Abstract
Psychological treatments are increasingly regarded as useful interventions for schizophrenia. However, a comprehensive evaluation of the available evidence is lacking and the benefit of psychological interventions for patients with current positive symptoms is still debated. The present study aimed to evaluate the efficacy, acceptability and tolerability of psychological treatments for positive symptoms of schizophrenia by applying a network meta‐analysis approach, that can integrate direct and indirect comparisons. We searched EMBASE, MEDLINE, PsycINFO, PubMed, BIOSIS, Cochrane Library, World Health Organization's International Clinical Trials Registry Platform and http://ClinicalTrials.gov for randomized controlled trials of psychological treatments for positive symptoms of schizophrenia, published up to January 10, 2018. We included studies on adults with a diagnosis of schizophrenia or a related disorder presenting positive symptoms. The primary outcome was change in positive symptoms measured with validated rating scales. We included 53 randomized controlled trials of seven psychological interventions, for a total of 4,068 participants receiving the psychological treatment as add‐on to antipsychotics. On average, patients were moderately ill at baseline. The network meta‐analysis showed that cognitive behavioural therapy (40 studies) reduced positive symptoms more than inactive control (standardized mean difference, SMD=−0.29; 95% CI: –0.55 to −0.03), treatment as usual (SMD=−0.30; 95% CI: –0.45 to −0.14) and supportive therapy (SMD=−0.47; 95% CI: –0.91 to −0.03). Cognitive behavioural therapy was associated with a higher dropout rate compared with treatment as usual (risk ratio, RR=0.74; 95% CI: 0.58 to 0.95). Confidence in the estimates ranged from moderate to very low. The other treatments contributed to the network with a lower number of studies. Results were overall consistent in sensitivity analyses controlling for several factors, including the role of researchers’ allegiance and blinding of outcome assessor. Cognitive behavior therapy seems to be effective on positive symptoms in moderately ill patients with schizophrenia, with effect sizes in the lower to medium range, depending on the control condition.
Keywords: Schizophrenia, positive symptoms, psychological interventions, cognitive behavioural therapy, network meta‐analysis
Psychological interventions for schizophrenia have been developed to address many aspects of the disorder and, according to guidelines from the National Institute for Health and Care Excellence (NICE)1 in the UK and the Schizophrenia Patient Outcomes Research Team (PORT)2 in the US, are regarded as useful interventions.
A number of systematic reviews of randomized studies have been conducted on these treatments3. However, findings are unclear and often contradictory. For example, while some reviews4, 5 have found a superiority of cognitive behavioural therapy (CBT) compared to usual care, other authors could not replicate this finding when non‐blinded randomized controlled trials (RCTs) were excluded6. A Cochrane review found CBT to be effective in the long term, but not in the short or medium term7, while another meta‐analysis did not find a benefit for CBT8.
Moreover, the current evidence presents several shortcomings. First, all the existing reviews have compared two interventions at a time using pairwise meta‐analysis. This method summarizes results only when two treatments have already been compared in existing studies, leaving open questions for all the other possible comparisons. Even in the review by Turner et al9, which included only studies comparing two “active psychological interventions”, pairwise meta‐analysis was applied to compare each intervention with the pooled others, again not providing information on the comparisons that were not already considered in a trial.
Furthermore, the existing reviews have included heterogeneous samples, pooling patients with different sets of symptoms. No review focused specifically on patients with current positive symptoms, which are – at least in the acute phase – at the core of the disorder. Also the review by Zimmermann et al5, aiming at evaluating the effect of CBT on positive symptoms, did not restrict its selection to studies on patients presenting these symptoms.
As a result of these limitations in the current evidence, it is still unclear whether there are efficacious and acceptable psychological interventions for treating positive symptoms in schizophrenia.
The aim of the present study was to overcome these limitations by conducting a network meta‐analysis, which integrates direct and indirect comparisons of interventions10, and informs about differences between treatments, even when direct comparisons are not available. Such a meta‐analysis requires a certain degree of homogeneity in the population, settings and methods across the studies. A careful definition of the target population of the intervention is therefore essential in order to produce information that is useful for clinical practice.
Our network meta‐analysis covered psychological interventions addressing positive symptoms of schizophrenia, in patients currently experiencing such symptoms, in order to generate results that will be relevant for this specific population.
METHODS
Study design and participants
The detailed methodology for this systematic review and network meta‐analysis is described in the study protocol, that was registered a priori at PROSPERO (no. CRD42017067795) and published3. In reporting results, we followed the PRISMA extension statement for network meta‐analyses11, 12.
We included studies in adult individuals with a diagnosis of schizophrenia or a related disorder (such as schizophreniform or schizoaffective disorder), presenting active positive symptoms, or in the phase of acute exacerbation, as defined by inclusion criteria of the trial, without restrictions on setting, gender or ethnicity. We optimized homogeneity of studies within and across treatment comparisons by excluding studies on patients with predominant negative symptoms or concomitant medical or psychiatric illness, and patients in their first psychotic episode or at risk of psychosis. Studies were included if at least 80% of the patients had schizophrenia or related disorders. In case of a mixed population, data about patients with schizophrenia were extracted, if available. We included the trials irrespective of the diagnostic criteria used.
Interventions and comparators
As defined a priori in our protocol3, interventions were any psychological treatments that occur through an interaction between therapist and patient, either face‐to‐face individually or in group, with the primary aim to reduce positive symptoms.
Comparators were classified as follows: a) interventions (e.g., cognitive remediation, psychoeducation) with a primary target different from improving positive symptoms (e.g., cognition, knowledge of the illness, adherence to medication, functioning), which were primarily analyzed as separate nodes, then combined in a sensitivity analysis; b) inactive controls, defined as interventions intended to control for non‐specific aspects of the therapy (befriending, recreation and support, social activity therapy, supportive counselling), also sometimes referred to as “psychological placebos”; c) treatment as usual (i.e., patients continue to receive standard psychiatric care); d) waiting list.
Outcomes
The primary outcome was the change in positive symptoms of schizophrenia, as measured by a rating scale such as the positive subscale of the Positive and Negative Syndrome Scale (PANSS)13, the positive subscale of the Brief Psychiatric Rating Scale (BPRS)14, or any other published scale.
Secondary outcomes were: study dropout for any reason (all‐cause discontinuation), effects on overall symptoms of schizophrenia, effects on negative symptoms, response (as defined in the study), relapse (operationalized by rating scales or, if not available, rehospitalization due to psychopathology), adherence and insight, changes in depressive symptoms, quality of life, functioning, adverse events that might be related to psychological treatment (according to Linden et al15), and mortality (measured as death for any reason, death due to natural causes, death due to suicide). All outcomes were measured at study endpoint, as defined in each study.
Search strategy and selection criteria
We searched EMBASE, MEDLINE, PsycINFO, PubMed, BIOSIS, Cochrane Library, World Health Organization's International Clinical Trials Registry Platform and http://ClinicalTrials.gov for RCTs published up to January 10, 2018, comparing psychological interventions with each other or with a non‐pharmacological control condition in people with schizophrenia who presented active positive symptoms. Additionally, we searched the reference lists of previous reviews.
