within the context of pharmacological, genetic, and behavioral manipulations used in these studies.
Recent work is also characterizing how individual differences in baseline Hcrt activity may pose resilience or susceptibility to stress. Rats that show low expression of preprohypocretin mRNA are resilient to social stress, and further manipulations show that chemogenetic inhibition of Hcrt reduces depressive-like behavior in otherwise stress-susceptible rats 98. Together, these data suggest that the activity of Hcrt on stress may be context or stressor specific but additionally that individual differences at baseline may influence stress resilience.
Motivation and addiction
The mesolimbic DA system, which originates in the VTA and projects to the striatum, is a key region for the processing of reward and reinforcement 99, 100. These processes necessitate and evoke arousal states to monitor reinforcers and facilitate learning 101. Reciprocally, motivational states impact arousal so as to facilitate the seeking of rewards and the avoidance of punishments 102, 103. As discussed above, LH-Hcrt neurons send excitatory projections to the VTA 13, 50, 51. Thus, the VTA may be an optimal region by which Hcrt can influence motivated arousal states. The majority of recent advances made in this field have investigated the effects of Hcrt manipulations on motivation for cocaine and ethanol (EtOH). To date, these studies suggest that Hcrt1 plays a role in motivation for drug reward, especially when drug presentation is dependent on effortful responses on the part of the animal. Here, we discuss the role of Hcrt in addiction and motivation, focusing on cocaine, alcohol, and opioids.
Hcrt knockdown attenuates cocaine self-administration under progressive ratio schedule (that is, Hcrt knockdown lowers cocaine breakpoint) but not under a fixed ratio schedule 104. Similarly, Hcrt-deficient mice show reduced cue-induced cocaine-seeking behavior following a period of abstinence, suggesting a role for Hcrt in relapse behavior 105. Additionally, these animals show blunted cocaine intake at the highest dose and reduced behavioral responses to cocaine after abstinence 105. Additional work from Navarro and colleagues further supports the role of Hcrt in relapse behavior 106. In particular, their work shows that cocaine acts at and alters activity of corticotropin-releasing factor receptor (CRF 1R)/OX 1R heterodimers within the VTA. Action of cocaine at these sites disrupts Hcrt/CRF crosstalk even 24 hours after a single systemic injection and may be a mechanism underlying stress-induced cocaine relapse 106.
Indeed, Hcrt may play a unique role in cue-reward associations, as OX 1R antagonism via SB-334867 only decreases cocaine demand in the presence of cues. SB-334867 treatment also blocks cue-induced reinstatement of drug seeking—an effect most pronounced in high-demand animals (animals with the greatest cue-dependent behavior). This suggests that OX 1R increases the reinforcing efficacy of cocaine-associated cues but not of cocaine alone. This supports the notion that Hcrt plays a role in the ability of conditioned cues to elicit motivational responses 107. Recent in vivo measurements of DA activity are beginning to inform the mechanisms that may underlie these observed effects on cocaine reinforcement. For example, Hcrt knockdown within the VTA delays acquisition of cocaine self-administration and reduces motivation for cocaine under a progressive ratio schedule while reducing DA release in the ventral striatum, DA uptake, and cocaine-induced DA reuptake inhibition at striatal terminals 108. Similarly, OX 1R blockade with RTIOX-276 attenuates motivation for cocaine and reduces the number of DA transients, DA release evoked by cocaine cues, and cocaine-induced DA reuptake inhibition as measured by fast scan cyclic voltammetry (FSCV) 109. Suvorexant, a DORA, attenuates the motivational properties of cocaine as measured by progressive ratio and place conditioning. Additionally, treatment with Suvorexant also reduces the hedonic properties of cocaine as measured by ultrasonic vocalizations. Additionally, DORA treatment reduced cocaine-induced elevations in ventral striatal DA 110. Work by Prince and colleagues suggests that effects of the DORA may be mediated by OX 1R, as blockade of OX 2R receptors alone has no effect on DA signaling or self-administration of cocaine 111. However, blocking of OX 1R or both OX 1R and OX 2R decreases motivation for cocaine as measured by self-administration under a progressive ratio schedule and reduces the effects of cocaine on DA signaling as measured by FSCV 111.
In the case of EtOH, Hcrt antagonism generally reduces EtOH consumption. In a voluntary EtOH intake model in zebrafish, it was seen that intake of EtOH increases Hcrt expression in the hypothalamus 112. OX 1R antagonism with SB-334867 reduces EtOH self-administration in alcohol-preferring rats 113. Similarly, the OX 1R antagonist GSK1059865 reduces EtOH drinking in EtOH-dependent mice 114. In a model of EtOH seeking and preference, activation of the LH is correlated with degree of seeking in context-induced reinstatement and degree of preference in home cage EtOH preference testing. Interestingly, cue-evoked reinstatement shows no correlation with Hcrt activation in any region. This suggests that there is a relationship between Hcrt activity in the LH and EtOH seeking and preference behavior but that cue-induced reinstatement for alcohol may be mediated by a different mechanism 115. Interestingly, EtOH consumption increases OX 2R mRNA within the anterior paraventricular nucleus of the thalamus and local antagonism of OX 2R reduces total EtOH intake 116.
The interactions of Hcrt with opioid rewards are particularly interesting, as the endogenous opioid dynorphin (Dyn) is expressed in 94% of Hcrt neurons and Hcrt and Dyn are thought to be co-released at Hcrt terminals within the VTA 117. The interactions of these neurotransmitters are beyond the scope of this review; however, of major relevance is the point that these neurotransmitters have opposing yet complementary actions on VTA cellular excitability 117– 121. OX 1R antagonism with SB-332867 modulates demand for the opioid drug remifentanil in low takers but not in high takers 122. Additionally, intra-VTA injections of the OX 1R antagonist SB-334867 attenuate morphine conditioned place preference (CPP) acquisition and expression. Interestingly, in the case of opioid reward, OX 2R antagonism via TCS-OX2-29 also significantly attenuates morphine CPP acquisition and expression, suggesting that both receptors within the VTA are important for expression of morphine reward 123. Similarly, systemic treatment with the OX 2R antagonist NBI-80713 dose-dependently reduces heroin self-administration in a long-access paradigm. Long-access heroin self-administration paradigms are thought to mimic compulsive drug taking; thus, OX 2R antagonism may be particularly effective at influencing drug-associated compulsivity. Similar effects have been observed in the hippocampal dentate gyrus (DG), which receives Hcrt projections from the LH and interacts with the VTA to play an important role in the linking of drug reward with contextual cues 124. In a stress- and drug-induced model of morphine reinstatement, intra-DG administration of OX 1R and OX 2R antagonists attenuates drug priming-induced reinstatement dose-dependently with no effect on stress-induced reinstatement 125. Similarly, morphine CPP increases Hcrt1 release in the DG and OX 1R antagonism via SB-334867 ameliorates morphine CPP. These findings suggest that Hcrt actions at the DG may influence the learning of drug-context associations 126.
Finally, additional work has begun to delineate the effect of Hcrt on motivation at VTA terminal sites such as the nucleus accumbens (NAc) 127. Blomeley and colleagues used optogenetics and electrophysiology to characterize a direct Hcrt→DA D 2 excitatory circuit that is necessary for the expression of risk avoidance behavior in mice 127. Indeed, increased DA D 2 neuron activation caused animals to avoid risks such as crossing a predator-scented chamber to attain a food reward and chemogenetic silencing of accumbal DA D 2 cells inhibited Hcrt-mediated avoidance. Importantly, these data showcase how Hcrt can influence adaptive behavioral inhibition even in the presence of rewards. These data open up new opportunities of research, such as characterizing the effects of Hcrt on different subregions of the NAc, which is a heterogeneous structure with distinct electrophysiological properties 128, 129. Additional lines of research should investigate how Hcrt-mediated motivation in the NAc is impacted by diurnal rhythms as well as sleep disturbance and how the Dyn system interacts in this region to modulate motivation 120, 130.
Cognitive function and memory
Studies suggest that Hcrt deficiency is associated with memory deficits. Hcrt deficiencies negatively impact working memory as tested in a non-matching-to-place T-maze task 131. Hcrt/ataxin-3 transgenic mice (a progressive model of narcolepsy), which become Hcrt deficient at 12 weeks old, show impaired avoidance memory in a two-way active avoidance paradigm in which an animal has to perform a specific motor response to avoid an aversive stimulus. Hcrt1 administration reverses memory deficits, suggesting that Hcrt plays a role in hippocampal-dependent consolidation of two-way active avoidance memory 132. Chemogenetic activation of Hcrt neurons improves short-term memory for novel locations, a function that putatively supports foraging and exploration 133.
Pain negatively influences memory processing in ways that may be influenced by Hcrt. In the Morris water maze (MWM) (a test of spatial learning and memory), orofacial pain-induced memory impairments are exacerbated by the OX 1R antagonist SB-334867 whereas administration of Hcrt1 prevented these spatial memory deficits 134. Importantly, injections were directed at the trigeminal nucleus caudalis, which is a central relay for orofacial pain. Thus, the observed effect on memory may be via alterations in the experience of pain itself rather than the formation of a pain-associated memory 134. In a similar study by Raoof and colleagues, orofacial pain memory was mediated by Hcrt at the level of the hippocampus (HPC). Intra-hippocampal injections of Hcrt1 inhibit pain-induced memory impairments as measured by the MWM. However, treatment with the OX 1R antagonist SB-334867 had no effect on learning and memory 135. Indeed, the HPC is a critical region for memory function and Hcrt action at this site may influence memory processes via its influence on the induction of long-term potentiation (LTP). In vitro studies show that OX 1R antagonists significantly decrease the firing rates of hippocampal CA1 neurons, showing that the effect of Hcrt on these neurons is excitatory 136. Additional in vitro electrophysiology studies demonstrate that Hcrt1 may bidirectionally modulate HPC CA1 function. Specifically, moderate doses of Hcrt1 inhibit LTP while subnanomolar concentrations result in re-potentiation via OX 1R and OX 2R 137. It is important to note that the Hcrt manipulations discussed here may have influenced sleep and therefore resulting memory problems may be sleep dependent and thus only indirectly dependent on Hcrt.