We applied no language restrictions, with the exception that we did not search Chinese databases. We contacted authors of included studies published in the last 30 years for missing or additional information about their studies.
Data extraction and risk of bias assessment
All abstracts identified by the search were reviewed independently by two researchers of the group. Disagreements were resolved by discussion, and in case of doubts the full paper was retrieved for further inspection. Full reports were obtained for all eligible papers, and again assessed by two independent reviewers. Disagreements were discussed with the senior author and, in case of need, study authors were contacted for further information.
Two researchers independently extracted data from the selected studies, considering main reports and supplementary materials, entered the relevant information into a Microsoft Access database especially created for this study, and assessed risk of bias using the Cochrane risk of bias tool16. The following domains of possible bias were considered: sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, incomplete outcome data, selective reporting, researchers’ allegiance17, 18, other bias. We also made a global risk of bias rating for each study based on criteria applied in a network meta‐analysis of antidepressants19.
Statistical analysis
We performed random effects pairwise meta‐analyses and network meta‐analysis in a frequentist framework using the netmeta package in R (version 3.4.3)20, 21. We calculated standardized mean differences (SMDs) for continuous outcomes, and risk ratios (RRs) for binary outcomes, both presented with their 95% confidence intervals (CIs). We also calculated the relative ranking for each intervention using the Surface Under the Cumulative Ranking curve (SUCRA), estimated within the frequentist framework (as P scores)22.
Before running the network meta‐analysis, we attempted to assess the transitivity assumption. This assumption implies that studies comparing different sets of interventions are sufficiently similar to provide valid indirect inferences, which we tried to ensure by applying narrow inclusion criteria and making populations as similar as possible within and across treatment comparisons. We also considered whether the potential effect modifiers (listed below) were distributed similarly across the available direct comparisons.
We assumed a common heterogeneity parameter across the various treatment comparisons, and presented the between study variance (tau2) for each outcome. We characterized the amount of heterogeneity as low, moderate or high, using the first and third quantiles of their empirical distributions23. Statistical inconsistency was evaluated separating direct evidence from indirect evidence provided by the entire network, and then testing the agreement of these two pieces of evidence24. The magnitude of inconsistency factors (the difference in direct and indirect SMD) and their respective p values were used to identify the presence of inconsistency. We also applied the design‐by‐treatment interaction model, that evaluates inconsistency in the network jointly25.
To explore potential sources of heterogeneity or inconsistency, we planned a priori subgroup analyses for the primary outcome on the following potential effect modifiers: number of sessions, study duration, setting (individual vs. group), expertise of the therapist, baseline severity. Sensitivity analyses were performed excluding open label studies, studies that presented only completer analyses, studies at overall high risk of bias19, studies with high risk of researchers’ allegiance, studies focused on treatment‐resistant patients, and studies with a non‐active comparison group. We also assessed small trial effects (potentially associated with publication bias) by examining funnel plots of pairwise meta‐analyses and comparison‐adjusted funnel plots, if ten or more studies were included26. Additionally, we assessed the confidence in estimates of the main outcome with Confidence in Network Meta‐Analysis (CINeMA), an adaptation of the Grading of Recommendations Assessment, Development and Evaluation framework (GRADE) specifically developed for network meta‐analysis27.
RESULTS
Characteristics of included studies
21,772 references were identified by the search (last update January 10, 2018), and 2,754 articles were retrieved in full text (Figure 1). We included 62 randomized controlled trials, of which 53 had usable data and were included in the network meta‐analysis (involving 4,068 participants) (Table 1).
Figure 1.
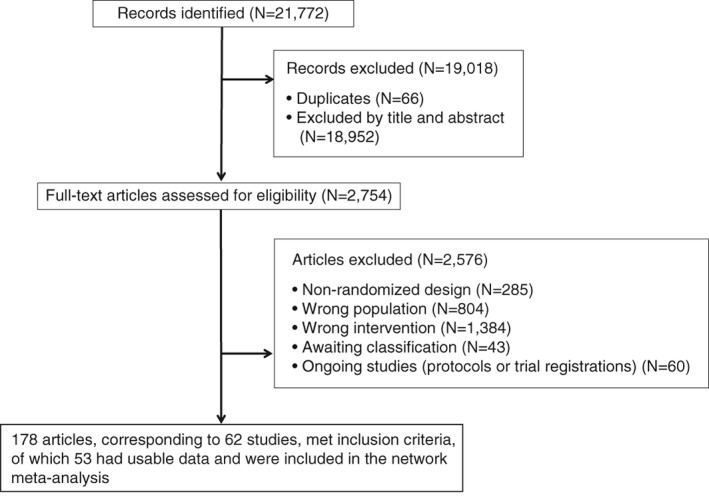
PRISMA flow chart of the study selection process
Table 1.
Characteristics of studies
| Study | Country | Treatments (N. patients) | Trial duration (weeks) | N. sessions | Diagnosis | Study design | Risk of bias (overall) |
|---|---|---|---|---|---|---|---|
| Barrowclough et al28 | UK | Cognitive behavioural therapy (N=57), TAU (N=56) | 26 | 10.4 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Bechdolf et al29 | Germany | Cognitive behavioural therapy (N=40), psychoeducation (N=48) | 8 | 11.9 (cognitive behavioural therapy), 6.4 (psychoeducation) | Episode of a schizophrenic or related disorder (ICD‐10) | SB | High |
| Birchwood et al30 | UK | Cognitive behavioural therapy (N=98), TAU (N=99) | 39 | 19 | Schizophrenia or schizoaffective disorder (ICD‐10) | SB | Moderate |
| Drury et al31 | UK | Cognitive therapy (N=30), recreation and support (N=32) | 12 | NA | Functional psychosis (DSM‐IV) | OL | High |
| Durham et al32 | UK | Cognitive behavioural therapy (N=22), supportive therapy (N=23), TAU (N=21) | 39 | 20 | Schizophrenia, schizoaffective disorder or delusional disorder (ICD‐10 and DSM‐IV) | SB | High |
| England33 | NA (author's affiliation in Canada) | Cognitive nursing intervention (N=44), TAU (N=21) | 18 | 12 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Foster et al34 | UK | Cognitive behavioural therapy (N=12), TAU (N=12) | 4 | 4 | Schizophrenia, schizoaffective disorder or delusional disorder (clinical diagnosis) | OL | High |
| Freeman et al35 | UK | Cognitive behavioural therapy (N=15), TAU (N=15) | 8 | 6 | Schizophrenia, schizoaffective disorder or delusional disorder (clinical diagnosis) | SB | Low |
| Freeman et al36 | UK | Cognitive behavioural therapy (N=73), TAU (N=77) | 8 | 5.5 | Schizophrenia, schizoaffective disorder or delusional disorder (clinical diagnosis) | SB | High |
| Freeman et al37 | UK | Cognitive behavioural therapy (N=24), TAU (N=26) | 12 | 7.3 | Schizophrenia, schizoaffective disorder or delusional disorder (clinical diagnosis) | SB | Moderate |
| Garety et al38 | UK | Cognitive behavioural therapy (N=27), family intervention (N=28), TAU (N=28) | 39 | 13.9 | Non‐affective psychosis (DSM‐IV and ICD–10) | SB | Moderate |
| Garety et al38 | UK | Cognitive behavioural therapy (N=106), TAU (N=112) | 39 | 14.3 | Non‐affective psychosis (DSM‐IV and ICD–10) | SB | Moderate |
| Gottlieb et al39 | US | Cognitive behavioural therapy (N=19), TAU (N=18) | 24 | 10 | Schizophrenia, schizoaffective disorder or psychosis not otherwise specified (NA) | SB | Moderate |
| Habib et al40 | Pakistan | Cognitive behavioural therapy (N=21), TAU (N=21) | 21 | 13 | Schizophrenia (DSM‐IV‐TR) | SB | High |
| Haddock et al41 | UK | Cognitive behavioural therapy (N=10), supportive counselling (N=11) | 5 | 10.2 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Haddock et al42 | UK | Cognitive behavioural therapy (N=38), social activity therapy (N=39) | 26 | 17 (cognitive behavioural therapy), 17.4 (social activity therapy) | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Hazell et al43 | UK | Cognitive behavioural therapy (N=15), waitlist (N=15) | 12 | 8 | Schizophrenia and related disorders (NA) | SB | Moderate |
| Krakvik et al44 | Norway | Cognitive behavioural therapy (N=23), waitlist (N=22) | 26 | 20 | Schizophrenia, schizoaffective disorder or persistent delusional disorder (ICD‐10) | OL | Moderate |
| Kuipers et al45 | UK | Cognitive behavioural therapy (N=28), TAU (N=32) | 39 | 18.6 | Paranoid schizophrenia (DSM‐III‐R) | OL | High |
| Lecomte et al46 | Canada | Cognitive behavioural therapy (N=48), social skills training (N=54), waitlist (N=27) | 13 | 24 | Schizophrenia spectrum disorder (NA) | SB | Moderate |
| Lee et al47 | South Korea | Cognitive behavioural social skills training (N=12), TAU (N=13) | 7 | 12 | Schizophrenia (DSM‐IV‐TR) | SB | Moderate |
| Lee et al48 | South Korea | Cognitive behavioural therapy (N=25), supportive therapy (N=25) | 32 | 20.1 | Schizophrenia (DSM‐IV) | SB | Moderate |
| Levine et al49 | NA (author's affiliation in Israel) | Cognitive therapy (N=6), supportive therapy (N=6) | 6 | 6 | Paranoid schizophrenia (DSM‐III‐R) | NA | High |
| Li et al50 | China | Cognitive behavioural therapy (N=96), supportive therapy (N=96) | 24 | 15 | Schizophrenia (DSM‐IV) | SB | Moderate |
| McLeod et al51 | UK | Cognitive behavioural therapy (N=10), waitlist (N=10) | 12 | 8 | Schizophrenia (DSM‐IV) | NA | High |
| Morrison et al52 | UK | Cognitive therapy (N=37), TAU (N=37) | 39 | 13.3 | Schizophrenia, schizoaffective disorder or delusional disorder (ICD‐10 or PANSS) | SB | Moderate |
| Penn et al53 | US | Cognitive behavioural therapy (N=32), supportive therapy (N=33) | 12 | 8.3 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Low |
| Pinninti et al54 | US | Cognitive behavioural therapy (N=18), TAU (N=15) | 12 | 11.93 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Pot‐Kolder et al55 | The Netherlands | Virtual reality based cognitive behavioural therapy (N=58), waitlist (N=58) | 12 | 16 | Psychotic disorder (DSM‐IV) | SB | Low |
| Rector et al56 | Canada | Cognitive behavioural therapy (N=24), TAU (N=21) | 26 | 20 | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | Moderate |
| Sensky et al57 | UK | Cognitive behavioural therapy (N=46), befriending (N=44) | 39 | 19 | Schizophrenia (ICD‐10 Research Criteria and DSM‐IV) | SB | Moderate |
| Startup et al58 | UK | Cognitive behavioural therapy (N=47), TAU (N=43) | 26 | 12.9 | Schizophrenia, schizophreniform disorder or schizoaffective disorder (DSM‐IV) | OL | High |
| Tarrier et al59 | UK | Cognitive behavioural therapy (N=33), supportive Counselling (N=26), TAU (N=28) | 10 | 20 | Schizophrenia, schizoaffective psychosis or delusional disorder (DSM‐III‐R) | SB | Moderate |
| Trower et al60 | UK | Cognitive behavioural therapy (N=18), TAU (N=20) | 26 | 16 | Schizophrenia or related disorder (ICD‐10) | SB | High |
| Turkington et al61 | UK | Cognitive behavioural therapy (N=13), befriending (N=6) | 8 | 6 | Schizophrenia (ICD‐10 Research Criteria) | SB | Moderate |
| Valmaggia et al62 | The Netherlands, Belgium | Cognitive behavioural therapy (N=36), supportive counselling (N=26) | 23 | 16 | Schizophrenia (DSM‐IV) | SB | Moderate |
| van der Gaag et al63 | The Netherlands | Cognitive behavioural therapy (N=110), TAU (N=106) | 26 | 13 | Schizophrenia or schizoaffective disorder (DSM‐IV‐TR) | SB | High |
| Velligan et al64 | US | Cognitive behavioural therapy (N=43), cognitive adaptation training (N=41), cognitive behavioural therapy + cognitive adaptation training (N=40), TAU (N=42) | 39 | 26.6 (cognitive behavioural therapy), 27.5 (cognitive adaptation training), 27.5 (cognitive behavioural therapy + cognitive adaptation training) | Schizophrenia or schizoaffective disorder (DSM‐IV) | SB | High |
| Wahass & Kent65 | Saudi Arabia | Cognitive behavioural therapy (N=3), TAU (N=3) | 9 | 25 | Schizophrenia (ICD‐10) | OL | Moderate |
| Wittorf et al66 | Germany | Cognitive behavioural therapy (N=50), supportive therapy (N=50) | 33 | 20 | Schizophrenia, schizophreniform disorder, schizoaffective disorder or delusional disorder (DSM‐IV) | SB | High |
| Wykes et al67 | UK | Cognitive behavioural therapy (N=45), TAU (N=40) | 10 | 7 | Schizophrenia (DSM‐IV) | OL | High |
| ACTRN1261600097648268 | Australia | Metacognitive training (N=28), cognitive remediation (N=28) | 4 | 4 | Schizophrenia spectrum disorder (DSM‐V) | SB | Moderate |
| Briki et al69 | France | Metacognitive training (N=35), supportive therapy (N=33) | 8 | 14.6 | Schizophrenia or schizoaffective disorders (DSM‐IV‐TR) | SB | High |