Part III: quantitative modeling of hypocretin circuits
Computational modeling of the Hcrt network remains a relatively unexplored frontier. Development of analytical models of Hcrt function will inform our interpretation of data gathered through empirical study and drive the development of testable hypotheses. In particular, computational modeling of Hcrt networks will prove essential for our understanding of the following three questions: (1) how do internal or external physiological states influence arousal? (2) How does the heterogeneity of the system (that is, genetic, afferent, and efferent diversity) contribute to network dynamics? (3) How does Hcrt function as a volume transmitter to produce both generalized and specific effects? Ultimately, integration of these models with experimental approaches will allow for understanding of the network as a whole as well as monosynaptic interactions.
Models of hypocretin network in arousal
Current models have described Hcrt as functioning within a “flip/flop” model where it stabilizes wakefulness, preventing aberrant switches between mutually exclusive states 138. This model, however, cannot account for overlapping states of arousal such as those observed in narcolepsy or RBD in which REM sleep can co-occur with conscious awareness 139, 140. Additionally, this model does not factor in the many systems that interact to influence arousal. These observations make it necessary to revise the binary nature of the flip/flop model. Studies have expanded the model by characterizing a circuit with hierarchical gating of additional neural circuits, feedback, and redundancy 141. This hierarchical model provides a framework on which to add motivational influences on arousal states. Indeed, animals can adapt their sleep on the basis of internal and external variables such as migration or predator avoidance or to increase the likelihood of mating 142– 144. Recently, an alternative has been proposed in which sleep-to-wake transitions are predicted on the basis of inputs with different “weights” onto an integrator neuron 145. An integrator neuron would continuously compute probabilities of wakefulness on the basis of functional connectivity of the system as well as physiological factors such as stress or circadian phase. Diversity of neuronal responses to stimuli can be integrated within this model to account for the heterogeneity of the system. In this vein, Schöne and Burdakov acknowledge the necessity of an adaptive behavioral control system that can respond to unpredictable changes in the environment 146. Thus, they propose a model of brain arousal control modules organized in a feedback loop by which Hcrt can gate relevant information on the basis of environmental and homeostatic needs 146. We look forward to the future advancement of this area of Hcrt research that will undoubtedly expand our understanding as an adaptable regulator of arousal.
Volume transmission
Volume transmission (VT) is a mechanism of neural signaling by which neurotransmitters can exert actions on cells in close proximity as well as distant targets. In VT, neurotransmitters signal via diffusion within extracellular fluid 147, 148. This type of release is thought to allow for modulation of neural activity via long time courses and greater distances 147– 149. VT may happen via cellular pores, diffusion through the plasma membrane, exocytosis, or reversal of transporter proteins 149. To date, actions of Hcrt at the dorsal lateral geniculate nucleus (DLG) and the DRN (aside from already-known synaptic actions) have been theorized to be exerted via VT 150, 151. Observations of Hcrt1 immunoreactivity in many non-synaptic varicosities located far from synapses with axons forming asymmetric synapses suggest that DRN excitation via Hcrt1 may be via this mechanism 150. Indeed, the DRN plays an important role in the regulation of arousal and both synaptic and VT mechanisms may support long-term cortical arousal 25, 36. In a separate set of findings, Hcrt was found to powerfully modulate neurons of the DLG despite only sparse expression of Hcrt nerve terminals in the region, suggesting that these actions are via VT 151. Additionally, a recent study of melanin-concentrating hormone (MCH), a hypothalamic peptide important for the regulation of feeding, shows that MCH neurons project to ventricular regions where they increase MCH levels in the cerebrospinal fluid (CSF) and stimulate feeding 152. MCH neurons are intermingled with Hcrt neurons in the LH, and the authors measure that 40% of Hcrt neurons also project to the CSF where they are poised to signal via VT to influence distal targets 152. Further investigations should determine whether Hcrt acts via VT and, if so, how its activity is influenced by (1) temporal and spatial release dynamics, (2) diffusion and dilution parameters, and (3) transporter kinetics in order to characterize its effective radius.
Future directions and conclusions
As reviewed here, the ever-growing database on Hcrt continues to broaden our conceptualization of these peptides as more than just regulators of sleep-to-wake transitions. Technical advances have allowed ever more precise measurement and manipulation of these circuits which will continue to inform our understanding of this circuit. To date, therapeutic advances have allowed the effective targeting of Hcrt circuitry for the treatment of narcolepsy and insomnia, and research discussed here provides evidence for the potential of this system for the treatment of anxiety, addiction, and memory deficits. Integration of these findings with analytical models will provide a novel means for explaining and interpreting biological observations so as to gain a holistic understanding of their role in physiology and behavior.