| Favrod et al70 | Switzerland | Metacognitive training (N=26), TAU (N=26) | 8 | 7 | Schizophrenia spectrum disorder (ICD‐10) | SB | Moderate |
| Kumar et al71 | India | Metacognitive training (N=8), TAU (N=8) | 4 | 8 | Paranoid schizophrenia (ICD‐10) | NA | High |
| So et al72 | Hong Kong | Metacognitive training (N=23), waitlist (N=21) | 4 | 3.15 | Schizophrenia spectrum disorder (clinical diagnosis) | SB | Moderate |
| van Oosterhout et al73 | The Netherlands | Metacognitive training (N=75), TAU (N=79) | 8 | 8 | Psychotic disorder in the DSM‐IV schizophrenia spectrum (DSM‐IV‐TR) | SB | Moderate |
| Chadwick et al74 | UK | Mindfulness (N=11), waitlist (N=11) | 10 | 10 | Psychotic disorder (NA) | OL | High |
| Chadwick et al75 | UK | Mindfulness (N=54), TAU (N=54) | 16 | 12 | Schizophrenia or schizoaffective disorder (ICD‐10) | SB | Low |
| Bach & Hayes76 | US | Acceptance and Commitment therapy (N=40), TAU (N=40) | 16 | 4 | Auditory hallucinations or delusions (clinical diagnosis) (81.25% diagnosed with schizophrenia, schizoaffective disorder or delusional disorder) | OL | High |
| Shawyer et al77 | Australia | Acceptance and commitment therapy (N=49), befriending (N=47) | 13 | 7 | Schizophrenia or schizoaffective disorder (DSM‐IV‐TR) | SB | Low |
| Schnackenberg et al78 | Germany | Experienced focused counselling (N=12), TAU (N=10) | 44 | NA | Schizophrenia and schizoaffective disorder (NA) | OL | High |
| Jenner et al79 | The Netherlands | Hallucination focused integrative treatment (N=39), TAU (N=39) | 39 | 11 | Non‐affective psychosis, including schizophrenia, schizoaffective or psychotic disorder not otherwise specified (DSM‐IV) | OL | High |
| Craig et al80 | UK | AVATAR therapy (N=75), supportive counselling (N=75) | 12 | 5.6 (AVATAR therapy), 5.1 (supportive counselling) | Schizophrenia spectrum disorder or affective disorder with psychotic symptoms (ICD‐10) | SB | Low |
TAU – treatment as usual, OL – open label, SB – single blind, NA – not available, PANSS – Positive and Negative Syndrome Scale
These trials provided comparisons of the following psychological treatments: CBT (N=40)28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, metacognitive training (N=6)68, 69, 70, 71, 72, 73, mindfulness (N=2)74, 75, acceptance and commitment therapy (N=2)76, 77, experience focused counselling (N=1)78, hallucination focused integrative treatment (N=1)79, and AVATAR therapy (N=1)80.
The mean sample size was 76.5 participants (range 6‐218), and the median trial duration was 13 weeks (range 4‐44 weeks). Of 3,941 participants whose gender was reported, 2,361 were men (59.9%). The mean duration of illness was 12.4 years, and the mean age of participants was 37.4 years. Nine studies included only inpatients, 15 only outpatients and 14 both, while 15 did not provide information on patients’ status. On average, patients had moderate schizophrenic symptoms, with a mean reported PANSS baseline score of 68.2681, 82. Thanks to collaboration of the authors, we were able to include unpublished data for some studies36, 37, 41, 42, 43, 57, 61, 68, 72.
Risk of bias assessment
Six, 27 and 21 of the included studies were considered to be at low, moderate and high overall risk of bias, respectively (see Table 1). The risk of bias was low in 26 studies (50%) concerning random sequence generation; in 13 studies (25%) concerning allocation concealment; in no study concerning blinding of participants and personnel; in 18 studies (34.6%) concerning blinding of outcome assessment; in seven studies (13.5%) concerning attrition bias; in 11 studies (21.1%) concerning selective reporting; in six studies (11.5%) concerning researchers’ alliance; and in 41 studies (78.8%) concerning other bias.
Primary outcome: positive symptoms
Figure 2 shows the network of treatments for the primary outcome. Two studies were not considered in the analyses, because they were not connected to the rest of the network, contributing neither direct nor indirect evidence29, 68.
Figure 2.
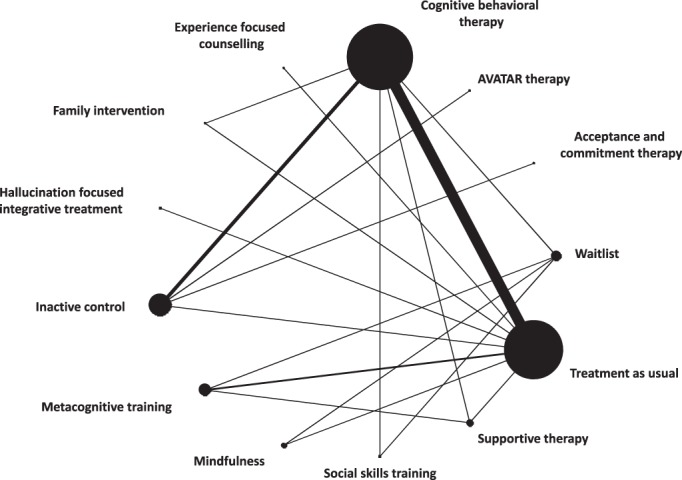
Network meta‐analysis of eligible comparisons for positive symptoms. Line width is proportional to the number of trials comparing every pair of treatments. Node size is proportional to the number of studies providing data for each treatment.
Network meta‐analysis results show that, for the primary outcome, CBT was associated with a higher decrease in positive symptoms than inactive control (SMD=−0.29; 95% CI: –0.55 to −0.03, seven RCTs contributing direct evidence to the network meta‐analysis, low confidence in the estimates), treatment as usual (SMD=−0.30; 95% CI: –0.45 to −0.14, 18 RCTs contributing direct evidence, moderate confidence in the estimates) and supportive therapy (SMD=−0.47; 95% CI: –0.91 to −0.03, two RCTs contributing direct evidence, low confidence in the estimates). The difference was not significant for the comparison with waitlist (SMD=−0.24; 95% CI: –0.65 to 0.16), but only two small trials (with 30 and 45 participants respectively43, 44) contributed direct evidence to this comparison (Figure 3).
Figure 3.
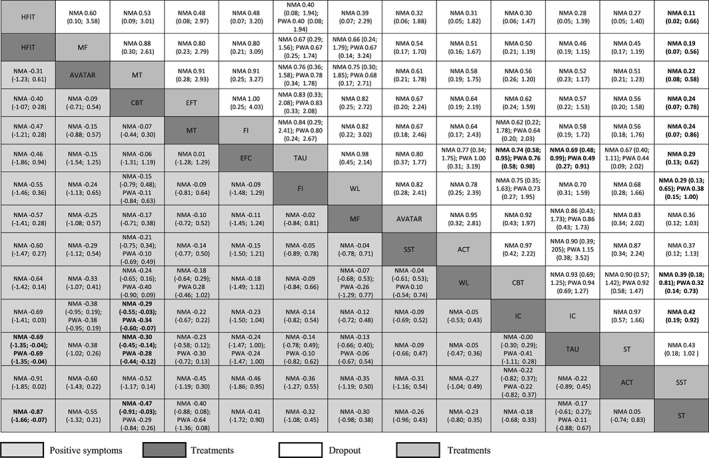
Comparisons between psychological treatments for positive symptoms and study dropouts. Results for positive symptoms are presented in the lower triangle; results for dropout are presented in the upper triangle. Significant results are presented in bold. Relative treatments effects are measured by standardized mean difference (SMD) for positive symptoms and risk ratio (RR) for study dropout along with their 95% confidence intervals (95% CIs). SMDs lower than 0 and RRs lower than 1 favour the column defining treatment. SMDs of –0.2 can be considered small, −0.5 medium, and –0.8 large. To obtain SMDs for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken. ACT – acceptance and commitment therapy, CBT – cognitive behavioral therapy, EFC – experience focused counselling, FI – family intervention, HFIT – hallucination focused integrative treatment, IC – inactive control, MT – metacognitive training, MF – mindfulness, SST – social skills training, ST – supportive therapy, TAU – treatment as usual, WL – waitlist, NMA – network meta‐analysis, PWA – pairwise meta‐analysis.
One study on hallucination focused integrative treatment showed a decrease in symptoms in comparison to treatment as usual and supportive therapy (moderate and low confidence in the estimate, respectively). All other relative treatment effects were very imprecise, but on average they favored the active psychological treatment over the inactive control interventions.
The heterogeneity variance (tau2) was 0.0514, hence considered to be low to moderate23. The design‐by‐treatment interaction test did not reveal significant inconsistency (p=0.35). By splitting direct and indirect evidence for each comparison, we found no evidence for disagreement between these two pieces of evidence for any of the comparisons. None of the methods we used suggested important inconsistency but, given the low number of studies for most of the comparisons, the power of these tests is low. The assessments of confidence in the estimates using CINeMA highlighted moderate to very low confidence, primarily due to study limitations (high risk of bias) and imprecision.
The interpretation of subgroup analyses is limited due to restricted number of studies available for the different subgroups. We did not detect any important indication that the advantage of CBT over treatment as usual is moderated by number of sessions, study duration, setting (individual vs. group), therapist's expertise and severity at baseline.
Similarly, exclusion of studies for the different sensitivity analyses left a low number of trials for most of the treatments. When excluding open label studies, results of CBT compared to treatment as usual and supportive therapy were consistent with the main analysis (SMD=−0.27; 95% CI: –0.41 to −0.13 and SMD=−0.47; 95% CI: –0.86 to −0.08, respectively), while the difference between CBT and inactive control was not significant anymore (SMD=−0.14; 95% CI: –0.37 to 0.09).
Sensitivity analyses excluding studies presenting only completer analyses, studies with high risk of bias, studies at high risk of bias for researchers’ allegiance, or studies focused on treatment resistant patients were overall consistent with the main analyses.
The results of a post‐hoc sensitivity analysis pooling the “active control” comparators did not differ from the main analysis.
Investigation of small study effect and publication bias with conventional funnel plot did not reveal any association between study precision and effect size (only possible for CBT versus treatment as usual). However, the comparison‐adjusted funnel plot suggests that small studies that did not show a benefit for the newer psychological treatment over the older treatment are underrepresented in our data (i.e., they possibly remain unpublished).
Secondary outcomes
CBT and inactive control were less acceptable than treatment as usual in terms of all‐cause discontinuation. All treatments had fewer dropouts than social skills training (with the exception of AVATAR therapy, acceptance and commitment therapy, and supportive therapy) (Figure 3).
CBT was associated with a higher reduction of overall symptoms compared to waitlist and treatment as usual, and with higher reduction in negative symptoms compared with treatment as usual (Figure 4). Hallucination focused integrative treatment and CBT were associated with larger probability of response compared with treatment as usual and inactive control.
Figure 4.
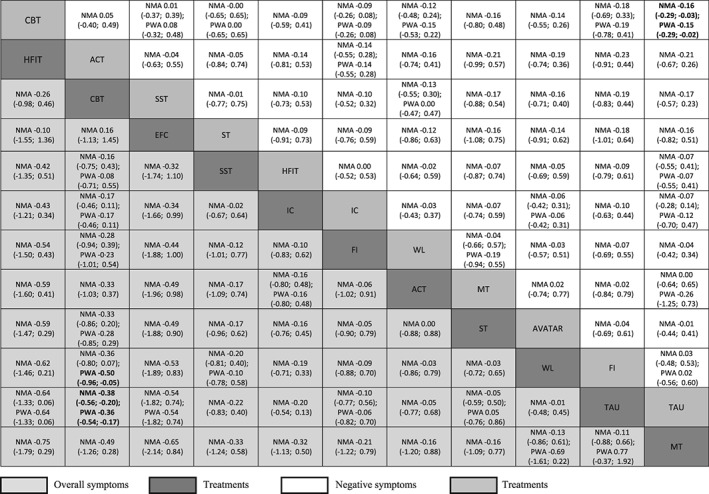
Results for overall symptoms are presented in the lower triangle; results for negative symptoms are presented in the upper triangle. Significant results are presented in bold. Relative treatments effects are measured by standardized mean difference (SMD) along with its 95% confidence intervals (95% CIs). SMDs lower than 0 favour the column defining treatment. SMDs of –0.2 can be considered small, −0.5 medium, and –0.8 large. To obtain SMDs for comparisons in the opposite direction, negative values should be converted into positive values, and vice versa. ACT – acceptance and commitment therapy, CBT – cognitive behavioral therapy, EFC – experience focused counselling, FI – family intervention, HFIT – hallucination focused integrative treatment, IC – inactive control, MT – metacognitive training, MF – mindfulness, SST – social skills training, ST – supportive therapy, TAU – treatment as usual, WL – waitlist, NMA – network meta‐analysis, PWA – pairwise meta‐analysis.
When looking at adherence and insight, metacognitive training, social skills training, CBT and treatment as usual produced a higher improvement in comparison to supportive therapy. For quality of life and functioning, CBT was more efficacious than treatment as usual. No significant differences were observed for depression. Mortality was in general a rare event, and did not differ between treatments. Very few data were available for relapse, adverse events and other mortality outcomes.
Heterogeneity variance assessed with tau2 ranged from 0 to 0.0649, being evaluated from none to low‐to‐moderate. The design‐by‐treatment interaction model revealed some inconsistency for the secondary outcome of depression (p=0.03).
DISCUSSION
To our knowledge, this is the first network meta‐analysis on psychological treatments for patients with positive symptoms of schizophrenia.
With 40 studies, CBT was the most represented among the included treatments. We found significant efficacy for CBT in comparison with treatment as usual in many outcomes (positive, overall and negative symptoms, response to treatment, quality of life and functioning), higher efficacy in comparison with inactive control for positive symptoms and response to treatment, and in comparison with supportive therapy for adherence. There was no convincing proof of efficacy of other treatments, probably due to the small number of studies.
CBT was also associated with higher dropout rates than treatment as usual (18.8% versus 12%). CBT might actually be less acceptable, and not all patients might be willing to engage in such a demanding treatment; however, we argue that to compare the dropout rates with those in treatment as usual could be misleading. Patients in this latter arm – by definition – continue their usual care, and they might have less reason to leave in comparison with patients assigned to a new intervention, that they could find demanding or challenging, or about which they may have high expectations, being discouraged if they do not see results in a few sessions. As a confirmation to this hypothesis, the inactive control condition (where patients participate to sessions like befriending and recreation activities) also had a higher dropout rate than treatment as usual.