Acknowledgments
The authors would like to thank Jeremy C. Borniger and Christopher C. Angelakos for their helpful comments on the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Thomas Scammell, Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
Jyrki P. Kukkonen, Biochemistry and Cell Biology, Department of Veterinary Biosciences, Faculty of Veterinary Medicine, and Department of Physiology, Institute of Biomedicine, Faculty of Medicine, University of Helsinki, Helsinki, Finland
Denis Burdakov, Neurophysiology Laboratory, Francis Crick Institute, London, UK
Funding Statement
Our work was supported by the National Institutes of health under grant numbers 5R01MH087592-07, 5R01AG047671-04 and 1R01MH102638-01A1.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. de Lecea L, Kilduff TS, Peyron C, et al. : The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai T, Amemiya A, Ishii M, et al. : Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 3. Kim CK, Adhikari A, Deisseroth K: Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18(4):222–35. 10.1038/nrn.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomez JL, Bonaventura J, Lesniak W, et al. : Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503–7. 10.1126/science.aan2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth BL: DREADDs for Neuroscientists. Neuron. 2016;89(4):683–94. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin MZ, Schnitzer MJ: Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016;19(9):1142–53. 10.1038/nn.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funato H, Miyoshi C, Fujiyama T, et al. : Forward-genetics analysis of sleep in randomly mutagenized mice. Nature. 2016;539(7629):378–83. 10.1038/nature20142 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Susaki EA, Ukai H, Ueda HR: Next-generation mammalian genetics toward organism-level systems biology. NPJ Syst Biol Appl. 2017;3: 15. 10.1038/s41540-017-0015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cong L, Ran FA, Cox D, et al. : Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Mali P, Yang L, Esvelt KM, et al. : RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Weber F, Dan Y: Circuit-based interrogation of sleep control. Nature. 2016;538(7623):51–9. 10.1038/nature19773 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Lőrincz ML, Adamantidis AR: Monoaminergic control of brain states and sensory processing: Existing knowledge and recent insights obtained with optogenetics. Prog Neurobiol. 2017;151:237–53. 10.1016/j.pneurobio.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Peyron C, Tighe DK, van den Pol AN, et al. : Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. 10.1523/JNEUROSCI.18-23-09996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida K, McCormack S, España RA, et al. : Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–61. 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Scammell TE, Arrigoni E, Lipton JO: Neural Circuitry of Wakefulness and Sleep. Neuron. 2017;93(4):747–65. 10.1016/j.neuron.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Eban-Rothschild A, de Lecea L: Neuronal substrates for initiation, maintenance, and structural organization of sleep/wake states [version 1; referees: 2 approved]. F1000Res. 2017;6:212. 10.12688/f1000research.9677.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thannickal TC, Moore RY, Nienhuis R, et al. : Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–74. 10.1016/S0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishino S, Ripley B, Overeem S, et al. : Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355(9197):39–40. 10.1016/S0140-6736(99)05582-8 [DOI] [PubMed] [Google Scholar]
- 19. Lin L, Faraco J, Li R, et al. : The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin ( orexin) receptor 2 gene. Cell. 1999;98(3):365–76. 10.1016/S0092-8674(00)81965-0 [DOI] [PubMed] [Google Scholar]
- 20. Chemelli RM, Willie JT, Sinton CM, et al. : Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–51. 10.1016/S0092-8674(00)81973-X [DOI] [PubMed] [Google Scholar]
- 21. de Lecea L: Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci. 2015;25:367–78. 10.1007/7854_2014_364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Didato G, Nobili L: Treatment of narcolepsy. Expert Rev Neurother. 2009;9(6):897–910. 10.1586/ern.09.29 [DOI] [PubMed] [Google Scholar]
- 23. Schoch SF, Werth E, Poryazova R, et al. : Dysregulation of Sleep Behavioral States in Narcolepsy. Sleep. 2017;40(12): zsx170. 10.1093/sleep/zsx170 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Branch AF, Navidi W, Tabuchi S, et al. : Progressive Loss of the Orexin Neurons Reveals Dual Effects on Wakefulness. Sleep. 2016;39(2):369–77. 10.5665/sleep.5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adamantidis AR, Zhang F, Aravanis AM, et al. : Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–4. 10.1038/nature06310 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Tsunematsu T, Tabuchi S, Tanaka KF, et al. : Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. 2013;255:64–74. 10.1016/j.bbr.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 27. Sasaki K, Suzuki M, Mieda M, et al. : Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6(5):e20360. 10.1371/journal.pone.0020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakurai T, Nagata R, Yamanaka A, et al. : Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Haas H, Panula P: The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–30. 10.1038/nrn1034 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi K, Lin JS, Sakai K: Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26(40):10292–8. 10.1523/JNEUROSCI.2341-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Fujita A, Bonnavion P, Wilson MH, et al. : Hypothalamic Tuberomammillary Nucleus Neurons: Electrophysiological Diversity and Essential Role in Arousal Stability. J Neurosci. 