Patients in the included studies were only moderately ill on the average, compared with those in a meta‐analysis of studies testing antipsychotic drugs vs. placebo, where they were markedly ill82. It seems that severely ill patients are usually not enrolled in psychotherapy studies. But this finding just reflects clinical practice: psychotherapy requires a minimum ability of patients to collaborate, and many patients do not have this ability when they are very acutely ill.
Interpretation of subgroup and sensitivity analyses was limited by the low number of studies available. However, results on CBT remained stable after all pre‐planned sensitivity analyses, corroborating the robustness of the results for this intervention. We also tested the potential role of researchers’ allegiance18, by excluding the studies in which the authors tested the efficacy of an intervention that was developed by themselves, and did not find significantly different results from the main analysis.
One open and increasingly relevant issue is whether psychological interventions might cause harm15. We collected all the available data about adverse events potentially connected with the psychological intervention, but we found this aspect very poorly reported in the trials. We believe that future studies should collect and report this information, in order to address this still unclear question83.
Our results are in agreement with findings from some previous pairwise meta‐analyses, where CBT was found to be efficacious for overall, positive and negative symptoms of schizophrenia in comparison with control conditions4, 5, 6, but not when compared with other psychological therapies7. However, the results of previous studies and reviews regarding the efficacy of CBT for schizophrenia have been conflicting.
In this context, the role of blinded studies may be particularly critical8. Here, our results are in contrast with the findings of Jauhar et al6: when excluding studies with a non‐blind outcome assessor, they found no differences between CBT and any control condition. On the contrary, we found that the superiority for CBT over treatment as usual and inactive control was maintained also in blinded studies. It was not maintained over supportive therapy and waiting list, but only very few studies (two and one, respectively) contributed direct evidence for these comparators.
However, our work cannot be directly compared with that of Jauhar et al6, because they included any patients with schizophrenia without a restriction to positive symptoms, they used somewhat different criteria for risk of bias, and they lumped all comparators together in their pairwise meta‐analysis.
Our findings have the following limitations. First, available data for other treatments than CBT and for CBT versus other nodes than treatment as usual are based on few studies only, leading to low power to detect possible differences. Therefore, results should be interpreted with caution, in particular when looking at sensitivity and subgroup analyses. For this reason we did not focus our interpretation on hierarchies (SUCRA rankings), that could be misleading when there are no statistically significant differences among active treatments.
Second, our focus was on the treatment of positive symptoms, and the findings observed for other outcomes might be secondary to the effect of the treatment on these symptoms. For example, a patient might experience withdrawal, lack of spontaneity, depressive symptoms or a lower functioning due to the difficulties connected with delusions or hallucinations. When these are treated, the quality of life and the other symptoms may benefit as well. For this reason, we focus our interpretations mainly on positive symptoms.
Third, patients in the included trials were also receiving antipsychotic medication. We collected the available information on the use of antipsychotics. However, this was rarely given and never provided for experimental and control arm separately. The only exception is the study of Morrison et al52, that included patients not receiving antipsychotic medication (a post‐hoc sensitivity analysis excluding this study did not materially change the results). As a result, it was not possible to assess the role of pharmacological treatment as a moderator. However, we assume that the intake of medications can be considered similar across study arms, due to randomization. Furthermore, we argue that the situation in the included studies resembles what happens in real‐life clinical practice, where psychological interventions are intended to be used as add‐on to pharmacological therapy, and participants usually continue their previous medication.
On the other hand, this work presents outstanding strengths. First, the study was carefully planned in agreements with PRISMA guidelines, and followed a sound methodology that was a priori published in the protocol3. This included comprehensive outcome measures and the evaluation of quality at study level (risk of bias) and confidence in results at outcome level (CINeMA). Second, the consideration of control conditions such as treatment as usual and waiting list as separate allowed to ascertain their relative efficacy. This is particularly important, as waitlist has been found to be connected with a nocebo effect83. Third, the strict selection criteria led to a homogenous population, as confirmed by very low heterogeneity, coherence across direct and indirect comparisons, and by side‐splitting test and design‐by‐treatment interaction test. This makes us confident that the results of this study are robust.
In conclusion, cognitive behavior therapy seems to be effective on positive symptoms in moderately ill patients with schizophrenia, with effect sizes in the lower to medium range, depending on the control condition.
ACKNOWLEDGEMENTS
This study was funded by European Union's Horizon 2020 Research and Innovation Programme, Marie Skłodowska‐Curie grant agreement no. 701717. The funder had no role in study design, data collection, analysis or interpretation, writing of the report, or decision to submit the paper for publication. The authors thank S. Roberts for help in the literature search, P. Kratochwill for assistance in full text acquisition and proof reading, Y. Zhu for help with screening and data extraction from Chinese studies, and C. Carmi for assistance in data extraction. They thank all authors of the included studies, especially the ones who provided additional information and data. On the website http://www.psykl.mri.tum.de/node/69, extensive information can be found about search strategy, included treatments, risk of bias assessment, results for secondary outcomes, SUCRA rankings, evaluation of heterogeneity and inconsistency, subgroup and sensitivity analyses, evaluation of confidence in the estimates. This information is also available upon request from the authors.
REFERENCES
- 1. National Collaborating Centre for Mental Health. Core interventions in the treatment and management of schizophrenia in adults in primary and secondary care (Clinical Guideline CG82). London: National Collaborating Centre for Mental Health, 2009. [Google Scholar]
- 2. Buchanan RW, Kreyenbuhl J, Kelly DL et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010;36:71‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bighelli I, Salanti G, Reitmeir C et al. Psychological interventions for positive symptoms in schizophrenia: protocol for a network meta‐analysis of randomised controlled trials. BMJ Open 2018;8:e019280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wykes T, Steel C, Everitt B et al. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmermann G, Favrod J, Trieu VH et al. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta‐analysis. Schizophr Res 2005;77:1‐9. [DOI] [PubMed] [Google Scholar]
- 6. Jauhar S, McKenna PJ, Radua J et al. Cognitive‐behavioural therapy for the symptoms of schizophrenia: systematic review and meta‐analysis with examination of potential bias. Br J Psychiatry 2014;204:20‐9. [DOI] [PubMed] [Google Scholar]
- 7. Jones C, Hacker D, Cormac I et al. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2012;4:CD008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynch D, Laws KR, McKenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta‐analytical review of well‐controlled trials. Psychol Med 2010;40:9‐24. [DOI] [PubMed] [Google Scholar]
- 9. Turner DT, van der Gaag M, Karyotaki E et al. Psychological interventions for psychosis: a meta‐analysis of comparative outcome studies. Am J Psychiatry 2014;171:523‐38. [DOI] [PubMed] [Google Scholar]
- 10. Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3:80‐97. [DOI] [PubMed] [Google Scholar]
- 11. Hutton B, Salanti G, Caldwell DM et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777‐84. [DOI] [PubMed] [Google Scholar]
- 12. Shamseer L, Moher D, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261‐76. [DOI] [PubMed] [Google Scholar]
- 14. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799‐812. [Google Scholar]
- 15. Linden M, Schermuly‐Haupt M‐L. Definition, assessment and rate of psychotherapy side effects. World Psychiatry 2014;13:306‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. JPT Higgins, Churchill R, Chandler J. et al (eds). Cochrane handbook for systematic reviews of interventions, Version 5.2.0, Cochrane, 2017.