2017;37(39):9574–92. 10.1523/JNEUROSCI.0580-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bayer L, Eggermann E, Serafin M, et al. : Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14(9):1571–5. 10.1046/j.0953-816x.2001.01777.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Eriksson KS, Sergeeva O, Brown RE, et al. : Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21(23):9273–9. 10.1523/JNEUROSCI.21-23-09273.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Huang ZL, Qu WM, Li WD, et al. : Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98(17):9965–70. 10.1073/pnas.181330998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen A, Singh C, Oikonomou G, et al. : Genetic Analysis of Histamine Signaling in Larval Zebrafish Sleep. eNeuro. 2017;4(1): pii: ENEURO.0286-16.2017. 10.1523/ENEURO.0286-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Carter ME, Adamantidis A, Ohtsu H, et al. : Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29(35):10939–49. 10.1523/JNEUROSCI.1205-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Yu X, Ye Z, Houston CM, et al. : Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron. 2015;87(1):164–78. 10.1016/j.neuron.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Eggermann E, Serafin M, Bayer L, et al. : Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108(2):177–81. 10.1016/S0306-4522(01)00512-7 [DOI] [PubMed] [Google Scholar]
- 39. Marcus JN, Aschkenasi CJ, Lee CE, et al. : Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- 40. España RA, Baldo BA, Kelley AE, et al. : Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106(4):699–715. 10.1016/S0306-4522(01)00319-0 [DOI] [PubMed] [Google Scholar]
- 41. Chen L, Yin D, Wang TX, et al. : Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology. 2016;41(8):2133–46. 10.1038/npp.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Anaclet C, Pedersen NP, Ferrari LL, et al. : Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6: 8744. 10.1038/ncomms9744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duque A, Balatoni B, Detari L, et al. : EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84(3):1627–35. 10.1152/jn.2000.84.3.1627 [DOI] [PubMed] [Google Scholar]
- 44. Jones BE: Principal cell types of sleep-wake regulatory circuits. Curr Opin Neurobiol. 2017;44:101–9. 10.1016/j.conb.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee MG, Hassani OK, Alonso A, et al. : Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25(17):4365–9. 10.1523/JNEUROSCI.0178-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Xu M, Chung S, Zhang S, et al. : Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18(11):1641–7. 10.1038/nn.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han Y, Shi Yf, Xi W, et al. : Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24(6):693–8. 10.1016/j.cub.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 48. Irmak SO, de Lecea L: Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37(12):1941–51. 10.5665/sleep.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim T, Thankachan S, McKenna JT, et al. : Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112(11):3535–40. 10.1073/pnas.1413625112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baldo BA, Daniel RA, Berridge CW, et al. : Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464(2):220–37. 10.1002/cne.10783 [DOI] [PubMed] [Google Scholar]
- 51. Fadel J, Deutch AY: Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. 10.1016/S0306-4522(02)00017-9 [DOI] [PubMed] [Google Scholar]
- 52. Korotkova TM, Sergeeva OA, Eriksson KS, et al. : Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. 10.1523/JNEUROSCI.23-01-00007.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Narita M, Nagumo Y, Hashimoto S, et al. : Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. 10.1523/JNEUROSCI.2761-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vittoz NM, Berridge CW: Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31(2):384–95. 10.1038/sj.npp.1300807 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Ichinose T, Tanimoto H, Yamagata N: Behavioral Modulation by Spontaneous Activity of Dopamine Neurons. Front Syst Neurosci. 2017;11:88. 10.3389/fnsys.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boutrel B, Koob GF: What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27(6):1181–94. 10.1093/sleep/27.6.1181 [DOI] [PubMed] [Google Scholar]
- 57. Trulson ME, Jacobs BL: Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163(1):135–50. 10.1016/0006-8993(79)90157-4 [DOI] [PubMed] [Google Scholar]
- 58. Trulson ME, Preussler DW: Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83(2):367–77. 10.1016/S0014-4886(84)90105-5 [DOI] [PubMed] [Google Scholar]
- 59. Miller JD, Farber J, Gatz P, et al. : Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–41. 10.1016/0006-8993(83)91101-0 [DOI] [PubMed] [Google Scholar]
- 60. Steinfels GF, Heym J, Strecker RE, et al. : Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258(2):217–28. 10.1016/0006-8993(83)91145-9 [DOI] [PubMed] [Google Scholar]
- 61. Eban-Rothschild A, Rothschild G, Giardino WJ, et al. : VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19(10):1356–66. 10.1038/nn.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Taylor NE, Van Dort CJ, Kenny JD, et al. : Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113(45):12826–12831. 10.1073/pnas.1614340113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Oishi Y, Suzuki Y, Takahashi K, et al. : Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D 2-like receptors in mice. Brain Struct Funct. 2017;222(6):2907–15. 10.1007/s00429-017-1365-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Aston-Jones G, Cohen JD: An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- 65. Berridge CW, Waterhouse BD: The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. 10.1016/S0165-0173(03)00143-7 [DOI] [PubMed] [Google Scholar]
- 66. Gompf HS, Aston-Jones G: Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res. 2008;1224:43–52. 10.1016/j.brainres.2008.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Carter ME, Brill J, Bonnavion P, et al. : Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109(39):E2635–44. 10.1073/pnas.1202526109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carter ME, Yizhar O, Chikahisa S, et al. : Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–33. 10.1038/nn.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Singh C, Oikonomou G, Prober DA: Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish. eLife. 2015;4:e07000. 10.7554/eLife.07000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu B, Yang N, Qiao QC, et al. : Roles of the orexin system in central motor control. Neurosci Biobehav Rev. 2015;49:43–54. 10.1016/j.neubiorev.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 71. Burgess CR, Scammell TE: Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci. 2012;32(36):12305–11. 10.1523/JNEUROSCI.2630-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blouin AM, Siegel JM: Relation of melanin concentrating hormone levels to sleep, emotion and hypocretin levels. Sleep. 2013;36(12):1777. 10.5665/sleep.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lammers GJ, Overeem S, Tijssen MA, et al. : Effects of startle and laughter in cataplectic subjects: a neurophysiological study between attacks. Clin Neurophysiol. 2000;111(7):1276–81. 10.1016/S1388-2457(00)00306-0 [DOI] [PubMed] [Google Scholar]
- 74. Wu MF, Nienhuis R, Maidment N, et al. : Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch Ital Biol. 2011;149(4):492–8. 10.4449/aib.v149i4.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Vetrivelan R, Chang C, Lu J: Muscle tone regulation during REM sleep: neural circuitry and clinical significance. Arch Ital Biol. 2011;149(4):348–66. 10.4449/aib.v149i4.1272 [DOI] [PubMed] [Google Scholar]
- 76. Vitrac C, Benoit-Marand M: Monoaminergic Modulation of Motor Cortex Function. Front Neural Circuits. 2017;11:72. 10.3389/fncir.2017.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rommelfanger KS, Edwards GL, Freeman KG, et al. : Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104(34):13804–9. 10.1073/pnas.0702753104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Luthman J, Fredriksson A, Sundström E, et al. : Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: motor behavior and monoamine alterations at adult stage. Behav Brain Res. 1989;33(3):267–77. 10.1016/S0166-4328(89)80121-4 [DOI] [PubMed] [Google Scholar]
- 79. Bickford P: Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res. 1993;620(1):133–8. 10.1016/0006-8993(93)90279-V [DOI] [PubMed] [Google Scholar]
- 80. Geyer MA, Segal DS, Mandell AJ: Effect of intraventricular infusion of dopamine and norepinephrine on motor activity. Physiol Behav. 1972;8(4):653–8. 10.1016/0031-9384(72)90090-X [DOI] [PubMed] [Google Scholar]
- 81. Larrosa O, de la Llave Y, Bario S, et al. : Stimulant and anticataplectic effects of reboxetine in patients with narcolepsy: a pilot study. Sleep. 2001;24(3):282–5. 10.1093/sleep/24.3.282 [DOI] [PubMed] [Google Scholar]
- 82. Hasegawa E, Maejima T, Yoshida T, et al. : Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc Natl Acad Sci U S A. 2017;114(17):E3526–E3535. 10.1073/pnas.1614552114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Mahoney CE, Agostinelli LJ, Brooks JN, et al. : GABAergic Neurons of the Central Amygdala Promote Cataplexy. J Neurosci. 2017;37(15):3995–4006. 10.1523/JNEUROSCI.4065-15.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Scammell TE, Winrow CJ: Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. 10.1146/annurev-pharmtox-010510-100528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Giardino WJ, de Lecea L: Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 2014;29:103–8. 10.1016/j.conb.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gao XB, Wang AH: Experience-dependent plasticity in hypocretin/orexin neurones: re-setting arousal threshold. Acta Physiol (Oxf). 2010;198(3):251–62. 10.1111/j.1748-1716.2009.02047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnson PL, Molosh A, Fitz SD, et al. : Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–61. 10.1016/B978-0-444-59489-1.00009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bonaventure P, Yun S, Johnson PL, et al. : A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015;352(3):590–601. 10.1124/jpet.114.220392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bonaventure P, Dugovic C, Shireman B, et al. : Evaluation of JNJ-54717793 a Novel Brain Penetrant Selective Orexin 1 Receptor Antagonist in Two Rat Models of Panic Attack Provocation. Front Pharmacol. 2017;8:357. 10.3389/fphar.2017.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Johnson PL, Federici LM, Fitz SD, et al. : OREXIN 1 AND 2 RECEPTOR INVOLVEMENT IN CO2 -INDUCED PANIC-ASSOCIATED BEHAVIOR AND AUTONOMIC RESPONSES. Depress Anxiety. 2015;32(9):671–83. 10.1002/da.22403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rasch B, Born J: About sleep's role in memory. Physiol Rev. 2013;93(2):681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cohen S, Ifergane G, Vainer E, et al. : The wake-promoting drug modafinil stimulates specific hypothalamic circuits to promote adaptive stress responses in an animal model of PTSD. Transl Psychiatry. 2016;6(10):e917. 10.1038/tp.2016.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vance MC, Kovachy B, Dong M, et al. : Peritraumatic distress: A review and synthesis of 15 years of research. J Clin Psychol. 2018. 10.1002/jclp.22612 [DOI] [PubMed] [Google Scholar]
- 94. Bahaaddini M, Khatamsaz S, Esmaeili-Mahani S, et al. : The role of trigeminal nucleus caudalis orexin 1 receptor in orofacial pain-induced anxiety in rat. Neuroreport. 2016;27(15):1107–13. 10.1097/WNR.0000000000000660 [DOI] [PubMed] [Google Scholar]