- 17. Munder T, Brütsch O, Leonhart R et al. Researcher allegiance in psychotherapy outcome research: an overview of reviews. Clin Psychol Rev 2013;33:501‐11. [DOI] [PubMed] [Google Scholar]
- 18. Lieb K. von der Osten‐Sacken J, Stoffers‐Winterling J et al. Conflicts of interest and spin in reviews of psychological therapies: a systematic review. BMJ Open 2016;6:e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furukawa TA, Salanti G, Atkinson LZ et al. Comparative efficacy and acceptability of first‐generation and second‐generation antidepressants in the acute treatment of major depression: protocol for a network meta‐analysis. BMJ Open 2016;6:e010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwarzer G. meta: an R package for meta‐analysis. R News 2007;7:40‐5. [Google Scholar]
- 21. Schwarzer G, Carpenter JR, Rücker G. Meta‐analysis with R (Use‐R!). Basel: Springer, 2015. [Google Scholar]
- 22. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhodes KM, Turner RM, Higgins JPT. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. J Clin Epidemiol 2015;68:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dias S, Welton NJ, Caldwell DM et al. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010;29:932‐44. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT, Jackson D, Barrett JK et al. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods 2012;3:98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Res Synth Methods 2012;3:161‐76. [DOI] [PubMed] [Google Scholar]
- 27. Salanti G, Del Giovane C, Chaimani A et al. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrowclough C, Haddock G, Lobban F et al. Group cognitive‐behavioural therapy for schizophrenia. Randomised controlled trial. Br J Psychiatry 2006;189:527‐32. [DOI] [PubMed] [Google Scholar]
- 29. Bechdolf A, Knost B, Kuntermann C et al. A randomized comparison of group cognitive‐behavioural therapy and group psychoeducation in patients with schizophrenia. Acta Psychiatr Scand 2004;110:21‐8. [DOI] [PubMed] [Google Scholar]
- 30. Birchwood M, Michail M, Meaden A et al. Cognitive behaviour therapy to prevent harmful compliance with command hallucinations (COMMAND): a randomized controlled trial. Lancet Psychiatry 2014;1:23‐33. [DOI] [PubMed] [Google Scholar]
- 31. Drury V, Birchwood M, Cochrane R et al. Cognitive therapy and recovery from acute psychosis: a controlled trial. I. Impact on psychotic symptoms. Br J Psychiatry 1996;169:593‐601. [DOI] [PubMed] [Google Scholar]
- 32. Durham RC, Guthrie M, Morton RV et al. Tayside‐Fife clinical trial of cognitive‐behavioural therapy for medication‐resistant psychotic symptoms. Results to 3‐month follow‐up. Br J Psychiatry 2003;182:303‐11. [DOI] [PubMed] [Google Scholar]
- 33. England M. Efficacy of cognitive nursing intervention for voice hearing. Perspect Psychiatr Care 2007;43:69‐76. [DOI] [PubMed] [Google Scholar]
- 34. Foster C, Startup H, Potts L et al. A randomised controlled trial of a worry intervention for individuals with persistent persecutory delusions. J Behav Ther Exper Psychiatry 2010;41:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freeman D, Pugh K, Dunn G et al. An early Phase II randomised controlled trial testing the effect on persecutory delusions of using CBT to reduce negative cognitions about the self: the potential benefits of enhancing self confidence. Schizophr Res 2014;160:186‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freeman D, Dunn G, Startup H et al. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single‐blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry 2015;2:305‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freeman D, Waite F, Startup H et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor‐blind, randomised controlled pilot trial. Lancet Psychiatry 2015;2:975‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garety PA, Fowler DG, Freeman D et al. Cognitive‐behavioural therapy and family intervention for relapse prevention and symptom reduction in psychosis: randomised controlled trial. Br J Psychiatry 2008;192:412‐23. [DOI] [PubMed] [Google Scholar]
- 39. Gottlieb JD, Gidugu V, Maru M et al. Randomized controlled trial of an internet cognitive behavioral skills‐based program for auditory hallucinations in persons with psychosis. Psychiatr Rehabil J 2017;40:283‐92. [DOI] [PubMed] [Google Scholar]
- 40. Habib N, Dawood S, Kingdon D et al. Preliminary evaluation of culturally adapted CBT for psychosis (CA‐CBTp): findings from developing culturally‐sensitive CBT project (DCCP). Behav Cogn Psychother 2015;43:200‐8. [DOI] [PubMed] [Google Scholar]
- 41. Haddock G, Tarrier N, Morrison AP et al. A pilot study evaluating the effectiveness of individual inpatient cognitive‐behavioural therapy in early psychosis. Soc Psychiatry Psychiatr Epidemiol 1999;34:254‐8. [DOI] [PubMed] [Google Scholar]
- 42. Haddock G, Barrowclough C, Shaw JJ et al. Cognitive‐behavioural therapy v. social activity therapy for people with psychosis and a history of violence: randomised controlled trial. Br J Psychiatry 2009;194:152‐7. [DOI] [PubMed] [Google Scholar]
- 43. Hazell CM, Hayward M, Cavanagh K et al. Guided self‐help cognitive behavioral intervention for VoicEs (GiVE): study protocol for a pilot randomized controlled trial. Trials 2016;17:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krakvik B, Grawe RW, Hagen R et al. Cognitive behaviour therapy for psychotic symptoms: a randomized controlled effectiveness trial. Behav Cogn Psychother 2013;41:511‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuipers E, Garety P, Fowler D et al. London‐East Anglia randomised controlled trial of cognitive‐behavioural therapy for psychosis. I: Effects of the treatment phase. Br J Psychiatry 1997;171:319‐27. [DOI] [PubMed] [Google Scholar]
- 46. Lecomte T, Leclerc C, Corbiere M et al. Group cognitive behavior therapy or social skills training for individuals with a recent onset of psychosis? Results of a randomized controlled trial. J Nerv Ment Dis 2008;196:866‐75. [DOI] [PubMed] [Google Scholar]
- 47. Lee DH, Ko SM, Choi YS et al. A randomized controlled pilot study of cognitive behavioral social skills training (Korean version) for middle‐ or older‐aged patients with schizophrenia: a pilot study. J Korean Neuropsychiatr Assoc 2012;51:192‐201. [Google Scholar]
- 48. Lee DE, Lee HJ, Yoon OS et al. The effect of cognitive behavioral therapy in drug‐resistant patients with schizophrenia. J Korean Neuropsychiatr Assoc 2013;52:26‐32. [Google Scholar]
- 49. Levine J, Barak Y, Granek I. Cognitive group therapy for paranoid schizophrenics: applying cognitive dissonance. J Cogn Psychother 1998;12:3‐12. [Google Scholar]
- 50. Li ZJ, Guo ZH, Wang N et al. Cognitive‐behavioural therapy for patients with schizophrenia: a multicentre randomized controlled trial in Beijing, China. Psychol Med 2015;45:1893‐905. [DOI] [PubMed] [Google Scholar]