- 95. Blume SR, Nam H, Luz S, et al. : Sex- and Age-dependent Effects of Orexin 1 Receptor Blockade on Open-Field Behavior and Neuronal Activity. Neuroscience. 2018;381:11–21. 10.1016/j.neuroscience.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Khalil R, Fendt M: Increased anxiety but normal fear and safety learning in orexin-deficient mice. Behav Brain Res. 2017;320:210–8. 10.1016/j.bbr.2016.12.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Abbas MG, Shoji H, Soya S, et al. : Comprehensive Behavioral Analysis of Male Ox1r -/- Mice Showed Implication of Orexin Receptor-1 in Mood, Anxiety, and Social Behavior. Front Behav Neurosci. 2015;9:324. 10.3389/fnbeh.2015.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grafe LA, Eacret D, Dobkin J, et al. : Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro. 2018;5(2): pii: ENEURO.0273-17.2018. 10.1523/ENEURO.0273-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Schultz W: Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. 10.1152/jn.1998.80.1.1 [DOI] [PubMed] [Google Scholar]
- 100. Berridge KC, Robinson TE: What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- 101. Oleson EB, Gentry RN, Chioma VC, et al. : Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci. 2012;32(42):14804–8. 10.1523/JNEUROSCI.3087-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mahler SV, Moorman DE, Smith RJ, et al. : Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–303. 10.1038/nn.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boutrel B, Cannella N, de Lecea L: The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–11. 10.1016/j.brainres.2009.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schmeichel BE, Matzeu A, Koebel P, et al. : Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology. 2018. 10.1038/s41386-018-0054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Steiner N, Rossetti C, Sakurai T, et al. : Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology. 2018;133:395–403. 10.1016/j.neuropharm.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 106. Navarro G, Quiroz C, Moreno-Delgado D, et al. : Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J Neurosci. 2015;35(17):6639–53. 10.1523/JNEUROSCI.4364-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bentzley BS, Aston-Jones G: Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41(9):1149–56. 10.1111/ejn.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bernstein DL, Badve PS, Barson JR, et al. : Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine. Addict Biol. 2017;23(5):1032–1045. 10.1111/adb.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Levy KA, Brodnik ZD, Shaw JK, et al. : Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology (Berl ). 2017;234(18):2761–76. 10.1007/s00213-017-4673-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Gentile TA, Simmons SJ, Barker DJ, et al. : Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol. 2018;23(1):247–55. 10.1111/adb.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Prince CD, Rau AR, Yorgason JT, et al. : Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6(1):138–46. 10.1021/cn500246j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sterling ME, Karatayev O, Chang GQ, et al. : Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav Brain Res. 2015;278:29–39. 10.1016/j.bbr.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moorman DE, James MH, Kilroy EA, et al. : Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2017;1654(Pt A):34–42. 10.1016/j.brainres.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 114. Lopez MF, Moorman DE, Aston-Jones G, et al. : The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016;1636:74–80. 10.1016/j.brainres.2016.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Moorman DE, James MH, Kilroy EA, et al. : Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 2016;43(5):710–20. 10.1111/ejn.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Barson JR, Ho HT, Leibowitz SF: Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: Role of orexin receptor 2. Addict Biol. 2015;20(3):469–81. 10.1111/adb.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li Y, van den Pol AN: Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26(50):13037–47. 10.1523/JNEUROSCI.3380-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Eriksson KS, Sergeeva OA, Selbach O, et al. : Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci. 2004;19(5):1278–84. 10.1111/j.1460-9568.2004.03243.x [DOI] [PubMed] [Google Scholar]
- 119. Thomas TS, Baimel C, Borgland SL: Opioid and hypocretin neuromodulation of ventral tegmental area neuronal subpopulations. Br J Pharmacol. 2018;175(14):2825–33. 10.1111/bph.13993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Muschamp JW, Hollander JA, Thompson JL, et al. : Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–55. 10.1073/pnas.1315542111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Baimel C, Lau BK, Qiao M, et al. : Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. 2017;18(6):1346–55. 10.1016/j.celrep.2017.01.030 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Porter-Stransky KA, Bentzley BS, Aston-Jones G: Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2017;22(2):303–17. 10.1111/adb.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Farahimanesh S, Zarrabian S, Haghparast A: Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides. 2017;66:45–51. 10.1016/j.npep.2017.08.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 124. Luo AH, Tahsili-Fahadan P, Wise RA, et al. : Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–7. 10.1126/science.1204622 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 125. Ebrahimian F, Naghavi FS, Yazdi F, et al. : Differential roles of orexin receptors within the dentate gyrus in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Behav Neurosci. 2016;130(1):91–102. 10.1037/bne0000112 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 126. Guo SJ, Cui Y, Huang ZZ, et al. : Orexin A-mediated AKT signaling in the dentate gyrus contributes to the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Addict Biol. 2016;21(3):547–59. 10.1111/adb.12236 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 127. Blomeley C, Garau C, Burdakov D: Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat Neurosci. 2018;21(1):29–32. 10.1038/s41593-017-0023-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 128. Wightman RM, Heien ML, Wassum KM, et al. : Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26(7):2046–54. 10.1111/j.1460-9568.2007.05772.x [DOI] [PubMed] [Google Scholar]