- 51. McLeod T, Morris M, Birchwood M et al. Cognitive behavioural therapy group work with voice hearers. Part 1. Br J Nurs 2007;16:248‐52. [DOI] [PubMed] [Google Scholar]
- 52. Morrison AP, Turkington D, Pyle M et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: a single‐blind randomised controlled trial. Lancet 2014;383:1395‐403. [DOI] [PubMed] [Google Scholar]
- 53. Penn DL, Meyer PS, Evans E et al. A randomized controlled trial of group cognitive‐behavioral therapy vs. enhanced supportive therapy for auditory hallucinations. Schizophr Res 2009;109:52‐9. [DOI] [PubMed] [Google Scholar]
- 54. Pinninti NR, Rissmiller DJ, Steer RA. Cognitive‐behavioral therapy as an adjunct to second‐generation antipsychotics in the treatment of schizophrenia. Psychiatr Serv 2010;61:940‐3. [DOI] [PubMed] [Google Scholar]
- 55. Pot‐Kolder RMCA, Geraets CNW, Veling W et al. Virtual‐reality‐based cognitive behavioural therapy versus waiting list control for paranoid ideation and social avoidance in patients with psychotic disorders: a single‐blind randomised controlled trial. Lancet Psychiatry 2018;5:217‐26. [DOI] [PubMed] [Google Scholar]
- 56. Rector NA, Seeman MV, Segal ZV. Cognitive therapy for schizophrenia: a preliminary randomized controlled trial. Schizophr Res 2003;63:1‐11. [DOI] [PubMed] [Google Scholar]
- 57. Sensky T, Turkington D, Kingdon D et al. A randomized controlled trial of cognitive‐behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Arch Gen Psychiatry 2000;57:165‐72. [DOI] [PubMed] [Google Scholar]
- 58. Startup M, Jackson MC, Bendix S. North Wales randomized controlled trial of cognitive behaviour therapy for acute schizophrenia spectrum disorders: outcomes at 6 and 12 months. Psychol Med 2004;34:413‐22. [DOI] [PubMed] [Google Scholar]
- 59. Tarrier N, Yusupoff L, Kinney C et al. Randomised controlled trial of intensive cognitive behaviour therapy for patients with chronic schizophrenia. BMJ 1998;317:303‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trower P, Birchwood M, Meaden A et al. Cognitive therapy for command hallucinations: randomised controlled trial. Br J Psychiatry 2004;184:312‐20. [DOI] [PubMed] [Google Scholar]
- 61. Turkington D, Kingdon D. Cognitive‐behavioural techniques for general psychiatrists in the management of patients with psychoses. Br J Psychiatry 2000;177:101‐6. [DOI] [PubMed] [Google Scholar]
- 62. Valmaggia LR, van der Gaag M, Tarrier N et al. Cognitive‐behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication. Randomised controlled trial. Br J Psychiatry 2005;186:324‐30. [DOI] [PubMed] [Google Scholar]
- 63. van der Gaag M, Stant AD, Wolters KJ et al. Cognitive‐behavioural therapy for persistent and recurrent psychosis in people with schizophrenia‐spectrum disorder: cost‐effectiveness analysis. Br J Psychiatry 2011;198:59‐65. [DOI] [PubMed] [Google Scholar]
- 64. Velligan DI, Tai S, Roberts DL et al. A randomized controlled trial comparing cognitive behavior therapy, cognitive adaptation training, their combination and treatment as usual in chronic schizophrenia. Schizophr Bull 2015;41:597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wahass S, Kent G. The modification of psychological interventions for persistent auditory hallucinations to an Islamic culture. Behav Cogn Psychother 1997;25:351. [Google Scholar]
- 66. Wittorf A, Jakobi UE, Bannert KK et al. Does the cognitive dispute of psychotic symptoms do harm to the therapeutic alliance? J Nerv Ment Dis 2010;198:478‐85. [DOI] [PubMed] [Google Scholar]
- 67. Wykes T, Hayward P, Thomas N et al. What are the effects of group cognitive behaviour therapy for voices? A randomised control trial. Schizophr Res 2005;77:201‐10. [DOI] [PubMed] [Google Scholar]
- 68. ACTRN12616000976482. Efficacy of individualised metacognitive therapy (MCT+) for delusions in psychosis. Australian New Zealand Clinical Trial Registry, 2016.
- 69. Briki M, Monnin J, Haffen E et al. Metacognitive training for schizophrenia: a multicentre randomised controlled trial. Schizophr Res 2014;157:99‐106. [DOI] [PubMed] [Google Scholar]
- 70. Favrod J, Rexhaj S, Bardy S et al. Sustained antipsychotic effect of metacognitive training in psychosis: a randomized‐controlled study. Eur Psychiatry 2014;29:275‐81. [DOI] [PubMed] [Google Scholar]
- 71. Kumar D, Zia Ul Haq M, Dubey I et al. Effect of meta‐cognitive training in the reduction of positive symptoms in schizophrenia. Eur J Psychother Couns 2010;12:149‐58. [Google Scholar]
- 72. So SH, Chan AP, Chong CS et al. Metacognitive training for delusions (MCTd): effectiveness on data‐gathering and belief flexibility in a Chinese sample. Front Psychol 2015;6:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Oosterhout B, Krabbendam L, de Boer K et al. Metacognitive group training for schizophrenia spectrum patients with delusions: a randomized controlled trial. Psychol Med 2014;44:3025‐35. [DOI] [PubMed] [Google Scholar]
- 74. Chadwick P, Hughes S, Russell D et al. Mindfulness groups for distressing voices and paranoia: a replication and randomized feasibility trial. Behav Cogn Psychother 2009;37:403‐12. [DOI] [PubMed] [Google Scholar]
- 75. Chadwick P, Strauss C, Jones AM et al. Group mindfulness‐based intervention for distressing voices: a pragmatic randomised controlled trial. Schizophr Res 2016;175:168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bach P, Hayes SC. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: a randomized controlled trial. J Consult Clin Psychol 2002;70:1129‐39. [DOI] [PubMed] [Google Scholar]
- 77. Shawyer F, Farhall J, Thomas N et al. Acceptance and commitment therapy for psychosis: randomised controlled trial. Br J Psychiatry 2016;210:140‐8. [DOI] [PubMed] [Google Scholar]
- 78. Schnackenberg J, Fleming M, Martin CR. A randomised controlled pilot study of Experience Focused Counselling with voice hearers. Psychosis 2017;9:12‐24. [Google Scholar]
- 79. Jenner JA, Nienhuis FJ, Wiersma D et al. Hallucination focused integrative treatment: a randomized controlled trial. Schizophr Bull 2004;30:133‐45. [DOI] [PubMed] [Google Scholar]
- 80. Craig TK, Rus‐Calafell M, Ward T et al. AVATAR therapy for auditory verbal hallucinations in people with psychosis: a single‐blind, randomised controlled trial. Lancet Psychiatry 2018;5:31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Adams CE, Coutinho E, Davis JM et al. Cochrane Schizophrenia Group. The Cochrane library. Chichester: Wiley, 2011. [Google Scholar]
- 82. Leucht S, Kane JM, Kissling W et al. What does the PANSS mean? Schizophr Res 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
- 83. Hutton P. Should people with psychosis be supported in choosing cognitive therapy as an alternative to antipsychotic medication: a commentary on a commentary. Schizophr Res (in press). [DOI] [PubMed] [Google Scholar]