- 129. Yang H, de Jong JW, Tak Y, et al. : Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron. 2018;97(2):434–449.e4. 10.1016/j.neuron.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. McGregor R, Wu MF, Barber G, et al. : Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31(43):15455–67. 10.1523/JNEUROSCI.4017-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 131. Dang R, Chen Q, Song J, et al. : Orexin knockout mice exhibit impaired spatial working memory. Neurosci Lett. 2018;668:92–7. 10.1016/j.neulet.2018.01.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 132. Mavanji V, Butterick TA, Duffy CM, et al. : Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol Learn Mem. 2017;146:21–30. 10.1016/j.nlm.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 133. Aitta-Aho T, Pappa E, Burdakov D, et al. : Cellular activation of hypothalamic hypocretin/orexin neurons facilitates short-term spatial memory in mice. Neurobiol Learn Mem. 2016;136:183–8. 10.1016/j.nlm.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Kooshki R, Abbasnejad M, Esmaeili-Mahani S, et al. : The role of trigeminal nucleus caudalis orexin 1 receptors in orofacial pain transmission and in orofacial pain-induced learning and memory impairment in rats. Physiol Behav. 2016;157:20–7. 10.1016/j.physbeh.2016.01.031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 135. Raoof R, Esmaeili-Mahani S, Abbasnejad M, et al. : Changes in hippocampal orexin 1 receptor expression involved in tooth pain-induced learning and memory impairment in rats. Neuropeptides. 2015;50:9–16. 10.1016/j.npep.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 136. Chen XY, Chen L, Du YF: Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. J Neurosci Res. 2017;95(7):1415–26. 10.1002/jnr.23975 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 137. Lu GL, Lee CH, Chiou LC: Orexin A induces bidirectional modulation of synaptic plasticity: Inhibiting long-term potentiation and preventing depotentiation. Neuropharmacology. 2016;107:168–80. 10.1016/j.neuropharm.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 138. Saper CB, Fuller PM, Pedersen NP, et al. : Sleep state switching. Neuron. 2010;68(6):1023–42. 10.1016/j.neuron.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Krueger JM, Rector DM, Roy S, et al. : Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9(12):910–9. 10.1038/nrn2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vyazovskiy VV, Olcese U, Hanlon EC, et al. : Local sleep in awake rats. Nature. 2011;472(7344):443–7. 10.1038/nature10009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. Sorooshyari S, Huerta R, de Lecea L: A Framework for Quantitative Modeling of Neural Circuits Involved in Sleep-to-Wake Transition. Front Neurol. 2015;6:32. 10.3389/fneur.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Acerbi A, Nunn CL: Predation and the phasing of sleep: An evolutionary individual-based model. Animal Behaviour. 2011;81(4):801–11. 10.1016/j.anbehav.2011.01.015 [DOI] [Google Scholar]
- 143. Rattenborg NC, Voirin B, Cruz SM, et al. : Evidence that birds sleep in mid-flight. Nat Commun. 2016;7: 12468. 10.1038/ncomms12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lesku JA, Rattenborg NC, Valcu M, et al. : Adaptive sleep loss in polygynous pectoral sandpipers. Science. 2012;337(6102):1654–8. 10.1126/science.1220939 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 145. Eban-Rothschild A, Appelbaum L, de Lecea L: Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology. 2018;43(5):937–52. 10.1038/npp.2017.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schöne C, Burdakov D: Orexin/Hypocretin and Organizing Principles for a Diversity of Wake-Promoting Neurons in the Brain. Curr Top Behav Neurosci. 2017;33:51–74. 10.1007/7854_2016_45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Agnati LF, Guidolin D, Guescini M, et al. : Understanding wiring and volume transmission. Brain Res Rev. 2010;64(1):137–59. 10.1016/j.brainresrev.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 148. Agnati LF, Zoli M, Strömberg I, et al. : Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69(3):711–26. 10.1016/0306-4522(95)00308-6 [DOI] [PubMed] [Google Scholar]
- 149. Trueta C, De-Miguel FF: Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol. 2012;3:319. 10.3389/fphys.2012.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Del Cid-Pellitero E, Garzón M: Medial prefrontal cortex receives input from dorsal raphe nucleus neurons targeted by hypocretin1/orexinA-containing axons. Neuroscience. 2011;172:30–43. 10.1016/j.neuroscience.2010.10.058 [DOI] [PubMed] [Google Scholar]
- 151. Chrobok L, Palus-Chramiec K, Chrzanowska A, et al. : Multiple excitatory actions of orexins upon thalamo-cortical neurons in dorsal lateral geniculate nucleus - implications for vision modulation by arousal. Sci Rep. 2017;7(1): 7713. 10.1038/s41598-017-08202-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 152. Noble EE, Hahn JD, Konanur VR, et al. : Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell Metab. 2018;28(1):55–68.e7. 10.1016/j.cmet.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation